Introduction
Thyroid carcinoma, the most common endocrine
carcinoma (1), is seeing an
increasingly high incidence worldwide in recent years (2), with a 10th-ranking incidence
among tumors in China (3). Recently
more scholars have a rising interest in the pathogenesis of thyroid
carcinoma. Phosphoinositide-dependent protein kinase 1 (PDK-1), a
monomeric polypeptide enzyme consisting of more than five hundred
amino acids including the N-terminal kinase domain and the
C-terminal PH domain, which is normally expressed in a variety of
peripheral tissues including the heart, stomach, spleen, kidney,
brain and other tissues, has a ligand that plays an important role
in the regulation of the immune system, tumor metastasis, embryonic
development and angiogenesis (4).
Studies showed that the expression level of deleted in malignant
brain tumors (DMBT1) was greatly decreased in breast carcinoma
(5), human oral squamous cell
carcinoma (6) compared with that in
the normal tissues, which suggested the potential ability of DMBT1
gene as a tumor suppressor gene to get involved in the regulation
of the occurrence and development of the carcinogenesis of tumor
cells. In recent years, few studies of the expression levels of
DMBT and PDK-1 gene in the thyroid tissue have been reported. In
the following parts, this study investigated the expression levels
of PDK-1 and DMBT1 genes in the thyroid tissue and the correlation
between the two, and further explored the relationship between the
expression levels of PDK-1 and DMBT1 and the molecular biological
mechanisms of the occurrence, development, metastasis,
proliferation and malignant transformation of the thyroid
carcinoma.
Patients and methods
General information
Eighty-seven patients (34 males and 53 females) with
thyroid carcinoma diagnosed in The Second People's Hospital of
Lianyungang (Lianyungang, China) from June 2016 to March 2018 were
selected as the research subjects. Inclusion criteria: i) adults
with no history of mental illness who were capable of normal
communication, self-care, and clear consciousness and ii) patients
(or their immediate families) that signed the informed consent.
Exclusion criteria: i) patients with permanent intracardiac
conduction disorder, cardiogenic shock and a history of severe
mental illness and ii) patients with acute inflammation,
hematological disease, and severe heart and liver dysfunction.
Patients in this study received no chemotherapy, radiotherapy or
immunotherapy ever. Immediately after the surgery, the resected
thyroid carcinoma tissues and normal tissues over 2 cm away from
the carcinoma with no observed cancer cell infiltration were stored
in the refrigerator at −80°C (Table
I).
 | Table I.Basic information of patients. |
Table I.
Basic information of patients.
| Factors | n=87 |
|---|
| Age (year) |
|
|
<35 | 31 (35.63) |
| ≥35 | 56 (64.37) |
| Sex |
|
| Male | 52 (59.77) |
|
Female | 35 (40.23) |
| Obesity |
|
| Yes | 47 (54.02) |
| No | 40 (45.98) |
| Clinical staging |
|
| Stage
I–II | 31 (35.63) |
| Stage
III–IV | 56 (64.37) |
| Pathological
type |
|
|
Undifferentiated | 51 (58.62) |
|
Differentiated | 36 (41.38) |
| Size of tumor |
|
|
Microcarcinoma | 52 (59.77) |
| Non-small
cell carcinoma | 35 (40.23) |
The study was approved by the Ethics Committee of
The Second People's Hospital of Lianyungang. Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Main reagents and instruments
DMBT1 ELISA test kit for human (article no.
IC-DMBT1-Hu; Shanghai Yu Bo Biotechnology Co., Ltd., Shanghai,
China), PDK-1 ELISA test kit for human (article no. EH15196; Wuhan
Fine Biotech Co., Ltd., Wuhan, China).
Experimental methods
This experiment was carried out in a sterile
environment, with all the instruments autoclaved and dried before
the use. The normal thyroid tissue and the thyroid carcinoma tissue
were taken out from the −80°C refrigerator and thawed. Then, 0.1 g
of normal thyroid tissue and 0.1 g of thyroid carcinoma were
weighted. With a small amount of liquid nitrogen, the tissues were
separately crushed into powder in the mortar quickly. Next, the
powder was transferred into the 2 ml-sized Ep tube, afterward got
mixed together with 1.2 ml of PBS (pH 7.3) at the speed of 1,500 ×
g and got centrifugalized at 4°C for 20 min to get the supernatant
liquid. Then the homogenate of normal thyroid tissue and thyroid
carcinoma tissue was detected in strict accordance with the
instructions of the DMBT1 ELISA kit for human and the PDK-1 ELISA
kit for human.
