Introduction
Cancer of the ovary is the seventh most common type
of cancer in females, accounting for almost 225,000 new cases and
140,000 mortalities annually (1).
Efficacy of first-line chemotherapy with paclitaxel and carboplatin
is impressive; however, 70% of patients will eventually succumb to
disease-related complications during long-term follow-up.
New chemotherapy agents and alternative dosing
schedules have been investigated to improve responses and survival
rates and to increase tolerability (2–5).
Standard 3-week dosing schedules have improved response rates and
progression-free survival, but long-term overall survival rates are
less impressive and relapses continue to exceed 70% (3).
Studies have analyzed the efficacy of primary
therapy, but data have not shown the superiority of a specific
standard triplet chemotherapy regimen for the treatment of ovarian
carcinoma (3).
Weekly chemotherapy regimens have been evaluated
with regard to improved prognosis and reduced or altered drug
toxicity. Promising activity and a favorable toxicity profile have
been reported (4,5). A higher total dose of paclitaxel
(dose-dense) may be achieved with weekly regimens and may
theoretically be superior to the standard 3-week schedule for
first-line therapy (6).
Docetaxel is an alternative to paclitaxel in
combination with a platinum agent in first-line chemotherapy, but
also for the treatment of recurrent ovarian cancer (7,8). The
standard three-week schedule appears to be of comparable efficacy
with paclitaxel but with a different toxicity profile (9). More dose-dense weekly schedules have
also been studied in small patient series of recurrent ovarian
cancer (10–16). New data on up-front weekly docetaxel
with regard to efficacy, toxicity and quality of life have recently
been presented (17,18).
The purpose of the present study was to compare the
toxicity profiles of weekly docetaxel administration and the
standard three-week schedule for primary therapy of advanced
ovarian carcinoma. In both schedules carboplatin was administered
every three weeks. Efficacy and quality-of-life data have been
presented previously and are not analyzed in this study (17,18).
Patients and methods
Eligibility
This was a retrospective comparative multicenter
study, including patients with histologically confirmed epithelial
ovarian carcinoma from the Gynecological Oncology departments at
two university hospitals (Örebro and Gothenburg) in Sweden. All
patients were in FIGO stage IIB–IV and underwent primary
cytoreductive surgery. The period of recruitment was from May 2003
to December 2008. In all, 167 patients were included in the study
and 108 patients received weekly docetaxel (Örebro) and 59 received
docetaxel every three weeks (Gothenburg) together with carboplatin
given every three weeks in both regimens. Of these patients, 147
(88%) completed 3 or more courses of chemotherapy. Clinical and
biochemical response rates were based on patients with measurable
disease and/or elevated CA-125 levels at the start of chemotherapy.
The results from a phase II study of weekly treatment have been
published previously (17).
Toxicity, which is the main topic of this study, was recorded in
all 167 patients (≥1 course of chemotherapy). Patient and tumor
characteristics are shown in Table
I.
 | Table IPatient characteristics for the series
of weekly vs. three-week docetaxel-carboplatin administrations
(n=167). |
Table I
Patient characteristics for the series
of weekly vs. three-week docetaxel-carboplatin administrations
(n=167).
|
Characteristics | One-week group | Three-week
group |
|---|
| Mean age,
years | 63.3 (range,
28–80) | 63.6 (range
47–79) |
| Body mass index
(BMI) | 23.8 | 23.8 |
| Body surface area
(BSA), m2 | 1.69 | 1.67 |
| Histological type,
n (%) | | |
| Papillary
serous | 95 (88.0) | 45 (76.3) |
| Mucinous | 2 (1.9) | 0 (0.0) |
| Endometrioid | 4 (3.7) | 9 (15.3) |
| Clear cell | 7 (6.5) | 3 (5.1) |
| Anaplastic | 0 (0.0) | 2 (3.4) |
| FIGO stage, n
(%) | | |
| IIB | 1 (0.9) | 1 (1.7) |
| IIC | 4 (3.7) | 4 (6.8) |
| IIIA | 3 (2.8) | 2 (3.4) |
| IIIB | 3 (2.8) | 8 (13.6) |
| IIIC | 63 (58.3) | 31 (52.5) |
| IV | 34 (31.5) | 13 (22.0) |
| Differentiation
grade, n (%) | | |
| Poor | 67 (62.0) | 37 (62.7) |
| Moderate | 29 (26.9) | 16 (27.1) |
| Well | 5 (4.6) | 3 (5.1) |
| Not graded (clear
cell) | 7 (6.5) | 3 (5.1) |
A chemotherapy regimen of weekly docetaxel 30
mg/m2 and carboplatin [area under the curve (AUC) 5] was
given every 3 weeks to 108 patients (17). Six cycles were administered during
18 weeks. Of these patients, 71 (66%) completed 6 cycles of
chemotherapy. The mean dose intensity of docetaxel was 29.8
mg/m2/week (95% CI, 29.6–29.9) and of carboplatin 105.7
mg/m2/week (95% CI, 102.0–109.5).
A chemotherapy regimen of docetaxel 75
mg/m2 and carboplatin (AUC 5) was given every 3 weeks to
59 patients (9). Six cycles were
administered during 18 weeks. Of these patients, 41 (70%) completed
6 cycles of chemotherapy. The mean dose intensity of docetaxel was
25.1 mg/m2/week (95% CI, 24.9–25.3) and of carboplatin
109.4 mg/m2/week (95% CI, 105.5–113.3).
Eligible patients had adequate bone marrow, renal
and hepatic function, and an absolute neutrophil count (ANC)
≥1.5×109/l, a platelet count ≥100×109/l,
serum-creatinine ≤1.25 times the normal level, serum ASAT/ALAT ≤1.5
times the normal level, no previous history of chemotherapy or
radiotherapy, and an Eastern Cooperative Oncology Group (ECOG)
performance status ≤2. Exclusion criteria included severe
infection, hypertension and myocardial infarction within the
previous 6 months, congestive heart failure, prior serious allergic
reactions and previous malignancy within 5 years. The study was
approved by the Ethics Committees of the participating University
Hospitals (Dnr 03-258). Informed consent was obtained from all
patients.
Drug administration
Patients were treated with intravenous docetaxel (30
mg/m2) and carboplatin (AUC 5) on day 1. Docetaxel was
repeated on days 8 and 15 and was administered via a ½-hour
infusion. Carboplatin was administered in accordance with the
Calvert formula (19) for 30 min on
day 1. In the group with standard chemotherapy, intravenous
docetaxel (75 mg/m2) and carboplatin (AUC 5) were both
given on day 1. The second course started on day 22. Before
docetaxel infusion, patients were premedicated with intravenous
dexamethasone, diphenhydramine and a histamine
H2-receptor antagonist, such as cimetidine. The
creatinine clearance was calculated by the method of Cockcroft and
Gault (20).
