Introduction
Cetuximab (Cmab), a monoclonal antibody that
specifically binds to and inhibits the activity of human epidermal
growth factor receptor (EGFR), is used as a molecularly targeted
anticancer agent. The antitumor activities of Cmab are believed to
be confined to wild-type KRAS tumors, and indications for
its use are therefore limited to such cases. Recently, however, a
number of studies have reported Cmab to also be effective against
KRAS p.G13D mutant-type tumors (1–3). We
recently encountered a case of metastatic ascending colon cancer
harboring a KRAS p.G13D mutation that responded well to
FOLFOX4 [folinic acid (FOL), fluorouracil (F) plus oxaliplatin
(OX)] + Cmab therapy, which we present in this study.
Case report
The patient was a 65-year-old female. Chest X-rays
performed at another hospital as part of an annual health checkup
revealed granular shadows in both lung fields. The patient was
therefore referred to our Department of Respiratory Medicine in
November 2010, and a detailed, whole-body examination was
performed.
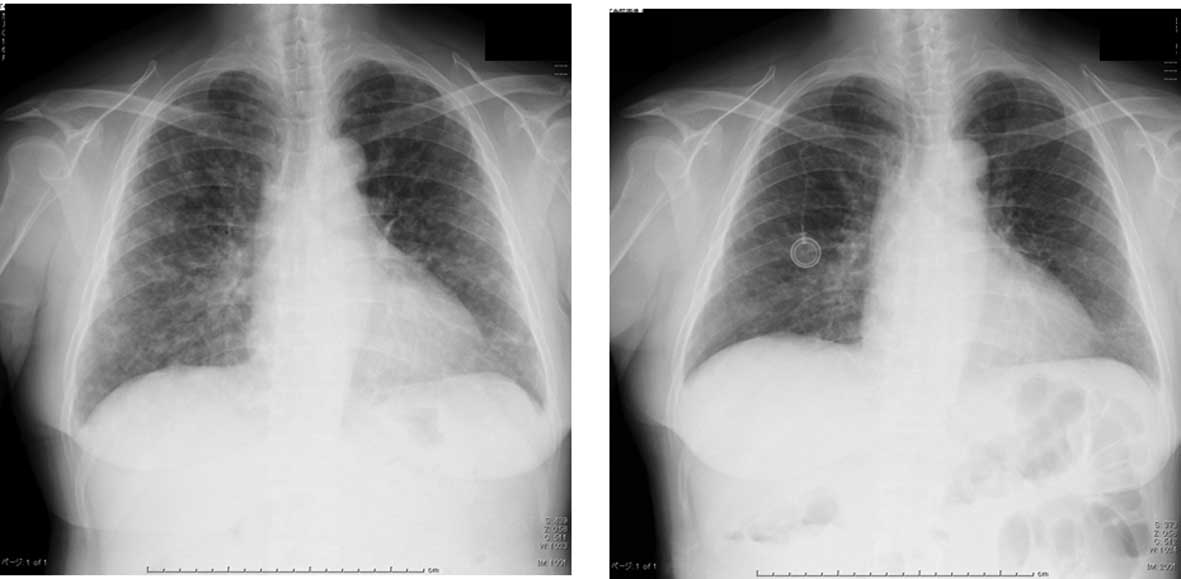
A chest X-ray confirmed numerous granular shadows in
both lung fields (Fig. 1A).