Statistical analysis
The SPSS 20.0 software (IBM Corp., Armonk, NY, USA)
was used to perform the statistical analysis. The basic count data
of patients were expressed as the percentage [n (%)]; the
expression levels of DMBT1 and PDK-1 were expressed as the mean ±
standard deviation (SD). Pearson's correlation was used for the
correlation between the expression of PDK-1 and DMBT1. The t-test
was used for statistical analysis and P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of DMBT1 and PDK-1
in thyroid carcinoma tissues and normal tissues adjacent to
them
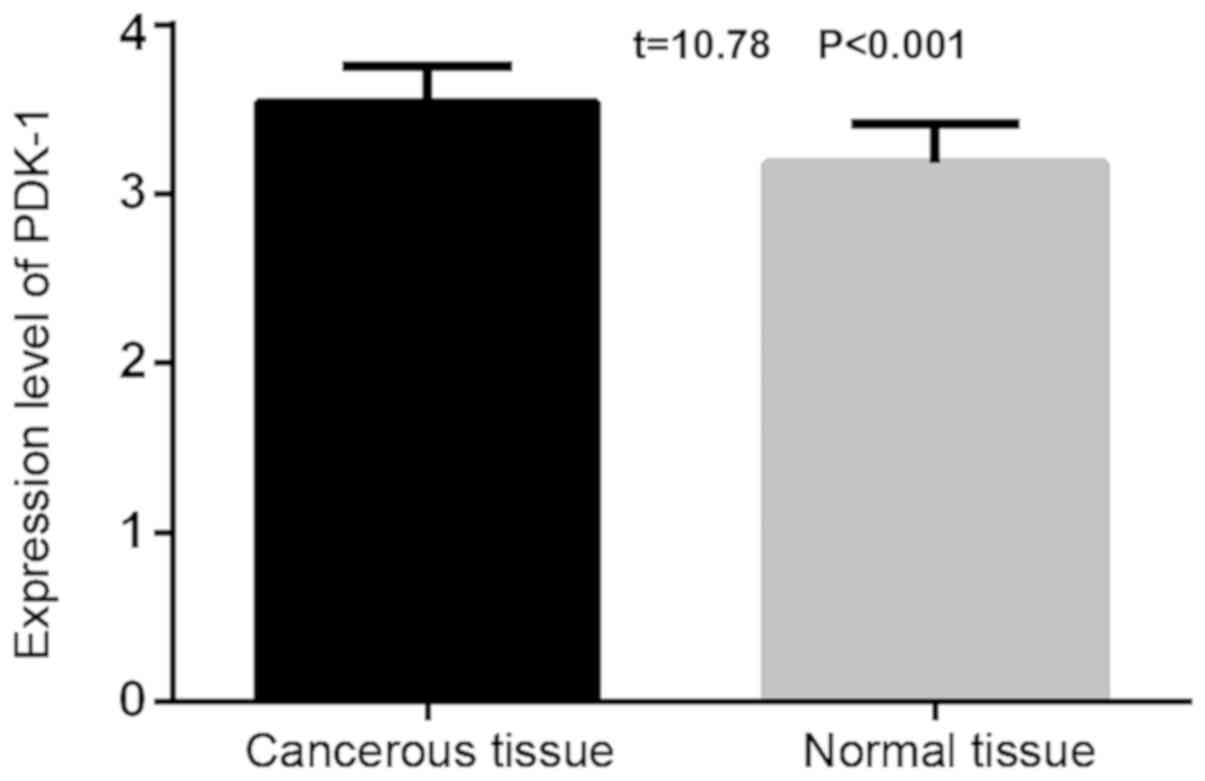
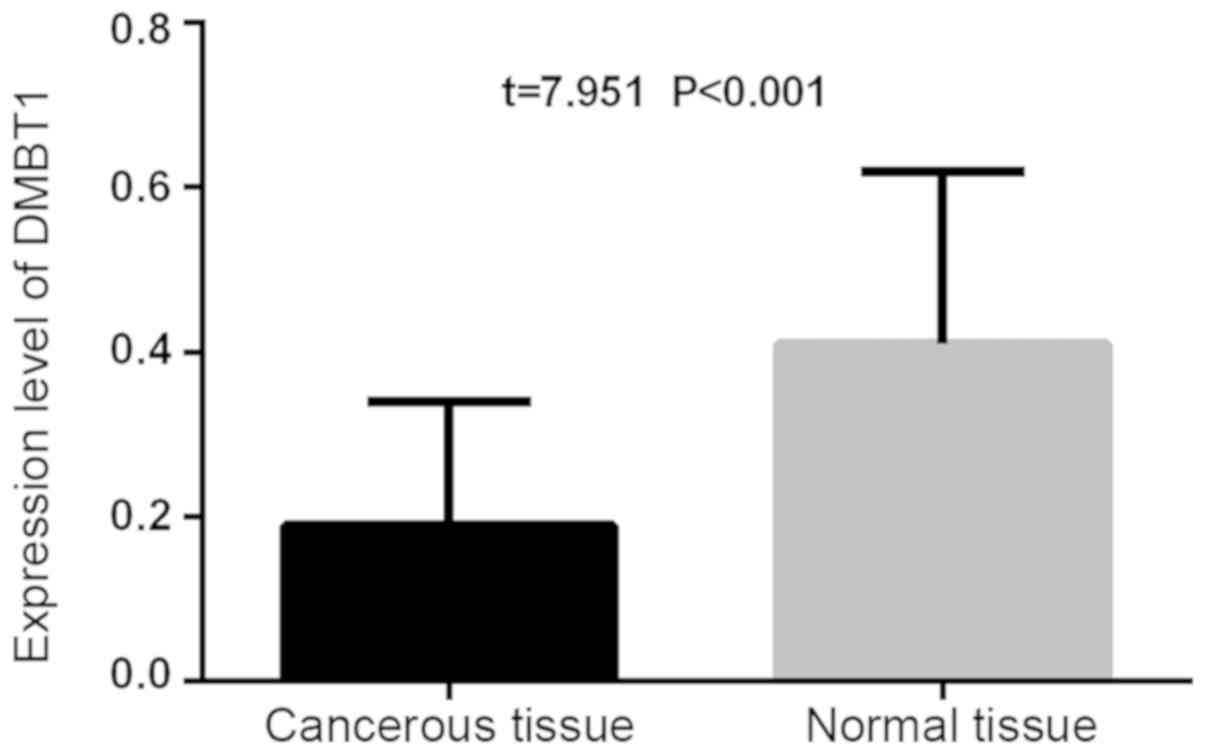
The expression level of PDK-1 in the thyroid
carcinoma tissue (3.54±0.21) was statistically higher than that of
the adjacent normal thyroid tissues (3.18±0.23; t=10.78,
P<0.001). The expression level of PDK-1 in the thyroid carcinoma
tissue (0.19±0.15) was lower than that of the adjacent normal
thyroid tissues (0.41±0.21), and the difference was statistically
significant (t=7.951, P<0.001; Figs.
