Introduction
Epithelial ovarian cancer is the leading cause of
mortality from gynecological cancers, and the majority of patients
present with advanced stage (1,2). The
5-year survival rates for stage IIIC and IV patients are 29 and
13%, respectively (3,4). Researchers have aimed to improve the
survival rates of epithelial ovarian cancer patients by studying
the effects of early diagnosis, cytoreductive surgery and
chemotherapeutic agents (5–8). Recent progress in understanding the
pathogenesis of epithelial ovarian cancer suggests that the
development and differentiation of ovarian carcinoma is affected by
certain factors that induced the spread of tumor cells from primary
to distal sites (9,10).
A recent study demonstrated that cofilin 1 is a
small ubiquitous protein (approximately 19 kD) that is able to bind
monomeric (G) and filamentous (F) actin (11). By severing actin filaments, cofilin
1 increases the number of filament ends for polymerization and
depolymerization (12). Cofilin 1
then promotes cytoskeletal dynamics by depolymerizing actin
filaments, which is critical for several processes, including
cytokinesis and cell motility (13). The activity status of cofilin 1 is
directly associated with invasion, intravasation, and metastasis of
mammary tumors (14). However,
there have been no studies demonstrating a correlation between
cofilin 1 expression and the progression and differentiation of
patients with ovarian cancer. The aim of the study was to
investigate the correlation between cofilin 1 expression and the
differentiation of epithelial ovarian cancer in patients, and
provide an experimental foundation for the treatment of ovarian
cancer.
Materials and methods
Clinical tissue samples
All clinical tissue samples were obtained from
patients at The Women’s Hospital of Zhejiang University School of
Medicine (Hangzhou, China). The tissues, including 30 primary
epithelial ovarian carcinoma (10 well-differentiated cases, 13
moderately differentiated cases and 7 poorly differentiated cases),
14 borderline epithelial ovarian tumors, 13 benign epithelial
ovarian tumors and 10 normal ovarian tissues, were collected from
patients who underwent surgery. Written/verbal consent was obtained
from the patient or the patient’s parent/carer, and the use of
tissue was approved by the Institutional Review Board of the School
of Medicine (Zhejiang University, Zhejiang, China; No.
201104120).
Histological staining and
immunohistochemistry
Tissues were preserved in 4% paraformaldehyde for 24
h. Histological staining and immunohistochemistry were conducted on
8 μm sections of paraffin-embedded tissues. All sections
were deparaffinized and rehydrated. Sections for histological
analysis were stained with 0.3% cresyl violet (VWR International,
Buffalo Grove, IL, USA). Other sections for immunohistochemistry
were treated with antigen retrieval containing 10.2 mmol/l sodium
citrate buffer (pH 6.1), for 20 min at 95°C. The sections were then
washed in 0.01 M PBS containing 0.3% Triton X-100 (pH 7.4; PBS-T),
immersed in 2% normal goat serum in PBS for 2 h at 37°C, and
incubated overnight at 4°C with a polyclone cofilin 1 antibody
(1:100; Bioss Inc., Woburn, MA, USA) in PBS containing 1% bovine
serum albumin. Once the sections were washed in PBS (3×5 min), they
were incubated in biotinylated goat-anti-rabbit IgG (1:200; Boster
Biological Technology, Ltd., Wuhan, China) in PBS for 4 h at room
temperature, washed in PBS (3×5 min), incubated in
avidin-biotin-peroxidase complex solution (ABC; 1:100; Boster
Biological Technology, Ltd.) for 2 h at room temperature, then
rinsed again in PBS (3×5 min). Immunolabeling was visualized using
0.05% diaminobenzidine (DAB) plus 0.3% H2O2
in PBS. After staining, the sections were counterstained using
hematoxylin, then dehydrated through ethanol and xylene, and
Permount was applied to the coverslips. Rat IgG (1:200; Biomeda
Corporation, Foster City, CA, USA) was used instead of a primary
antibody as a negative control. Relative cofilin 1 protein
expression was determined as the product of the immunostaining
intensity and the percent of cells stained.
