Introduction
Primary testicular lymphoma (PTL) is an uncommon and
aggressive form of extranodal non-Hodgkin lymphoma (NHL) (1). PTL is the most common testicular
malignancy in men >60 years of age (2). PTL accounts for <5% of testicular
malignancies and 1–2% of NHL (3).
Overall, 60–79% of patients present with an early stage of disease
(stage I/II), but the outcome is poor (4). In recent years, immunotherapy has
become a promising and effective treatment strategy for several
types of malignancy.
Programmed cell death-ligand 1 (PD-L1), also known
as B7-H1 or CD274, is an inhibitory ligand of programmed cell death
1 (PD-1). PD-L1 is expressed on the surface of tumor cells, T cells
and other immune cells (5,6). The binding of PD-L1 to PD-1 suppresses
the activation and effector function of T cells, thereby inducing
T-cell exhaustion and functioning as a crucial checkpoint in the
regulation of cellular and humoral immune responses (7–9).
Targeting the PD-1/PD-L1 signaling pathway has marked clinical
therapeutic efficacy, not only in solid tumors (10) but also in Hodgkin lymphoma and NHL
(11,12).
PTL, characterized by tumors arising in an
immune-privileged site and under the selective pressure of immune
surveillance, may develop an immune escape phenotype (13). Furthermore, a nascent PTL clone may
benefit from developing in an immune-privileged site behind the
blood-testis barrier (14). Genetic
alterations in 9p24.1, resulting in increased expression of PD-L1,
have been demonstrated in PTL (15).
Diffuse large B cell lymphoma (DLBCL), the predominant
histopathological type of PTL, accounts for 80–98% of PTL cases
(16). Previous studies have
reported the expression of PD-L1 in DLBCL (17,18);
however, studies of PD-L1 expression in primary testicular DLBCL
(PT-DLBCL) are lacking. In the present study, the expression of
PD-L1 in PT-DLBCL was investigated retrospectively. Using a
well-annotated cohort of patients, the immunohistochemical
expression of PD-L1 on tumor cells and in the tumor
microenvironment (TME) was evaluated, and its association with
clinical data was analyzed.
Materials and methods
Patients and samples
In total, 30 patients, aged 33–66 years, were
diagnosed with PT-DLBCL at Peking University First Hospital
(Beijing, China) between August 2006 and July 2017, and were
included in the present study. Patients with clear pathological
diagnosis and complete clinical data were included in the study,
and patients whose pathological diagnosis was not PT-DLBCL and
whose clinical data were incomplete were excluded. All patients
underwent orchiectomy for pathological diagnosis. Formalin-fixed
paraffin-embedded blocks from 30 PT-DLBCL specimens were retrieved
from the Department of Urological Pathology, Peking University
First Hospital. Clinicopathological and follow-up data were
collected and entered into a database. The Ann Arbor staging
classification system was used for staging, and the International
Prognostic Index (IPI) was used for risk stratification (14). The algorithm of Hans et al
(19) was used to determine germinal
center or non-germinal center classification. Immunohistochemistry
was used to determine the expression of B cell leukemia 2 (BCL-2).
BCL-2 expression was assessed by BCL-2 score. The scoring criteria
were: 0 points (no lymphoma cells stained); 1 point (1–10% stained
lymphoma cells); 2 points (11–30%); 3 points (31–70%); and 4 points
(>70%) (20). Pathologists
determined the BCl-2 score as part of the postoperative pathology
to determine PT-DLBCL diagnosis, and the BCL-2 scores were
collected from postoperative pathology reports of the patients.
Overall survival (OS) was calculated from the time of diagnosis to
the time of mortality or the last follow-up. Progression-free
survival (PFS) was calculated from the time of diagnosis to the
time of disease progression, mortality or the last follow-up. The
study was approved by the Ethics Committee of Peking University
First Hospital [(Beijing, China); ethics no., 2018 (197)]. A waiver
of written informed consent was granted from the Ethics Committee
of Peking University First Hospital, since the study was a
retrospective analysis of routine data.
Immunohistochemistry
All tumor specimens were acquired by orchiectomy
prior to chemotherapy and radiotherapy (RT). The expression of
PD-L1 in the PT-DLBCL samples was evaluated according to standard
immunohistochemistry protocols. Briefly, 4 µm-thick sections from
formalin-fixed paraffin-embedded specimens were deparaffinized in
xylene, rehydrated in decreasing concentrations of ethanol (100,
95, 95 and 85%) and washed in distilled water. Heat-induced antigen
retrieval at 120°C for 20 min was performed with Tris-EDTA buffer
(pH 8.0). Following the use of 3% hydrogen peroxidase to block
endogenous peroxidase, sections were incubated with 10% normal
blocking serum in Tris-buffered saline at room temperature for 20
min. The sections were then incubated with anti-human PD-L1 rabbit
monoclonal antibody (1:50; E1L3N; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C for 16 h, followed by incubation with the
secondary antibody (goat anti-rabbit IgG/HRP polymer; PV-6001;
OriGene Technologies, Inc., Beijing, China) at 37°C for 40 min.
