Introduction
Osteosarcoma is a malignant tumor of skeletal
origin, and its incidence remains high among primary malignant bone
tumors (1). Patients are primarily
adolescents, and osteosarcoma is associated with a high degree of
malignancy, particularly metastasis to the lungs during the early
stages. Cases of osteosarcoma, in particular cases with evidence of
lung metastasis, are associated with a poor prognosis (2). Osteosarcoma exerts a large toll on
mental health on patients and their families. In recent years,
surgical treatment and neoadjuvant chemotherapy have improved the
treatment of this disease (3,4).
Radiotherapy and chemotherapy combined with limb salvage surgery
have replaced amputation surgery and have become the primary
surgical method for treating patients with osteosarcoma (5). As therapeutic radiotherapy and
chemotherapy regimens may not effectively remove all tumor cells,
20–40% of patients undergoing this treatment may still die of
metastasis, and metastases are one of the primary factors affecting
the survival rate of patients with osteosarcoma (6). With the discovery of an increasing
number of molecular mechanisms that can mediate the invasion and
metastasis of osteosarcoma (7–9),
identifying novel drugs or therapeutic regimens to inhibit these
processes and improve the survival rate of patients has become the
primary task for improving the current clinical treatment options
available.
Diosgenin is a hydrolysate of dioscin, which is an
important basic raw material necessary for the production of
steroid hormone drugs (10).
Diosgenin is an important steroidal sapogenin and is widely
distributed in plants of the Dioscoreae, Liliaceae, Rosaceae
and Caryophyllaceae genera and a number of other plants
(11). Diosgenin is one of the
active ingredients in a variety of Chinese medicines, and it has
anti-inflammatory, antidiabetic, antithrombotic, antiallergic and
antiviral properties (12–16). A number of studies have shown that
diosgenin inhibits tumor cells by interfering with apoptosis and
autophagy of tumor cells (17–22).
However, to the best of our knowledge, there are no studies
investigating the role of diosgenin in the invasion and migration
of osteosarcoma cells, and the exact mechanisms underlying its
effects remain unknown., and the exact mechanisms underlying its
effects remain unknown.
The epithelial-mesenchymal transition (EMT) is a
process cancerous cells undergo in which cells with epithelial-like
morphologies undergo morphological and molecular changes to attain
a mesenchymal-like morphology and thus becoming more migratory
(23). Cells lose their polarity,
contact with surrounding cells, the extracellular matrix is reduced
and cellular migration and motility are increased. In addition, the
phenotype of these cells changes, and characteristics associated
with interstitial cells appear (24). These changes enhance the invasive and
migratory capacity of tumor cells (25). EMT is one of the transformations by
which tumor cells can acquire the ability to migrate and is an
important process in tumor cell infiltration and metastasis
(26,27). An increasing number of experimental
studies have shown that the initiation of EMT serves a critical
role in the invasion and metastasis of osteosarcoma (9,28,29). The
present study applied diosgenin to two different osteosarcoma cell
lines to observe the effects of this drug on the invasion and
migration of the cells, and the mechanism of action was further
explored in relation to the inhibition of EMT initiation in tumor
cells.
Materials and methods
Chemicals and reagents
Diosgenin, purity >90% was identified in Nanjing
Zelang Technology Co., Ltd. by HPLC and was purchased from Nanjing
Zelang Medical Technology Co., Ltd. (cat. no. ZL20170702014). A
First Strand cDNA Synthesis kit was obtained from Thermo Fisher
Scientific, Inc., fetal bovine serum (FBS) was purchased from
ExCell Biology, Inc., TRIzol® was purchased from
Invitrogen; Thermo Fisher Scientific, Inc., chloroform and
isopropanol were purchased from Nanjing Chemical Reagent Co., Ltd.
and 0.25% trypsin-EDTA, PBS, total protein extraction kit, Braford
assay kit, 5X SDS-PAGE protein loading buffer solution, SDS-PAGE
gel preparation kit, prestained protein molecular weight ladder,
10X Tris-glycine protein electrophoresis buffer, Coomassie blue
staining protein detection kit, phosphorylated p38 (pP38) inhibitor
SB203580, 10X electrotransfer buffer solution, Ponceau staining
solution, western blotting primary antibody diluent, a western
blotting secondary antibody diluent, enhanced chemiluminescent
detection kit, internal reference primary antibody (anti-GAPDH;
cat. no. KGAA002-1; dilution, 1:200), secondary antibody, and
developing and fixing reagents were all purchased from KeyGen
Biotechnology Co., Ltd.
Cell culture
Human osteosarcoma MG63 and U2OS cells were donated
by Jiangsu Health Vocational College (Nanjing, China). The MG63 and
U2OS cells were treated with 90% minimal essential medium
supplemented with 10% FBS or 90% complete DMEM supplemented with
10% FBS, respectively. The cells were cultured at 37°C in 5%
CO2. The culture medium was replaced every 2 days.
MTT assay and calculation of the
cellular IC50
Cells in the logarithmic growth phase were
collected, and the two cell lines were prepared in cell suspensions
at a concentration of 5×104 cells/ml. The cells were
added to 96-well cell culture plates (100 µl per well) and placed
in a 37°C, 5% CO2 incubator for 24 h. Diosgenin diluted
to various concentrations in complete medium (200, 100, 50, 25,
12.5 and 6.25 µM) was added to the 96-well medium. Untreated cells
were the negative control group. The culture plates were placed in
a 37°C, 5% CO2 incubator for 24 h after which MTT
staining was performed. DMSO was used to dissolve the purple
formazan and the optical density (OD) value was measured at λ=490
nm using a BioTek ELx800 plate reader (BioTek Instruments, Inc.).
The inhibition rate and 50% inhibitory concentration
(IC50) of diosgenin at each concentration was
calculated. Inhibition rate and IC50 were calculated
using the following formula: Inhibition rate (%) = [(Negative
control group-Experimental group)/Negative control group] ×
100.
Scratch test for the detection of cell
migration
Cells in the logarithmic growth phase were prepared
at 1×105 cells/ml and transferred to a 6-well plate, and
the corresponding diosgenin containing medium, MG63 (80 µM) and
U2OS (40 µM), was added. The next day, when the cell confluence was
>60%, a sterile pipette tip was used to evenly scratch the
6-well plate. The floating cells were washed away with PBS, and
serum-free medium was used for culture in a cell culture incubator.
After 24 h, the cells were imaged (magnification, ×100), and the
cell wound healing area was measured using Adobe Photoshop CS6
(Adobe Systems Europe, Ltd.) using an Olympus IX51 light microscope
(Olympus Corporation). Relative wound closure was calculated using
the following formula: relative wound closure (%)= (0 h time point
area-each time point by the area)/0 h time point area × 100.
Transwell invasion assay
Diosgenin was added to cells in the logarithmic
growth phase (MG63, 80 µM; U2OS 40 µM). Matrigel was thawed at 4°C
overnight and diluted 1:2 using serum-free medium. In the upper
chamber of the Transwell insert, 30 µl diluted Matrigel was added
at 37°C for 120 min. The cell density was adjusted to
5×105 cells/ml using serum-free medium. A total of 100
µl of the cells at a concentration of 5×105 cells/ml was
added to the upper chamber of a Transwell insert and 500 µl
complete medium supplemented with 20% FBS was added to the lower
chamber. The cells were then cultured in an incubator at 37°C and
5% CO2. After 24 h, the chamber was removed, washed with
PBS, fixed in absolute ethanol for 20 min at 37°C, stained with
crystal violet for 20 min at 37°C, washed with PBS and air-dried.
