Introduction
Endometrial cancer is the most common malignancy of
the female genital tract in Western countries, with an estimated
43,470 cases and 7,950 mortalities due to the disease expected in
the United States in 2010 (1). Of
these cancers, 80–90% are endometrioid endometrial cancer (EEC).
Phosphatase and tensin homolog deleted from chromosome-10 (PTEN) is
a tumor-suppressor gene. Functional inactivation of PTEN has been
associated with EEC initiation and progression. A previous study
reported a 34–55% somatic mutation frequency of the PTEN gene with
a 50–83% frequency of loss or decrease of the PTEN protein
(2) in EEC. Thus, PTEN inactivation
in EEC and in several other tumor types cannot be explained solely
by observed mutations (3). However,
the mechanism involved in the inactivation of PTEN is largely
unknown.
microRNAs (miRNAs), whose final product is a 19–22
nt functional RNA molecule, are a class of short non-coding RNAs
and play important regulatory roles by sequence-specific base
pairing on the 3′ untranslated region (3′-UTR) of target messenger
RNAs (mRNAs), promoting mRNA degradation or inhibiting translation
(4). In addition, a widespread
dysregulation of miRNAs is commonly observed in human cancer and
promotes cellular transformation and tumorigenesis. miRNAs may
function as oncogenes or tumor suppressors in tumor development
(5). Specifically, miR-21 has been
found to be overexpressed in numerous different solid tumors.
Altered miR-21 has been found to be associated with the
proliferation, invasion and apoptosis of malignant cells by
targeting and downregulating a number of tumor suppressors,
including PTEN (6,7), programmed cell death 4 (PDCD4)
(8–11), bcl-2 and tropomyosin l (TPM1)
(12–13), bone morphogenetic protein receptor
II (BMPRII) (14) and sprouty 2
(SPRY2) (15). These findings imply
that miR-21 plays a fundamental role in malignant behavior and
transformation. A recent study reported that upregulated miR-21 was
correlated with EEC (16). However,
the authors did not show evidence of the role that miR-21 may play
in EEC and whether or not this occurs through the PTEN pathway.
Therefore, we designed this study with the aim of investigating the
oncogenic role of miR-21 in the process of EEC and in the possible
regulation of PTEN expression.
Materials and methods
Tissues and cell line
Paired fresh tumor tissue and matched adjacent
non-tumor tissues (>2 cm from the tumor) were obtained from 16
untreated EEC patients (aged from 33 to 72 years, with a mean age
of 53.63±9.79) who underwent surgery in our hospital. The samples
were snap-frozen in liquid nitrogen, and appropriate informed
consent and approval from the Human Research Ethics Committee were
obtained. The risk factors of these patients included obesity and
anovulation. The tissues were carefully dissected by the
pathologist.
The PTEN-positive KLE cell line was obtained from
the Type Culture Collection of the Chinese Academy of Sciences
(Beijing, China) and was cultured in Dulbecco’s Modified Eagle’s
Medium (DMEM; Hyclone, South Logan, UT, USA) supplemented with 10%
fetal bovine serum (Hyclone) and 100 U/ml penicillin/streptomycin.
The cultures were maintained at 37°C in a 5% CO2
humidified chamber.
Transfection with miR-21 mimic and
inhibitor
Mature hsa-miR-21 mimic, hsa-miR-21 inhibitor and
their respective miRNA scrambled control (SC; Table I) were designed and chemically
synthesized by GenePharma (Shanghai, China). 5x105 KLE
cells were seeded in 6-well plates (Corning, Lowell, MA, USA) and
cultured in antibiotic-free medium for 48 h to achieve >70%
confluence on the day of transfection. The miR-21 mimic, inhibitor
or their respective SC (40 nM) was transfected into cells using
Lipofectamine™ 2000 Reagent (Invitrogen, Carlsbad, CA, USA) in
serum-free conditions for 6 h before changing to complete medium.
The efficiency of transfection was determined using a Leica DMIRE2
microscope system (Leica Microsystemes, Montreal, QC, Canada). RNA
and protein were collected 48 and 72 h after transfection,
respectively.
 | Table I.Sequences of miR-21 mimic, inhibitor
and scrambled controls. |
Table I.
Sequences of miR-21 mimic, inhibitor
and scrambled controls.
| Name | Sequence
(5′-3′) |
|---|
| hsa-miR-21
mimic | |
| Sense |
UAGCUUAUCAGACUGAUGUUGA |
| Antisense |
AACAUCAGUCUGAUAAGCUAUU |
| SC for miR-21
mimic | |
| Sense |
UUCUCCGAACGUGUCACGUTT |
| Antisense |
ACGUGACACGUUCGGAGAATT |
| hsa-miR-21
inhibitor |
UCAACAUCAGUCUGAUAAGCUA |
| SC for miR-21
inhibitor |
CAGUACUUUUGUGUAGUACAA |
RNA extraction and qRT-PCR assay
To test the expression of miRNA-21 and PTEN mRNA in
EEC tissues and the KLE cell line, qRT-PCR was carried out using
the ABI 7500 Real Time PCR system (Applied Biosystems, Foster City,
CA, USA). For detecting mature miR-21, reverse transcription and
quantitative PCR were performed using the Hairpin-it™ miRNAs qPCR
Quantitation kit (GenePharma). U6 small nuclear RNA (snRNA)
expression was assayed for normalization. The thermal cycling
conditions for miR-21 and U6 snRNA consisted of 95°C for 3 min, 40
cycles of 95°C for 12 sec and 62°C for 1 min. To detect PTEN mRNA,
the RevertAid™ First Strand cDNA Synthesis kit (MBI Fermentas,
Burlington, ON, Canada) was used and SYBR® Premix Ex
Taq™ II (Takara, Dalian, China) was used (primers shown in Table II). The conditions for the PTEN and
GAPDH reaction mixtures were 95°C for 2 min, 40 cycles of 95°C for
15 sec and 60°C for 1 min and finally 95°C for 15 sec, 60°C for 1
min, 95°C for 15 sec and 60°C for 15 sec. All experiments were
performed in triplicate. Analysis of relative miR-21 and PTEN mRNA
expression was performed using the comparative CT method with U6
and GAPDH snRNA as endogenous controls, respectively.