1 and 2).
Relationship between the expression
levels of DMBT1 and PDK-1 and the clinicopathological features of
patients with thyroid carcinoma
Relationship between the expression
level of PDK-1 and the clinicopathological features of patients
with thyroid carcinoma
The expression level of PDK-1 in the thyroid
carcinoma tissues was significantly lower than that in the adjacent
normal thyroid tissues, and the difference was statistically
significant (P<0.001). The expression of PDK-1 in thyroid
carcinoma was proved to be greatly correlated with factors like
pathological type, clinical stage, tumor size and lymph node
metastasis (P<0.001), in no significant correlation with factors
such as sex and age (P>0.05). In the thyroid carcinoma tissues,
the expression of PDK-1 in the lymph node metastasis (3.94±1.56)
was statistically greatly higher than that in the places without
lymph node metastasis (3.14±1.41; P<0.001). The PDK-1 expression
in the undifferentiated tissues (4.56±1.71) was much higher than
that in the differentiated thyroid carcinoma tissues (2.52±1.43),
with a statistical difference (P<0.001). The expression level of
PDK-1 in the thyroid carcinoma tissues at stage I to stage II
(3.21±1.47) was statistically significantly lower than that in the
thyroid carcinoma tissues at stage III to stage IV (3.87±1.68;
P<0.05). The expression level of PDK-1 in the microcarcinoma
tissues (2.30±1.32) was greatly lower than that in the non-small
carcinoma thyroid tissues (4.88±1.64), and the difference was
statistically significant (P<0.001; Table II).
 | Table II.Relationship between the expression
level of PDK-1 and clinicopathological features of patients with
thyroid carcinoma. |
Table II.
Relationship between the expression
level of PDK-1 and clinicopathological features of patients with
thyroid carcinoma.
| Factors | PDK-1 | t | P-value |
|---|
| Age (year) |
|
<35 | 3.41±1.52 | 1.166 | 0.245 |
| ≥35 | 3.67±1.42 |
|
|
| Gender |
| Male | 3.41±1.79 | 0.988 | 0.325 |
|
Female | 3.67±1.68 |
|
|
| Clinical staging |
| Stages
I–II | 3.21±1.47 | 2.758 | 0.007 |
| Stages
III–IV | 3.87±1.68 |
|
|
| Lymph node
metastasis |
| Yes | 3.94±1.56 | 3.549 | <0.001 |
| No | 3.14±1.41 |
|
|
| Pathological
type |
|
Undifferentiated | 4.56±1.71 | 8.536 | <0.001 |
|
Differentiated | 2.52±1.43 |
|
|
| Size of tumors |
|
Microcarcinoma | 2.30±1.32 | 11.43 | <0.001 |
| Non-small
carcinoma | 4.88±1.64 |
|
|
Relationship between the expression
level of DMBT1 and the clinicopathological features of patients
with thyroid carcinoma
The expression level of DMBT1 in the thyroid
carcinoma tissues was significantly lower than that that in the
adjacent normal thyroid tissues, and the difference was
statistically significant (P<0.001). The expression of DMBT1 in
thyroid carcinoma was proved to be greatly correlated with factors
like pathological type, clinical stage, tumor size and lymph node
metastasis (P<0.001), in no significant correlation with factors
such as sex and age (P>0.05). In the thyroid carcinoma tissues,
the expression of DMBT1 in the lymph node metastasis (0.17±0.14)
was statistically lower than that in the places without lymph node
metastasis (0.21±0.11; P<0.001). The DMBT1 expression in the
differentiated tissues (0.23±0.14) was much higher than that in the
undifferentiated thyroid carcinoma tissues (0.15±0.08), with a
statistical difference (P<0.001). The expression level of DMBT1
in the thyroid carcinoma tissues at stage I to stage II (0.22±0.14)
was statistically significantly higher than that in the thyroid
carcinoma tissues at stage III to stage IV (0.16±0.09; P<0.05).