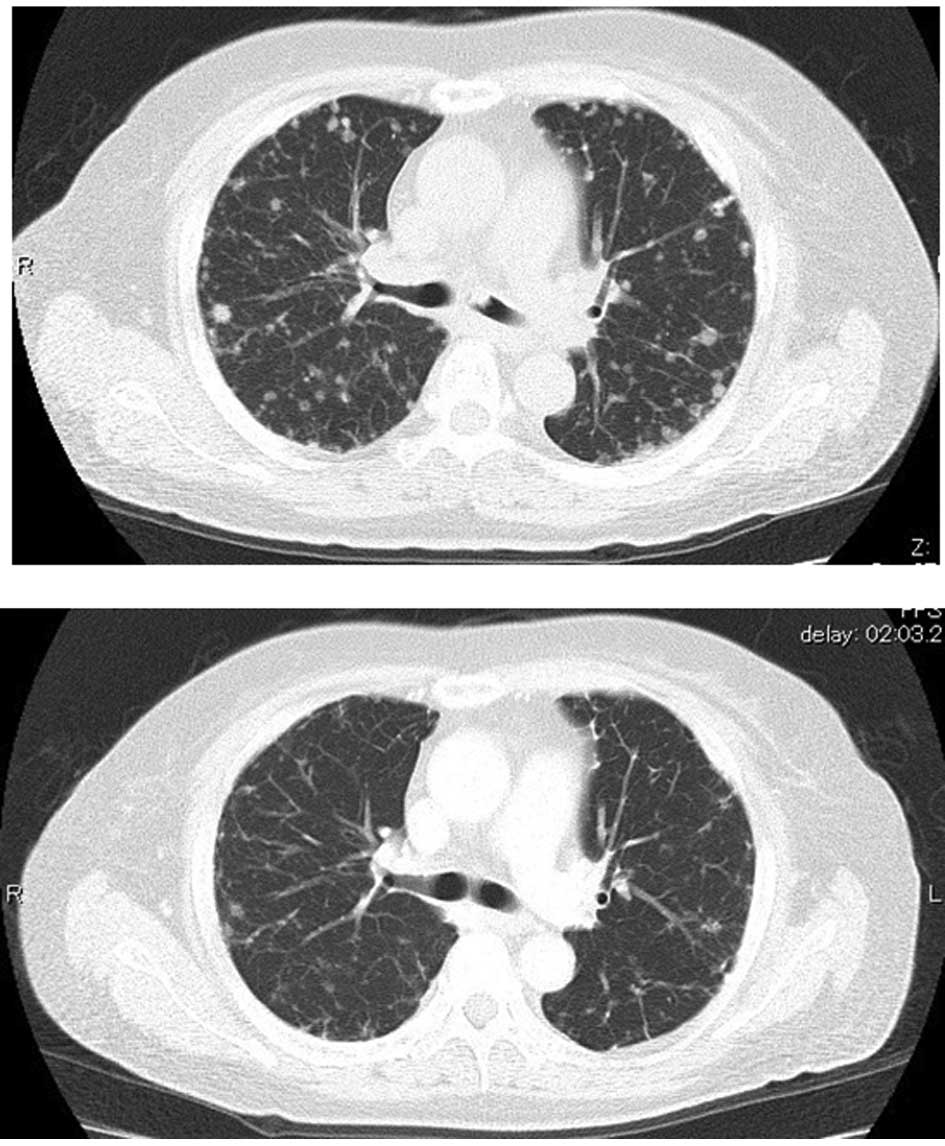
Computed tomography of the thorax revealed numerous
nodular lesions in both lung fields and a nodular lesion at the
entrance to the left main bronchus (Fig. 2A).
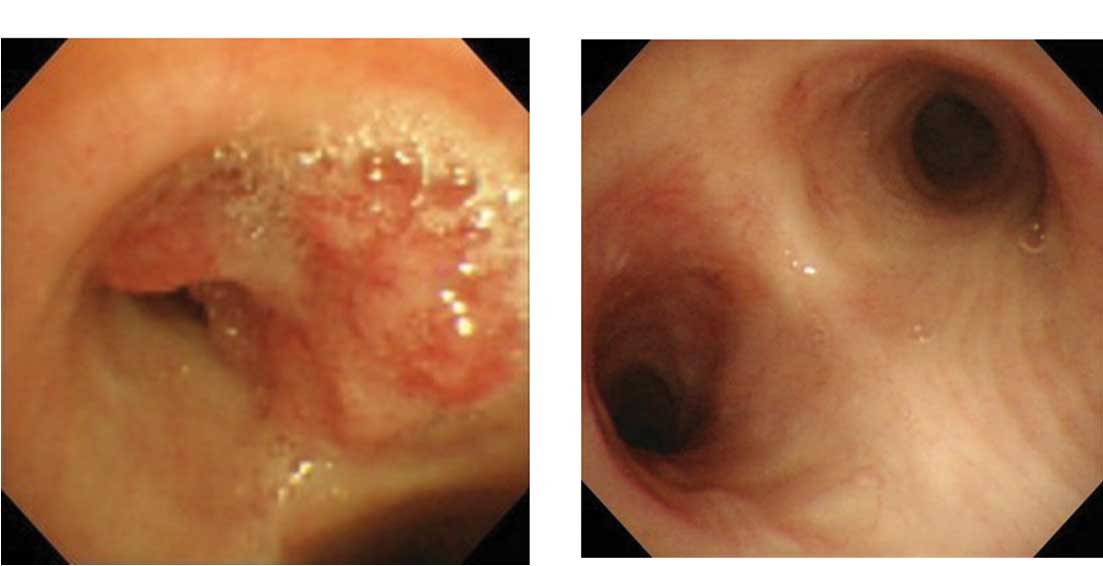
Bronchoscopy revealed a polypoid mass at the
entrance to the left main bronchus. The biopsy specimen was
pathologically rated as adenocarcinoma, suggesting metastasis of
colorectal tubular adenocarcinoma (Fig.