 | Table II.Primers used in the qRT-PCR
assay. |
Table II.
Primers used in the qRT-PCR
assay.
| Primer name | Sequence
(5′-3′) |
|---|
| PTEN (accession no.
NM_000314) |
| Forward |
GCGTGCAGATAATGACAAGG |
| Reverse |
GGATTTGACGGCTCCTCTAC |
| GAPDH (accession
no. NM_002046) |
| Forward |
CAAGGTCATCCATGACAACTTTG |
| Reverse |
GTCCACCACCCTGTTGCTGTAG |
Western blot analysis
Protein was extracted from the tissue samples and
cells 72 h after transfection using a protein extraction reagent
(Beyotime, Shanghai, China) and protein concentration was measured
using the BCA Protein Assay kit (Beyotime). The proteins (60
μg) were separated on 10% SDS-polyacrylamide gels and
transferred to nitrocellulose filter membranes (Hybond, Escondido,
CA, USA). The membrane was blocked with Tris-buffered saline
Tween-20 (TBST) containing 5% skimmed milk powder for 1 h at room
temperature, followed by incubation in TBST containing 5% BSA
(Sigma, St. Louis, MO, USA) and primary antibodies overnight at
4°C. Primary antibodies were detected using a peroxidase-coupled
goat anti-rabbit secondary antibody (1:8000, ZSBio, Beijing, China)
and EZ-ECL chemiluminescence Detection kit for HRP (Biological
Industries, Beit-Haemek, Israel). The following primary antibodies
were used: rabbit mAb PTEN (1:1000, Cell Signaling Technology,
Danvers, MA, USA) and rabbit pAb β-actin (1:1000, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Cell proliferation assay
Aberrant cell proliferation is a hallmark of cancer.
For proliferation assays, KLE cells were seeded in 96-well plates
at 4,000 cells/well and, after 48 h, were transfected with one of
the following oligonucleotides: hsa-miR-21 mimic, hsa-miR-21
inhibitor and their respective SCs, using Lipofectamine 2000
Reagent. Cell proliferation was measured at 24, 48 and 72 h after
transfection, respectively, using the Cell Counting Kit-8 assay
(CCK-8, Boster, Wuhan, China). The proliferation analysis of each
group in each individual experiment and the whole experiment
process were performed in triplicate.
pGL3-PTEN-3′-UTR luciferase reporter
assay
Using the computational algorithm RNA22 microRNA
target detection (http://cbcsrv.watson.ibm.com/rna22.html), we
characterized the putative binding sites in the 3′-UTR of PTEN
mRNA. The fragment of the 3′-UTR of PTEN containing the putative
miR-21 binding site was amplified from human genomic DNA by PCR,
cloned into the Xba1 site downstream of the luciferase
reporter gene of the pGL3-Control vector (Promega, Madison, WI,
USA) and named wt-PTEN-3′-UTR-pGL3. For the control, we introduced
three point mutations into the seed region of the miR-21 binding
sites and named the vector mu-PTEN-3′-UTR-pGL3. KLE cells were
seeded in 24-well plates (1×105 cells/well). After 48 h,
the cells were co-transfected with wt-PTEN-3′-UTR-pGL3 or
mu-PTEN-3′-UTR-pGL3 vectors, pRL-TK Renilla Luciferase Reporter
Vector (Promega), hsa-miR-21 mimic, hsa-miR-21 inhibitor or their
respective SC using Lipofectamine 2000 Reagent. Luciferase
activities were measured using the Dual-Luciferase®
Reporter Assay system (Promega) 48 h after transfection. The
firefly luciferase activity was normalized to renilla luciferase
actvity for each sample. All the experiments were performed in
triplicate
Statistical analysis
Statistical analysis was performed using SPSS 13.0.
Data are expressed as the mean ± standard error of the mean (SEM)
of three independent experiments. Pearson’s product-moment
correlation coefficient was used to assess the correlation between
PTEN mRNA and protein levels and miR-21 levels in EEC. A paired
Student’s t-test was used to evaluate differences between two
groups. Multiple group comparisons were analyzed using ANOVA with a
post hoc test. P<0.05 was considered to indicate a statistically
significant result.