Response evaluation
Clinical response was assessed at the completion of
6 chemotherapy cycles (or after at least 3 completed cycles) via
clinical, radiographic and serologic means in accordance with the
WHO response criteria (21) and the
Rustin criteria (22). Patients
with residual disease at the start of chemotherapy and who
completed at least 3 cycles of chemotherapy (n=85) were evaluable
for clinical response evaluation. Efficacy data of weekly therapy
have been presented in an earlier report (17). In the present study efficacy data
were not further analyzed.
Toxicity analysis
Toxicity was graded according to the Common
Terminology Criteria for Adverse Events (CTCAE v3.0, 2003)
(23). Patients were required to
have an ANC ≥1.5×109/l and a platelet count
≥100×109/l on day 1 to receive chemotherapy. Complete
blood cell values were obtained weekly until the conclusion of
cycle 6 and then subsequently, every 3 weeks. Adequate renal
function was defined as serum creatinine <1.25 times the upper
normal limit, and liver function of bilirubin < upper normal
limit, AST/ALT <1.5 times the upper normal limit, and ALP <3
times the upper normal limit. Symptomatic peripheral neuropathy ≥
CTCAE grade 2 was an exclusion criterion. All subjects who
completed at least 1 cycle of chemotherapy were included in the
toxicity analysis.
A quality-of-life measurement questionnaire (EORTC
QOL-C30, version 3) was used in the evaluation of the symptoms
recorded during the six courses of treatment (24). The compliance rate was high and
93–99% of the patients had evaluable data. The results from the
weekly schedule were analyzed and presented in a prior publication
(18).
The median follow-up time of all patients alive was
33 months (range, 1–60 months).
Statistical analysis
According to the inclusion and exclusion criteria,
108 patients were included in the one-week group and 59 in the
three-week group. Originally the patients were recruited into two
separate phase II studies during the same period of time. T-test,
Pearson’s Chi-square test and Fisher’s exact test were used to
compare continuous and non-continuous data. ANOVA (repeated
measurements) was used to compare symptom scores during the whole
period of treatment (cycles 1–6). A P-value <0.05 was considered
to indicate a statistically significant result. STATISTICA software
(StatSoft, Inc., Tulsa, OK, USA) version 10.0 was used for all
statistical analyses in this study.
Results
Response and survival rates
In the weekly schedule, 38 patients demonstrated a
clinical complete response (44.7%), and 29 patients exhibited a
partial response (34.1%), resulting in a total clinical response
rate of 78.8% (95% CI, 70.1–87.5%). Data from the three-week
schedule showed a clinical response rate of 88.7% (95% CI,
80.2–97.2%).
In the weekly schedule the median overall survival
time was 35.3 months and the progression-free survival time was
12.0 months. The corresponding figures in the three-week schedule
were 54.1 and 20.0 months, respectively.
Since this was not a randomized study, response and
survival data could not be compared compared between the weekly and
the three-week schedule due to differences in the study
populations; this was not the purpose of the study.
Toxicity
Hematological toxicity
Grade 3-4 neutropenia was recorded in 8 patients
(11.3%) in the one-week group and in 32 patients (78.1%) in the
three-week group after six completed courses of chemotherapy. This
was a highly significant difference (Table II). Grade 1–2 neutropenia was
recorded in 29 patients (40.9%) and 6 patients (14.6%) in the two
groups, respectively.
 | Table IIHematological toxicity in the
one-week and three-week groups. |
Table II
Hematological toxicity in the
one-week and three-week groups.
| Toxicity | One-week group | Three-week
group | P-valuea |
|---|
| Neutropeniab | | | |
| Grade 1 | 7 (9.9) | 1 (2.4) | |
| Grade 2 | 22 (31.0) | 5 (12.2) | |
| Grade 3 | 7 (9.9) | 15 (36.6) | |
| Grade 4 | 1 (1.4) | 17 (41.5) | <0.000001 |
|
Thrombocytopeniab | | | |
| Grade 1 | 31 (43.7) | 5 (9.8) | |
| Grade 2 | 5 (7.0) | 2 (3.9) | |
| Grade 3 | 1 (1.4) | 0 (0.0) | |
| Grade 4 | 0 (0.0) | 0 (0.0) | 0.0002 |
| Anemiab | | | |
| Grade 1 | 49 (69.0) | 18 (35.3) | |
| Grade 2 | 19 (26.8) | 12 (23.5) | |
| Grade 3 | 0 (0) | 0 (0.0) | |
| Grade 4 | 0 (0) | 0 (0.0) | <0.000001 |
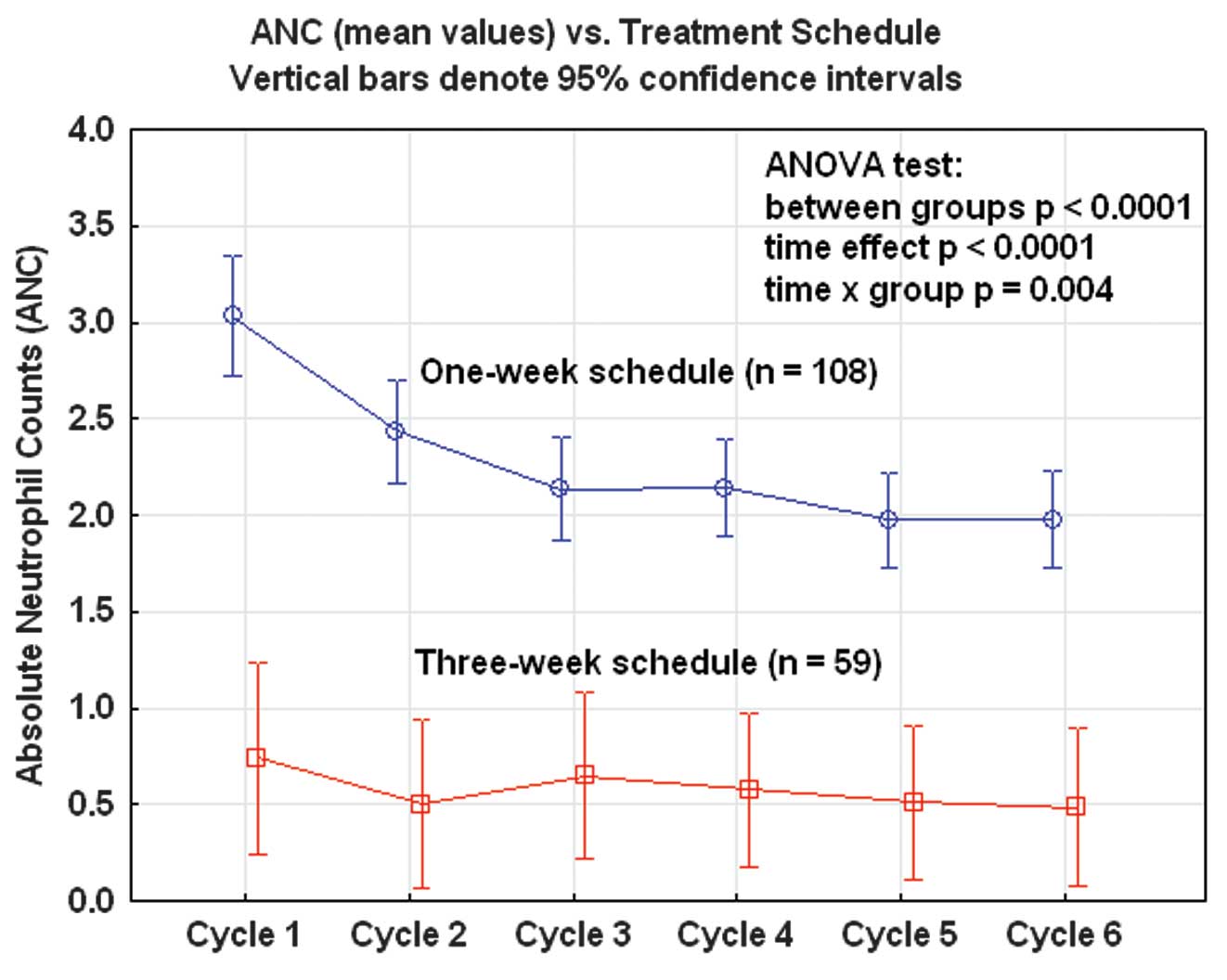
During all six courses of chemotherapy the mean
neutrophil count was significantly lower in the three-week group
compared with the one-week group (ANOVA; p<0.0001). A
time-dependent effect was also observed in the one-week group with
successively decreasing mean values from cycle 1 to cycle 5. This
time-dependent pattern was not observed in the three-week group,
with low but stable mean values from cycles 1–6 (Fig. 1).