An inverted microscope was used for observation, and the
transmembrane cells in each group were counted using an Olympus
IX51 light microscope.
Immunofluorescence assay for the
observation of protein expression changes
Cells in the logarithmic growth phase were harvested
and plated at a density of 5×104 cells/ml. Diosgenin was
added to each cell line (MG63, 80 µM; U2OS, 40 µM) and cultured for
24 h, after which the cells were air dried at room temperature
(20°C), fixed with 4% paraformaldehyde for 30 min at 20°C, and
subsequently washed with PBS three times. Two drops of a 3%
H2O2-methanol solution and 75 µl ready-to-use
goat serum (cat. no. AR0009; Wuhan Boster Biological Technology,
Ltd.) were added dropwise and then incubated for 20 min at room
temperature. The primary antibodies used were rabbit anti-human
TGF-β1 (cat. no. KG22744-1; dilution, 1:100; Nanjing KeyGen Biotech
Co., Ltd.) and incubated for 2 h at 37°C, after which the samples
were washed with PBS three times. A FITC-conjugated secondary
antibody (1:200 dilution) was added and incubated for 1 h in the
dark at 37°C, and the samples were washed with PBS three times.
After dropwise addition of a DAPI solution 0.2 µg/ml, antiquench
gel (cat. no. KGF028; Nanjing KeyGen Biotech Co., Ltd.) was used
for mounting. A total of three high-expression regions of the cells
were observed under a fluorescence microscope and photographed for
preservation. The integrated optical density (IOD) under the field
of view was calculated using Image-Pro Plus version 6.0 (Media
Cybernetics, Inc.), and the mean density was calculated as mean
density = IOD/area of the field.
Western blotting for the detection of
protein expression
Cells in the logarithmic growth phase were harvested
and plated at a density of 5×105 cells/ml. The following
day, after the cells had adhered to the wells, the medium was
replaced and diosgenin was added to each cell line (MG63, 80 µM;
U2OS, 40 µM). In the SB203580 group, SB203580 (cat. no. KGR0067
Nanjing KeyGen Biotech Co., Ltd.) was co-cultured with cells for 1
h. After 24 h, total cellular protein was extracted using a total
protein extraction kit (cat. no. KGP250; Nanjing KeyGen Biotech
Co., Ltd.), and the protein concentration was measured with a
bicinchoninic acid protein concentration assay kit. Proteins were
resolved for 90 min by 10% SDS-PAGE (30 µg/well). The resolved
proteins were transferred to PVDF membranes using a Bio-Rad semidry
transfer system (Bio-Rad Laboratories, Inc.). The PVDF membrane was
subsequently blocked with 5% skimmed milk powder overnight at 4°C
and then incubated with the primary antibody overnight at 4°C. The
following primary antibodies were used: Rabbit anti-human
E-cadherin (cat. no. KG22195-2; 1:200); rabbit anti-human vimentin
(cat. no. KG22794-2; 1:200); rabbit anti-human ERK1/2 (cat. no.
KG30107-2; 1:200); rabbit anti-human jun N-terminal kinase (JNK)
1/2 (cat. no. KG22481-2; 1:200); rabbit anti-human P38 (cat. no.
KG30244-2; 1:200); rabbit anti-human pERK1/2 (cat. no. KG30246-2;
1:200); rabbit anti-human pJNK1/2 (cat. no. KG11504-2; 1:200); and
rabbit anti-human pP38 (cat. no. KG11253-2; 1:200). The secondary
antibodies used were goat anti-rabbit immunoglobulin G (IgG; cat.
no. KGAA25; 1:200) and goat anti-mouse IgG (cat. no. KGAA37;
1:4,000). Both the primary and secondary antibodies were obtained
from Nanjing KeyGen Biotech Co., Ltd. The following day, the
membrane was incubated with the corresponding secondary antibody
(1:4,000) for 1 h at room temperature, and the signal was
visualized using enhanced chemiluminescent reagent (cat. no.
KGP1121; Nanjing KeyGen Biotech Co., Ltd.). A gel imager and
Gel-Pro32 version 4.4.0.36 (Bio-Rad, Inc.) were used for collecting
images and analyzing the gray scale values relative to the GAPDH
signal.
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells in the logarithmic growth phase were harvested
and digested with 0.25% trypsin and a trypsin digestive solution
containing 0.02% EDTA to prepare a single-cell suspension.
Subsequently 1 ml TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and 200 µl chloroform were added, incubated at
4°C for 10 min, mixed gently and centrifuged at centrifuged at
12,000 × g for 5 min at 4°C. The supernatant was transferred to a
new microcentrifuge tube. A total 800 µl precooled methanol (4°C)
was added, and the samples were placed at 4°C for 5 min with gentle
agitation. The supernatant was aspirated and discarded after
centrifugation at 12,000 × g for 10 min at 4°C. Subsequently, 1 ml
precooled 75% ethanol (4°C) was added, and the sample was
centrifuged at 12,000 × g for 5 min at 4°C. The supernatant was
aspirated and discarded, and 30 µl diethyl pyrocarbonate water was
added and dissolved at 4°C for 5 min. RNA was prepared for storage
in a −70°C freezer. A total of 5 µl RNA sample and 495 µl 1X
Tris-EDTA buffer were mixed. The concentration and purity of the
RNA were determined by measuring the absorption values at 260 and
280 nm. An OD260/OD280 of 1.8–2.0 was considered to indicate RNA of
good quality that could be used for subsequent experiments. The
extracted RNA was reverse transcribed into cDNA using a First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) using
the following incubation conditions: 25°C for 5 min, 42°C for 60
min, and on ice for 5 min. Next, qPCR was performed using a
fluorescence qPCR instrument (ABI StepOne Plus; Thermo Fisher
Scientific, Inc.). Primers were synthesized by Jiangsu Health
Vocational College. The primer sequences were: GAPDH (90 bp)
forward, 5-AGATCATCAGCAATGCCTCCT-3 and reverse,
5-TGAGTCCTTCCACGATACCAA-3; and E-cadherin (108 bp) forward,
5-CCAAGCAGCAGTACATTCTACA-3 and reverse, 5-CATTCACATCCAGCACATCCA-3.
The amplification conditions were as follows: Predenaturation at
95°C for 3 min, followed by 45 cycles of denaturation at 95°C for
10 sec, annealing at 60°C for 20 sec and extension at 72°C for 35
sec. The specificity of the amplified products was monitored by a
dissolution curve. Relative gene expression was analyzed and
calculated using the 2−ΔΔCq method (30), and the expression levels of the
target genes were normalized to GAPDH (ABI StepOne version 2.3;
Applied Biosystems; Thermo Fisher Scientific, Inc.).
Statistical analysis
All the data are presented as the mean ± standard
deviation and experiments were performed in triplicate. Statistical
analyses were performed in SPSS software version 16.0 (SPSS, Inc.).