Next, the sections were counterstained with hematoxylin at room
temperature for 3 min, dehydrated, covered with a coverslip and
viewed under a light microscope (magnification, ×40).
A total of two independent pathologists assessed the
expression of PD-L1 on tumor cells and the expression of PD-L1 in
the TME, without any prior knowledge of the clinical data of this
cohort.
Positive PD-L1 expression on tumor cells was defined
as ≥5% of lymphoma cells exhibiting distinct membranous and/or
cytoplasmic staining for PD-L1, regardless of the PD-L1-positivity
of nonmalignant stromal cells. Positive PD-L1 expression in the TME
was defined as positive staining of stromal cells representing ≥20%
of the total tissue (18).
Statistical analysis
The experiment was repeated 3 times. The data were
expressed as the mean ± standard deviation or n (%) as appropriate.
Patients were divided into subgroups according to the expression of
PD-L1 on tumor cells or the expression of PD-L1 in the TME
(positive or negative). The association of PD-L1 expression with
clinicopathological characteristics was examined using Fisher's
exact test. Survival curves for OS and PFS were prepared using the
Kaplan-Meier method and analyzed using the log-rank test. SPSS
software (version 14.0; SPSS, Inc., Chicago, IL, USA) was used for
the statistical analysis of all data, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
The demographic and clinicopathological
characteristics are presented in Table
I. The study included 30 patients with a mean age of 62.2±15.0
years (range, 33–66 years). Of these patients, 14 (46.7%) had PTL
in the left testicle, 12 (40.0%) patients had PTL in the right
testicle and 4 (13.3%) patients had PTL in both testicles. Lactate
dehydrogenase (LDH) was assessed in 21 patients, and 6 (28.6%) had
increased levels; β2-microglobulin (β2-MG) was assessed
in 13 patients, and 5 (38.5%) had increased levels; 4 (13.3%) of
the 30 patients had B symptoms, including unexplained fever,
drenching night sweats and weight loss >10% of normal body
weight; 10 (33.3%) had advanced-stage (stage III/IV) disease; and
14 (46.7%) had an IPI ≥3. Inguinal orchiectomy was performed as a
diagnostic procedure and initial treatment, and DLBCL was confirmed
in all 30 patients following histopathological examination. Of 19
patients that were assessed for subtype, nine (47.4%) had germinal
center B cell-like (GCB) subtype disease and 10 (52.6%) had non-GCB
subtype disease. BCL-2 expression was assessed in 15 patients, with
scores of 2–3 in 6 (40.0%) patients.
 | Table I.Demographic and clinicopathological
characteristics of 30 patients with primary testicular diffuse
large B cell lymphoma. |
Table I.
Demographic and clinicopathological
characteristics of 30 patients with primary testicular diffuse
large B cell lymphoma.
| Variable | n (%) |
|---|
| Age, years |
|
|
≤60 | 12 (40.0) |
|
>60 | 18 (60.0) |
| Laterality |
|
|
Left | 14 (46.7) |
|
Right | 12 (40.0) |
|
Bilateral | 4 (13.3) |
| LDHa, U/l |
|
|
≤245 | 15 (71.4) |
|
>245 | 6 (28.6) |
| β2-MGb, mg/l |
|
|
≤2.52 | 8 (61.5) |
|
>2.52 | 5 (38.5) |
| Clinical stage |
|
|
I–II | 20 (66.7) |
|
III–IV | 10 (33.3) |
| IPI |
|
|
<3 | 16 (53.3) |
| ≥3 | 14 (46.7) |
| GCB
subtypec |
|
|
Non-GCB | 10 (52.6) |
|
GCB | 9 (47.4) |
| BCL-2 scored |
|
|
0-1 | 9 (60.0) |
|
2-3 | 6 (40.0) |
Treatments and outcomes
The final follow-up date was July 2018. The median
follow-up time following orchiectomy was 23.5 months (range, 2–143
months). Disease progression occurred in 10 (33.3%) patients; among
these patients, one experienced central nervous system (CNS)
relapse, and one experienced contralateral testis relapse. Overall,
11 (36.7%) patients succumbed. Following orchiectomy, 23 (76.7%)
patients received chemotherapy, including a doxorubicin-containing
regimen in all 23 patients and a rituximab-containing regimen in 15
patients. The median number of cycles was 6 (range, 1–8). A total
of 11 patients received RT; 9 of these received RT at the
contralateral testis alone, and 2 received RT at the contralateral
testis and the abdominal lymph nodes. A total of 18 patients
received CNS prophylaxis. In total, 10 patients received multimodal
therapy (surgery+chemotherapy+radiotherapy+CNS prophylaxis)
(Table II).
 | Table II.Treatments and outcomes of 30
patients with primary testicular diffuse large B cell lymphoma. |
Table II.
