Introduction
Although there has been considerable progress in
recent years in the development of anti-cancer therapies, including
chemotherapy, radiation therapy and biological targeted therapy,
the mortality of patients with advanced non-small cell lung cancer
(NSCLC) remains high due to the inability to treat the condition
surgically (1). As a consequence,
the research into novel prognostic biomarkers and therapeutic
target structures in advanced NSCLC remains a focus of
attention.
The human trophoblast cell-surface antigen, Trop-2,
is a transmembrane glycoprotein originally found to be expressed at
high levels on the surface of trophoblastic cells (2). More recent studies have identified
Trop-2 to be highly expressed in a number of human epithelial
tumors, including colorectal cancer (3), oral squamous cell carcinoma (4) and pancreatic cancer (5), and high expression is often associated
with a poor prognosis. By contrast, Trop-2 expression is minimal or
absent in normal epithelial tissues. This means that Trop-2 may
promote tumor cell proliferation and aggressiveness (6). Trop-2 expression has also been
detected in the early stage of NSCLC, but its clinical significance
in operative NSCLC remains controversial (7,8). To
the best of our knowledge, Trop-2 expression in advanced NSCLC and
its association with prognosis has not yet been reported. In the
present retrospective study, Trop-2 antigen expression and its
correlation with clinicopathological features was evaluated in
advanced NSCLC.
Patients and methods
Patients
The clinical records of 87 patients (61 males and 26
females; mean age 63.4 years; range, 45–71 years) with advanced
NSCLC who were admitted to the Taizhou People’s Hospital (Taizhou,
Jiangsu, China) between June 2008 and June 2010 were
retrospectively evaluated. In total, 37 cases of squamous-cell
carcinoma (SCC), 50 cases of adenocarcinoma (AdC) and 17
tumor-adjacent normal tissue samples were obtained. All cases were
confirmed using CT-guided percutaneous or bronchoscopic lung
biopsies. The patients were divided into stage IIIb and stage IV
tumor groups, according to the TNM system (9). The standard procedure that was used
for the inoperable stage IIIb patients was that of sequential
chemo-radiation. Platinum-based doublets in two to three cycles
were administered prior to irradiation. The platinum doublets were
similar to those used for stage IV tumors. Radiation was
administered using a linear accelerator (≥6 MeV) or cobalt-60, for
a total dose of 50–60 Gy, delivered in 25–30 fractions of 2 Gy/day,
5 days/week. Patients were excluded from the study if they had
received prior chemotherapy or radiotherapy, had no definitive
histological diagnosis, had a bad performance status (PS; ECOG ≥3),
had brain tumor metastasis or if they had a disease other than lung
cancer that may have affected survival, including cardiac
dysfunction, renal insufficiency, liver cirrhosis or concomitant
malignancy. This study was approved by the Ethics Committee of
Taizhou People’s Hospital, Jiangsu, China and was performed
according to the Declaration of Helsinki. Written informed consent
was obtained from each patient’s family.
Immunohistochemistry
Paraffin-embedded tissue blocks were cut into
4-μm sections and analyzed immunohistochemically (EliVision™
Plus IHC kit; Wuhan Boster Biological Engineering Co., Ltd., Wuhan,
Hubei, China) for Trop-2 expression (1:50; goat polyclonal
antibody; R&D Systems, Minneapolis, MN, USA). The sections were
dewaxed in xylene and rehydrated using graded concentrations of
ethanol. The endogenous peroxidase activity was blocked by
incubating the sections in 5% hydrogen peroxide in absolute
methanol at room temperature for 10 min. Antigen retrieval was
performed in a microwave oven for two cycles of 10 min each. The
primary antibodies were applied for 1 h at room temperature and the
sections were washed three times using 0.05M Tris-buffered saline
(TBS, pH 7.2), prior to 50μl IgG/HRP secondary antibody
(Wuhan Boster Biological Engineering Co., Ltd.) being added,
followed by incubation for 30 min at room temperature. The sections
were washed three times with TBS and the reaction products were
visualized with diaminobenzidine (DAB kit; Wuhan Boster Biological
Engineering Co., Ltd.). The sections were counterstained with
hematoxylin and eosin, dehydrated and evaluated under a light
microscope.