Statistical comparisons between two groups were performed using an
unpaired Student's t-test and comparisons between multiple groups
were performed using a one-way ANOVA followed by a post hoc Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
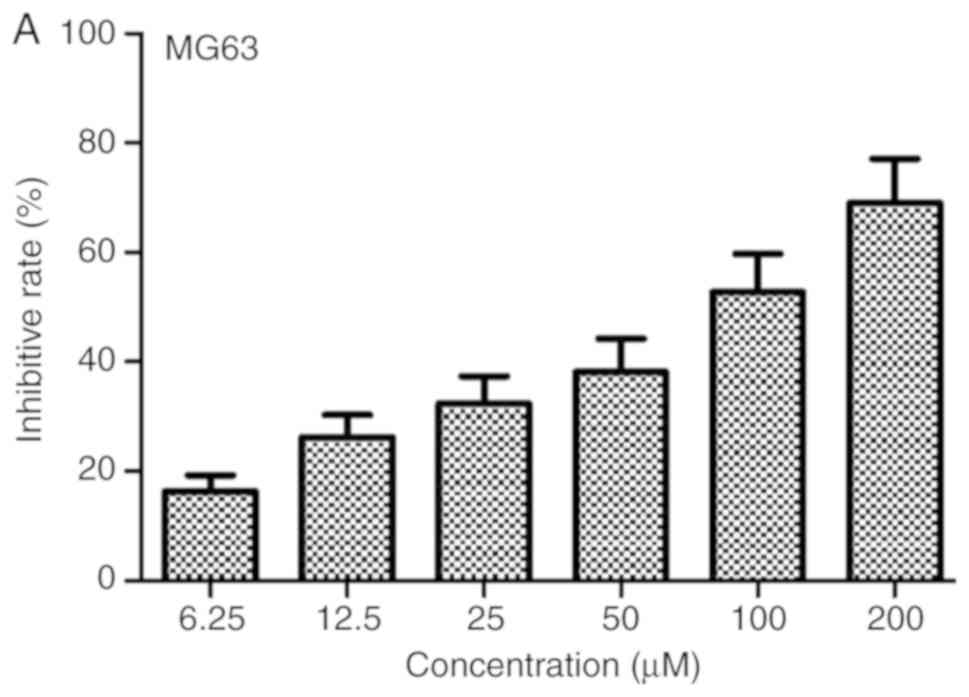
Diosgenin inhibits the proliferation
of osteosarcoma MG63 and U2OS cells
MG63 and U2OS cells were treated with diosgenin at
different molar mass concentrations (200, 100, 50, 25, 12.5 and
6.25 µM) for 24 h. The results shown in Fig. 1 revealed that diosgenin had
inhibitory effects on both cell lines in a dose-dependent manner.
The 24-h IC50 of this drug in MG63 and U2OS cells were
76.2 and 40.15 µM, respectively.
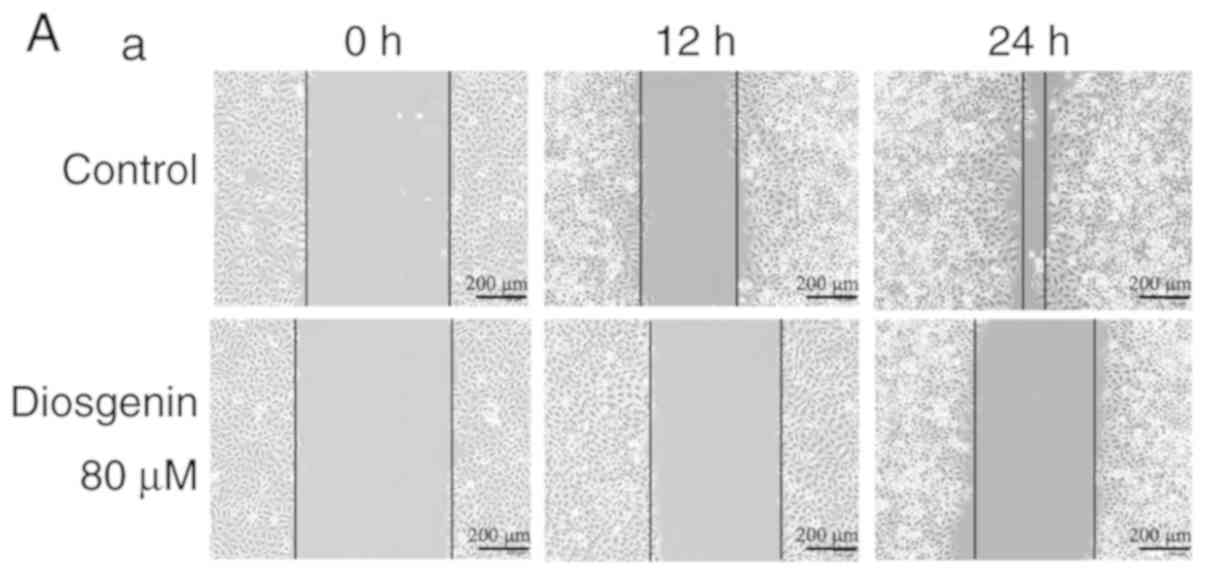
Diosgenin inhibits the migratory
ability of osteosarcoma cells
The cell scratch test is a method for detecting cell
movement and can be used to detect the invasive and metastatic
capacities of tumor cells (31).
Fig. 2 shows that relative wound
closure (%) of the MG63 cells in the control group were 34.03±3.42
and 77.45±4.50 after 12 and 24 h, respectively, while relative
wound closure (%) of the U2OS cells in the control group were
48.16±3.18 and 67.71±4.22, respectively. However, upon treatment
with the IC50 dose of diosgenin, relative wound closure
(%) of MG63 cells (80 µM) were 18.83±2.61 and 28.75±2.11 after 12
and 24 h, respectively. Similarly, when U2OS cells were treated
with 40 µm diosgenin, relative wound closure (%) were 27.36±3.26
and 36.02±3.74, respectively. Compared to those of the cells in the
control group, the in vitro migratory abilities of the two
types of osteosarcoma cells in the group treated with diosgenin
were decreased (P<0.01).