Treatments and outcomes of 30
patients with primary testicular diffuse large B cell lymphoma.
| Variable | n (%) |
|---|
| Chemotherapy |
|
| No | 7 (23.3) |
|
Yes | 23 (76.7) |
|
Rituximaba |
|
| No | 8 (34.8) |
|
Yes | 15 (65.2) |
| Radiotherapy |
|
| No | 19 (63.3) |
|
Yes | 11 (36.7) |
| CNS
prophylaxis |
|
| No | 12 (40.0) |
|
Yes | 18 (60.0) |
| Multimodal therapy
(surgery+chemotherapy+radiotherapy+CNS prophylaxis) |
|
| No | 20 (66.7) |
|
Yes | 10 (33.3) |
| Disease
progression |
|
| No | 20 (66.7) |
|
Yes | 10 (33.3) |
| Mortality |
|
| No | 19 (63.3) |
|
Yes | 11 (36.7) |
PD-L1 expression on tumor cells and in
the TME
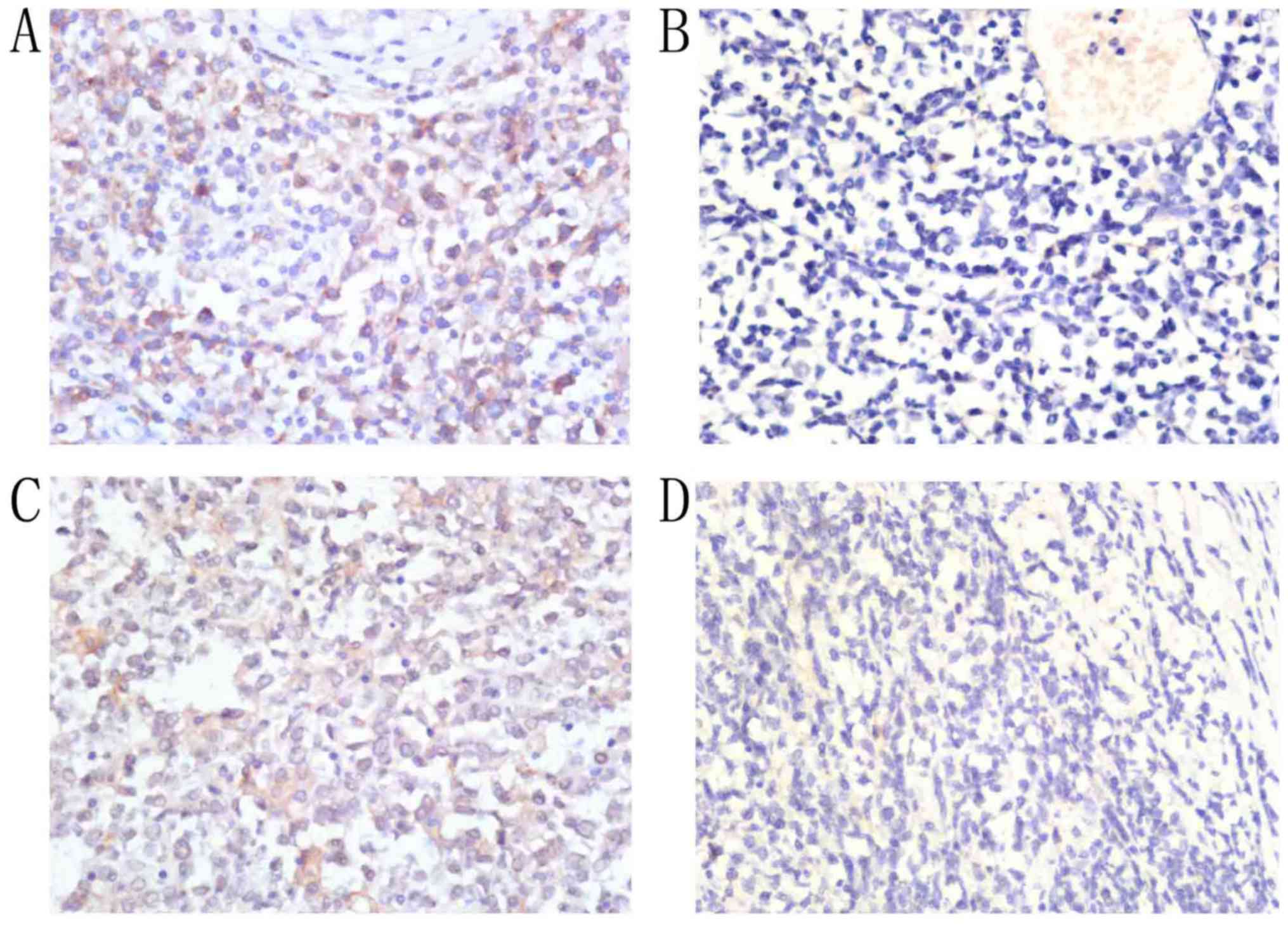
Among the 30 patients with PT-DLBCL, positive PD-L1
expression on tumor cells was detected in 20 (66.7%), and a lack of
PD-L1 expression on tumor cells was detected in 10 (33.3%).