Image and statistical analysis
Immunostaining intensity was scored as follows: 0,
no staining; 1, light staining; 2, moderate staining; and 3, heavy
staining. The percent of cell staining was measured as follows: 0,
no detectable staining; 1, 1–33%; 2, 34–66%; and 3, 67–100%. The
final staining score was the product of the immunostaining
intensity score multiplied by the percent of cells stained score,
allowing for a maximal score of 9 and a minimal score of 0
(15). The mean ± SD for all data
were calculated. Statistical analysis was performed using SPSS
version 14.0 statistical software (SPSS Inc., St. Louis, MO, USA).
The correlation between all the groups was evaluated using the
Pearson’s correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
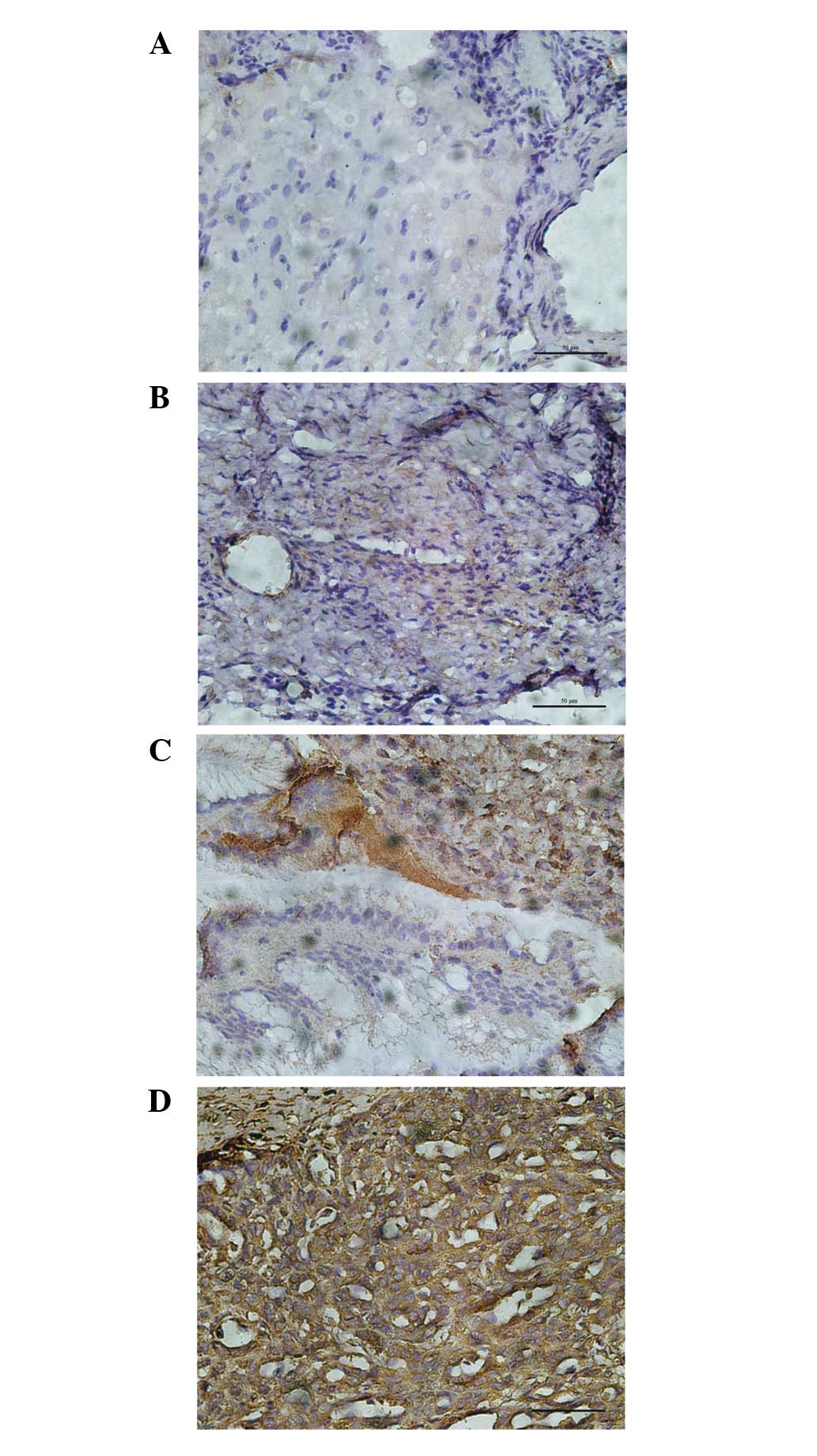
Diffuse cytoplasmic staining for cofilin 1 with
moderate intensity was observed in various proportions of tumor
cells. Cofilin 1 was scored for the cytoplasmic immunostaining
intensity and the percent of cells stained.