Febrile neutropenia and septicemia were also more
frequent in the three-week group (Table
III).
 | Table IIINon-hematological toxicity in the
one-week and three-week groups. |
Table III
Non-hematological toxicity in the
one-week and three-week groups.
| Type of
toxicitya | No. of patients (%)
| P-valueb |
|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| Sensory
neuropathy | | | | | 0.237 |
| One-week
group | 25 (35.2) | 3 (4.2) | 0 | 0 | |
| Three-week
group | 12 (22.6) | 1 (1.9) | 0 | 1 (1.9) | |
| Nausea | | | | | 0.626 |
| One-week
group | 11 (15.5) | 2 (2.8) | 1 (1.4) | 0 | |
| Three-week
group | 6 (11.8) | 3 (5.9) | 2 (3.9) | 0 | |
| Mucositis | | | | | 0.403 |
| One-week
group | 10 (14.1) | 1(1.4) | 0 | 0 | |
| Three-week
group | 4 (7.8) | 2 (3.9) | 0 | 0 | |
| Nail changes | | | | | 0.125 |
| One-week
group | 13 (18.3) | 8 (11.3) | 0 | 0 | |
| Three-week
group | 8 (15.7) | 1 (2.0) | 0 | 0 | |
| Diarrhea | | | | | 0.033 |
| One-week
group | 9 (12.7) | 0 | 0 | 0 | |
| Three-week
group | 1 (2.0) | 0 | 0 | 0 | |
| Myalgia | | | | | 0.163 |
| One-week
group | 3 (4.2) | 0 | 1 (1.4) | 0 | |
| Three-week
group | 5 (9.8) | 2 (3.9) | 0 | 0 | |
| Dyspnea | | | | | 0.042 |
| One-week
group | 9 (12.7) | 4 (5.6) | 1 (1.4) | 0 | |
| Three-week
group | 0 | 2 (3.9) | 0 | 0 | |
| Cardiac | | | | | 0.576 |
| One-week
group | 2 (2.8) | 1 (1.4) | 1 (1.4) | 2 (2.8) | |
| Three-week
group | 3 (5.9) | 1 (2.0) | 0 | 0 | |
| Fever | | | | | 0.040 |
| One-week
group | 2 (2.8) | 0 | 0 | 0 | |
| Three-week
group | 0 | 3 (5.9) | 2 (3.9) | 1 (2.0) | |
| Infection | | | | | 0.824 |
| One-week
group | 1 (1.4) | 3 (4.2) | 2 (2.8) | 0 | |
| Three-week
group | 1 (2.0) | 4 (8.0) | 1 (2.0) | 0 | |
| Fatigue | | | | | 0.496 |
| One-week
group | 26 (36.1) | 7 (9.7) | 1 (1.4) | 0 | |
| Three-week
group | 15 (29.4) | 3 (5.9) | 0 | 0 | |
| Tearing eyes | | | | | 0.000 |
| One-week
group | 37 (52.1) | 7 (9.9) | 0 | 0 | |
| Three-week
group | 1 (2.0) | 0 | 0 | 0 | |
| Taste
disturbances | | | | | 0.425 |
| One-week
group | 26 (36.3) | 6 (8.5) | 0 | 0 | |
| Three-week
group | 14 (27.5) | 3 (5.9) | 0 | 0 | |
Grade 3–4 thrombocytopenia was recorded in one
patient in the one-week group and in no patients in the three-week
group. However, grade 1–2 thrombocytopenia was more frequent in the
one-week group (50.7%) than in the three-week group (14.6%). The
thrombocytic toxicity was more pronounced in the one-week-group and
significantly increased with every chemotherapy cycle administered.
In the three-week group this pattern was not observed.
None of the patients exhibited grade 3–4 anemia.
Grade 1–2 anemia was more frequent in the one-week group (95.8%)
than in the three-week group (73.2%). The degree of anemia
increased with every successive course of chemotherapy and in a
similar manner for both treatment groups. All of these differences
were statistically significant (Table
II). Despite the different pattern of hematological toxicity,
the compliance rate with the chemotherapy regimens was similar in
the two groups, and 65.5 and 69.5% of the patients, respectively,
completed the planned 6 cycles.