Positive PD-L1 expression in the TME was detected in 13 patients
(43.3%), and a lack of PD-L1 expression in the TME was detected in
10 patients (56.7%; Fig. 1). Among
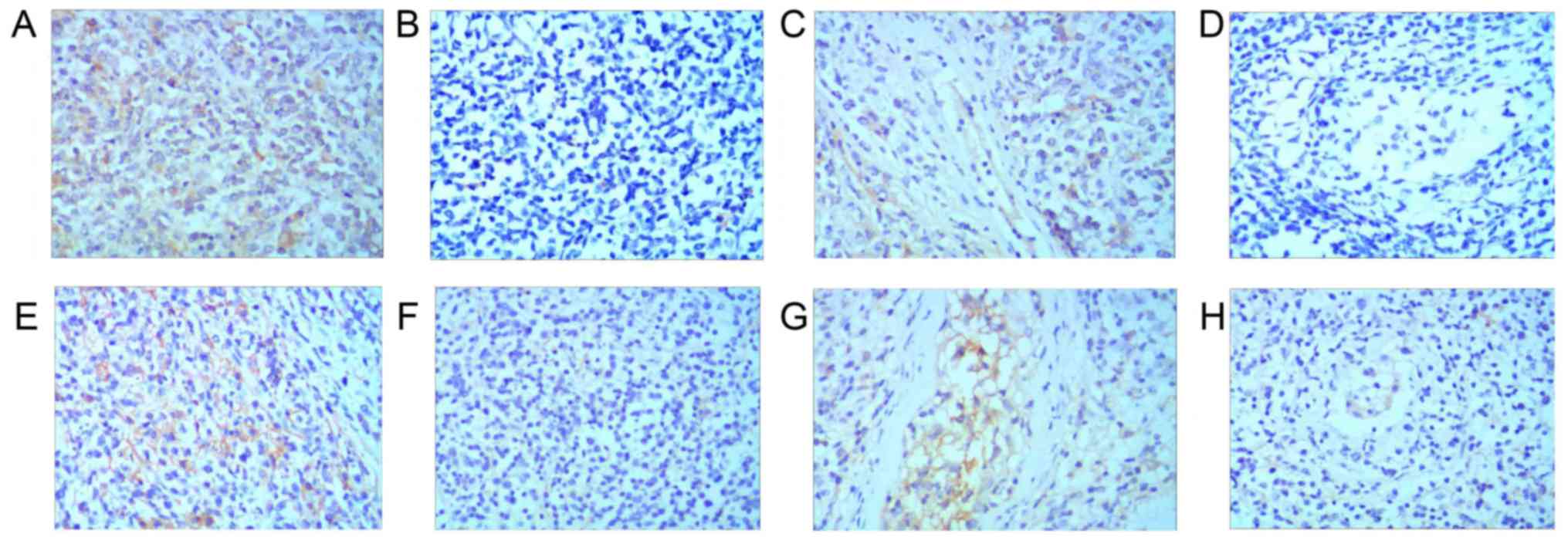
the 19 patients assessed for subtype, positive PD-L1 expression on
tumor cells was detected in 5 (55.6%) with the GCB subtype, and a
lack of PD-L1 expression on tumor cells was detected in 4 (44.4%)
with the GCB subtype. Positive PD-L1 expression in the TME was
detected in 3 patients (33.3%) with the GCB subtype, and a lack of
PD-L1 expression in the TME was detected in 6 patients (66.7%) with
the GCB subtype. Positive PD-L1 expression on tumor cells was
detected in 8 patients (80.0%) with the non-GCB subtype, and a lack
of PD-L1 expression on tumor cells was detected in 2 patients
(20.0%) with the non-GCB subtype. Positive PD-L1 expression in the
TME was detected in 5 patients (50.0%) with the non-GCB subtype,
and a lack of PD-L1 expression in the TME was detected in 5
patients (50.0%) with the non-GCB subtype (Fig. 2).
Association of PD-L1 expression with
clinicopathological characteristics
Overall, early-stage (stage I/II) and advanced-stage
(stage III/IV) disease was identified in 20 (66.7%) and 10 (33.3%)
patients, respectively. PD-L1 expression on tumor cells was
significantly higher in patients at an early stage compared with
those at an advanced stage (16/20, 80.0% vs. 4/10, 40.0%; P=0.045),
and there was a significant difference in PD-L1 expression in the
TME between these two groups (60.0 vs. 10.0%; P=0.017). IPIs <3
and ≥3 were identified in 16 (53.3%) and 14 (46.7%) patients,
respectively. PD-L1 expression on tumor cells was significantly
higher in patients with an IPI <3 compared with patients with an
IPI ≥3 (87.5 vs. 42.9%; P=0.019); however, there was no significant
difference in PD-L1 expression in the TME between these two groups
(50.0 vs. 35.7%; P=0.484). According to the postoperative
pathological results, 9 (47.4%) patients had a GCB subtype, and 10
(52.6%) patients had a non-GCB subtype. No significant differences
were observed between the subtypes in terms of PD-L1 expression on
tumor cells or in the TME (P=0.35 and 0.65, respectively). In
addition, age, laterality, B symptoms, LDH level, β2-MG level and
BCL-2 expression were not significantly associated with PD-L1
expression on either tumor cells or in the TME (Table III).
 | Table III.Association between PD-L1 expression
and clinicopathological characteristics in 30 patients with primary
testicular diffuse large B cell lymphoma. |
Table III.