3A).
Colonoscopy revealed a tumorous lesion in the lumen
of the ascending colon (Fig. 4A).
The biopsy specimen was pathologically rated as a moderately
differentiated tubular adenocarcinoma.
Based on these findings, ascending colon cancer with
multiple lung metastases was diagnosed and the patient was referred
to the Department of Surgery, where surgical resection of the
primary cancer was initially considered. However, considering the
absence of symptoms at the time of referral and the post-operative
risk of respiratory complications, anticancer drug therapy was
selected as first-line therapy, and FOLFOX4 was started in
mid-December. Four days after the first session of FOLFOX4 therapy,
the patient was urgently hospitalized for severe nausea. Treatment
with aprepitant alleviated these symptoms, allowing the patient to
be discharged from the hospital on the fourth hospital day. Later,
a KRAS test revealed a KRAS p.G13D mutation.
Therefore, with informed consent, FOLFOX4 therapy was combined with
weekly Cmab early in January 2011. The response to chemotherapy was
evaluated following 2 sessions of FOLFOX therapy and 2 subsequent
sessions of FOLFOX4 + Cmab therapy.
A chest X-ray revealed the presence of numerous
granular shadows in both lung fields. There was, however,
disappearance or reduction in size of certain granular shadows
compared with the previous X-ray (Fig.
1B).
A thoracic CT scan revealed the continued presence
of numerous nodular lesions in both lung fields. There was,
however, disappearance or reduction in size of certain granular
shadows compared with the previous CT scan. The nodular lesion
observed at the entrance to the left main bronchus prior to
treatment had disappeared (Fig.
2B).
Bronchoscopy revealed only mild redness and no
tumorous lesion in the left main bronchus (Fig. 3B).
Colonoscopy revealed that the tumorous lesion in the
lumen of the ascending colon had disappeared. Converging mucosal
folds accompanied by redness were, however, noted in this area
(Fig. 4B), yielding a pathological
rating of granulation in the colonic mucosa.