Non-hematological toxicity
Fatigue was the most frequently recorded
non-hematological side-effect associated with the one-week regimen
and was significantly more frequent than with the three-week
regimen (ANOVA; p<0.0001). This difference was noted for every
individual cycle of the weekly schedule and the time-effect was
also present with increasing fatigue during the treatment period
(Table IV).
 | Table IVNon-hematological toxicity in the
one-week group and the three-week group during treatment (cycle
1–6). The toxicity grading is converted to a 0–100 linear scale
according to the technique used for quality-of-life analysis (EORTC
QLQ-C30 symptom scores). |
Table IV
Non-hematological toxicity in the
one-week group and the three-week group during treatment (cycle
1–6). The toxicity grading is converted to a 0–100 linear scale
according to the technique used for quality-of-life analysis (EORTC
QLQ-C30 symptom scores).
| Toxicity | Cycle
| P-values (ANOVA)
|
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Time | Group | TxG |
|---|
| Neuropathy
sensory | | | | | | | | | |
| One-week group
(A) | 2.2 | 3.2 | 4.5 | 7.3 | 9.0 | 10.9 | | | |
| Three-week group
(B) | 0.0 | 1.9 | 2.8 | 3.8 | 6.1 | 8.5 | | | |
| P-value A vs.
B | 0.023 | 0.311 | 0.274 | 0.102 | 0.227 | 0.402 | 0.000 | 0.125 | 0.971 |
| Fatigue | | | | | | | | | |
| One-week group
(A) | 8.1 | 11.5 | 13.7 | 14.0 | 15.1 | 14.9 | | | |
| Three-week group
(B) | 0.0 | 1.4 | 1.9 | 1.0 | 1.9 | 10.3 | | | |
| P-value A vs.
B | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 |
| Nausea and
vomiting | | | | | | | | | |
| One-week group
(A) | 14.0 | 12.3 | 8.6 | 7.9 | 9.0 | 6.3 | | | |
| Three-week group
(B) | 15.0 | 15.6 | 12.0 | 9.9 | 5.3 | 8.8 | | | |
| P-value A vs.
B | 0.750 | 0.293 | 0.238 | 0.428 | 0.226 | 0.417 | 0.000 | 0.277 | 0.610 |
| Myalgia | | | | | | | | | |
| One-week group
(A) | 0.7 | 1.2 | 1.3 | 1.5 | 1.9 | 2.5 | | | |
| Three-week group
(B) | 5.9 | 6.9 | 7.4 | 7.1 | 7.7 | 7.4 | | | |
| P-value A vs.
B | 0.000 | 0.000 | 0.000 | 0.006 | 0.014 | 0.032 | 0.626 | 0.000 | 0.995 |
| Mucositis | | | | | | | | | |
| One-week group
(A) | 2.5 | 5.4 | 5.2 | 4.3 | 4.2 | 4.2 | | | |
| Three-week group
(B) | 6.5 | 8.8 | 13.4 | 12.7 | 12.5 | 9.8 | | | |
| P-value A vs.
B | 0.025 | 0.180 | 0.001 | 0.000 | 0.002 | 0.013 | 0.001 | 0.000 | 0.106 |
| Taste
disturbances | | | | | | | | | |
| One-week group
(A) | 5.6 | 10.3 | 13.0 | 13.4 | 12.8 | 13.4 | | | |
| Three-week group
(B) | 0.0 | | 1.9 | 0.9 | 1.9 | 1.4 | 5.9 | | |
| P-value A vs.
B | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 | 0.000 | 0.000 | 0.004 |
| Nail changes | | | | | | | | | |
| One-week group
(A) | 0.7 | 1.7 | 4.2 | 4.6 | 9.6 | 10.2 | | | |
| Three-week group
(B) | 0.0 | 0.0 | 0.0 | 0.0 | 1.4 | 4.9 | | | |
| P-value A vs.
B | 0.201 | 0.085 | 0.005 | 0.006 | 0.000 | 0.001 | 0.000 | 0.003 | 0.012 |
| Dyspnea | | | | | | | | | |
| One-week group
(A) | 1.5 | 1.5 | 2.1 | 3.4 | 3.6 | 7.0 | | | |
| Three-week group
(B) | 0.0 | 0.0 | 0.0 | 3.3 | 0.5 | 2.0 | | | |
| P-value A vs.
B | 0.115 | 0.169 | 0.079 | 0.981 | 0.058 | 0.046 | 0.002 | 0.010 | 0.113 |
| Cardiac
toxicity | | | | | | | | | |
| One-week group
(A) | 0.2 | 1.5 | 3.6 | 3.0 | 2.6 | 5.3 | | | |
| Three-week group
(B) | 0.5 | 1.9 | 2.3 | 4.7 | 1.4 | 2.5 | | | |
| P-value A vs.
B | 0.658 | 0.808 | 0.577 | 0.533 | 0.547 | 0.342 | 0.210 | 0.809 | 0.399 |
| Epiphora | | | | | | | | | |
| P-value A vs.
B | 0.201 | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | | | |
| One-week group
(A) | 0.7 | 3.7 | 7.6 | 12.8 | 15.1 | 18.0 | | | |
| Three-week group
(B) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.000 | 0.000 | 0.000 |
| Diarrhea | | | | | | | | | |
| One-week group
(A) | 6.1 | 7.1 | 3.4 | 2.7 | 3.5 | 3.2 | | | |
| Three-week group
(B) | 1.4 | 3.8 | 2.8 | 1.4 | 1.5 | 0.5 | | | |
| P-value A vs.
B | 0.016 | 0.142 | 0.729 | 0.334 | 0.176 | 0.033 | 0.023 | 0.018 | 0.690 |
| Fever | | | | | | | | | |
| One-week group
(A) | 0.7 | 0.2 | 1.3 | 0.6 | 1.9 | 0.7 | | | |
| Three-week group
(B) | 0.0 | 6.5 | 7.9 | 3.3 | 4.8 | 7.8 | | | |
| P-value A vs.
B | 0.465 | 0.003 | 0.005 | 0.014 | 0.119 | 0.010 | 0.059 | 0.000 | 0.024 |
| Infection | | | | | | | | | |
| One-week group
(A) | 2.5 | 1.7 | 3.9 | 1.8 | 1.9 | 4.6 | | | |
| Three-week group
(B) | 0.0 | 7.4 | 7.9 | 4.7 | 6.3 | 6.0 | | | |
| P-value A vs.