Results
Inverse correlation of miR-21 levels with
PTEN protein expression in EEC
miR-21 was overexpressed in all 16 EEC tumor tissues
compared with the matched adjacent non-tumor tissues (Fig. 1A, Table
III). The average fold change of miR-21 was 2.985-fold (range,
1.021–7.402-fold; SEM, 0.419) higher in EEC tumor tissue versus
non-tumor tissues. Compared with the non-tumor tissues, the EEC
tumor tissue expressed a significantly lower level of PTEN protein
(1.02±0.06 vs. 0.57±0.08, P=0.022, paired Student’s t-test,
Fig. 1B). Fig.1C reveals representative gels for PTEN
protein levels in 8 pairs of tumor tissues and matched non-tumor
tissues from EEC patients. A significantly lower level of PTEN mRNA
was observed (7.84±0.82x10−3 vs.
5.75±0.58x10−3, P=0.047, paired Student’s t-test,
Fig. 1D). Furthermore, a
significant inverse correlation was observed between miR-21 and
PTEN protein expression (Fig. 1E),
but not between miR-21 and PTEN mRNA expression (Pearson’s
correlation=0.453, P=0.078).
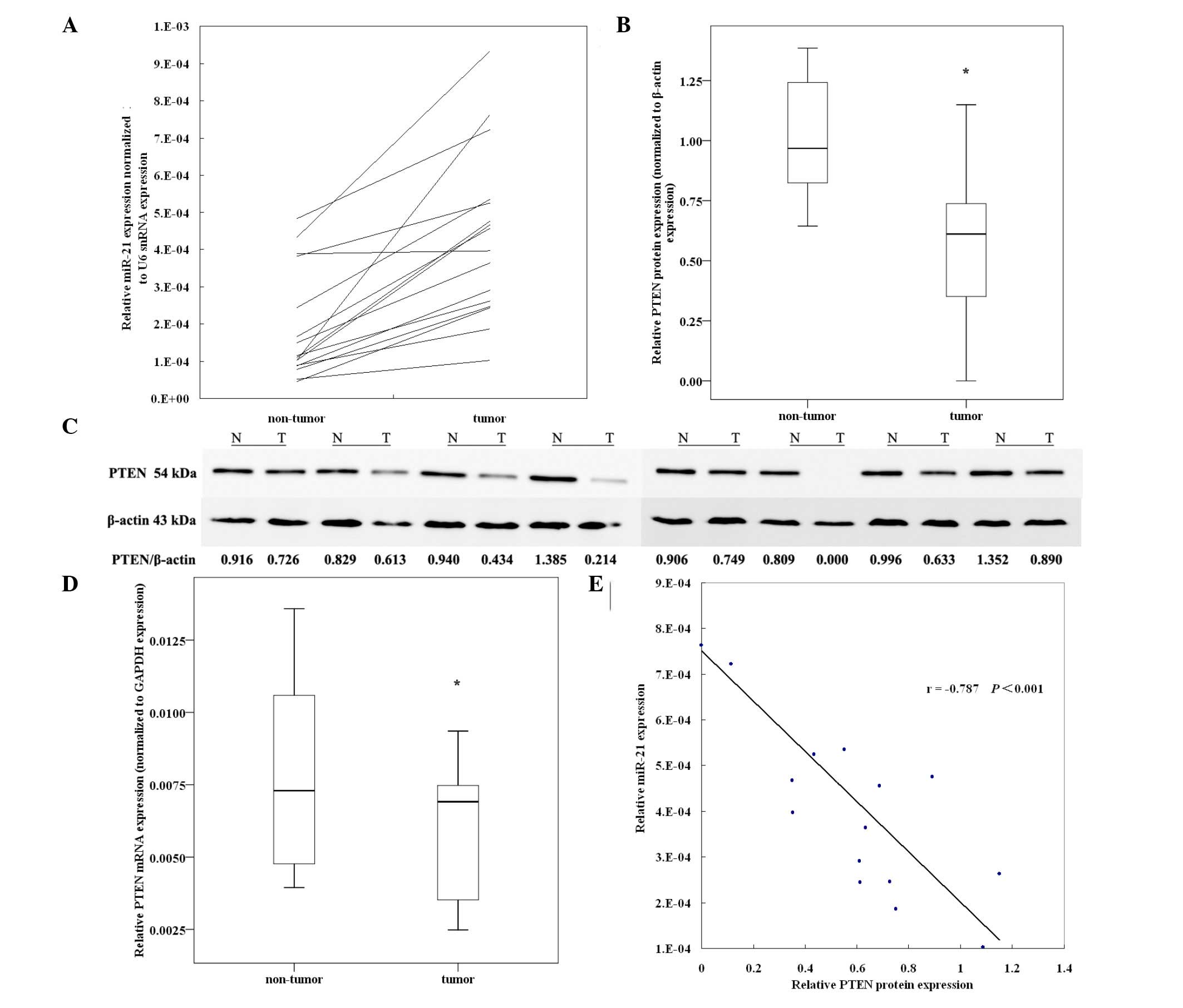 | Figure 1.Expression of miR-21 and its inverse
correlation with PTEN protein in EEC tissues. Sixteen pairs of
matched EEC specimens were detected to determine the expression
levels of PTEN mRNA and protein. (A) miR-21 was found to be
overexpressed in EEC tumor tissues compared with paired non-tumor
tissues detected by qRT-PCR. Relative miR-21 expression was
normalized to U6 snRNA expression. (B) Relative expression of PTEN
protein in EEC tumor tissues compared with matched non-tumor
tissues (n=16, P=0.022, paired Student’s t-test). PTEN protein
expression was detected by western blot analysis and normalized to
β-actin protein expression. (C) Representative gels for PTEN
protein levels in 8 pairs of tumor tissues and matched non-tumor
tissues. N, non-tumor tissue; T, tumor tissue. (D) Relative
expression of PTEN mRNA in EEC tumor tissues compared with matched
non-tumor tissues (n=16, P=0.047, paired Student’s t-test). The
PTEN-mRNA levels were evaluated by qRT-PCR and normalized to GAPDH
mRNA expression. (E) Correlation between relative miR-21 expression
and PTEN protein levels in EEC tissues (Pearson’s correlation =
−0.787, P<0.001). *P<0.01. miR, microRNA, EEC,
endometrioid endometrial cancer; snRNA, small nuclear RNA. |
 | Table III.miR-21 expression and
clinicopathological factors. |
Table III.