Among the 10 normal ovarian tissues, 5 patients
(50%) did not express cofilin 1 (score=0), and 5 patients (50%)
expressed cofilin 1 in 1–33% of cells (score=1). In the 13 benign
epithelial ovarian tumor patients, 8 (61.5%), 4 (30.8%) and 1
(7.7%) patients expressed cofilin 1 in 1–33%, 34–66% and 67–100% of
cells, respectively. Among the 14 borderline epithelial ovarian
tumor patients, 1–33% of cells expressed cofilin 1 in 8 patients
(57.1%) and 34–66% in 6 patients (42.9%). In the 30 primary
epithelial ovarian carcinoma patients, no patients expressed 1–33%
cell staining of cofilin 1, but 21 patients (70.0%) expressed
34–66% and 9 patients (30.0%) expressed 67–100% cell staining. The
staining score of cofilin 1 gradually increased from normal ovarian
to benign tumor, borderline tumor and ovarian carcinoma tissues,
respectively (r= 0.94, P<0.05; Fig.
1 and Table I).
 | Table I.Immunohistochemistry staining scores
for cofilin 1 in normal ovarian, benign ovarian tumor, bordline
ovarian tumor and ovarian carcinoma tissues (r=0.94,
P<0.05). |
Table I.
Immunohistochemistry staining scores
for cofilin 1 in normal ovarian, benign ovarian tumor, bordline
ovarian tumor and ovarian carcinoma tissues (r=0.94,
P<0.05).
| Clinical cases | No. of cases | Staining scores |
|---|