B | 0.117 | 0.016 | 0.190 | 0.208 | 0.091 | 0.641 | 0.058 | 0.009 | 0.143 |
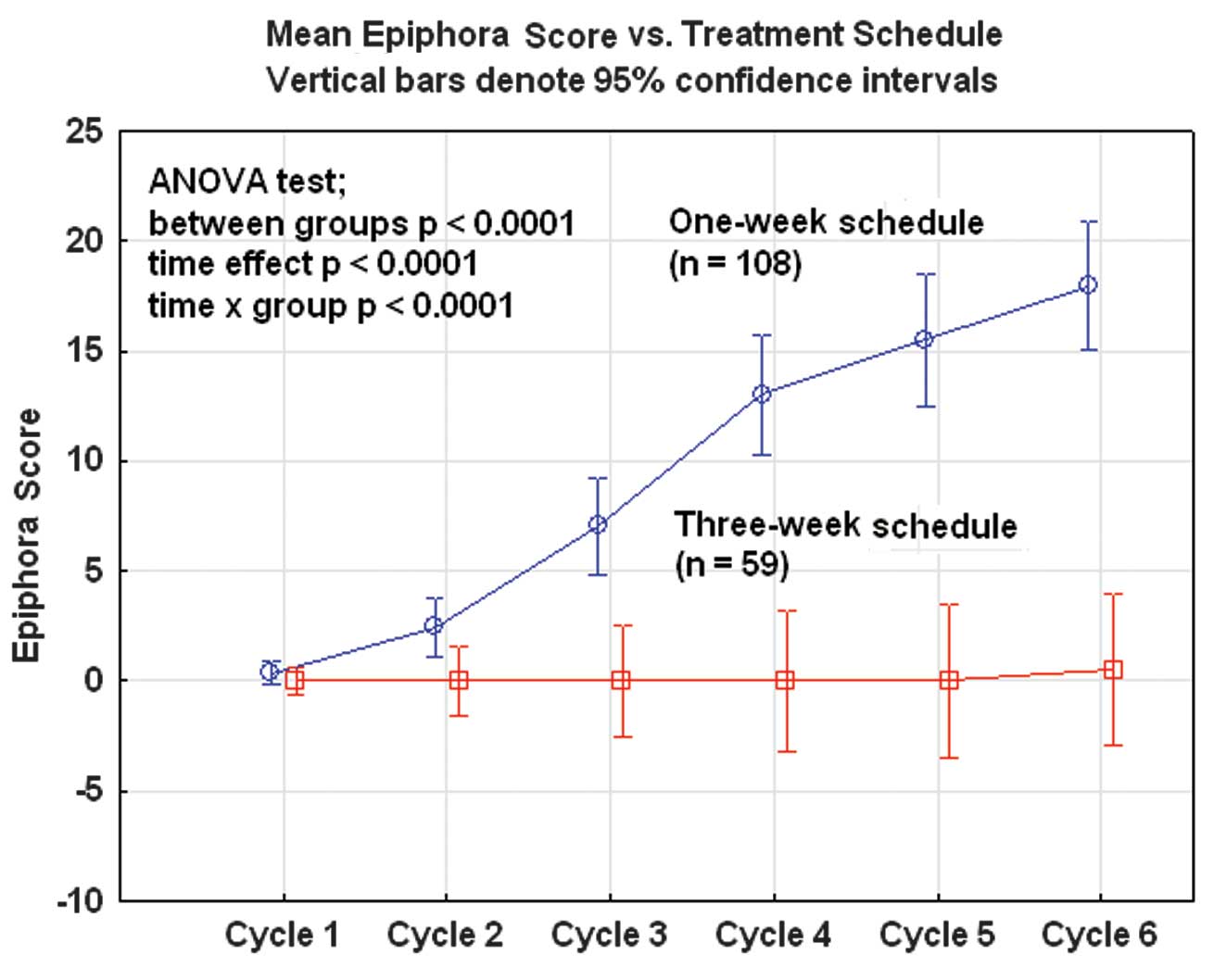
The second most common adverse event was watery eyes
and tearing (epiphora), affecting 55 patients (50.9%) in the
one-week group but only one patient (1.7%) in the three-week group.
Thus, this side-effect was very specific for the weekly regimen and
the frequency increased for every consecutive treatment cycle
(Fig. 3).
Nail changes were relatively common with the weekly
schedule and were reported in 30 cases (27.8%) compared with 9
cases (15.3%) with the three-week schedule (Fisher’s exact test; p=
0.049). Analyzed with ANOVA repeated test, significant differences
(p<0.005) were shown after 3 courses of chemotherapy and until 6
completed courses as well as for the complete treatment (course
1–6). A significant time-dependent effect was noted after 3 cycles
in the one-week group and after 5 cycles in the three-week group
(p<0.0001).
With regard to peripheral neurotoxicity, no
significant differences (ANOVA; p=0.125) were reported between the
two treatment schedules, but a very pronounced time-effect was
observed in both groups (ANOVA; p<0.0001; Table IV). After 6 completed courses of
chemotherapy, 28 patients (39.4%) had grade 1–2 neurotoxicity in
the weekly group and 13 patients (24.5%) in the three-week group.
No patients exhibited grade 3 or higher sensory neuropathy
(Table III).
Oral mucositis was significantly more common with
the three-week schedule (ANOVA; p<0.0001) and increased in
frequency from cycle 1 to cycle 3 and then reached a plateau and
slightly decreased up to cycle 6. In the one-week group this was a
less common problem but showed a slight increase with every
consecutive cycle of treatment.
Taste disturbances were significantly more common
after weekly treatment (ANOVA; p<0.0001) and showed a clear cut
increase in frequency from the first to the fourth docetaxel cycle
and then reached a plateau. In the three-week group this was a
minor problem with a different time pattern (Table IV).
Myalgia following treatment was more pronounced with
the three-week schedule than with weekly administration of
docetaxel. The difference was highly significant (ANOVA;
p<0.0001) during the complete period of treatment. There was no
significant change in myalgia with time and this was true for both
treatment groups (ANOVA; p=0.626).
Cardiac toxicity was extremely rare in the two
groups. No significant differences were noted during the treatment
period of six administered cycles of docetaxel-carboplatin (ANOVA;
p=0.809). No significant time-dependent effect was noted in either
group (ANOVA; p=0.210).
The mean score of nausea and vomiting was 14–15 on
the scale of 1–100 in both groups after the first course of
chemotherapy and then significantly decreased to ∼6 after five
courses of therapy (ANOVA; p<0.0001). There were no significant
differences between the two chemotherapy regimens (ANOVA; p=
0.277). Dyspnea and diarrhea were slightly more common after weekly
administration of docetaxel but of limited clinical
significance.