miR-21 expression and
clinicopathological factors.
| Clinicopathological
factors | n | miR-21/U6 snRNA
(mean ± SEM; x10−4) |
|---|
| Clinical stage | | |
| I | 8 | 2.62±0.33 |
| II | 4 | 4.81±0.15 |
| III | 4 | 7.38±0.82 |
| Histological
grade | | |
| G1 | 3 | 1.78±0.43 |
| G2 | 9 | 4.54±0.48 |
| G3 | 4 | 5.88±1.55 |
| Lymph node
metastasis | | |
| Negative | 14 | 3.80±1.06 |
| Positive | 2 | 8.28±0.46 |
| Myometrial
invasion | | |
| <1/2
myometrial thickness | 10 | 3.45±0.58 |
| >1/2
myometrial thickness | 6 | 5.86±0.90 |
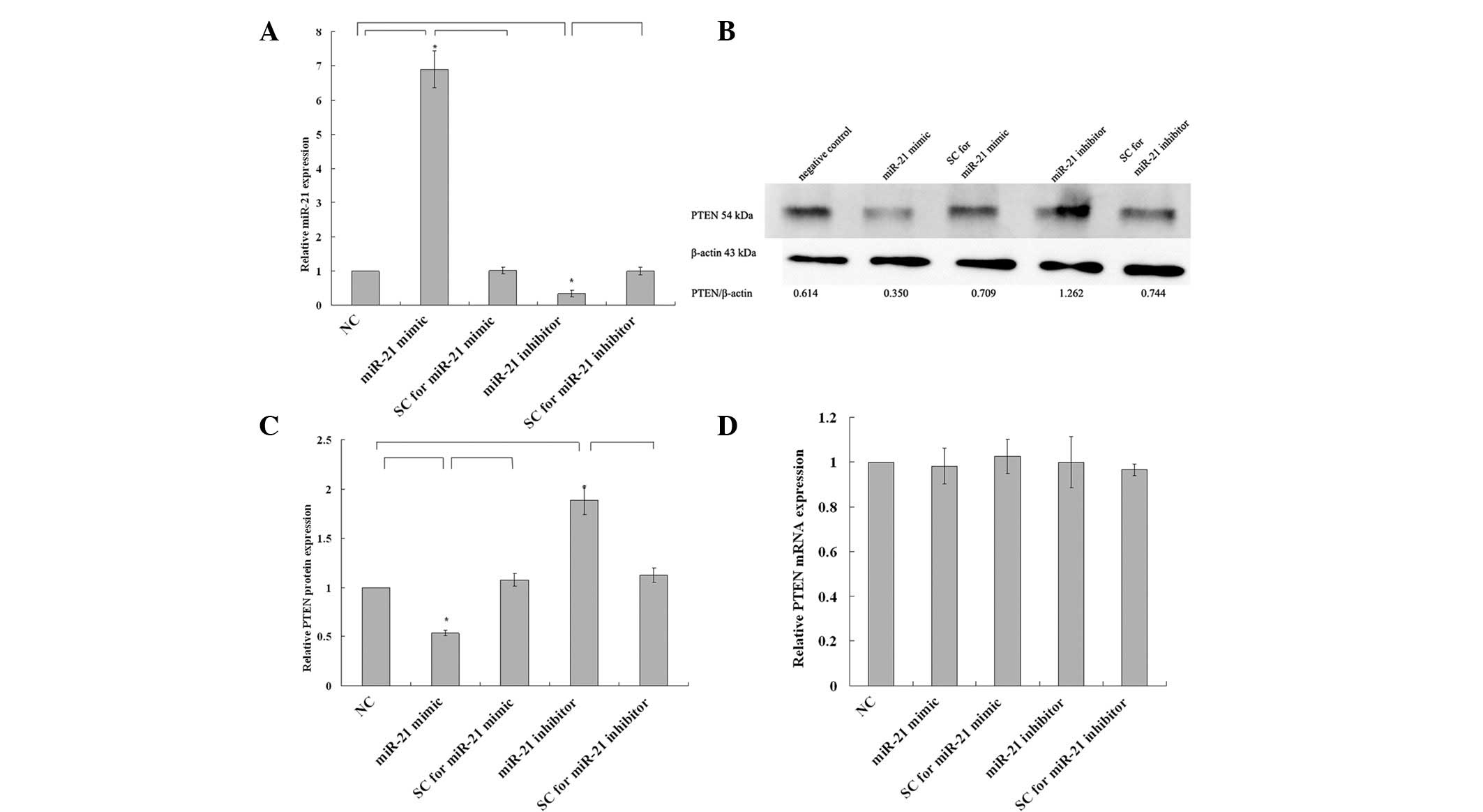
miR-21 mimic suppresses and miR-21
inhibitor enhances PTEN protein levels without affecting mRNA
levels in EEC
To investigate the regulation of miR-21 to PTEN, we
designed experiments of gain- and loss-of-function of miR-21 in the
KLE cell line. An miR-21 mimic, inhibitor or their respective SC
were transfected into KLE cells and the efficiency of transfection
was ∼90%. As shown in Fig. 2A, the
administration of miR-21 mimic resulted in a 6.898±0.312-fold
upregulation (P=0.003) and administration of the miR-21 inhibitor
resulted in a 33.4±5.4% decrease (P=0.007) in miR-21 expression.