| Normal ovarian | 10 | 0.5±0.5 |
| Benign ovarian
tumour | 13 | 1.5±0.7 |
| Bordline ovarian
tumor | 14 | 2.5±0.9 |
| Ovarian
carcinoma | 30 | 6.0±1.9 |
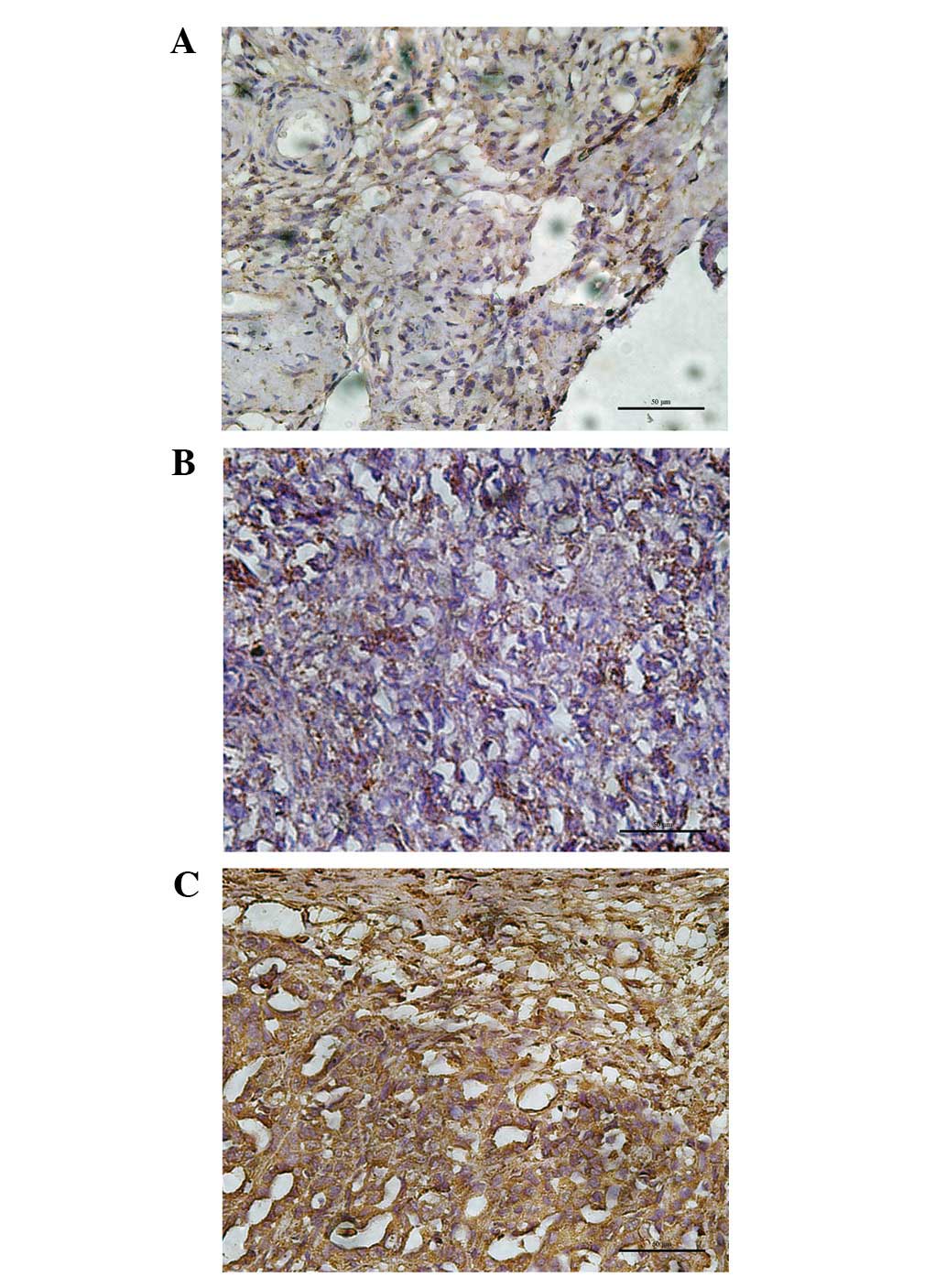
The cofilin 1 expression was also associated with
tumor differentiation. The results of a multivariate logistic
regression analysis suggested that cofilin 1 was significantly
associated with the differentiation grade. Patients with poorly
differentiated ovarian carcinoma were more likely to express
cofilin 1 compared to those who were moderately and
well-differentiated. A total of 34–66% of cells expressed cofilin 1
in 7 well-differentiated tumor patients (70% of 10 patients),
67–100% in 3 well-differentiated tumor patients (30.0% of 10
patients), 34–66% in 7 moderately differentiated tumor patients
(53.8% of 13 patients), 67–100% in 6 moderately differentiated
tumor patients (46.2% of 13 patients) and 67–100% in 7 poorly
differentiated ovarian carcinoma patients (100.0% of 7 patients).
The staining score of cofilin 1 gradually increased in well-,
moderately and poorly differentiated ovarian carcinoma,
respectively (r=0.97, P<0.05; Fig.
2 and Table II).
 | Table II.Immunohistochemistry staining scores
for cofilin 1 in ovarian carcinoma tissues with tumor
differentiation (r=0.97, P<0.05). |
Table II.