The expression level of DMBT1 in the microcarcinoma tissues
(0.27±0.13) was greatly higher than that in the non-small carcinoma
thyroid tissues (0.11±0.09), and the difference was statistically
significant (P<0.001; Table
III).
 | Table III.Relationship between the expression
level of DMBT1 and the clinicopathological features of patients
with thyroid carcinoma. |
Table III.
Relationship between the expression
level of DMBT1 and the clinicopathological features of patients
with thyroid carcinoma.
| Factors | DMBT1 | t | P-value |
|---|
| Age (year) |
|
<35 | 0.20±0.12 | 1.244 | 0.215 |
| ≥35 | 0.18±0.09 |
|
|
| Gender |
| Male | 0.20±0.12 | 1.146 | 0.253 |
|
Female | 0.18±0.11 |
|
|
| Clinical staging |
| Stages
I–II | 0.22±0.14 | 3.363 | 0.001 |
| Stages
III–IV | 0.16±0.09 |
|
|
| Lymph node
metastasis |
| No | 0.21±0.11 | 2.096 | 0.037 |
| Yes | 0.17±0.14 |
|
|
| Pathological
type |
|
Differentiated | 0.23±0.14 | 4.628 | <0.001 |
|
Undifferentiated | 0.15±0.08 |
|
|
| Size of tumors |
|
Microcarcinoma | 0.27±0.13 | 9.439 | <0.001 |
|
Non-small carcinoma | 0.11±0.09 |
|
|
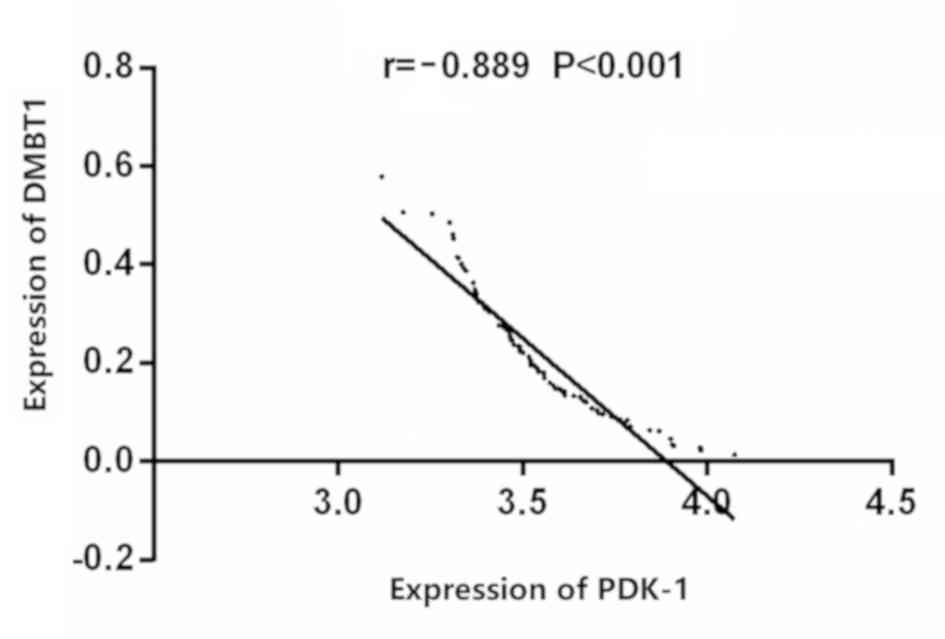
Correlation between the expression of
DMBT1 and the expression of PDK-1 in the thyroid carcinoma
Correlation analysis showed that the expressions of
DMBT1 and PDK-1 were negatively correlated in the thyroid carcinoma
(r=−0.889, P<0.001) (Fig. 3).
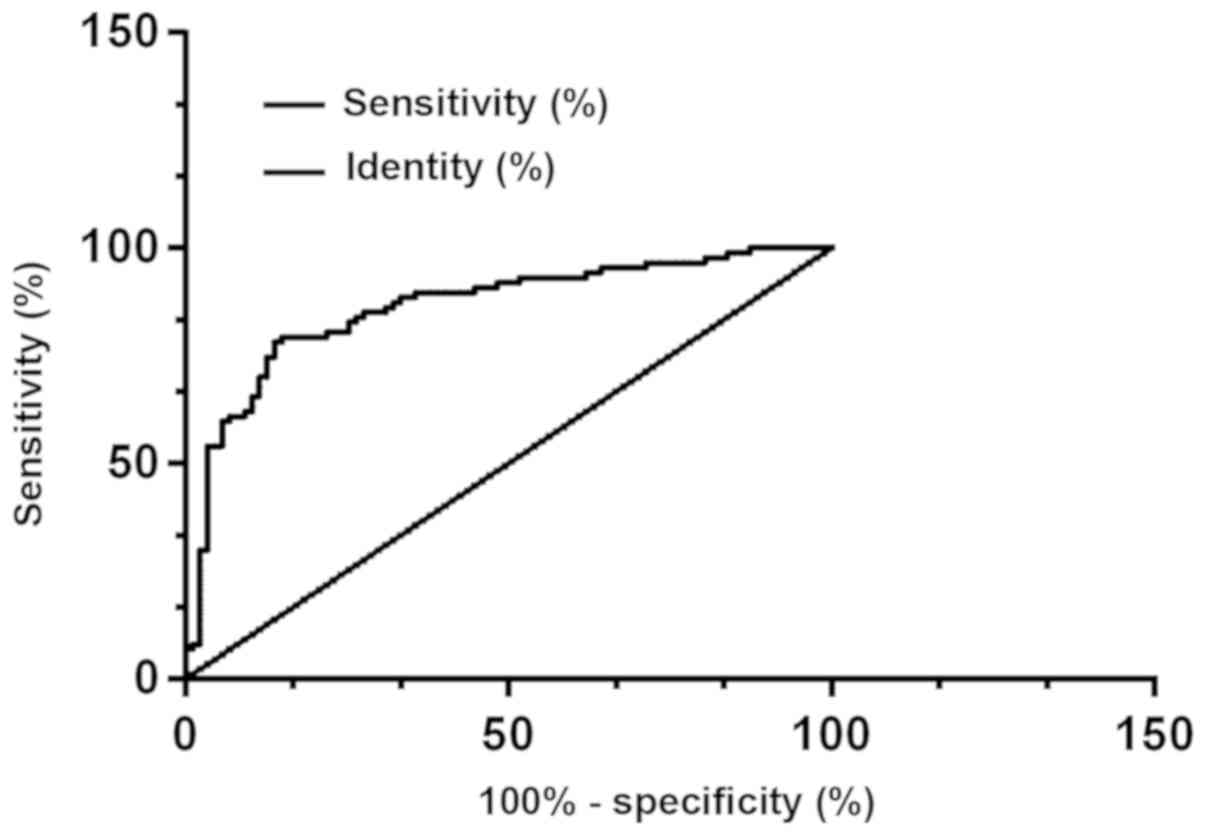
Clinical diagnostic value of DMBT1 and
PDK-1
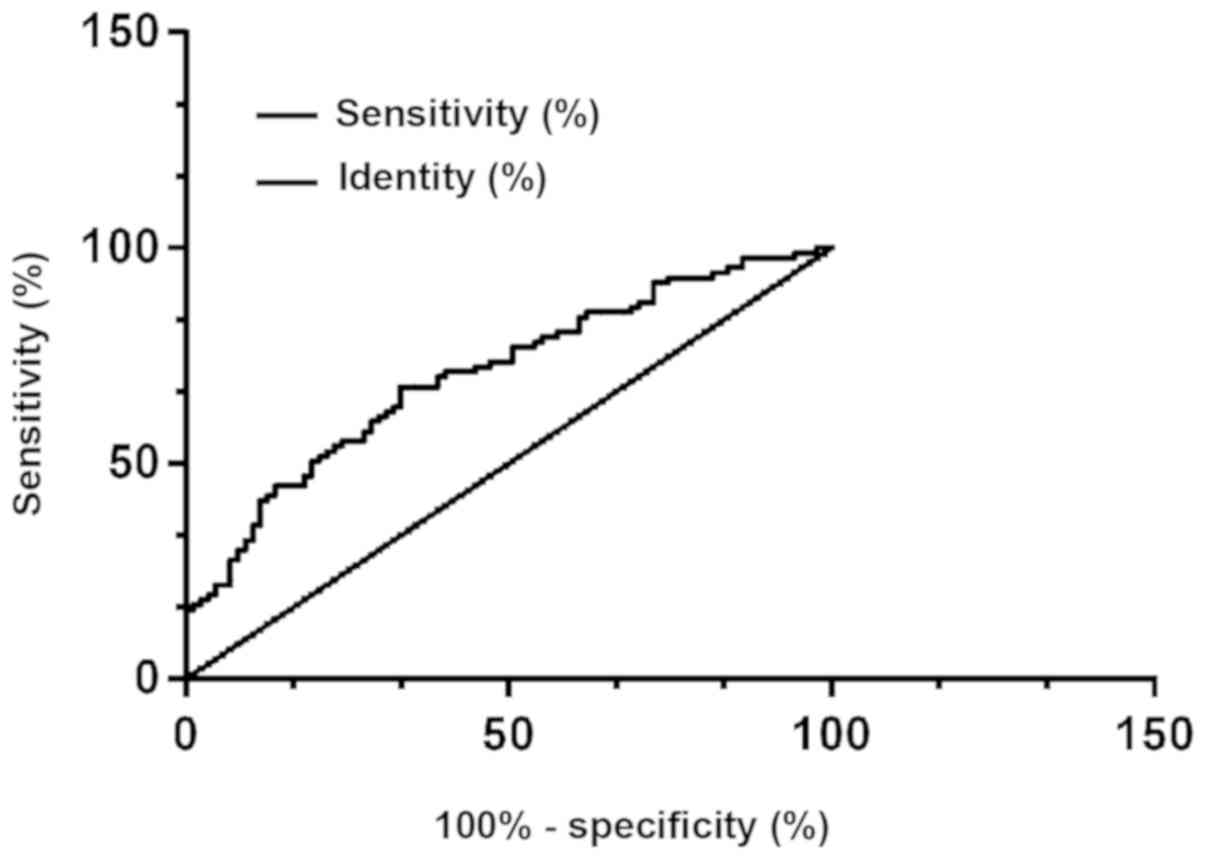