However, there was no significant difference between SCs and
negative control (P=0.862 for miR-21 mimic SC compared with
negative control and P=0.953 for miR-21 inhibitor SC compared with
negative control).
In western blot analysis, the upregulation of miR-21
in the miR-21 mimic-transfected group led to a significant decrease
in PTEN protein expression level, to 53.8±1.6% of that of the
control (P=0.007), and the downregulation of miR-21 in the miR-21
inhibitor-transfected group led to a significant increase in PTEN
protein level, to 1.888±0.085-fold that of the control (P=0.002,
Fig. 2B and C); however, there was
no significant difference between SC and negative control P=0.517
for the miR-21 mimic SC compared with negative control and P=0.315
for miR-21 inhibitor SC compared with negative control). In qRT-PCR
analysis, no statistically significant change in PTEN mRNA
expression was observed among those groups (P>0.05, Fig. 2D). These results indicated that the
expression of PTEN protein, but not mRNA, was regulated by miR-21
in KLE cells.
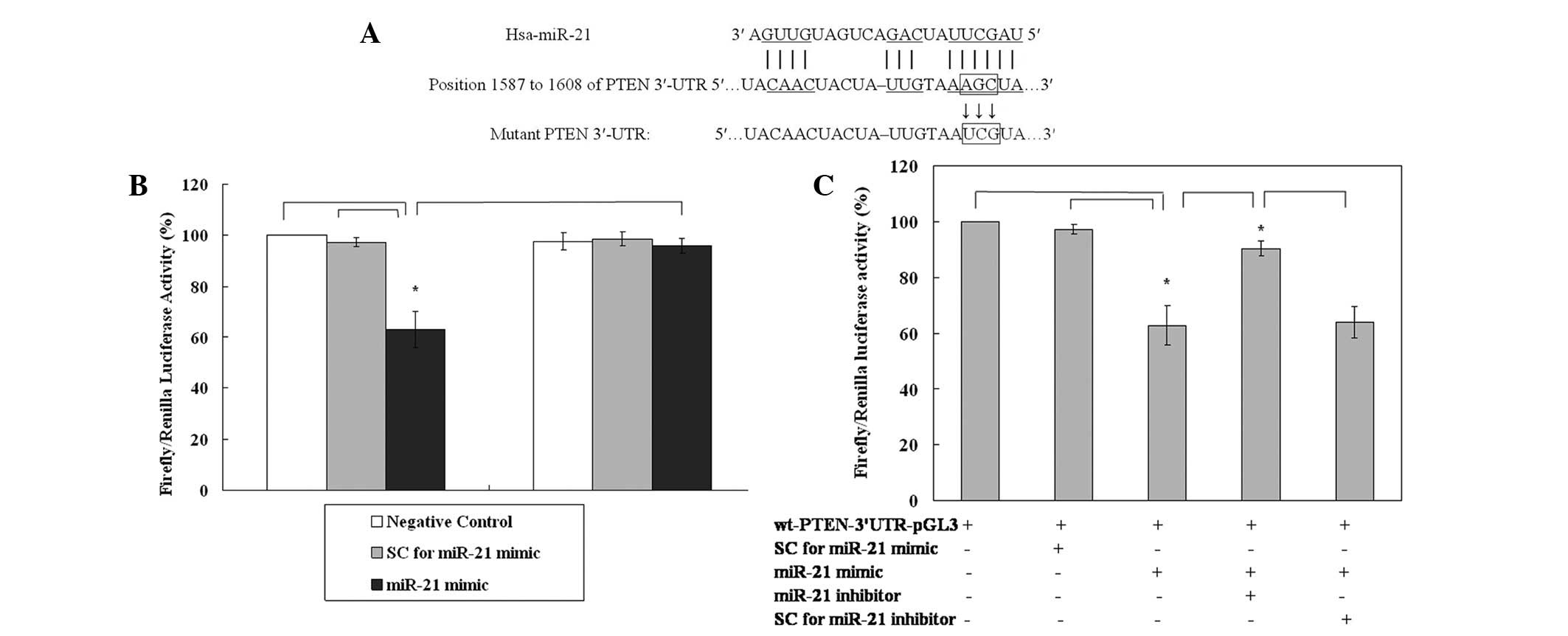
miR-21 directly targets the 3′-UTR of
PTEN mRNA
To validate the putative direct binding site of
miR-21 in the 3′-UTR of PTEN mRNA, a dual-luciferase reporter assay
was carried out. The putative binding sites characterized by the
computational algorithm RNA22 microRNA target detection are shown
in Fig. 3A. The results of the
luciferase assay revealed that, compared with negative control and
SCs, the expression of luciferase in the wt-PTEN-3′-UTR-pGL3 group
was downregulated to 62.93% of that of the control in the presence
of the miR-21 mimic (both P=0.001, Fig.