Immunohistochemistry staining scores
for cofilin 1 in ovarian carcinoma tissues with tumor
differentiation (r=0.97, P<0.05).
| Clinical stage | No. of cases | Staining
scores |
|---|
|
Well-differentiated | 10 | 4.6±1.0 |
| Moderately
differentiated | 13 | 5.8±1.7 |
| Poorly
differentiated | 7 | 8.6±1.1 |
Discussion
Cell migration is the result of a multi-step process
initiated by the formation of membrane protrusions in response to
migratory and chemotactic stimuli (16). The driving force for membrane
protrusion is the localized polymerization of submembrane actin
filaments (12). Recently, several
studies have revealed that the actin cytoskeleton and its
regulatory proteins are dynamically remodeled and are the driving
force for cell migration. Cofilin 1 is specifically targeted to
filopodia upon stalling of protrusion and during their retraction.
Subsequent electron tomography identified that the filopodial actin
filament and/or bundle fragmentation may precisely correlate with
cofilin 1 accumulation (17).
Inhibition of cofilin 1 activity in carcinoma cells with small
interfering RNA inhibits cell motility and the invasiveness of
carcinoma cells by reducing the assembly and stability of
invadopodia (13,18). The overexpression of cofilin
enhances the motility of glioblastoma tumor cells in a
concentration-dependent fashion, which is likely to contribute to
their invasiveness (19). The
present study demonstrates that cofilin 1 expression in borderline
ovarian tumor tissues was slightly greater compared with that of
benign ovarian tissue, and the expression of cofilin 1 in primary
ovarian carcinoma tissues was significantly greater compared with
that of the borderline ovarian tumor tissues. The results suggest
that cofilin 1 may enhance cell motility and metastasis, and may
cause poor prognosis.
As the leading cause of mortality from gynecologic
cancers, ovarian cancer cells migrate rapidly and eventually spread
throughout the whole peritoneal cavity. The present study revealed
that a diffuse cytoplasmic staining for cofilin 1 with moderate
intensity was observed in various proportions of tumor cells. No
cells expressed cofilin 1 in 7 normal ovarian tissues and only
1–33% of cells expressed cofilin 1 in 3 normal ovarian tissues. The
staining score of cofilin 1 gradually increased from normal ovarian
to benign ovarian, ovarian borderline tumor and ovarian carcinoma
tissues, respectively.
The invasiveness of malignant cancer cells depends
on the altered regulation of cell migration. However, we identified
the differential expression of cofilin 1 in tumor differentiation.
The data revealed that with the differentiation of ovarian cancers,
the level of cofilin 1 expression increased. Meanwhile, the
expression of cofilin 1 was positively correlated with the progress
of human ovarian cancer differentiation. The present study suggests
that the activation of cofilin 1 may promote the proliferation and
invasion of cancer cells, leading the development of ovarian
cancer.
Our results demonstrate that increased cofilin 1
expression may result in an increase in the progression of ovarian
cancer, and indicate that targeting the activities in cancer cells
is sufficient to significantly inhibit tumor cell invasiveness.
This study provides an experimental foundation for therapeutic
strategy, and suggests that RNA Interference Technology or other
relevant methods may be used to decrease the level of cofilin 1 and
lead to the inhibition of ovarian cancer cell invasion. However,
further studies of cofilin 1 in the clinical outcomes of ovarian
cancer for are required to clarify these mechanisms.
Acknowledgements
The study was supported by the Science
and Technology Department of Zhejiang province (Zhejiang, China;
Grant No. 2011C23093) and the Zhejiang Education Bureau (Zhejiang,
China; Grant No. Y201225312).