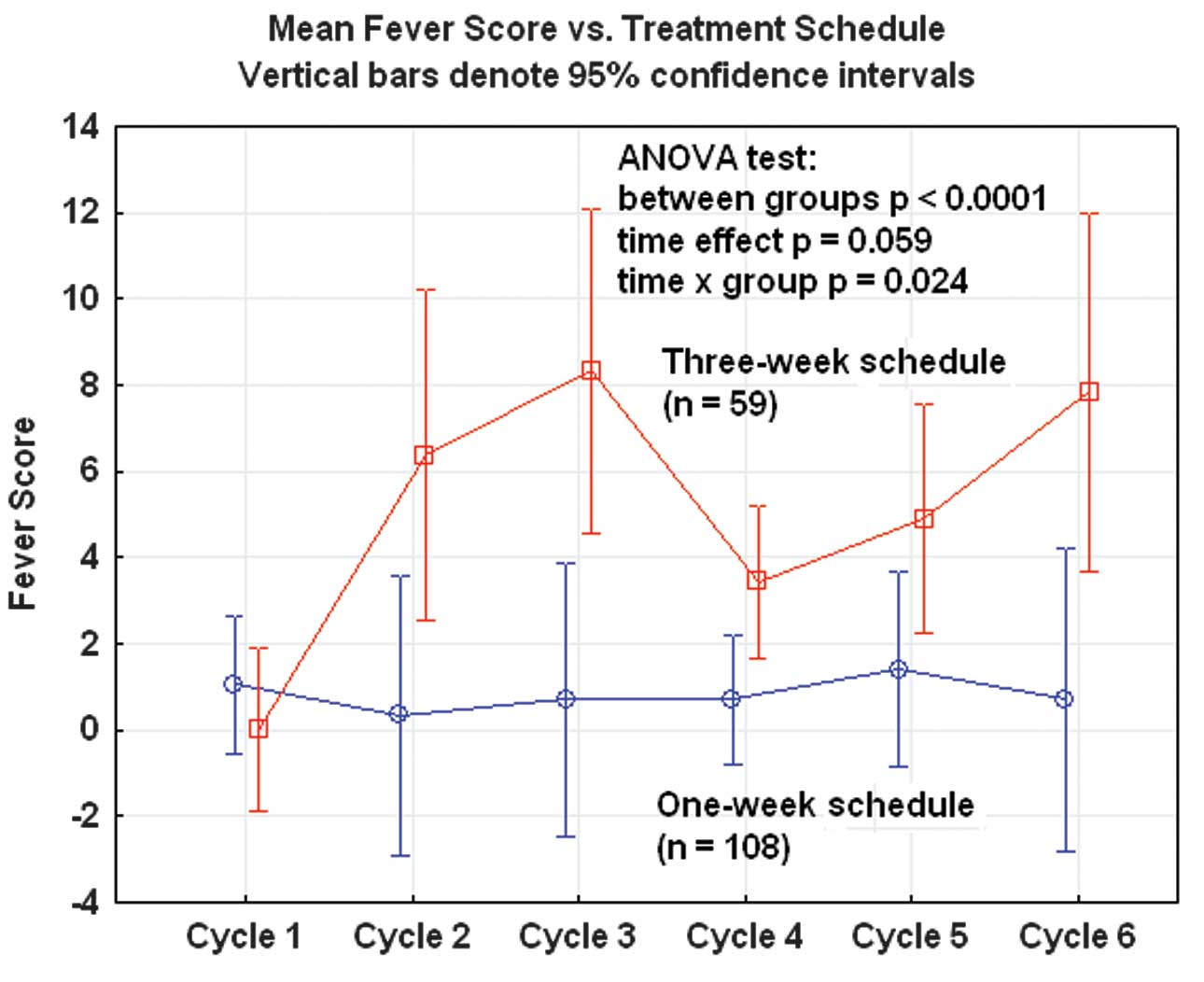
Fever and clinical infections were significantly
more frequent after three-week administration of docetaxel than
after weekly administration (ANOVA; p<0.0001; Fig. 2). This was possibly associated with
neutropenia for the three-week schedule.
Discussion
In order to improve the efficacy and tolerability of
standard 3-week regimens of the combination of carboplatin and a
taxane, the activity of weekly administration of docetaxel and
3-weekly carboplatin has been studied (17). This has not been performed
previously in a first-line setting in primary advanced ovarian
cancer. Katsumata et al(6)
have investigated the efficacy of the combination of weekly
dose-dense paclitaxel (80 mg/m2) and carboplatin (AUC=6)
compared with paclitaxel (180 mg/m2) and carboplatin
(AUC=6) every 3 weeks in patients with previously untreated ovarian
cancer. The two regimens had relatively similar toxicity, but
progression-free survival was significantly improved in the
patients who received the dose-dense regimen (median 28 versus 17
months; p=0.0014). Weekly chemotherapy data have been reported in a
number of previous studies (4,5). In
our prior study (17) we
encountered an overall response rate of 79%, which is superior to
the 56% reported by Katsumata et al(8). Our data and results are in line with
those of Micha et al(25)
and Penson et al(26) who
reported response rates of 80 and 76%, respectively, when adding
bevacizumab to paclitaxel and carboplatin in the treatment of
advanced-stage ovarian cancer.
The present study focuses on hematological and
non-hematological toxicity when docetaxel is administered weekly
compared with the standard 3-week schedule in combination with
carboplatin given every three weeks. The dose-intensity of
docetaxel was higher in the weekly schedule with 29.8
mg/m2/week compared to 25.1 mg/m2/week in the
three-week schedule (t-test; p<0.001). The dose intensity of
carboplatin was similar in the two regimens (t-test; p=0.211) with
105.7 and 109.4 mg/m2/week, respectively. Thus,
differences in toxicity should be associated with the docetaxel
treatment schedule. This was not a randomized study, but two
separate first-line phase II studies of docetaxel in combination
with carboplatin in the primary treatment of advanced ovarian
cancer. The two study cohorts were similar with regard to the
patients’ characteristics, but with regard to the tumors there were
slightly more endometrioid and more stage IIIB tumors in the
three-week group, and more papillary serous and stage IV tumors in
the one-week group. Distribution of the tumor grade was similar in
the two groups. Response rates and survival data for the weekly
schedule have been presented in a previous study (17). No comparison was made between the
two regimens with regard to response rate and survival in the
current study.
In terms of hematological toxicity, grade 3–4
neutropenia was recorded in 11% of the patients after six courses
and weekly administration and in 78% after the standard three-week
schedule. This was a highly significant difference and it was true
during the whole course of chemotherapy from cycle 1 to cycle 6.
The higher frequency of fever and infections recorded in the
three-week group was probably explained by this myelosuppression.
This difference in neutropenia (ANC) was the most important and
clinically relevant difference between the two regimens. In a study
from Germany, Sehouli et al(5) reported that 28% of their patients with
advanced ovarian cancer developed grade 3–4 neutropenia after
weekly paclitaxel (100 mg/m2) and carboplatin (AUC 2).
Thrombocytopenia was infrequent and grade 3–4 was recorded in only
one patient in the weekly group. However, grade 1–2
thrombocytopenia was more frequent in the one-week group (51%) than
in the three-week group (17%), but this difference in laboratory
readings had no clinical implications in this series of patients.
None of the patients experienced grade 3–4 anemia. Grade 1–2 anemia
was recorded in 96% of the weekly group and in 73% of the
three-week administration group. This difference was statistically
significant (Pearson Chi-square; p<0.0001), but clinically of
minor importance. Overall, the hematological toxicity was quite
manageable in both groups, but was more so in the weekly schedule
with regard to the risk of neutropenia, fever and infections.