3B). The effect was reversed (luciferase expression 95.89% of
that of the control) when three point mutations were introduced to
the seed region of the miR-21 binding sites (Fig. 3B) and rescued (90.43%) by
administration of the miR-21 inhibitor (Fig. 3C). Taken together, these results
confirm that PTEN is directly regulated by miR-21 in the KLE cell
line.
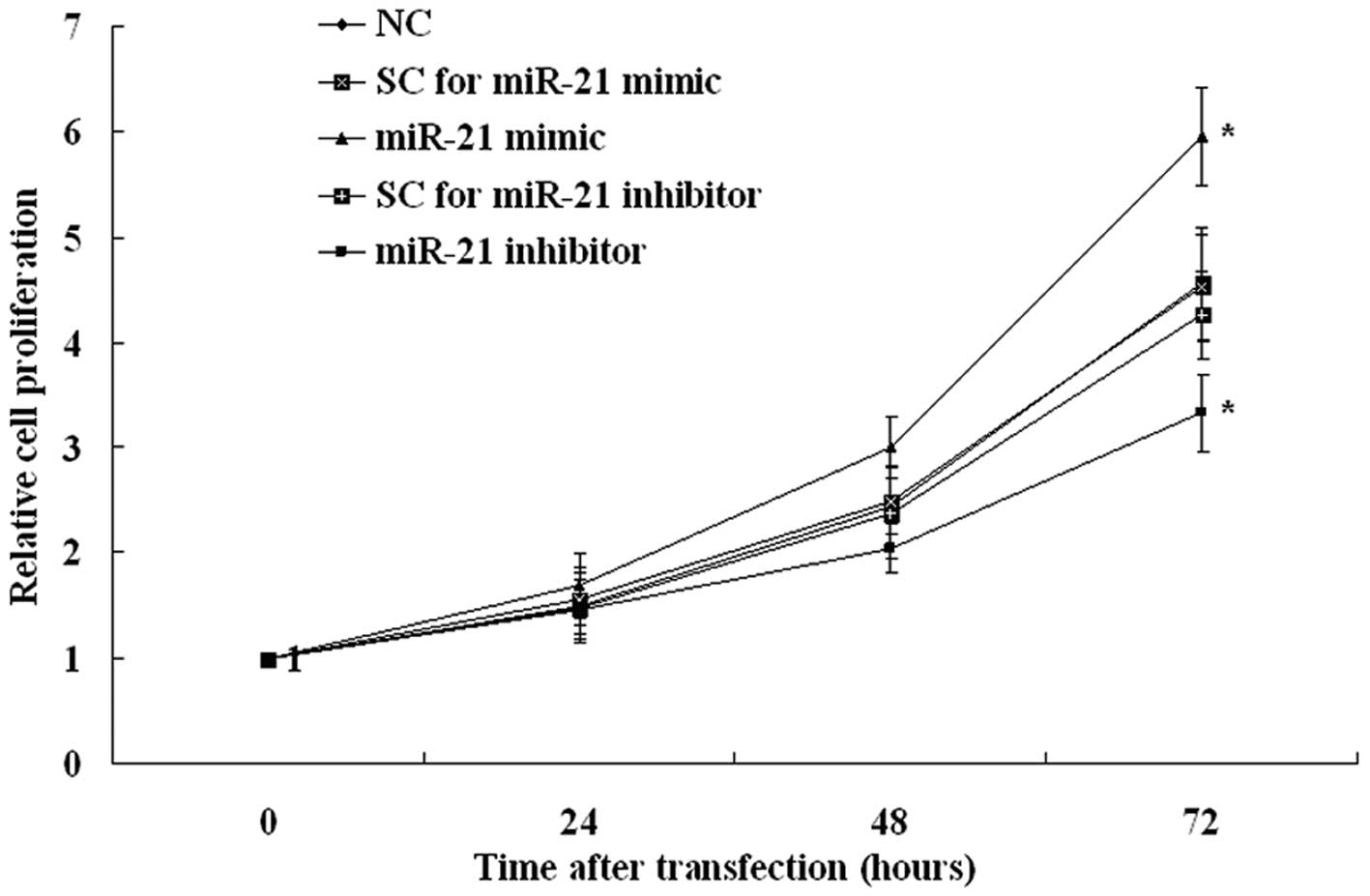
Overexpression of miR-21 increases and
inhibition of miR-21 decreases cell proliferation
To evaluate the potential oncogenic effect of miR-21
on endometrial tumor cell proliferation, we performed an
overexpression study, using an miR-21 mimic, and an inhibition
study, with an miR-21 inhibitor. In the KLE cell line, the miR-21
mimic increased and miR-21 inhibitor decreased cell proliferation
significantly (Fig. 4). These
results indicate that the expression of miR-21 modulates
endometrial tumor cell proliferation.