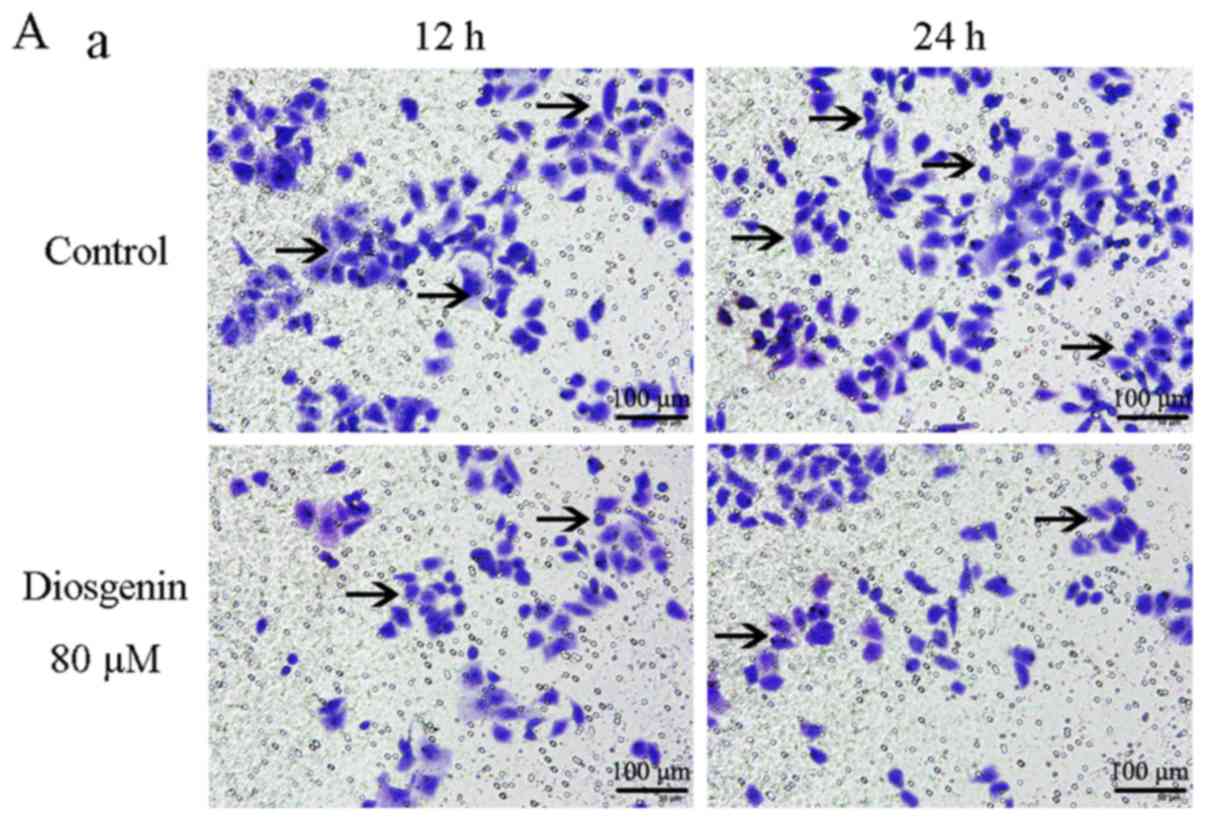
Diosgenin inhibits osteosarcoma cell
invasion
We next employed a Transwell test, which detects the
invasive ability of cells based on their ability to pass through
Matrigel (32). As shown in Fig. 3, the number of MG63 cells passing
through the Matrigel after 12 and 24 h in the control group was
97±2.59 and 122±1.58/field of view, respectively. For U2OS cells,
the counts were 84.2±3.27 and 204.6±2.51/field of view,
respectively. However, the numbers of MG63 cells passing through
the Matrigel after 12 and 24 h in the drug-treated group was only
41±2.07 and 53±1.82/field of view, respectively, and the number of
U2OS cells was 45.4±2.7 and 55.6±2.3/field of view, respectively.
Compared with that of the respective control for each cell line,
the invasive ability of the diosgenin-treated osteosarcoma cells
was decreased (P<0.05).
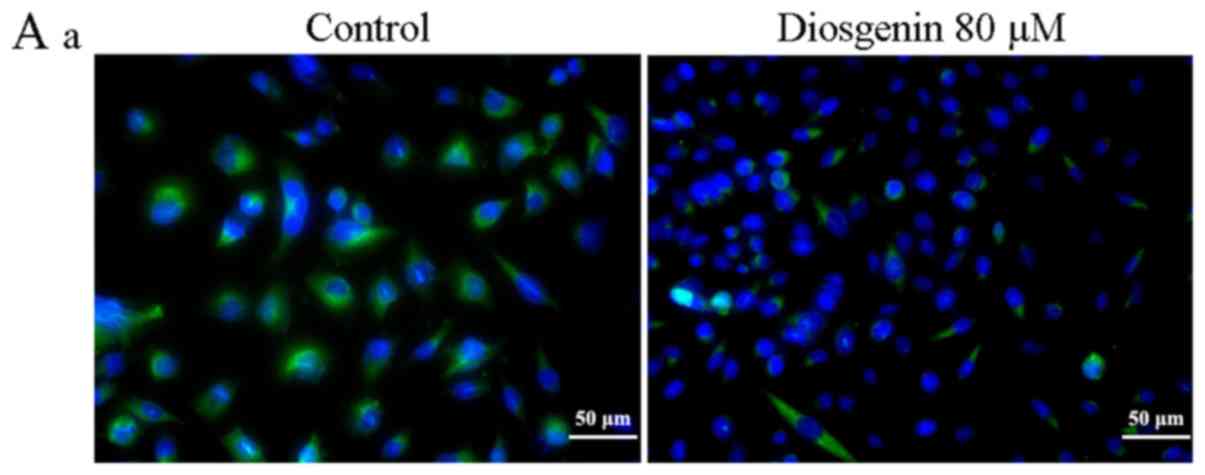
Diosgenin inhibits TGF-β1 protein
expression in the osteosarcoma cell lines
As shown in Fig. 4,
following treatment with diosgenin, the ODs were 1.25±0.03 and
1.07±0.04/pixel in the MG63 and U2OS cells, respectively. Compared
with the respective control group, the average TGF-β1 ODs of MG63
and U2OS cells were 1.33±0.02 and 1.25±0.04/pixel. TGF-β1 protein
expression in the two treated osteosarcoma cell line groups was
decreased (P<0.05).
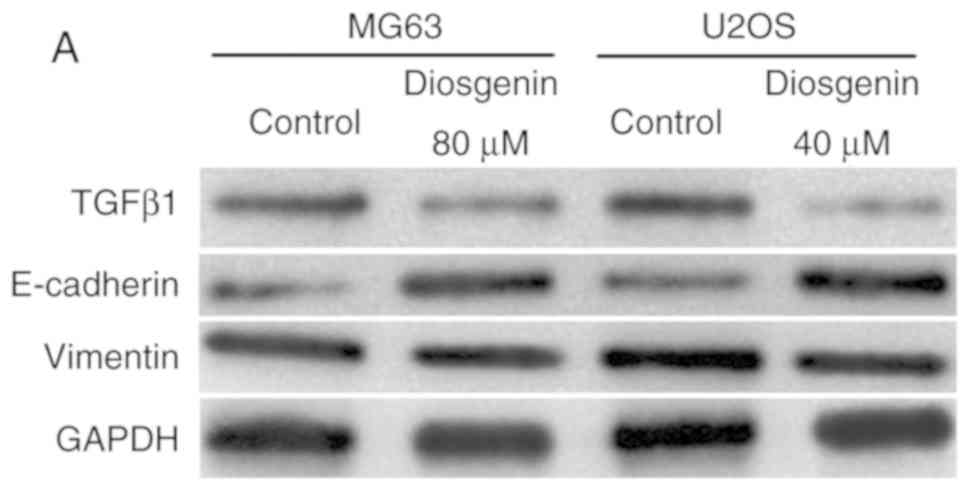
Diosgenin downregulates TGF-β1 and
upregulates E-cadherin protein expression in in the osteosarcoma
cell lines
To determine whether the effect of diosgenin on the
invasion and migration of the two osteosarcoma cell lines was
associated with EMT, western blotting was used to detect changes in
TGF-β1, E-cadherin and vimentin protein expression in the
osteosarcoma cell lines prior to and following diosgenin
administration. As shown in Fig. 5,
after diosgenin treatment, the protein expression levels of TGF-β1
were decreased, while those of E-cadherin were increased in both
cell types (P<0.01), suggesting that the invasive and migratory
capacities of the cells were inhibited. However, diosgenin had no
effect on vimentin protein expression.
Diosgenin inhibits the ERK/JNK/P38
signaling pathway in the osteosarcoma cell lines
Western blot analysis was used to assess the
ERK/JNK/P38 pathway in the osteosarcoma cell lines before and after
diosgenin treatment. As shown in Fig.