Hematological toxicity (neutropenia) is also a common reason for
not completing all planned courses of a chemotherapy regimen.
Colony-stimulating factors were normally not used in these two
phase II studies.
In the present study, sensory neuropathy grades 1
and 2 developed in 39.4% of patients in the one-week group and in
24.5% of those in the three-week group after six completed cycles
of chemotherapy. Neuropathy significantly increased with time in
both groups, but there were no significant differences between the
two treatment regimens. None of the patients exhibited severe
neurotoxicity grade 3. These results are comparable to those of
Micha et al(25) and Sehouli
et al(5) who reported low
rates of severe peripheral neuropathy (2.3% grade 3). One would
suspect that lower, weekly doses of taxanes would mitigate toxicity
(4). However, Seidman et
al(27) reported that
neurotoxicity was a dose-limiting factor after weekly paclitaxel
(80 mg/m2) for the treatment of metastatic breast
cancer. Thus, docetaxel appears to have a lower rate of disabling
neurotoxicity than paclitaxel when administered on a weekly
schedule. However, the weekly schedule of docetaxel did not appear
to be superior to a 3-week schedule of the same drug.
Epiphora was a type of toxicity recorded in 51% of
the patients treated with the weekly schedule, but only in 1.7% of
the patients with the three-week schedule. This toxicity appears to
be specifically associated with weekly administration of docetaxel.
Esmaeli et al(28) from the
MD Anderson Cancer Center reported on 148 patients with this type
of side-effect. Thirty of 71 patients given weekly docetaxel needed
surgery to correct epiphora. Of the patients who received docetaxel
every 2 or 3 weeks, only 3 required a surgical intervention to
correct epiphora. A schedule of docetaxel given every 2 weeks has
shown a favorable outcome and toxicity profile, also with regard to
epiphora, and should be further evaluated in larger series of
advanced ovarian cancer patients (29).
Fatigue was the most frequently reported side-effect
and was particularly associated with the weekly regimen. The short
interval (one week) between the administrations of docetaxel may
explain this difference and time for recovery is therefore limited.
Nail changes were also a common side-effect of the weekly schedule
and were twice as common as in the three-week schedule. This
side-effect has also been reported for weekly treatment with
paclitaxel and is sometimes quite serious (30).
Oral mucositis may be a problem when higher doses of
docetaxel are administered every three weeks (31). This was also confirmed in this study
with significantly more mucositis in the three-week group. The
incidence of mucositis reached a maximum after the third
chemotherapy cycle with docetaxel. On the other hand, taste
disturbances were significantly more common after weekly
administration than after three-week administration.
Diarrhea and dyspnea were more frequent with the
weekly schedule, but these side-effects appeared to be of less
clinical significance and few patients reported these symptoms.
Cardiac toxicity was also very rare and no differences between the
two groups were recorded.
Fever and infections were significantly more
frequent in the three-week group and this was a clinically
important difference between the two schedules, favoring the weekly
schedule. The more pronounced neutropenia after the standard
three-week schedule is the probable reason for this difference
(32).
In the current study the technique from the
quality-of-life analysis (EORTC QLQ-C30) was used to calculate a
symptom score on a linear scale from 0 to 100 for each symptom item
(33). These scores were also used
in the repeated ANOVA analyses when the two treatment schedules
were compared over time. In these analyses the differences may be
compared between the groups, as well as differences and patterns
over time, and the combined effect of group and time.
Quality-of-life measurements were not part of this
study, but data from the weekly schedule have been presented
previously (18). The results from
the quality-of-life data showed similar levels as for the standard
carboplatin-paclitaxel regimen administered every three weeks
(34). Peripheral neuropathy was
one the most notable side-effects affecting quality of life
(35). Peripheral neuropathy is a
minor problem for docetaxel compared with paclitaxel regimens in
the treatment of ovarian cancer. This is the principal advantage of
this taxane. One-third of patients undergoing cisplatin and
paclitaxel treatment experienced long-term toxicity (36).
The two docetaxel schedules studied showed different
toxicity profiles favoring weekly administration with regard to
neutropenia, fever and infections as well as problems with oral
mucositis and myalgia. Fatigue, epiphora, taste disturbances and
nail changes were more specific side-effects of the weekly schedule
and in a number of cases a clinical problem. Peripheral sensory
neuropathy is a more limited problem with docetaxel compared with
paclitaxel but no significant differences were noted between the
two regimens studied.
Docetaxel is an alternative to paclitaxel in
first-line and second-line chemotherapy regimens for advanced
ovarian cancer. Dose-dense schedules with weekly or twice-weekly
administrations of the drug should be further explored to improve
and optimize the efficacy and the toxicity profile of docetaxel
chemotherapy combinations.
Acknowledgements
The authors thank Sanofi-Aventis AB,
Bromma, Sweden, for the financial support by grants.
References
|
1
|
Ferlay J, Shin HR, Bray F, et al: GLOBOCAN
2008, Cancer incidence and mortality worldwide: IARC CaseBase No.
10. Lyon, France: International Agency for Research on Cancer;
2010
|
|
2
|
McGuire WP, Hoskins WJ, Brady MF, et al:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Copeland LJ, Bookman M and Trimble E:
Gynecologic Oncology Group Protocol GOG 182-ICON5. Clinical trials
of newer regimens for treating ovarian cancer: the rationale for
Gynecologic Oncology Group Protocol GOG 182-ICON5. Gynecol Oncol.
90:S1–S7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Safra T, Menczer J, Bernstein RM, et al:
Combined weekly carboplatin and paclitaxel as primary treatment of
advanced epithelial ovarian carcinoma. Gynecol Oncol. 114:215–218.
2009. View Article : Google Scholar
|
|
5
|
Sehouli J, Stengel D, Mustea A, et al:
Weekly paclitaxel and carboplatin (PC-W) for patients with primary
advanced ovarian cancer: results of a multicenter phase-II study of
the NOGGO. Cancer Chemother Pharmacol. 61:243–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katsumata N, Yasuda M, Takahashi F, et al:
Dose-dense paclitaxel once a week in combination with carboplatin
every 3 weeks for advanced ovarian cancer: a phase 3, open-label,
randomised controlled trial. Lancet. 374:1331–1338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kavanagh JJ: Docetaxel in the treatment of
ovarian cancer. Oncology. 16:73–81. 2002.PubMed/NCBI
|
|
8
|
Katsumata N: Docetaxel: an alternative
taxane in ovarian cancer. Br J Cancer. 89:S9–S15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vasey PA, Jayson GC, Gordon A, et al:
Phase III randomized trial of docetaxel-carboplatin versus
paclitaxel-carboplatin as first-line chemotherapy for ovarian
carcinoma. J Natl Cancer Inst. 96:1682–1691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kushner DM, Connor JP, Sanchez F, et al:
Weekly docetaxel and carboplatin for recurrent ovarian and
peritoneal cancer: a phase II trial. Gynecol Oncol. 105:358–364.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tinker AV, Gebski V, Fitzharris B, et al:
Phase II trial of weekly docetaxel for patients with relapsed
ovarian cancer who have previously received paclitaxel - ANZGOG
02-01. Gynecol Oncol. 104:647–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta D, Owers RL, Kim M, et al: A phase
II study of weekly topotecan and docetaxel in heavily treated
patients with recurrent uterine and ovarian cancers. Gynecol Oncol.