Immunohistochemistry scoring
Positive staining for Trop-2 expression was assessed
in 10 high-power fields of each tumor by two independent
pathologists using light microscopy in a blinded fashion. Trop-2
expression was evaluated for each tissue sample by calculating a
total immunostaining score as the product of a proportion and
intensity score (5). The proportion
score described the estimated fraction of positively-stained tumor
cells (0, none; 1, ≤10%; 2, 10–50%; 3, 51–80%; and 4, ≥80%). The
intensity score represented the estimated staining intensity (0, no
staining; 1, weak; 2, moderate; and 3, strong). Thus, the total
score ranged from 0–12. The positive and negative expression of
Trop-2 were defined as a score of >4 and ≤4, respectively.
Follow-up
The patients were followed up from the date of the
pathological diagnosis until the date of mortality or the last
follow-up at the outpatient department. At the time of the last
follow-up, 80 patients (92%) had succumbed to the tumor and 7
patients (8%) were lost to follow-up or succumbed to other
causes.
Statistical analysis
The statistical analysis was performed using the
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). The associations
between Trop-2 immunostaining and the clinicopathological
parameters (gender, age, histologic grade, lymph node metastasis,
TNM stage and ECOG-PS) were analyzed using χ2 and
Fisher’s exact tests. Overall survival (OS) was calculated from the
date of diagnosis to the date of the last follow up or mortality.
The cases of patients that were lost to follow-up or had succumbed
from any other cause were defined as censored data for the analysis
of survival rates. The survival curves were plotted using the
Kaplan-Meier method, and P-values were calculated using the
log-rank test. A multivariate analysis was performed using the
Cox-proportional hazards model to identify independent prognostic
factors. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Trop-2 expression in tumor-adjacent
normal tissues
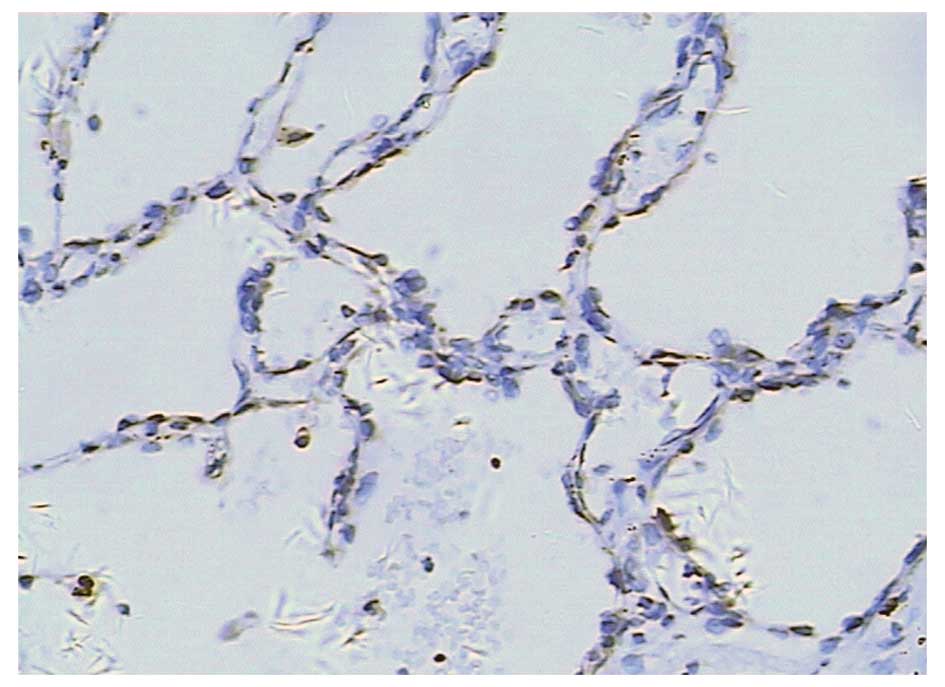
Trop-2 expression was absent or infrequent in the
tunica mucosa bronchiorum. No positive expression was observed in
the alveolar wall. Trop-2 overexpression was detected in 5.9%
(1/17) of tumor adjacent normal tissues (Fig. 1).