6, among the ERK/JNK/P38 pathway molecules, only the pP38
protein exhibited a decreased level in both osteosarcoma cell lines
following administration of diosgenin (P<0.01), while changes in
the levels of other proteins in the pathway were not statistically
significant.
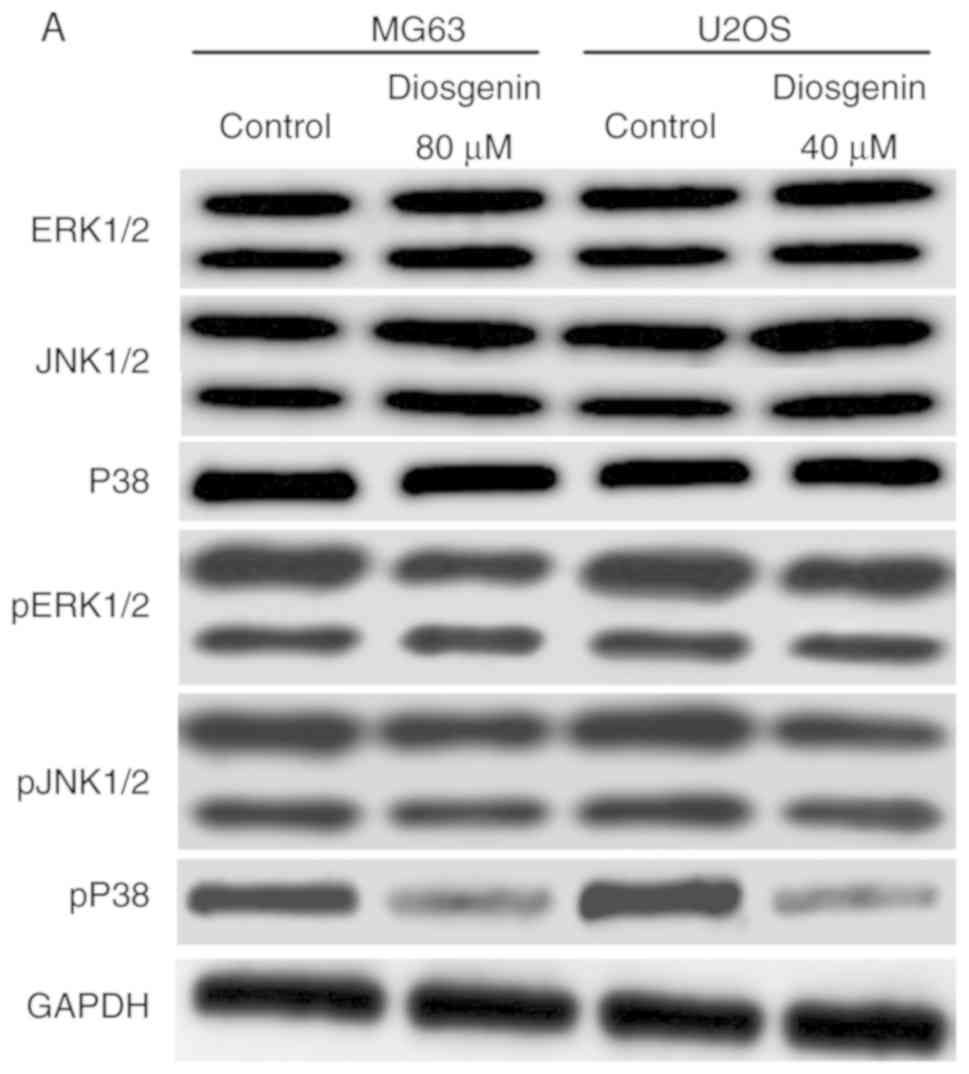 | Figure 6.Western blot analysis of the effects
of diosgenin on the expression of ERK1/2, JNK1/2, P38 and pP38. (A)
Western blot analysis of ERK1/2, JNK1/2, P38, pERK1/2 and pJNK1/2
in the osteosarcoma cell lines prior to and following diosgenin
treatment. (B-a) Quantification of expression in ERK1/2, JNK1/2,
P38 relative to GAPDH. (B-b) Quantification of expression levels in
pERK1/2, pJNK1/2, pP38 relative to total ERK1/2, total JNK1/2,
total P38. There was a significant reduction in the quantity of
pP38, although the levels of total P38 were not affected by
diosgenin treatment. **P<0.01. ERK1/2, extracellular
signal-regulated kinase; JNK, c Jun N-terminal kinase; p,
phospho. |
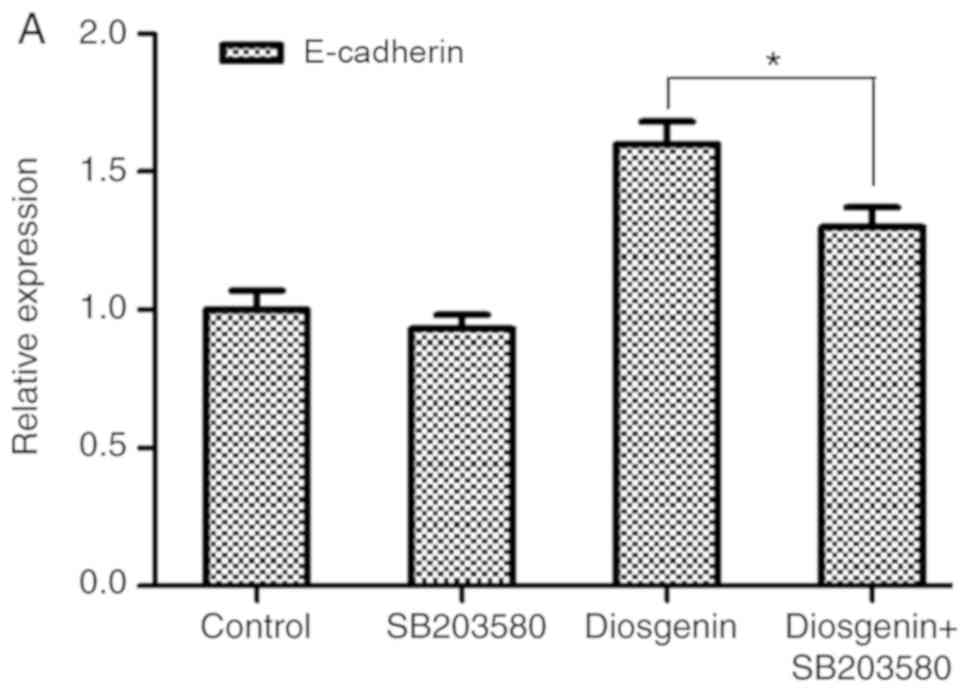
Diosgenin inhibits the invasion and
migration of the two osteosarcoma cell lines by reducing the
protein expression levels of the pP38 in the MAPK pathway
Diosgenin treatment was combined with a pP38 protein
inhibitor and the expression levels of a downstream indicator of
EMT, E-cadherin was observed (33).
As shown in Fig. 7, the cellular
expression level of E-cadherin in the group treated with the pP38
inhibitor SB203580 (20 µM) was not statistically different compared
with the control group; however, the expression level of E-cadherin
in the osteosarcoma cell lines treated with diosgenin in
combination with SB203580 group was decreased compared with the
cells in the diosgenin group (MG63, P<0.05; U2OS,
P<0.01).
Discussion
In the present study, an MTT assay was used to
determine the IC50 dose of diosgenin on the MG63 and
U2OS cell lines. The MTT assay also demonstrated that the drug had
a concentration-dependent inhibitory effect on the proliferation of
both osteosarcoma cell lines.