Association between PD-L1 expression
and clinicopathological characteristics in 30 patients with primary
testicular diffuse large B cell lymphoma.
|
| PD-L1 expression in
tumor cells, n |
| PD-L1 expression in
tumor microenvironment, n |
|
|---|
|
|
|
|
|
|
|---|
| Features | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age, years |
|
| 0.694 |
|
| >0.999 |
|
≤60 | 3 | 9 |
| 7 | 5 |
|
|
>60 | 7 | 11 |
| 10 | 8 |
|
| Laterality |
|
| 0.127 |
|
| 0.733 |
|
Left | 7 | 7 |
| 9 | 5 |
|
|
Right | 3 | 9 |
| 6 | 6 |
|
|
Bilateral | 0 | 4 |
| 2 | 2 |
|
| B symptoms |
|
| 0.584 |
|
| 0.113 |
| No | 8 | 18 |
| 13 | 13 |
|
|
Yes | 2 | 2 |
| 4 | 0 |
|
| LDHa, U/l |
|
| 0.262 |
|
| >0.999 |
|
≤245 | 5 | 10 |
| 7 | 8 |
|
|
>245 | 0 | 6 |
| 3 | 3 |
|
| β2-MGb, mg/l |
|
| >0.999 |
|
| >0.999 |
|
≤2.52 | 2 | 6 |
| 4 | 4 |
|
|
>2.52 | 2 | 3 |
| 3 | 2 |
|
| Clinical stage |
|
| 0.045 |
|
| 0.017 |
|
I–II | 4 | 16 |
| 8 | 12 |
|
|
III–IV | 6 | 4 |
| 9 | 1 |
|
| IPI |
|
| 0.019 |
|
| 0.484 |
|
<3 | 2 | 14 |
| 8 | 8 |
|
| ≥3 | 8 | 6 |
| 9 | 5 |
|
| GCB
subtypec |
|
| 0.35 |
|
| 0.650 |
|
Non-GCB | 2 | 8 |
| 5 | 5 |
|
|
GCB | 4 | 5 |
| 6 | 3 |
|
| BCL-2
scored |
|
| >0.999 |
|
| 0.315 |
|
0-1 | 3 | 6 |
| 3 | 6 |
|
|
2-3 | 2 | 4 |
| 4 | 2 |
|
Association of PD-L1 expression with
PFS and OS
The median follow-up time after orchiectomy was 23.5
months (range, 2–143 months). During this time, 10 (33.3%) patients
experienced disease progression, and 11 (36.7%) patients succumbed.
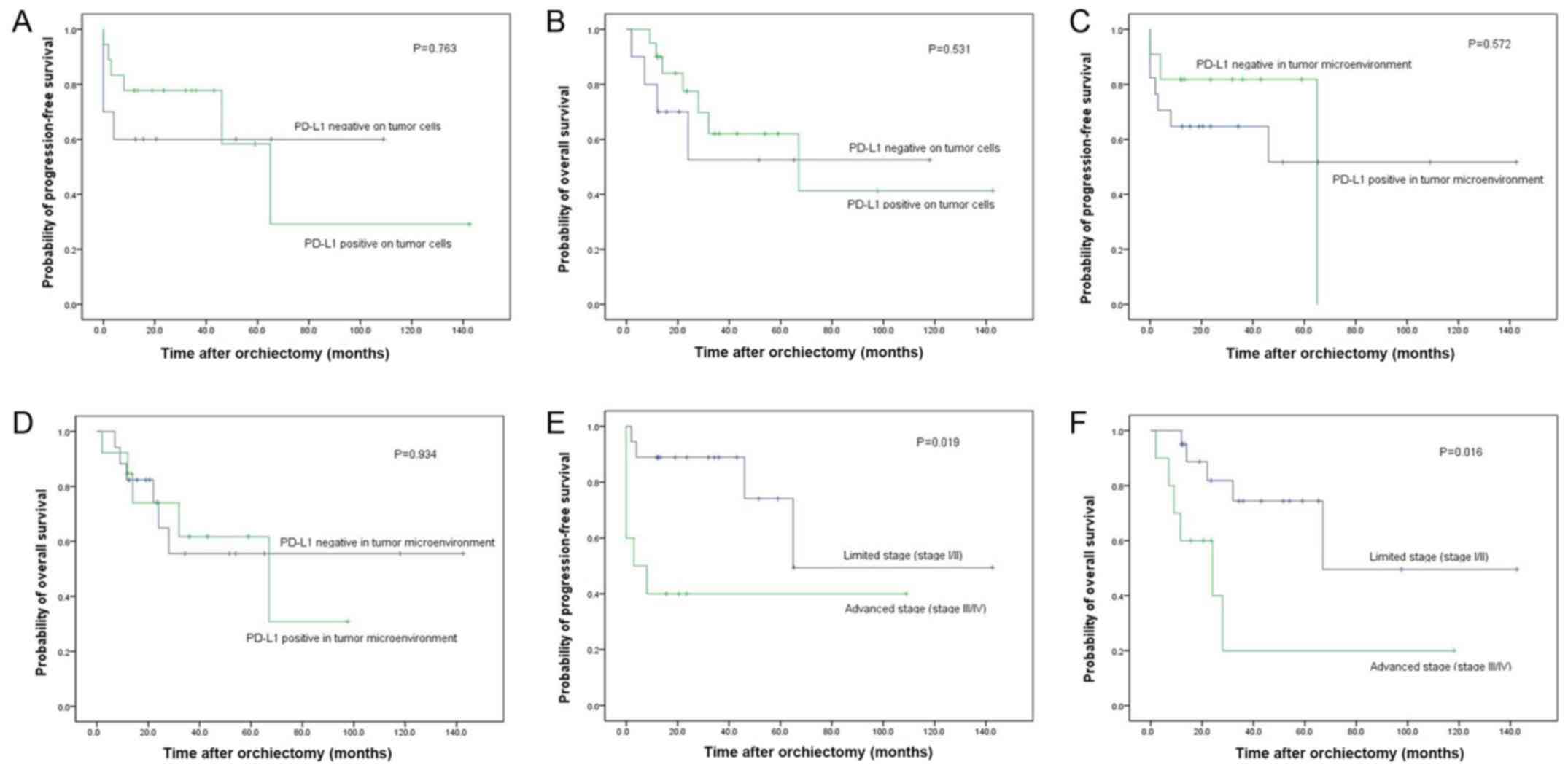
A Kaplan-Meier analysis indicated that PD-L1 expression on tumor
cells was not associated with PFS (P=0.763) or OS (P=0.531;
Fig. 3A and B) and that PD-L1
expression in the TME was not associated with PFS (P=0.572) or OS
(P=0.934; Fig. 3C and D). Following
division of the patients into subgroups (GCB subtype and non-GCB
subtype), a Kaplan-Meier analysis revealed that PD-L1 expression
was not associated with PFS or OS in the GCB subtype subgroup or in
the non-GCB subtype subgroup (Fig.
S1). Following division of the patients into subgroups
according to BCL-2 expression, a Kaplan-Meier analysis also
revealed that PD-L1 expression was not associated with PFS or OS in
the subgroups with BCL-2 expression scores of 0–1 and BCL-2
expression scores of 2–3 (Fig. S2).
However, the Kaplan-Meier analysis demonstrated that an early stage
of disease was associated with longer PFS (P=0.019) and OS
(P=0.016; Fig. 3E and F). In the
multivariate Cox model adjusting for clinical stage, PD-L1
expression on tumor cells or in the TME was not an independent risk
factor for PFS or OS.
Discussion
Previous studies have demonstrated that PD-L1 is