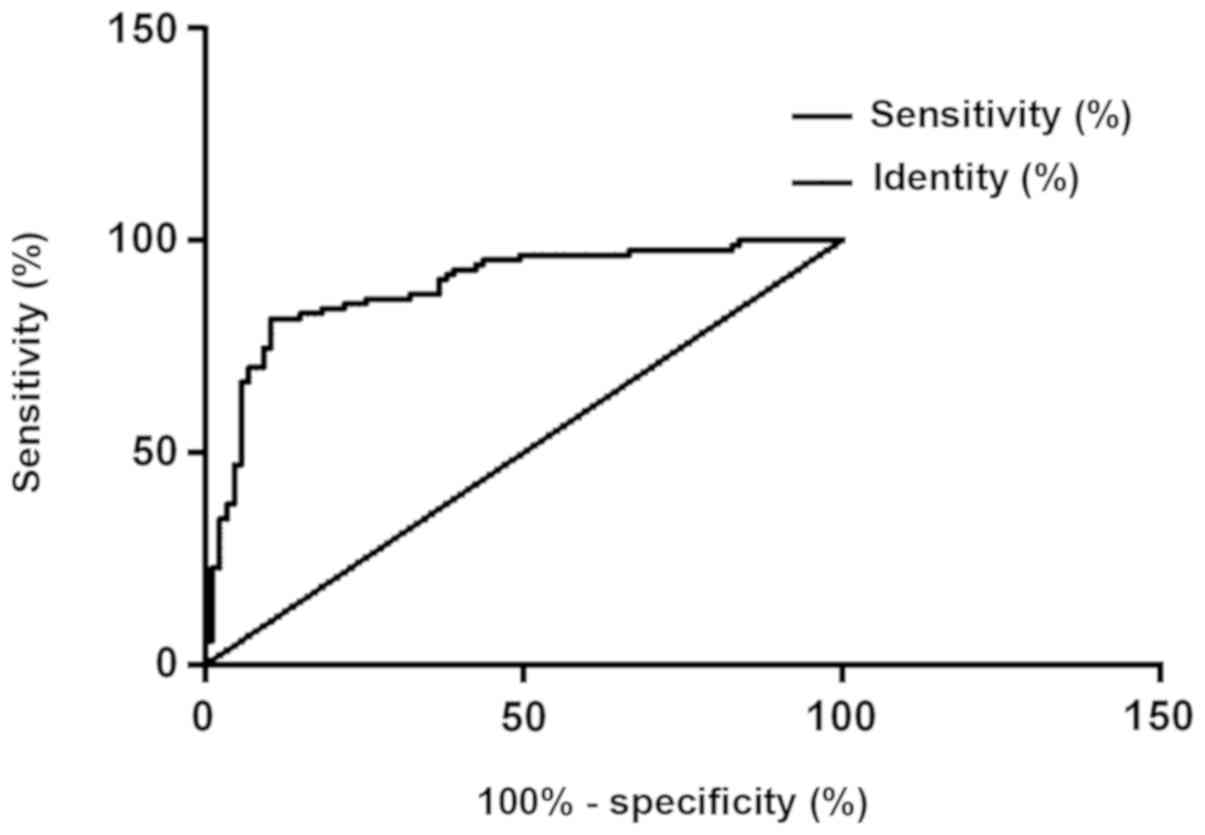
According to the ROC curve, the AUG, the
specificity, and the sensitivity of the protein PDK-1 in diagnosing
thyroid carcinoma were 0.862, 86.21% and 78.16%, respectively, with
the best cut-off point for diagnosing thyroid carcinoma of 199; the
AUG, the specificity, and the sensitivity of the protein DMBT1 in
diagnosing thyroid carcinoma were 0.708, 66.67% and 67.82%,
respectively, with the best cut-off point for diagnosing thyroid
carcinoma of 199; and the AUG, the specificity, and the sensitivity
of the combination of protein PDK-1 and protein DMBT1 in diagnosing
thyroid carcinoma were 0.888, 89.66% and 81.61%, respectively, with
the best cut-off point for diagnosing thyroid carcinoma of 199
(Figs. 4, 5 and 6).