A wound healing and Transwell assay was used to
observe the effect of diosgenin on migration and invasion in the
osteosarcoma cells at 12 and 24 h after diosgenin administration
and confirmed that the invasive and migratory abilities of these
osteosarcoma cells were decreased to different degrees upon
diosgenin administration in a time-dependent manner.
TGF-β is a protein widely recognized for its role in
EMT regulation (34). It primarily
regulates downstream transcription factors through both
Smad-dependent and Smad-independent signaling pathways and thereby
participates in the regulation of EMT (35). TGF-β was one of the first factors
discovered to be able to induce EMT in tumor cells (36). There are three primary subtypes,
TGF-β1-3, and among these, the TGFβ1 has been reported to be
associated with EMT initiation (37). TGF β1 can promote the metastasis of
tumor cells in the late stage of tumor cell growth, and its
cancer-promoting effects usually occur at the same time as the
induction of tumor cell EMT (38). A
number of experiments have shown that the levels of TGF-β1 in tumor
cells are positively correlated with the promotion of tumor cell
migration and invasion (39–41). However, TGF-β-induced apoptosis and
EMT may be independent cellular events that are mutually exclusive
but associated with each other and occur on a similar timeframe
(42).
E-cadherin is the primary molecule that maintains
the polarity and intercellular adhesion of epithelial cells. Its
primary function is to form a protein complex which links to the
actin cytoskeleton and prevents the metastasis and invasion of
tumor cells (43). However, EMT can
reduce or eliminate the expression of epithelial cell adhesion
molecules and cytoskeletal components to achieve a stromal cell
phenotype. Therefore, the reduction or loss of E-cadherin
expression is an important marker for EMT initiation in tumor cells
(44). Experiments have shown that a
decrease in the E-cadherin protein level can promote invasion and
metastasis in tumor cells (45–47).
Vimentin belongs to a family of cellular
intermediate filament proteins and is an important component of the
cytoskeleton. Studies have found that tumor cells can exhibit high
invasiveness and strong migratory abilities when vimentin is highly
expressed, whereas these abilities are reduced after knocking out
or reducing vimentin levels in these cells (48,49). In
some invasive tumors, such as colon cancer, prostate cancer and
breast cancer, the expression of vimentin is also positively
correlated with the degree of malignancy of the tumor (50–52).
To determine whether the ability of diosgenin to
inhibit invasion and migration in osteosarcoma cells was associated
with EMT, TGF-β1 expression was analyzed using immunofluorescence
prior to and following treatment. TGF-β1 expression decreased to
varying degrees in the two osteosarcoma cell lines following
treatment with diosgenin. To further observe whether the effects of
diosgenin were associated with EMT and to simultaneously verify the
results of the immunofluorescence experiment, western blotting was
used to measure the expression levels of three proteins associated
with EMT which showed that diosgenin reduced the protein expression
levels of TGF-β1 and increased those of E-cadherin but had no
effect on vimentin. These results suggested that diosgenin may
inhibit the initiation of EMT in osteosarcoma cells by reducing TGF
β1 protein expression levels and increasing E-cadherin protein
levels.
The MAPK signaling pathway is one of the numerous
molecular signaling pathways that can affect the initiation of EMT
(53). It has a synergistic effect
with TGF-β in the initiation of EMT in tumor cells. The ERK, JNK
and P38 proteins in the MAPK signaling pathway are key factors for
initiation of EMT (54). It has been
shown that once phosphorylated, these three MAPK pathway proteins
can bind to the serine and threonine sites of Smad2/3-linked
polypeptide, thereby promoting the activation of the Smad pathway
and initiating EMT in tumor cells (55). The tumor cells can thereby become
more migratory and invasive (56).
In addition, elevating ERK phosphorylation can upregulate TGF-β1
protein levels in tumor cells. ERK phosphorylation is one of the
primary drivers of EMT in tumor cells in vitro (57); in addition, TGF-β1 can activate
TGF-β-activated kinase 1, which promotes the phosphorylation of P38
and further drives EMT (58).
In the present study, all the proteins associated
with the MAPK signaling pathway were analyzed by western blotting
and it was demonstrated that diosgenin had no effect on the total
ERK1/2, JNK1/2 and P38 protein levels in the two osteosarcoma cell
lines. Diosgenin also did not significantly alter the levels of
phosphorylated ERK1/2 or JNK1/2. However, diosgenin did decrease
the levels of pP38. Therefore, diosgenin may have inhibited the
MAPK signaling pathway in both osteosarcoma cell lines by
decreasing the protein expression levels of pP38.
However, the MAPK signaling pathway is involved in a
number of functions associated with cancerous behaviors. In
addition to the initiation of EMT, the MAPK signaling pathway
additionally serves roles in cell growth, apoptosis and
inflammation (58–61). A previous study has shown that
diosgenin can inhibit the cell cycle of osteosarcoma cells through
the P38 protein (62). To confirm
that the observed diosgenin-mediated inhibition of the MAPK pathway
in osteosarcoma cells was associated with EMT, cells were treated
with a combination of an inhibitor of the p38MAPK pathway with
diosgenin treatment, and the levels of E-cadherin, a downstream EMT
molecule, were measured by RT-qPCR and western blotting. The
results showed that the E-cadherin levels in osteosarcoma cells
were decreased after diosgenin administration in combination with
the pathway inhibitor. The above experiments thus suggested that
the pP38 protein was a target of diosgenin-mediated inhibition of
EMT initiation in osteosarcoma cells.
In conclusion, the present study examined the
ability of diosgenin to inhibit the invasion and migration of two
different osteosarcoma cell lines in vitro and confirmed
that this drug can inhibit EMT initiation in osteosarcoma cells by
decreasing TGF-β1 expression and increasing E-cadherin protein
levels. Additionally, the MAPK signaling pathway was involved in
the diosgenin-mediated inhibition of EMT in osteosarcoma cells. The
target of this drug was the pP38 protein in the MAPK signaling
pathway. As a highly efficient plant extract with low toxicity,
diosgenin may have utility as an auxiliary drug for the clinical
reduction of osteosarcoma metastasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. SBK2019020703),
Wuxi Science and Technology Development Project (grant no.
CSZ0N1619) and Qinglan Project of Excellent Teaching Team in
Jiangsu (grant no. TD2019).
Availability of data and materials
The datasets used and/or analyzed during the present
study is available from the corresponding author on reasonable
request.