expressed in various types of cancer, including DLBCL, and
correlates with both favorable and unfavorable prognoses (21,22). In
a previous study on DLBCL, PD-L1 expression on tumor cells or in
the TME was reported to correlate with pathological tumor stage,
grade and prognosis (17). DLBCL is
the predominant histopathological type of PTL, and PD-L1
overexpression in DLBCL has been reported previously (23). Therefore, whether the clinical
significance of PD-L1 expression in DLBCL can be equally applied in
PT-DLBCL remains unknown and warrants further study. To the best of
our knowledge, the present study is the first to investigate the
association of PD-L1 expression with clinicopathological
characteristics and oncological outcomes in patients with PT-DLBCL.
PD-L1 can be expressed on both tumor cells and tumor-infiltrating
immune cells (24). PD-L1 expression
is induced endogenously by genetic aberrations or oncogenic
signaling, and exogenously by cytokines secreted by immune cells
(25). However, the underlying
molecular mechanism of PD-L1 expression in PT-DLBCL is unclear and
requires further investigation.
Of particular note is the threshold for identifying
PD-L1 positivity, which has varied widely. In the present study, 5%
was used as the cut-off for PD-L1 positivity on tumor cells, and
20% as the cut-off for PD-L1 positivity in the TME, in accordance
with a previous study (18). Among
the 30 patients with PT-DLBCL included in the present study, 67%
patients were positive for PD-L1 on tumor cells and 43% were
positive for PD-L1 in the TME. To put these results into context,
they were compared with those of published studies concerning PD-L1
expression in DLBCL. According to the reviewed literature, the
expression rates of PD-L1 on tumor cells and in the TME fluctuated
from 26 to 75% and from 30 to 66%, respectively (26,27). The
results from the present study were comparable with those observed
in previous studies. Several studies have investigated the
expression of PD-L1 in DLBCL using other standards. Kiyasu et
al (17) reported that the rates
of positive PD-L1 expression on tumor cells and in the TME were
10.5 and 15.3% using thresholds of 30.0 and 20.0%, respectively.
Xing et al (28) reported
that the rates of positive PD-L1 expression on tumor cells and in
the TME were 16.0 and 27.0% using thresholds of 30.0 and 5.0%,
respectively. The higher rates of PD-L1 expression in our cohorts
may be due to the nature of testicles as immunologically privileged
sites, or to differences in race, sample size, antibody type,
immunohistochemical detection system and experimental
standards.