Discussion
The altered expression of miRNAs, including miR-21,
has been found to be associated with various disorders, most
specifically with cancer (6–15). The
over- or underexpression of miRNAs is thought to result in down- or
upregulation of the protein product of the target genes, thus
affecting various biological behaviors of tumorigenesis (4). miRNA microarray analyses have shown
that a series of miRNAs are highly expressed in EEC. For example,
the miR-200 family has been reported to be highly expressed in EEC
compared with normal endometrial tissues and may play an important
role in cancer growth (17). In
uterine disorders, miR-21 has been found to be associated with
endometriosis, leiomyoma and cervical cancer (18,19).
However, the exact role of miR-21 in EEC is not fully
understood.
In the present study, quantitative RT-PCR assay
confirmed that miR-21 was overexpressed in EEC tissues compared
with their adjacent matched non-tumor endometrium. This was
consistent with the results of the study by Torres et al
(16). However, the results were
not supported by previous miRNA profiling studies showing the
dysregulated miRNAs in tumorous endometrium (20). This may be due to most of the
studies not including paired self-controls and there may be
individual differences in miRNA expressions. Our self-control study
overcame this disadvantage. Moreover, the qRT-PCR technique only
detects mature miRNAs, but the microarray technique detects both
precursor and mature miRNAs (21).
We also revealed a trend that miR-21 expression may be correlated
with advanced clinical stages, deep myometrial invasion and high
histological grade, which was not consistent with the results of
the study by Torres et al (16). However, larger sample numbers and
further studies are required to reveal the clinical relevance of
these results. Furthermore, miR-21 has been shown to be
overexpressed in a variety of solid tumors (22). Correlations between altered miR-21
expression and malignancy-related cellular processes, including
proliferation, migration and apoptosis, have been well demonstrated
(6,10,19).
These correlations indicate that the upregulation of miR-21 in EEC
participates in the pathogenesis of endometrial cancer.
In the present study, we found that PTEN protein and
mRNA expression were decreased in EEC tumor tissues compared with
matched non-tumor tissues. Although several studies have revealed
various frequencies of somatic PTEN mutation in EEC (2), the values are not adequate to explain
PTEN inactivation in EEC and in several other tumor types (3). Other mechanisms participate in the
loss of PTEN protein. PTEN has been validated as a target gene of
miR-21 in a variety of malignancies (5,6,9,23)
but has not been reported in EEC. In the present study, matched
analysis of miR-21 and PTEN protein expression in tumor tissues
showed a significantly negative correlation. Thus, we postulated
that PTEN was also an important target of miR-21 in EEC. Although
the negative correlation between miR-21 and PTEN levels suggests a
regulatory role for miR-21 in PTEN expression, a conclusion cannot
be drawn until direct evidence showing that the reduction or
overexpression of miR-21 alters PTEN expression is found. In this
regard, synthetic miR-21 mimic or inhibitor was transfected into
the KLE cell line to test the effect of overexpression and
inhibition of miR-21 on PTEN expression in EEC tumor cells. Our
results showed direct evidence that miR-21 regulates PTEN
expression at the protein, but not the mRNA, level. To further
confirm that miR-21 directly targets the 3′-UTR of PTEN mRNA, we
constructed luciferase expression plasmids containing the putative
binding sites or mutated seed region sequences in the 3′-UTR of
PTEN mRNA for miR-21. Our results verified that the PTEN 3′-UTR had
target binding sites for miR-21.
In the present study, transfection of miR-21
increased the cell proliferation of the KLE cell line and
transfection of the miR-21 inhibitor inhibited it. Given that PTEN
expression and cell proliferation are regulated by miR-21 and that
PTEN has been shown to repress tumor cell proliferation by blocking
the PI3K/AKT pathway (24), it may
be inferred that the alteration of cell growth modulated by the
expression of miR-21 may be partially mediated by the PTEN/PI3K/AKT
pathway. Other putative targets besides PTEN or other
miR-21-mediated mechanisms may also participate in the alteration
of cell proliferation of the KLE cell line. Further research should
be performed to elucidate those underlining mechanisms.
Several aspects of the mechanism of miR-21
dysregulation have been shown. In gastric cancer cells, the
suppression of nuclear factor κ-light-chain-enhancer of activated B
cells (NF-κB) activation has been reported to enhance miR-21
expression and it has previously been found that NF-κB may be
activated by estrogen in endometrial cancer (25). The regulation of miRNA is a complex
network. Further research is needed on the definite mechanism
leading to the dysregulation of miR-21.
Above all, our study demonstrated that miR-21 was
overexpressed in EEC and determined that this miRNA downregulates
PTEN expression via binding to the 3′-UTR of PTEN mRNA. In
addition, we found that miR-21 promoted cell proliferation in EEC
cells. The characterization of miR-21 as an oncogene provides
evidence of its potential as a therapeutic target in EEC.
Acknowledgements
This study was supported by a grant
from the Scientific Research Foundation for Shandong Provincial
Scientific and Technological Projects (2009GG10002056) and a grant
from the Shandong Natural Science Foundation (no. ZR2010HM005).