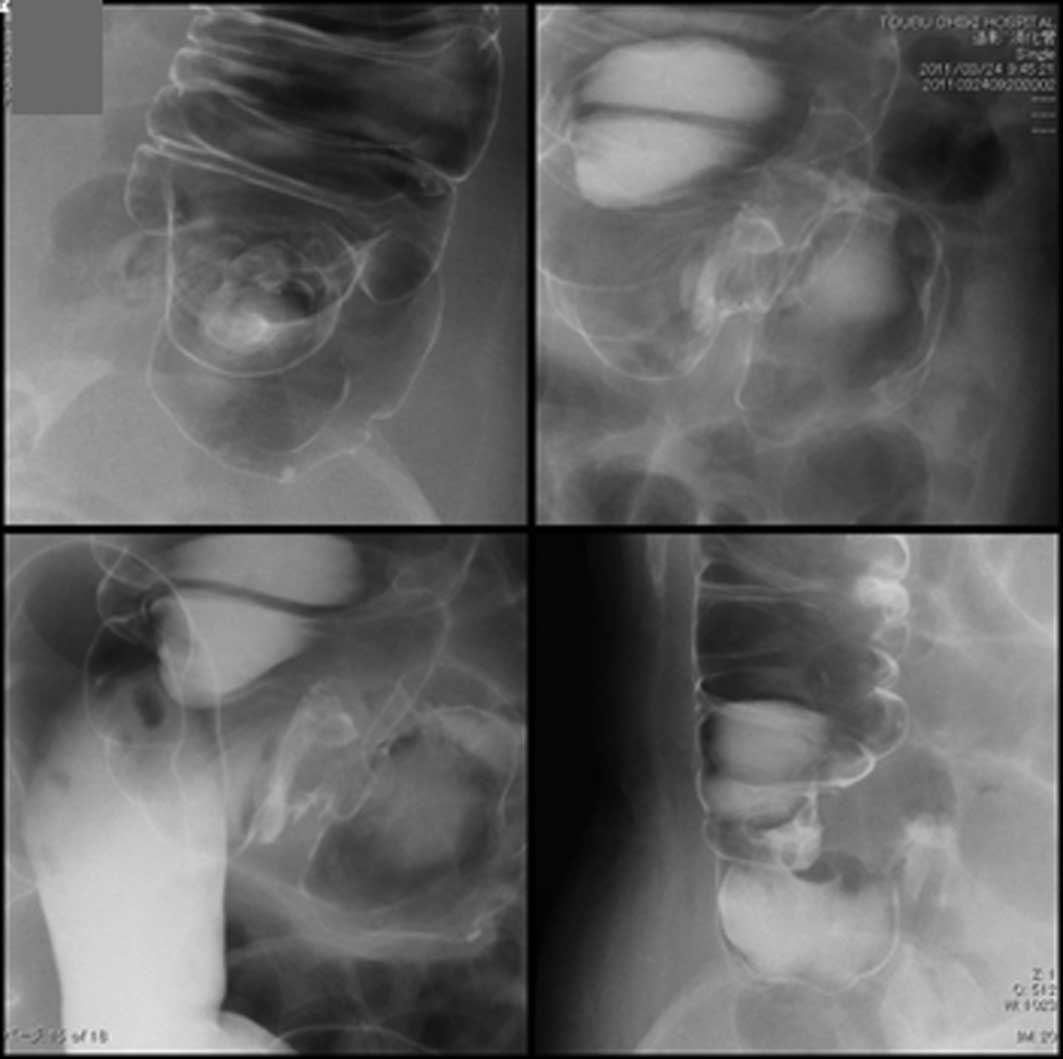
A barium enema revealed irregularities in a section
of the wall of the ascending colon (Fig. 5).
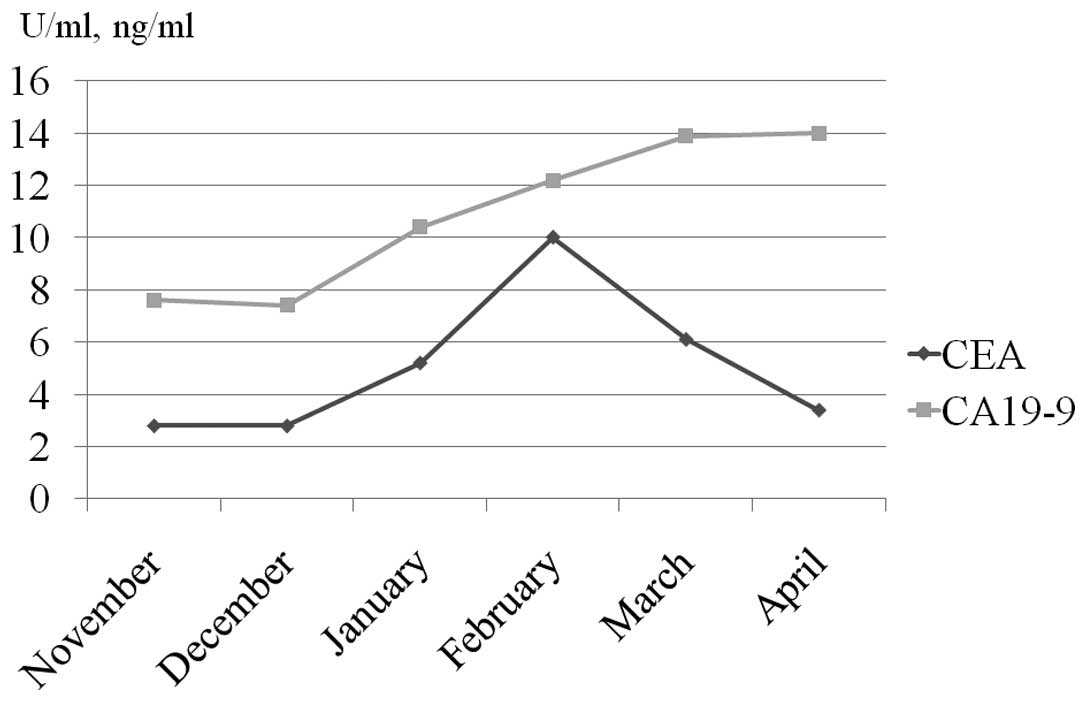
Changes in tumor marker levels are shown in Fig. 6. Carcinoembryonic antigens, which
had shown a transient increase, had returned to normal levels.
These findings suggest that the patient responded
well to FOLFOX4 + Cmab therapy, with the focus of metastasis in the
left main bronchus (a lesion of high concern at the start of