The results of the present study indicated that
patients with an early stage of disease (stage I/II) presented with
higher PD-L1 expression on tumor cells compared with those at an
advanced stage (stage III/IV). The results also indicated that
patients at an early (stage I/II) presented with higher PD-L1
expression in the TME than those at an advanced stage (stage
III/IV). Furthermore, it was observed that patients with a low IPI
presented with higher PD-L1 expression on tumor cells compared with
those with a high IPI. However, no significant differences were
observed in PD-L1 expression on tumor cells or in the TME between
subtypes. Pollari et al (27)
reported that patients with PTL with limited stage I–II disease
presented with higher PD-L1 expression in the TME compared with
those with advanced stage III–IV disease. Pollari et al
(27) also described how PD-L1 is
also expressed on tumor-infiltrating non-malignant cells, primarily
macrophages, and PD-1 is expressed on tumor-infiltrating
lymphocytes (TILs). The interaction of PD-L1+
macrophages and PD-1+ TILs may modify the TME and
promote an antitumor immune response. Ishii et al (21) reported that the high expression of
PD-L1 on tumor cells was correlated with an early disease stage in
patients with small cell lung cancer. However, the majority of
studies concerning PD-L1 expression have revealed that PD-L1
expression on tumor cells is associated with an unfavorable
prognosis (17,18,22). The
mechanism is as follows: The PD-L1 expression on tumor cells can
lead to T-cell exhaustion and a state of non-responsiveness, and
can enable tumor cells to escape the immune response (29,30). In
PT-DLBCL, there are PD-L1-positive tumor cells, PD-L1-positive
macrophages and PD-1-positive TILs, indicating that the PD-1/PD-L1
signaling pathway is much more complex (27). Nevertheless, the mechanism underlying
the reciprocal effects remains unclear and requires further
investigation.
To date, studies on the prognostic impact of PD-L1
expression in DLBCL have concentrated on forms of the disease other
than PTL. Kiyasu et al (17)
were the first to report that PD-L1 expression on tumor cells is
associated with a shorter OS in patients with DLBCL. Hu et
al (18) reported that PD-L1
expression predicts poor survival in patients with DLBCL in China.
However, the present study did not identify that PD-L1 expression
in tumor cells or in the TME was associated with PFS or OS in
patients with PT-DLBCL.
There are several limitations to the present study.
First, this was a single-center retrospective study with a small
sample size of 30 patients; therefore, prospective studies with
more patients are warranted to validate the status and prognostic
value of PD-L1 expression in patients with PT-DLBCL. Secondly,
immunohistochemistry is a semiquantitative technique and is
influenced by multiple factors, such as antibody concentrations and
cut-off criteria. However, positive and negative control slides
were used in the present study to ensure the reliability of the
protocol used. Thirdly, because the ideal treatment for PTL-DLBCL
remains under debate, the patients in the present study received a
variety of treatments, which made it difficult to identify relevant
prognostic factors.
In conclusion, PD-L1 is differentially expressed in
tumor cells and in the TME in PT-DLBCL. No significant association
was identified with age, laterality, B symptoms, LDH, β2-MG, GCB
subtype or BCL-2 expression. However, PD-L1 expression in tumor
cells and in the TME was higher in patients at an early stage of
disease compared with in those at an advanced stage, and PD-L1
expression on tumor cells was higher in patients with a low IPI
compared with those with a high IPI. Furthermore, PD-L1 expression
on tumor cells and in the TME was not associated with PFS or
OS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CS, WY and JJ designed the study and revised the
manuscript. DDZ, JZ and PH contributed to the writing of the
manuscript and analyzing the patient data. YF contributed to the
collection and analysis of the data. JL and QH contributed to the
assessment of PD-L1 expression. WKH and ZYZ designed the study and
revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Peking University First Hospital (ethics no., 2018 [197]). A waiver
of written informed consent was granted from the Ethics Committee
of Peking University First Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BCL-2
|
B cell leukemia 2
|
|
β2-MG
|
β2-microglobulin
|
|
CNS
|
central nervous system
|
|
DLBCL
|
diffuse large B cell lymphoma
|
|
GCB
|
germinal center B cell-like
|
|
IPI
|
International Prognostic Index
|
|
LDH
|
lactate dehydrogenase
|
|
NHL
|
non-Hodgkin lymphoma
|
|
non-GCB
|
non-germinal center B cell-like
|
|
OS
|
overall survival
|
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
programmed cell death-ligand 1
|
|
PFS
|
progression-free survival
|
|
PT-DLBCL
|
primary testicular DLBCL
|
|
PTL
|
primary testicular lymphoma
|
|
RT
|
radiotherapy
|
|
TME
|
tumor microenvironment
|
References
|
1
|
Kemal Y, Teker F, Demirag G and Yucel I:
Primary testicular lymphoma: A single centre experience. Exp Oncol.