Discussion
Citing from the American Carcinoma Society, 37,340
new cases (about 0% women) of thyroid carcinoma were diagnosed in
the United States in 2018, making the thyroid carcinoma the sixth
destructive carcinoma among women (7). Thyroid carcinoma, a common malignant
tumor in the head and neck (8), is
prone to spread and metastasis in its early stage (9). PDK1, as the main regulator of the
upstream AGG family that plays an important role in the regulation
of pathological processes such as cell growth, differentiation, and
apoptosis (10,11), is found in recent years to be highly
expressed in non-small cell carcinoma, breast carcinoma, and
pancreatic carcinoma tissues (12–14). The
close relation between the deterioration and the occurrence of
tumors is publicly known, and the PDK1, as an important kinase in
cells, has a significant regulatory effect on the cell reproduction
(15,16). Located on the long arm of the human's
number l0 chromosome (17), the
DMBT1 gene has its sequence that is mainly composed of repeated
highly homologous exons and introns (18). Some studies discovered that the
expression level of DMBT1 gene in tumors like esophageal carcinoma
(19), colon carcinoma (20), bladder carcinoma (21), prostate carcinoma (5), and non-small cell carcinoma (22) was significantly decreased compared
with its expression in normal tissues, suggesting that DMBT1 might
be able to inhibit the development of oncogenes. According to the
study of Mollenhauer's team (23),
DMBT1 might play an important role in the development of one
unclear part of the human body. It's likely that DMBT1 may inhibit
the occurrence of tumors by promoting cell differentiation since
the formation of tumors can lead to cell disorders. This study is
the first one to detect the expression of PDK-1 and DMBT1 proteins
in the thyroid carcinoma tissues and in their adjacent normal
tissues more than 2 cm away with no carcinoma cells infiltrating
using the ELISA method and analyze the correlation between the two
and the pathological significance of the thyroid carcinoma. The
results of this study revealed that the expression level of PDK-1
in the thyroid carcinoma tissues was statistically significantly
higher than that in the normal tissues adjacent to the carcinoma
(t=10.78, P<0.001), while the expression level of DMBT1 in the
thyroid carcinoma tissues was statistically significantly lower
than that in the normal tissues adjacent to carcinoma (t=7.491,
P<0.05). Previous studies showed that the positive expression of
DMBT1 in breast carcinoma was significantly lower than that in the
normal tissues (24), which
helpfully supports the results of this study. By now no relevant
study of the expression level of PDK-1 in the thyroid carcinoma
tissues has been reported. This study also analyzed the
relationship between the expression levels of DMBT1 and PDK-1 and
the clinicopathological features of patients with thyroid
carcinoma. The results showed that the expression of PDK-1 in
thyroid carcinoma was proved to be greatly correlated with factors
like pathological type, clinical stage, tumor size and lymph node
metastasis (P<0.001), in no significant correlation with factors
such as sex and age (P>0.05); in the thyroid carcinoma tissues,
the expression of PDK-1 in the lymph node metastasis was
statistically greatly higher than that in the places without lymph
node metastasis (P<0.001); the PDK-1 expression in the
undifferentiated tissues was much higher than that in the
differentiated thyroid carcinoma tissues, with a statistical
difference (P<0.001); the expression level of PDK-1 in the
thyroid carcinoma tissues at stage III to stage IV was
statistically significantly higher than that in the thyroid
carcinoma tissues at stage I to stage II (P<0.05); the
expression level of PDK-1 in the non-small carcinoma thyroid
tissues was greatly higher than that in the microcarcinoma tissues,
and the difference was statistically significant (P<0.001). The
expression of DMBT1 in the thyroid tissues was in a contrary
situation: in the thyroid carcinoma tissues, the expression of
DMBT1 in the lymph node metastasis was statistically greatly lower
than that in the places without lymph node metastasis; the DMBT1
expression in the undifferentiated tissues was much lower than that
in the differentiated thyroid carcinoma tissues, with a statistical
difference (P<0.001); the expression level of DMBT1 in the
thyroid carcinoma tissues at stage III to stage IV was
statistically significantly lower than that in the thyroid
carcinoma tissues at stage I to stageII (P<0.05); the expression
level of DMBT1 in the non-small carcinoma thyroid tissues was
greatly lower than that in the microcarcinoma tissues, and the
difference was statistically significant (P<0.001). Finally,
referring to the results of the correlation and partial correlation
between the expression of DMBT1 and the expression PDK-1 in the
thyroid carcinoma that DMBT1 and PDK-1 were negatively correlated
in the thyroid carcinoma (r=−0.889, P<0.001), this study made a
speculation that the pathological features of thyroid carcinoma
were closely related to the expression of DMBT1 and PDK-1, with a
highly expressed PDK-1 in it to promote the development of thyroid
carcinoma, and with a lowly expressed DMBT1 in it to possibly
inhibit the thyroid carcinoma. Despite the few reports about the
relationship between the expression of DMBT1 and PDK-1 and the
clinicopathological features of thyroid carcinoma in patients, some
previous reports on the thyroid carcinoma are still helpful to this
study. Following the detection of the expression of DMBT1 and
PDK-1, the ROC curve was drawn to investigate the diagnostic value
of DMBT1 alone, PDK-1 alone and the combination of the two for
thyroid carcinoma, leading to the results that the sensitivity and
specificity of the combined detection of DMBT1 and PDK-1 for
thyroid carcinoma detection were significantly higher than the
single detection of DMBT1 or PDK-1. No study of the diagnostic
value of DMBT1 and PDK-1 for the thyroid carcinoma has been
reported so far, so this study which specifically explored the
correlation between the expression of DMBT1 or PDK-1 and the
occurrence and development of the thyroid carcinoma, is of certain
significance for the diagnosis and clinical treatment of thyroid
carcinoma. Finally, with the research results and some supportive
views by other studies on hand, this study draw the conclusion that
monitoring the expression of DMBT1 or PDK-1 in the protein had
certain clinical diagnostic and therapeutic significance for the
occurrence and development of the thyroid carcinoma, and that the
combined detection of the expression changes of DMBT1 and PDK-1
could diagnose the thyroid carcinoma conveniently, quickly, and
accurately, worthy of clinical promotion.
Considering the limited resources and the small
number of research subjects, contingency was possible in this
study. In the future, more time and energy will be invested in the
improvement of this research to achieve better study results.