treatment) disappearing. Therefore, right hemicolectomy was
performed in mid-April 2011 following 2 sessions of FOLFOX4 therapy
and 4 subsequent sessions of FOLFOX4 + Cmab therapy.
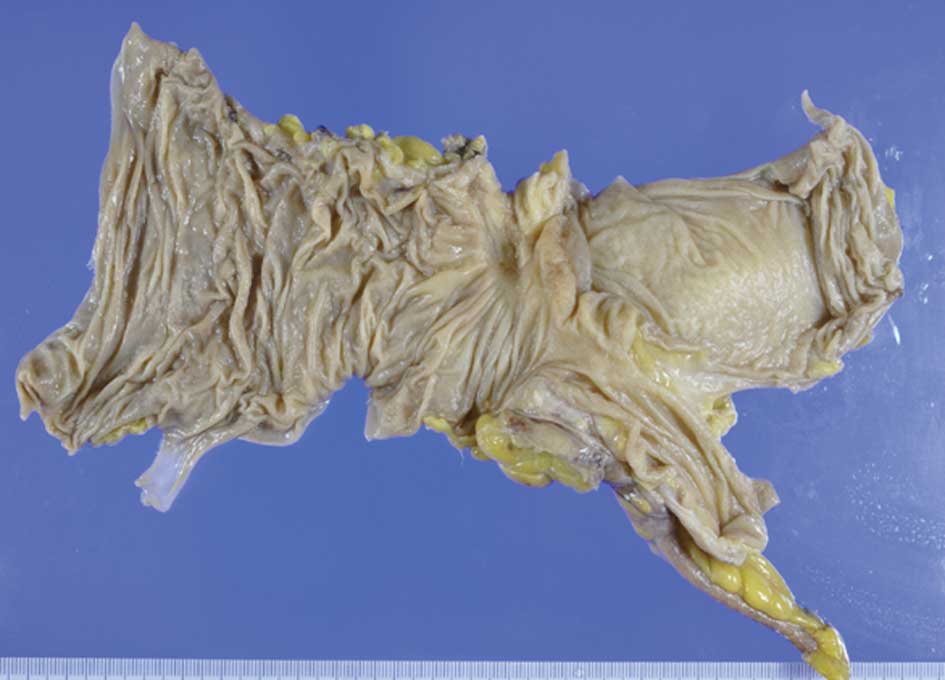
The resected specimen was found to harbor an
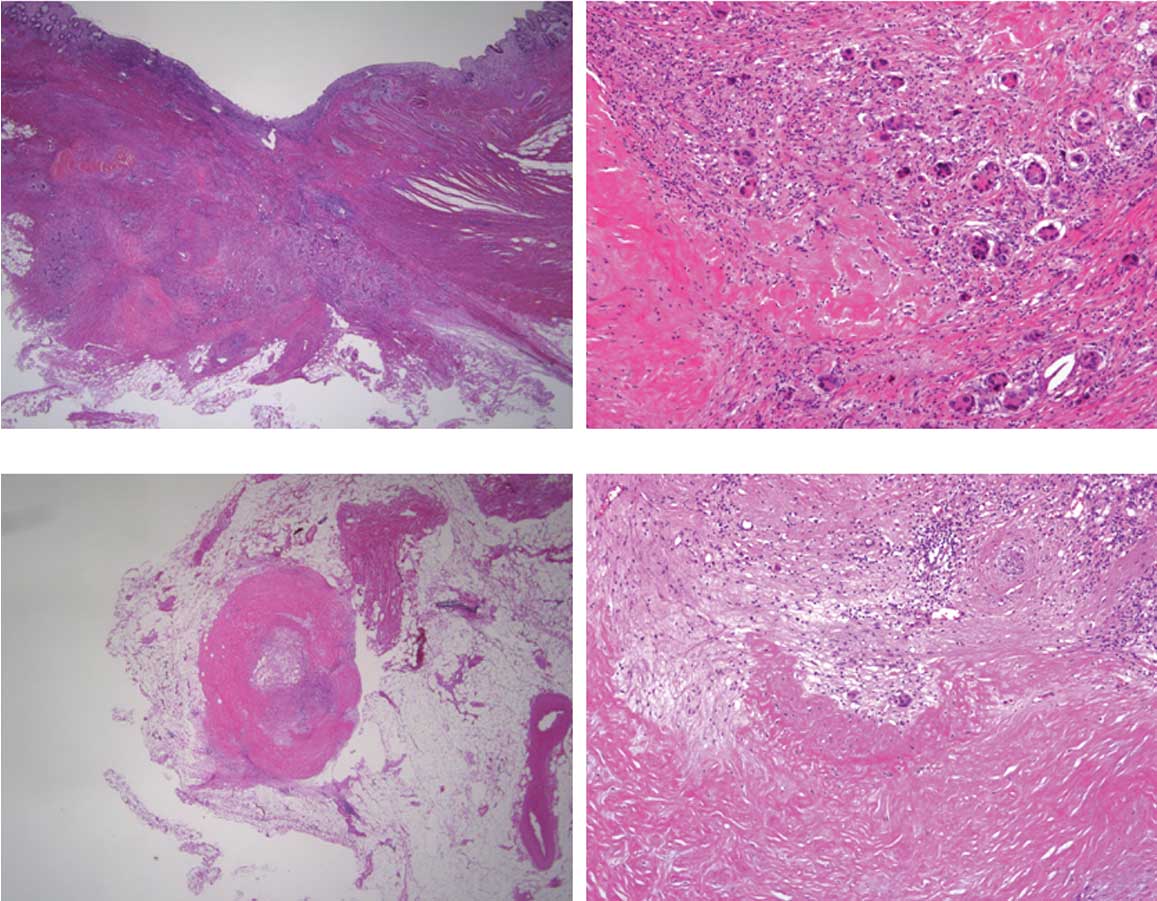
ulcerative lesion accompanied by converging folds (Fig. 7). Histopathological examination
revealed a tubular adenocarcinoma with marked degeneration and
fibrosis, indicating the effect of chemotherapy in the primary
tumor (pT3N1). However, viable cancer cells were also observed
(Fig. 8A and B). Residual cancer
cells with marked degeneration and fibrosis were noted in the lymph
nodes (Fig. 8C and D).
No postoperative complications occurred and the
patient was discharged from the hospital on the 16th hospital day.
The patient is currently being managed as an outpatient (mFOLFOX6 +
Cmab, biweekly).
Discussion
EGFR is found in a number of locations, including
the lungs, skin and gastrointestinal epithelium, is involved in the
proliferation, invasion and metastasis of cancer cells.