113:327–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Safra T, Bernstein Molho R, Menzcher J, et
al: A feasibility study of weekly docetaxel with capecitabine in
ovarian cancer: a promising combination of two active drugs with a
potential for synergism. Chemotherapy. 55:298–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terauchi F, Hirano T, Taoka H, et al:
Weekly docetaxel for patients with
platinum/paclitaxel/irinotecan-resistent relapsed ovarian cancer: a
phase I study. Int J Clin Oncol. 8:348–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berkenblit A, Seiden MV, Matulonis UA, et
al: A phase II trial of weekly docetaxel in patients with
platinum-resitent epithelial ovarian, primary peritoneal serous
cancer, or fallopian tube cancer. Gynecol Oncol. 95:624–631. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komiyama S, Tsuji H, Asai S, et al: A
pilot study of weekly docetaxel therapy for recurrent ovarian
cancer, tubal cancer, and primary peritoneal cancer. Eur J Gynaecol
Oncol. 6:299–302. 2005.PubMed/NCBI
|
|
17
|
Sorbe B, Graflund M, Horvath G, Swahn M,
Boman K, Bangshöj R, Lood M and Malmström H: Phase II study of
docetaxel weekly in combination with carboplatin every 3 weeks as
first-line chemotherapy in stage IIB to stage IV epithelial ovarian
cancer. Int J Gynecol Cancer. 22:47–53. 2012. View Article : Google Scholar
|
|
18
|
Sorbe B, Graflund M, Horvath G, Swahn M,
Boman K, Bangshöj R, Lood M and Malmström H: A phase II study of
docetaxel weekly in combination with carboplatin every three weeks
as first line chemotherapy in stage IIB-IV epithelial ovarian
cancer: neurological toxicity and quality-of-life evaluation. Int J
Oncol. 40:773–781. 2012.
|
|
19
|
Calvert AH, Newell DR, Gunbrell LA, et al:
Carboplatin dosage: prospective evaluation of a simple formula
based on renal function. J Clin Oncol. 7:1748–1756. 1989.PubMed/NCBI
|
|
20
|
Cockcroft DW and Gault MH: Prediction of
creatinine clearance from serum creatinine. Nephron. 16:31–41.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Green S and Weiss GR: Southwest oncology
group standard response criteria, endpoint definitions and toxicity
criteria. Invest New Drugs. 10:239–253. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rustin GJ, Marples M and Nelstrop AE: Use
of CA-125 in clinical trial evaluation of new therapeutic drugs for
ovarian cancer. Clin Cancer Res. 10:3919–3926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Institute of Health: Common
Terminology Criteria for Adverse Events (Version 3.0). National
Institute of Health; Washington, DC: 2003
|
|
24
|
Fayers P, Aaronson N, Bjordal K, et al:
EORTC QLQ-C30 Scoring Manual. 3rd edition. EORTC publications;
Brussels, Belgium: 2001
|
|
25
|
Micha JP, Goldstein BH, Rettenmaier MA, et
al: A phase II study of outpatient first-line paclitaxel,
carboplatin, and bevacizumab for advanced-stage epithelial ovarian,
peritoneal, and fallopian tube cancer. Int J Gynecol Cancer.
17:771–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Penson RT, Dizon DS, Cannistra SA, et al:
Phase II study of carboplatin, paclitaxel, and bevacizumab with
maintenance bevacizumab as first-line chemotherapy for advanced
mullerian tumors. J Clin Oncol. 28:154–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seidman AD, Berry D, Cirrincione C, et al:
Randomized phase III trial of weekly compared with every-3-weeks
paclitaxel for metastatic breast cancer, with trastuzumab for all
HER-2 overexpressors and random assignment to trastuzumab or not in
HER-2 nonoverexpressors: final results of Cancer and Leukemia Group
B protocol 9840. J Clin Oncol. 26:1642–1649. 2008.
|
|
28
|
Esmaeli B, Hidaji L, Adinin RB, et al:
Blockage of the lacrimal drainage apparatus as a side effect of
docetaxel therapy. Cancer. 98:504–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oishi T, Kigawa J, Fujiwara K, et al: A
feasibility study on biweekly administration of docetaxel for
patients with recurrent ovarian cancer. Gynecol Oncol. 90:421–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paul LJ and Cohen PR:
Paclitaxel-associated subungual pyogenic granuloma: report in a
patient with breast cancer receiving paclitaxel and review of
drug-induced pyogenic granulomas adjacent and beneath the nail. J
Drugs Dermatol. 11:262–268. 2012.
|
|
31
|
Bezjak A, Tu D, Bacon M, et al: Quality of
life in ovarian cancer patients: Comparison of paclitaxel plus
cisplatin, with cyclophosphamide plus cisplatin in randomized
study. J Clin Oncol. 22:4595–4603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Souglakos J, Kotsakis A, Kouroussis C, et
al: Nonneutropenic febrile episodes associated with docetaxel-based
chemotherapy in patients with solid tumors. Cancer. 95:1326–1333.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Greimel ER, Bjelic-Radisic V, Pfisterer J,
et al: Randomized study of the Arbeitsgemeinschaft Gynaekologische
Onkologie Ovarian Cancer Study Group comparing quality of life in
patients with ovarian cancer treated with cisplatin/paclitaxel
versus carboplatin/paclitaxel. J Clin Oncol. 24:579–586. 2006.
View Article : Google Scholar
|
|
34
|
Schwarz R and Hinz A: Reference data for
the quality of life questionnaire EORTC QLQ-C30 in the general
German population. Eur J Cancer. 37:1345–1351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Almadrones L, McGuire DB, Walczak JR, et
al: Psychometric evaluation of two scales assessing functional
status and peripheral neuropathy associated with chemotherapy for
ovarian cancer: A Gynecologic Oncology Group study. Oncol Nurs
Forum. 31:615–623. 2004. View Article : Google Scholar
|
|
36
|
Wenzel L, Huang HQ, Monk BJ, et al:
Quality-of-life comparisons in a randomized trial of interval
secondary cytoreduction in advanced ovarian carcinoma: A
Gynecologic Oncology Group study. J Clin Oncol. 23:5605–5612. 2005.
View Article : Google Scholar : PubMed/NCBI
|