In summary, PDK-1 was highly expressed in the
thyroid carcinoma, while the DMBT1 was lowly expressed. The
combined detection of DMBT1 and PDK-1 could improve the accuracy of
diagnosing the thyroid carcinoma to reduce the rate of
misdiagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS analyzed and interpreted the general data of
patients and drafted this paper. ZS and LX were responsible for
ELISA. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second People's Hospital of Lianyungang (Lianyungang, China).
Patients who participated in this research had complete clinical
data. The signed informed consents were obtained from the patients
or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jabbour E, Deininger M and Hochhaus A:
Management of adverse events associated with tyrosine kinase
inhibitors in the treatment of chronic myeloid leukemia. Leukemia.
25:201–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braidotti P, Nuciforo PG, Mollenhauer J,
Poustka A, Pellegrini C, Moro A, Bulfamante G, Coggi G, Bosari S
and Pietra GG: DMBT1 expression is down-regulated in breast cancer.
BMC Cancer. 4:462004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imai MA, Moriya T, Imai FL, Shiiba M,
Bukawa H, Yokoe H, Uzawa K and Tanzawa H: Down-regulation of DMBT1
gene expression in human oral squamous cell carcinoma. Int J Mol
Med. 15:585–589. 2005.PubMed/NCBI
|
|
7
|
Hernandez BY, Green MD, Cassel KD,
Pobutsky AM, Vu V and Wilkens LR: Preview of Hawaii cancer facts
and figures 2010. Hawaii Med J. 69:223–224. 2010.PubMed/NCBI
|
|
8
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gagliardi PA, Puliafito A and Primo L:
PDK1: At the crossroad of cancer signaling pathways. Semin Cancer
Biol. 48:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leroux AE, Schulze JO and Biondi RM: AGC
kinases, mechanisms of regulation and innovative drug development.
Semin Cancer Biol. 48:1–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arsenic R: Immunohistochemical analysis of
PDK1 expression in breast cancer. Diagn Pathol. 9:822014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han L, Zhang G, Zhang N, Li H, Liu Y, Fu A
and Zheng Y: Prognostic potential of microRNA-138 and its target
mRNA PDK1 in sera for patients with non-small cell lung cancer. Med
Oncol. 31:1292014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferro R and Falasca M: Emerging role of
the KRAS-PDK1 axis in pancreatic cancer. World J Gastroenterol.
20:10752–10757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng N, Ding X, Sun A and Jahan R: PDK1
activity regulates proliferation, invasion and growth of
hemangiomas. Cell Physiol Biochem. 36:1903–1910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lian S, Shao Y, Liu H, He J, Lu W, Zhang
Y, Jiang Y and Zhu J: PDK1 induces JunB, EMT, cell migration and
invasion in human gallbladder cancer. Oncotarget. 6:29076–29086.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mollenhauer J, Holmskov U, Wiemann S,
Krebs I, Herbertz S, Madsen J, Kioschis P, Coy JF and Poustka A:
The genomic structure of the DMBT1 gene: evidence for a region with
susceptibility to genomic instability. Oncogene. 18:6233–6240.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mollenhauer J, Wiemann S, Scheurlen W,
Korn B, Hayashi Y, Wilgenbus KK, von Deimling A and Poustka A:
DMBT1, a new member of the SRCR superfamily, on chromosome
10q25.3–26.1 is deleted in malignant brain tumours. Nat Genet.
17:32–39. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith HA and Kang Y: The
metastasis-promoting roles of tumor-associated immune cells. J Mol
Med (Berl). 91:411–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng H, Gao YB, Wang HF, Jin XL and Xiao
JC: Expression of deleted in malignant brain tumours 1 (DMBT1)
relates to the proliferation and malignant transformation of
hepatic progenitor cells in hepatitis B virus-related liver
diseases. Histopathology. 60:249–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du J, Guan M, Fan J and Jiang H: Loss of
DMBT1 expression in human prostate cancer and its correlation with
clinical progressive features. Urology. 77:509.e9–509.e13. 2011.
View Article : Google Scholar
|
|
22
|
Zöchbauer-Müller S, Fong KM, Virmani AK,
Geradts J, Gazdar AF and Minna JD: Aberrant promoter methylation of
multiple genes in non-small cell lung cancers. Cancer Res.
61:249–255. 2001.PubMed/NCBI
|
|
23
|
Mollenhauer J, Herbertz S, Helmke B,
Kollender G, Krebs I, Madsen J, Holmskov U, Sorger K, Schmitt L,
Wiemann S, et al: Deleted in Malignant Brain Tumors 1 is a
versatile mucin-like molecule likely to play a differential role in
digestive tract cancer. Cancer Res. 61:8880–8886. 2001.PubMed/NCBI
|
|
24
|
Ridnour LA, Cheng RY, Switzer CH, Heinecke
JL, Ambs S, Glynn S, Young HA, Trinchieri G and Wink DA: Molecular
pathways: toll-like receptors in the tumor microenvironment-poor
prognosis or new therapeutic opportunity. Clin Cancer Res.
19:1340–1346. 2013. View Article : Google Scholar : PubMed/NCBI
|