References
|
1.
|
L YangA KlintM LambePredictors of ovarian
cancer survival: a population-based prospective study in SwedenInt
J Cancer123672679200810.1002/ijc.2342918498135
|
|
2.
|
PS KimS DjazayeriR ZeineldinNovel
nanotechnology approaches to diagnosis and therapy of ovarian
cancerGynecol
Oncol120393403201110.1016/j.ygyno.2010.11.02921168905
|
|
3.
|
SS ChenA MichaelSA Butler-ManuelAdvances
in the treatment of ovarian cancer: a potential role of
antiinflammatory phytochemicalsDiscov Med13717201222284780
|
|
4.
|
DS ChiEL EisenhauerO ZivanovicImproved
progression-free and overall survival in advanced ovarian cancer as
a result of a change in surgical paradigmGynecol
Oncol1142631200910.1016/j.ygyno.2009.03.018
|
|
5.
|
AM LutzJK WillmannCW DrescherEarly
diagnosis of ovarian carcinoma: is a solution in
sight?Radiology259329345201110.1148/radiol.1109056321502390
|
|
6.
|
G BalbiA MonteverdeI LandinoMA ManganaroC
FranzeseCytoreductive surgery and ovarian carcinomaActa
Biomed80230233200920578416
|
|
7.
|
BJ MonkRL ColemanChanging the paradigm in
the treatment of platinum-sensitive recurrent ovarian cancer: from
platinum doublets to nonplatinum doublets and adding
antiangiogenesis compoundsInt J Gynecol Cancer19Suppl
2S63S67200910.1111/IGC.0b013e3181c104fa
|
|
8.
|
Q LiJ ZhuF SunL LiuX LiuY YueOncostatin M
promotes proliferation of ovarian cancer cells through signal
transducer and activator of transcription 3Int J Mol
Med281011082011
|
|
9.
|
V LutzU ReuningA KrugerHigh level
synthesis of recombinant soluble urokinase receptor (CD87) by
ovarian cancer cells reduces intraperitoneal tumor growth and
spread in nude miceBiol
Chem382789798200110.1515/bchm.2001.382.5.789
|
|
10.
|
H HallerO MamulaM KrasevicFrequency and
distribution of lymph node metastases in epithelial ovarian
cancerInt J Gynecol Cancer21245250201121721192
|
|
11.
|
S OnoMechanism of depolymerization and
severing of actin filaments and its significance in cytoskeletal
dynamicsInt Rev
Cytol258182200710.1016/S0074-7696(07)58001-017338919
|
|
12.
|
H YamaguchiJ CondeelisRegulation of the
actin cytoskeleton in cancer cell migration and invasionBiochim
Biophys Acta1773642652200710.1016/j.bbamcr.2006.07.00116926057
|
|
13.
|
P HotulainenE PaunolaMK VartiainenP
LappalainenActin-depolymerizing factor and cofilin-1 play
overlapping roles in promoting rapid F-actin depolymerization in
mammalian nonmuscle cellsMol Biol
Cell16649664200510.1091/mbc.E04-07-0555
|
|
14.
|
W WangG MouneimneM SidaniThe activity
status of cofilin is directly related to invasion, intravasation,
and metastasis of mammary tumorsJ Cell
Biol173395404200610.1083/jcb.20051011516651380
|
|
15.
|
RE ShackelfordMM BuiD CoppolaA
HakamOver-expression of nicotinamide phosphoribosyltransferase in
ovarian cancersInt J Clin Exp Pathol3522527201020606733
|
|
16.
|
CM TsangEP LauK DiBerberine inhibits Rho
GTPases and cell migration at low doses but induces G2 arrest and
apoptosis at high doses in human cancer cellsInt J Mol
Med241311382009
|
|
17.
|
D BreitsprecherSA KoestlerI ChizhovCofilin
cooperates with fascin to disassemble filopodial actin filamentsJ
Cell Sci12433053318201110.1242/jcs.08693421940796
|
|
18.
|
H YamaguchiM LorenzS KempiakMolecular
mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3
complex pathway and cofilinJ Cell
Biol168441452200510.1083/jcb.20040707615684033
|
|
19.
|
CT YapTI SimpsonT PrattDJ PriceSK
MaciverThe motility of glioblastoma tumour cells is modulated by
intracellular cofilin expression in a concentration-dependent
mannerCell Motil Cytoskeleton60153165200510.1002/cm.20053
|