Additionally, its excessive expression is associated with poor
prognosis in patients with various types of cancer, including
colorectal cancer (4). Cetuximab, a
human/mouse chimerized monoclonal antibody (a type of IgG1),
specifically binds to EGFR, inhibiting EGFR-mediated signal
transduction in cancer cells. A molecularly targeted drug, Cmab
exhibits antitumor activity through induction of apoptosis
(5). The KRAS gene, an
oncogene located on the short arm of chromosome 12, encodes p21, a
protein with GTPase activity that is capable of binding to GTP and
GDP. Cetuximab is believed to exert no antitumor activities in
cancer cells harboring a KRAS gene mutation due to the
constitutive activation of RAS located downstream of EGFR.
Cetuximab has, therefore, been reported to manifest antitumor
activities against wild-type KRAS tumors (6), and its use in the treatment of
patients with wild-type KRAS tumors is recommended in Japan.
KRAS gene mutations were observed in 37.6% of all colorectal
cancer patients (7), and mutations
of codons 12 or 13 (exon 2 region) account for 94% of all
KRAS gene mutations (8).
Wild-type codon 13 is known to be GGC (glycine: G), and its known
mutants include GAC (aspartic acid: D), TGC (cysteine: C) and CGC
(arginine: R). Aspartic acid mutation (p.G13D) has been reported to
account for approximately 94% of all codon 13 mutations and to
occupy approximately 21% of all KRAS gene mutations
(8). Recent reports that Cmab also
exerts antitumor activities against KRAS p.G13D mutant-type
cases have receved much attention (1,3).
KRAS p.G13D mutations, which are often observed in females
and in the right half of the colon, have a reported incidence of
7.7% in Japan (7).
In Japan, Cmab was previously used in combination
with FOLFIRI [folinic acid (FOL), fluorouracil (F) plus irinotecan
(IRI)] as second-line therapy and in combination with irinotecan or
in single-drug administration as third-line therapy. However, its
use as a first-line drug or in combination with FOLFOX has been
permitted since 2010. In the present case, resection of the primary
cancer was considered initially. However, taking into account the
absence of symptoms arising from the primary cancer and potential
respiratory complications arising from surgical intervention, such
as postoperative atelectasis caused by metastatic obstruction of
the left main bronchus, first-line anticancer drug therapy was
finally selected. Therefore, with informed consent, the patient was
treated with FOLFOX4 + Cmab therapy, although the tumor was a
KRAS p.G13D-mutant type. Response was evaluated following 2
sessions of FOLFOX4 therapy and 2 sessions of FOLFOX4 + Cmab
therapy. The results revealed the disappearance of metastatic foci
from the left main bronchus, size reduction in multiple lung
metastases and normalization of tumor marker levels. Therefore,
anticancer drug therapy was continued and surgery was carried out
following 2 sessions of FOLFOX4 therapy and 4 sessions of FOLFOX4 +
Cmab therapy. Although subsequent histopathological examination of
the resected tissue specimen revealed residual cancer cells, it
also showed the marked efficacy of the chemotherapy regimen used.
We believe that these results validated the decision to select
anticancer drug therapy as the first-line therapy in this case.
Resection of the primary cancer is often possible,
even when metastases are present, as in the present case. In Japan,
resection of the primary cancer is often performed at the beginning
of treatment, and the foci of metastasis are subsequently treated
with anticancer drugs. This is followed, if possible, by resection
of the metastatic foci. By contrast, in Western countries, a number
of reports have shown that treatment often commences with drug
therapy using FOLFOX/FOLFIRI + molecularly targeted drugs, followed
by resection of the primary cancer. The foci of metastasis are then
treated with anticancer drugs followed by surgical resection where
possible (9–11). Each of these therapeutic strategies
has advantages and disadvantages. However, performing resection of
the primary cancer first in cases presenting with symptoms, such as
stenosis and bleeding, attributable to the resectable primary
cancer is preferred.
The clinical response rate to treatment with
FOLFOX/FOLFIRI + molecularly targeted drugs is between 70 and 80%
(12–14). First-line therapy with
FOLFOX/FOLFIRI + molecularly targeted drugs should allow more
appropriate selection of treatment (including conversion therapy)
and lead to better prognoses in symptom-free cases. We believe that
FOLFOX/FOLFIRI + molecularly targeted drug therapy is likely to
become widely accepted in Japan as a first-line therapy in advanced
colorectal cancer patients with metastasis.