Authors' contributions
LZ supervised and directed this study. HH and LZ
performed the majority of the experiments. LZ contributed to the
conception and design of the experiments the project design. CN and
JZ contributed to the cell culture and RNA extraction. XQ helped
with interpretation of data for the experiments. CN analyzed the
data and H wrote this manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujiwara T, Oda M, Yoshida A, Ogura K,
Chuman H, Kusumoto M and Kawai A: Atypical manifestation of lung
metastasis 17 years after initial diagnosis of low-grade
centralosteosarcoma. J Orthop Sci. 22:357–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, He Z, Li Y, Yang Y, Shi J, Liu X,
Yuan T, Xia J, Li D, Zhang J and Yang Z: Selection of surgical
methods in the treatment of upper tibia osteosarcoma and prognostic
analysis. Oncol Res Treat. 40:528–532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng ZP, Liu BY, Sun Y, Jin T, Li B, Ding
Y and Niu XH: Transition from tumor tissue to bone marrow in
patients with appendicular osteosarcoma after neoadjuvant
chemotherapy. Chin Med J (Engl). 130:2215–2218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao K, Yang SY, Geng J, Gong X, Gong W,
Shen L and Ning B: Combination of anginex gene therapy and
radiation decelerates the growth and pulmonary metastasis of human
osteosarcoma xenografts. Cancer Med. 7:2518–2529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fenger JM, London CA and Kisseberth WC:
Canine osteosarcoma: A naturally occurring disease to inform
pediatric oncology. ILAR J. 55:69–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Hu Q, Li G, Li L, Liang S, Zhang
Y, Liu J, Fan Z, Li L, Zhou B, et al: ONZIN upregulation by mutant
p53 contributes to osteosarcoma metastasis through the CXCL5-MAPK
signaling pathway. Cell Physiol Biochem. 48:1099–1111. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuo B, Li Y, Gu F, Li Z, Sun Q, Shi Y,
Shen Y, Zhang F, Wang R and Wang X: Overexpression of CD155 relates
to metastasis and invasion in osteosarcoma. Oncol Lett.
15:7312–7318. 2018.PubMed/NCBI
|
|
9
|
Liu P, Yang P, Zhang Z, Liu M and Hu S:
Ezrin/NF-κB pathway regulates EGF-induced epithelial-mesenchymal
transition (EMT), metastasis, and progression of osteosarcoma. Med
Sci Monit. 24:2098–2108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Tang YM, Yu SL, Han YW, Kou JP,
Liu BL and Yu BY: Advances in the pharmacological activities and
mechanisms of diosgenin. Chin J Nat Med. 13:578–587.
2015.PubMed/NCBI
|
|
11
|
Yan W, Ji L, Hang S and Shun Y: New ionic
liquid-based preparative method for diosgenin from Rhizoma
dioscoreae nipponicae. Pharmacogn Mag. 9:250–254. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiasalari Z, Rahmani T, Mahmoudi N,
Baluchnejadmojarad T and Roghani M: Diosgenin ameliorates
development of neuropathic pain in diabetic rats: Involvement of
oxidative stress and inflammation. Biomed Pharmacother. 86:654–661.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hua S, Li Y, Su L and Liu X: Diosgenin
ameliorates gestational diabetes through inhibition of sterol
regulatory element-binding protein-1. Biomed Pharmacother.
84:1460–1465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei Z, Xin G, Wang H, Zheng H, Ji C, Gu J,
Ma L, Qin C, Xing Z, Niu H and Huang W: The diosgenin prodrug
nanoparticles with pH-responsive as a drug delivery system uniquely
prevents thrombosis without increased bleeding risk. Nanomedicine.
14:673–684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CH, Wang CC, Lin YC, Hori M and Jan
TR: Oral administration with diosgenin enhances the induction of
intestinal T helper 1-like regulatory T cells in a murine model of
food allergy. Int Immunopharmacol. 42:59–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YJ, Pan KL, Hsieh TC, Chang TY, Lin
WH and Hsu JT: Diosgenin, a plant-derived sapogenin, exhibits
antiviral activity in vitro against hepatitis C virus. J Nat Prod.
74:580–584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pons-Fuster López E, Wang QT, Wei W and
López Jornet P: Potential chemotherapeutic effects of diosgenin,
zoledronic acid and epigallocatechin-3-gallate on PE/CA-PJ15 oral
squamous cancer cell line. Arch Oral Biol. 82:141–146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhuvanalakshmi G, Basappa, Rangappa KS,
Dharmarajan A, Sethi G, Kumar AP and Warrier S: Breast cancer
stem-like cells are inhibited by diosgenin, a steroidal saponin, by
the attenuation of the Wnt β-catenin signaling via the Wnt
antagonist secreted frizzled related protein-4. Front Pharmacol.
8:1242017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nie C, Zhou J, Qin X, Shi X, Zeng Q, Liu
J, Yan S and Zhang L: Diosgenin-induced autophagy and apoptosis in
a human prostate cancer cell line. Mol Med Rep. 14:4349–4359. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai B, Liao A, Lee KK, Ban JS, Yang HS, Im
YJ and Chun C: Design, synthesis of methotrexate-diosgenin
conjugates and biological evaluation of their effect on
methotrexate transport- resistant cells. Steroids. 116:45–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghosh S, More P, Derle A, Kitture R, Kale
T, Gorain M, Avasthi A, Markad P, Kundu GC, Kale S, et al:
Diosgenin functionalized iron oxide nanoparticles as novel
nanomaterial against breast cancer. J Nanosci Nanotechnol.
15:9464–9472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding W, Jiang Y, Jiang Y, Zhu T, Xu Y,
Jiang W, Zhu W, Tang Z, Ge Z, Ma T and Tan Y: Role of SB203580 in
the regulation of human esophageal cancer cells under the effection
of diosgenin. Int J Clin Exp Med. 8:2476–2479. 2015.PubMed/NCBI
|
|
23
|
Goossens S, Vandamme N, Van Vlierberghe P
and Berx G: EMT transcription factors in cancer development
re-evaluated: Beyond EMT and MET. Biochim Biophys Acta Rev Cancer.
1868:584–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Z, Livas T and Kyprianou N: Anoikis
and EMT: Lethal ‘Liaisons’ during cancer progression. Crit Rev
Oncog. 21:155–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Illam SP, Narayanankutty A, Mathew SE,
Valsalakumari R, Jacob RM and Raghavamenon AC: Epithelial
mesenchymal transition in cancer progression: Prev entive
phytochemicals. Recent Pat Anticancer Drug Discov. 12:234–246.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zahedi A, Phandthong R, Chaili A, Remark G
and Talbot P: Epithelial-to-mesenchymal transition of A549 lung
cancer cells exposed to electronic cigarettes. Lung Cancer.
122:224–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Liang X, Liang H and Wang B:
SENP1/HIF-1α feedback loop modulates hypoxia-induced cell
proliferation, invasion and EMT in human osteosarcoma cells. J Cell
Biochem. 119:1819–1826. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Zhang K, Li Y and He Q:
Estrogen-related receptor α participates transforming growth
factor-β (TGF-β) induced epithelial-mesenchymal transition of
osteosarcoma cells. Cell Adh Migr. 11:338–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Weng Y, He F, Liang D and Cai L:
LncRNA MALAT-1 competitively regulates miR-124 to promote EMT and
development of non-small-cell lung cancer. Anticancer Drugs.
29:628–636. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen C, Liang QY, Chen HK, Wu PF, Feng ZY,
Ma XM, Wu HR and Zhou GQ: DRAM1 regulates the migration and
invasion of hepatoblastoma cells via autophagy-EMT pathway. Oncol
Lett. 16:2427–2433. 2018.PubMed/NCBI
|
|
33
|
Cheng G, Gao F, Sun X, Bi H and Zhu Y:
Paris saponin VII suppresses osteosarcoma cell migration and
invasion by inhibiting MMP-2/9 production via the p38 MAPK
signaling pathway. Mol Med Rep. 14:3199–3205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhan L, Chen L and Chen Z: Knockdown of
FUT3 disrupts the proliferation, migration, tumorigenesis and TGF-β
induced EMT in pancreatic cancer cells. Oncol Lett. 16:924–930.
2018.PubMed/NCBI
|
|
35
|
Kaur G, Li CG, Chantry A, Stayner C,
Horsfield J and Eccles MR: SMAD proteins directly suppress PAX2
transcription downstream of transforming growth factor-beta 1
(TGF-β1) signalling in renal cell carcinoma. Oncotarget.