References
|
1.
|
A JemalR SiegelE WardJ XuCancer
statistics, 2010CA Cancer J Clin60277300201010.3322/caac.20073
|
|
2.
|
H TashiroMS BlazesR WuMutations in PTEN
are frequent in endometrial carcinoma but rare in other common
gynecological malignanciesCancer Res573935394019979307275
|
|
3.
|
A PerrenLP WengAH BoagImmunohistochemical
evidence of loss of PTEN expression in primary ductal
adenocarcinomas of the breastAm J
Pathol15512531260199910.1016/S0002-9440(10)65227-310514407
|
|
4.
|
DP BartelMicroRNAs: genomics, biogenesis,
mechanism, and
functionCell116281297200410.1016/S0092-8674(04)00045-514744438
|
|
5.
|
YS LeeA DuttaMicroRNAs in cancerAnnu Rev
Pathol4199227200910.1146/annurev.pathol.4.110807.092222
|
|
6.
|
F MengR HensonM LangInvolvement of human
micro-RNA in growth and response to chemotherapy in human
cholangiocarcinoma cell
linesGastroenterology13021132129200610.1053/j.gastro.2006.02.05716762633
|
|
7.
|
F MengR HensonH Wehbe-JanekMicroRNA-21
regulates expression of the PTEN tumor suppressor gene in human
hepatocellular
cancerGastroenterology133647658200710.1053/j.gastro.2007.05.02217681183
|
|
8.
|
IA AsanganiSA RasheedDA
NikolovaMicroRNA-21 (miR-21) post-transcriptionally downregulates
tumor suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal
cancerOncogene2721282136200810.1038/sj.onc.1210856
|
|
9.
|
LB FrankelNR ChristoffersenA
JacobsenProgrammed cell death 4 (PDCD4) is an important functional
target of the microRNA miR-21 in breast cancer cellsJ Biol
Chem28310261033200810.1074/jbc.M70722420017991735
|
|
10.
|
S ZhuH WuF WuMicroRNA-21 targets tumor
suppressor genes in invasion and metastasisCell
Res18350359200810.1038/cr.2008.2418270520
|
|
11.
|
Q YaoH XuQQ ZhangMicroRNA-21 promotes cell
proliferation and down-regulates the expression of programmed cell
death 4 (PDCD4) in HeLa cervical carcinoma cellsBiochem Biophys Res
Commun388539542200910.1016/j.bbrc.2009.08.04419682430
|
|
12.
|
ML SiS ZhuH WumiR-21-mediated tumor
growthOncogene2627992803200710.1038/sj.onc.121008317072344
|
|
13.
|
S ZhuML SiH WuYY MoMicroRNA-21 targets the
tumor suppressor gene tropomyosin 1 (TPM1)J Biol
Chem2821432814336200710.1074/jbc.M61139320017363372
|
|
14.
|
W QinB ZhaoY ShiBMPRII is a direct target
of miR-21Acta Biochim Biophys Sin
(Shanghai)41618623200910.1093/abbs/gmp04919578724
|
|
15.
|
D SayedS RaneJ LypowyMicroRNA-21 targets
Sprouty2 and promotes cellular outgrowthsMol Biol
Cell1932723282200810.1091/mbc.E08-02-015918508928
|
|
16.
|
A TorresK TorresT PaszkowskiHighly
increased maspin expression corresponds with up-regulation of
miR-21 in endometrial cancer: a preliminary reportInt J Gynecol
Cancer21814201110.1097/IGC.0b013e318200050e21330826
|
|
17.
|
J SnowdonX ZhangT ChildsThe microRNA-200
family is upregulated in endometrial carcinomaPLoS
One6e22828201110.1371/journal.pone.002282821897839
|
|
18.
|
LA RamónA Braza-BoïlsJ
Gilabert-EstellésmicroRNAs expression in endometriosis and their
relation to angiogenic factorsHum Reprod2610821090201121335415
|
|
19.
|
Q PanX LuoN CheginimicroRNA 21: response
to hormonal therapies and regulatory function in leiomyoma,
transformed leiomyoma and leiomyosarcoma cellsMol Hum
Reprod16215227201010.1093/molehr/gap093
|
|
20.
|
DE CohnM FabbriN ValeriComprehensive miRNA
profiling of surgically staged endometrial cancerAm J Obstet
Gynecol202656 e18201020400061
|
|
21.
|
EJ LeeY GusevJ JiangExpression profiling
identifies microRNA signature in pancreatic cancerInt J
Cancer12010461054200710.1002/ijc.2239417149698
|
|
22.
|
S VoliniaGA CalinCG LiuA microRNA
expression signature of human solid tumors defines cancer gene
targetsProc Natl Acad Sci
USA10322572261200610.1073/pnas.051056510316461460
|
|
23.
|
WJ MaGD LvA TuersunRole of microRNA-21 and
effect on PTEN in Kazakh’s esophageal squamous cell carcinomaMol
Biol Rep3832533260201121104017
|
|
24.
|
PK VogtM GymnopoulosJR HartPI 3-kinase and
cancer: changing accentsCurr Opin Genet
Dev191217200910.1016/j.gde.2008.11.01119185485
|
|
25.
|
KH SeoHS LeeB JungEstrogen enhances
angiogenesis through a pathway involving platelet-activating
factor-mediated nuclear factor-kappaB activationCancer
Res6464826488200410.1158/0008-5472.CAN-03-2774
|