In conclusion, in this study, we presented a case of
metastatic ascending colon cancer harboring a KRAS p.G13D
mutation in which the patient responded well to first-line therapy
with FOLFOX4 + Cmab.
Acknowledgements
The authors thank Associate Professor Jeremy
Williams, Tokyo Dental College, for his assistance with the English
of the manuscript.
References
|
1
|
De Roock W, Jonker DJ, Di Nicolantonio F,
et al: Association of KRAS p. G13D mutation with outcome in
patients with chemotherapy-refractory metastatic colorectal cancer
treated with cetuximab. JAMA. 304:1812–1820. 2010.PubMed/NCBI
|
|
2
|
Bando H, Yoshino T, Shinozaki E, et al:
Clinical outcome in patients with metastatic colorectal cancer
harboring KRAS p. G13D mutation treated with cetuximab. J Clin
Oncol. 29:4482011.
|
|
3
|
Tejpar S, Bokemeyer C, Celik I, et al:
Influence of KRAS G13D mutations on outcome in patients with
metastatic colorectal cancer (mCRC) treated with first-line
chemotherapy with or without cetuximab. J Clin Oncol.
29:35112011.
|
|
4
|
Mayer A, Takimoto M, Fritz E, et al: The
prognostic significance of proliferating cell nuclear antigen,
epidermal growth factor receptor, and mdr gene expression in
colorectal cancer. Cancer. 71:2454–2460. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Nowacki M, Lang I, et al:
Randomized phase III study of irinotecan and 5-FU/FA with or
without cetuximab in the first-line treatment of patients with
metastatic colorectal cancer (mCRC): The CRYSTAL trial. J Clin
Oncol. 25:40002007.PubMed/NCBI
|
|
7
|
Yoshino T, Watanabe T, Yamazaki K, et al:
Japan Study Group of KRAS Mutation in Colorectal Cancer:
Clinicopathological features in metastatic colorectal cancer
patients with KRAS wild type compared with codon 12 and codon 13
mutant: Results from a multicenter, cross-sectional study by the
Japan study group of KRAS mutation in colorectal cancer. J Clin
Oncol. 29:4072011.
|
|
8
|
Brink M, De Goeij AF, Weijenberg MP, et
al: K-ras oncogene mutations in sporadic colorectal cancer in The
Netherlands Cohort Study. Carcinogenesis. 24:703–710. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarela AI, Guthrie JA, Seymour MT, et al:
Non-operative management of the primary tumor in patients with
incurable stage IV colorectal cancer. Br J Surg. 88:1352–1356.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muratore A, Zorzi D, Bouzari H, et al:
Asymptomatic colorectal cancer with un-resectable liver metastasis:
immediate colorectal resection or up-front systemic chemotherapy?
Ann Surg Oncol. 14:766–770. 2006. View Article : Google Scholar
|
|
11
|
Mentha G, Majno PE, Andres A, et al:
Neoadjuvant chemotherapy and resection of advanced synchronous
liver metastasis before treatment of the colorectal primary. Br J
Surg. 93:872–878. 2006. View
Article : Google Scholar
|
|
12
|
Van Cutsem E, Köhne CH, Láng I, et al:
Cetuximab plus irinotecan, fluorouracil, and leucovorin as
first-line treatment for metastatic colorectal cancer: updated
analysis of overall survival according to tumorKRAS and BRAF
mutation status. J Clin Oncol. 33:50912010.PubMed/NCBI
|
|
13
|
Bokemeyer C, Bondarenko I, Hartmann JT, et
al: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
the OPUS study. Ann Oncol. doi: 10.1093.annonc.mdq632. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Cutsem E, Lang I, D’Haens G, et al:
KRAS status and efficacy in the Crystal study: 1st line treatment
for patients with metastatic colorectal cancer receiving FOLFIRI
with or without cetuximab. Ann Oncol. 19:7102008.
|