37:223–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vitolo U, Ferreri AJ and Zucca E: Primary
testicular lymphoma. Crit Rev Oncol Hematol. 65:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Møller MB, d'Amore F and Christensen BE:
Testicular lymphoma: a population-based study of incidence,
clinicopathological correlations and prognosis The Danish Lymphoma
Study Group, LYFO. Eur J Cancer 30A. 1760–1764. 1994. View Article : Google Scholar
|
|
4
|
Zucca E, Conconi A, Mughal TI, Sarris AH,
Seymour JF, Vitolo U, Klasa R, Ozsahin M, Mead GM, Gianni MA, et
al: Patterns of outcome and prognostic factors in primary
large-cell lymphoma of the testis in a survey by the International
Extranodal Lymphoma Study Group. J Clin Oncol. 21:20–27. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong Y, Sun Q and Zhang X: PD-1 and its
ligands are important immune checkpoints in cancer. Oncotarget.
8:2171–2186. 2017.PubMed/NCBI
|
|
6
|
Goodman A, Patel SP and Kurzrock R:
PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev
Clin Oncol. 14:203–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen BJ, Chapuy B, Ouyang J, Sun HH,
Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA and Rodig
SJ: PD-L1 expression is characteristic of a subset of aggressive
B-cell lymphomas and virus-associated malignancies. Clin Cancer
Res. 19:3462–3473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachy E and Coiffier B: Anti-PD1 antibody:
A new approach to treatment of lymphomas. Lancet Oncol. 15:7–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheah CY, Wirth A and Seymour JF: Primary
testicular lymphoma. Blood. 123:486–493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merryman RW, Armand P, Wright KT and Rodig
SJ: Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood
Adv. 1:2643–2654. 2017.PubMed/NCBI
|
|
16
|
Menter T, Ernst M, Drachneris J, Dirnhofer
S, Barghorn A, Went P and Tzankov A: Phenotype profiling of primary
testicular diffuse large B-cell lymphomas. Hematol Oncol. 32:72–81.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kiyasu J, Miyoshi H, Hirata A, Arakawa F,
Ichikawa A, Niino D, Sugita Y, Yufu Y, Choi I, Abe Y, et al:
Expression of programmed cell death ligand 1 is associated with
poor overall survival in patients with diffuse large B-cell
lymphoma. Blood. 126:2193–2201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu LY, Xu XL, Rao HL, Chen J, Lai RC,
Huang HQ, Jiang WQ, Lin TY, Xia ZJ and Cai QQ: Expression and
clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse
large B cell lymphoma: A retrospective study. Chin J Cancer.
36:942017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gascoyne RD, Adomat SA, Krajewski S,
Krajewska M, Horsman DE, Tolcher AW, O'Reilly SE, Hoskins P,
Coldman AJ, Reed JC and Connors JM: Prognostic significance of
Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse
aggressive non-Hodgkin's lymphoma. Blood. 90:244–251.
1997.PubMed/NCBI
|
|
21
|
Ishii H, Azuma K, Kawahara A, Yamada K,
Imamura Y, Tokito T, Kinoshita T, Kage M and Hoshino T:
Significance of programmed cell death-ligand 1 expression and its
association with survival in patients with small cell lung cancer.
J Thorac Oncol. 10:426–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Yu W, Feng X, Zhao Z, Fan Y, Meng
Y, Hu S, Cui Y, He Q, Zhang H, et al: Prognostic significance of
PD-L1 expression on tumor cells and tumor-infiltrating mononuclear
cells in upper tract urothelial carcinoma. Med Oncol. 34:942017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgiou K, Chen L, Berglund M, Ren W, de
Miranda NF, Lisboa S, Fangazio M, Zhu S, Hou Y, Wu K, et al:
Genetic basis of PD-L1 overexpression in diffuse large B-cell
lymphomas. Blood. 127:3026–3034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andorsky DJ, Yamada RE, Said J, Pinkus GS,
Betting DJ and Timmerman JM: Programmed death ligand 1 is expressed
by non-hodgkin lymphomas and inhibits the activity of
tumor-associated T cells. Clin Cancer Res. 17:4232–4244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik
JH, Kim YA, Kim TM, Heo DS, Kim CW and Jeon YK: Clinicopathological
analysis of programmed cell death-1 and programmed cell
death-ligand 1 expression in the tumor microenvironments of diffuse
large B-cell lymphomas. Histopathology. 68:1079–1089. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu-Monette ZY, Zhou J and Young KH: PD-1
expression and clinical PD-1 blockade in B-cell lymphomas. Blood.
131:68–83. 2018.PubMed/NCBI
|
|
27
|
Pollari M, Brück O, Pellinen T, Vähämurto
P, Karjalainen-Lindsberg ML, Mannisto S, Kallioniemi O,
Kellokumpu-Lehtinen PL, Mustjoki S, Leivonen SK and Leppä S: PD-L1+
tumor-associated macrophages and PD-1+ tumor infiltrating
lymphocytes predict survival in primary testicular lymphoma.
Haematologica. 103:1908–1914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xing W, Dresser K, Zhang R, Evens AM, Yu
H, Woda BA and Chen BJ: PD-L1 expression in EBV-negative diffuse
large B-cell lymphoma: Clinicopathologic features and prognostic
implications. Oncotarget. 7:59976–59986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|