9:26852–26867. 2018.PubMed/NCBI
|
|
36
|
David CJ, Huang YH, Chen M, Su J, Zou Y,
Bardeesy N, Iacobuzio-Donahue CA and Massagué J: TGF-β tumor
suppression through a lethal EMT. Cell. 164:1015–1030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen CL, Chen YH, Tai MC, Liang CM, Lu DW
and Chen JT: Resveratrol inhibits transforming growth
factor-β2-induced epithelial-to-mesenchymal transition in human
retinal pigment epithelial cells by suppressing the smad pathway.
Drug Des Devel Ther. 11:163–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang TW, Li ST, Fang KM and Young TH:
Hyaluronan antagonizes the differentiation effect of TGF-β1 on
nasal epithelial cells through down-regulation of TGF-β type I
receptor. Artif Cells Nanomed Biotechnol. 46 (Suppl 3):S254–S263.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan W, Qian W, Zhou C, Cao J, Qin T, Xiao
Y, Cheng L, Li J, Chen K, Li X, et al: Metformin suppresses the
invasive ability of pancreatic cancer cells by blocking autocrine
TGF-β1 signaling. Oncol Rep. 40:1495–1502. 2018.PubMed/NCBI
|
|
40
|
Ohtani H, Terashima T and Sato E: Immune
cell expression of TGFβ1 in cancer with lymphoid stroma: Dendritic
cell and regulatory T cell contact. Virchows Arch. 472:1021–1028.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tao Y, Sturgis EM, Huang Z, Wang Y, Wei P,
Wang JR, Wei Q and Li G: TGFβ1 genetic variants predict clinical
outcomes of HPV-positive oropharyngeal cancerpatients after
definitive radiotherapy. Clin Cancer Res. 24:2225–2233. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song J and Shi W: The concomitant
apoptosis and EMT underlie the fundamental functions of TGF-β. Acta
Biochim Biophys Sin (Shanghai). 50:91–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang YP, Wang QY, Li CH and Li XW: COX-2
inhibition by celecoxib in epithelial ovarian cancer attenuates
E-cadherin suppression through reduced Snail nuclear translocation.
Chem Biol Interact. 292:24–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Chen H, Liu Z, Ye Z, Gou S and Wang
C: Overexpression of MIST1 reverses the epithelial-mesenchymal
transition and reduces the tµmorigenicity of pancreatic cancer
cells via the Snail/E-cadherin pathway. Cancer Lett. 431:96–104.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Shen J, Yan D, Yuan B, Zhang S,
Wei J and Du T: Euchromatic histone lysine methyltransferase 1
regulates cancer development in human gastric cancer by regulating
E-cadherin. Oncol Lett. 15:9480–9486. 2018.PubMed/NCBI
|
|
46
|
Zhu S, Deng S, He C, Liu M, Chen H, Zeng
Z, Zhong J, Ye Z, Deng S, Wu H, et al: Reciprocal loop of
hypoxia-inducible factor-1α (HIF-1α) and metastasis-associated
protein 2 (MTA2) contributes to the progression of pancreatic
carcinoma by suppressing E-cadherintranscription. J Pathol.
245:349–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma L, Liu L, Ma Y, Xie H, Yu X, Wang X,
Fan A, Ge D, Xu Y, Zhang Q and Song C: The role of
E-cadherin/β-catenin in hydroxysafflor yellow a inhibiting
adhesion, invasion, migration and lung metastasis of hepatoma
cells. Biol Pharm Bull. 40:1706–1715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Karim NA, Eldessouki I, Yellu M, Namad T,
Wang J and Gaber O: A case study in advanced lung cancer patients
with vimentin over expression. Clin Lab. 63:1575–1579. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Noh H, Yan J, Hong S, Kong LY,
Gabrusiewicz K, Xia X, Heimberger AB and Li S: Discovery of cell
surface vimentin targeting mAb for direct disruption of GBM tumor
initiating cells. Oncotarget. 7:72021–72032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lou L, Yu Z, Wang Y, Wang S and Zhao Y:
c-Src inhibitor selectively inhibits triple negative breast cancer
overexpressed vimentin in vitro and in vivo. Cancer Sci.
109:1648–1659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu CY, Qin MB, Tan L, Liu SQ and Huang JA:
NIBP impacts on the expression of E-cadherin, CD44 and vimentin in
colon cancer via the NF-κB pathway. Mol Med Rep. 13:5379–5385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vyas AR and Singh SV: Functional relevance
of D, L-sulforaphane-mediated induction of vimentin and plasminogen
activator inhibitor-1 in human prostate cancer cells. Eur J Nutr.
53:843–852. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang M, Wang YP, Zhu LQ, Cai Q, Li HH and
Yang HF: MAPK pathway mediates epithelial-mesenchymal transition
induced by paraquat in alveolar epithelial cells. Environ Toxicol.
31:1407–1414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xiao K, Cao S, Jiao L, Song Z, Lu J and Hu
C: TGF-β1 protects intestinal integrity and influences Smads and
MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate
Immun. 23:276–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park JH, Yoon J, Lee KY and Park B:
Effects of geniposide on hepatocytes undergoing
epithelial-mesenchymal transition in hepatic fibrosis by targeting
TGFβ/Smad and ERK-MAPK signaling pathways. Biochimie. 113:26–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang Y, Wu C, Boye A, Wu J, Wang J, Yang
X and Yang Y: MAPK inhibitors modulate Smad2/3/4 complex
cyto-nuclear translocation in myofibroblasts via Imp7/8 mediation.
Mol Cell Biochem. 406:255–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kang HM, Park BS, Kang HK, Park HR, Yu SB
and Kim IR: Delphinidin induces apoptosis and inhibits
epithelial-to-mesenchymal transition via the ERK/p38 MAPK-signaling
pathway in human osteosarcoma cell lines. Environ Toxicol.
33:640–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W
and Peng Y: AEG-1 participates in TGF-beta1-induced EMT through p38
MAPK activation. Cell Biol Int. 37:1016–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lyu Z, Cao J, Wang J and Lian H:
Protective effect of vitexin reduces sevoflurane-induced neuronal
apoptosis through HIF-1α, VEGF and p38 MAPK signaling pathway in
vitro and in newborn rats. Exp Ther Med. 15:3117–3123.
2018.PubMed/NCBI
|
|
60
|
Xiang S, Xiang T, Xiao Q, Li Y, Shao B and
Luo T: Zinc-finger protein 545 is inactivated due to promoter
methylation and functions as a tumor suppressor through the
Wnt/β-catenin, PI3K/AKT and MAPK/ERK signaling pathways in
colorectal cancer. Int J Oncol. 51:801–811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kello M, Kulikova L, Vaskova J, Nagyova A
and Mojzis J: Fruit peel polyphenolic extract induced apoptosis in
human breast cancer cells is associated with ROS production and
modulation of p38MAPK/Erk1/2 and the Akt signaling pathway. Nutr
Cancer. 69:920–931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Long C, Chen J, Zhou H, Jiang T, Fang X,
Hou D, Liu P and Duan H: Diosgenin exerts its tumor suppressive
function via inhibition of Cdc20 in osteosarcoma cells. Cell Cycle.
18:346–358. 2019. View Article : Google Scholar : PubMed/NCBI
|