Trop-2 expression in advanced NSCLC
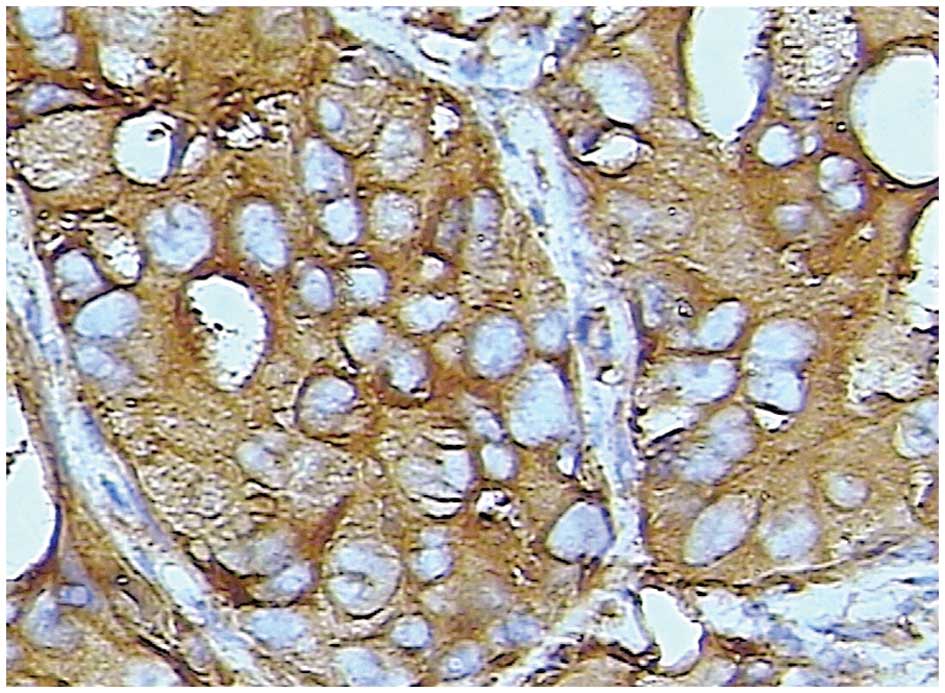
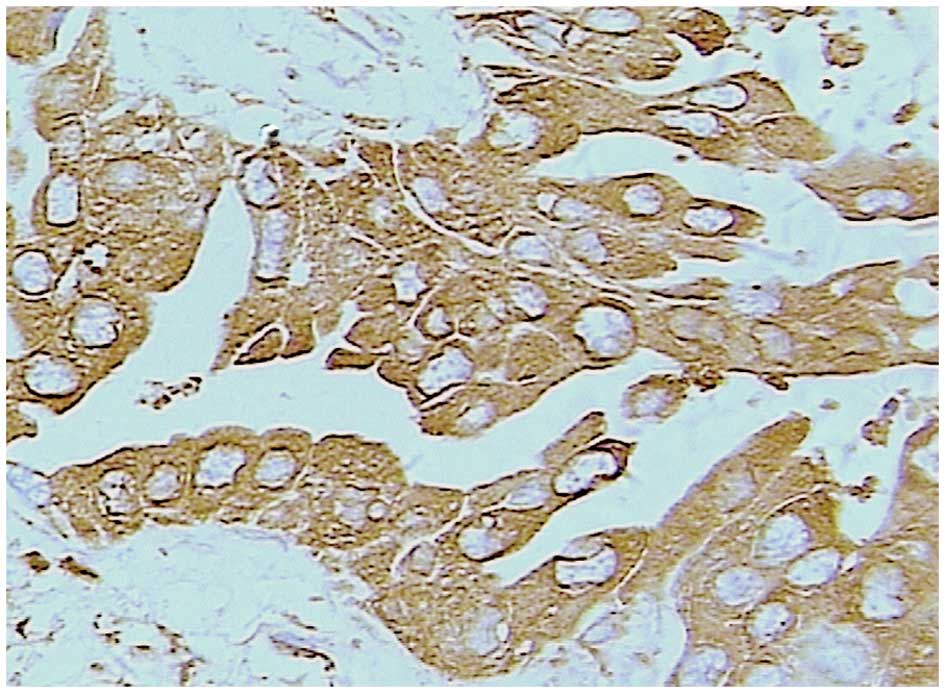
Staining for Trop-2 occurred in a diffuse pattern
localized mainly in the membrane of the cancer cells, although
staining was occasionally identified in the nucleus and cytoplasm
(Figs. 2 and 3). Trop-2 overexpression was detected in
52.9% (46/87) of the tumors. Trop-2 expression was higher in the
advanced NSCLC tissues than in the tumor-adjacent normal tissues
(P=0.000), and higher in the SCC cases [67.6% (25/37)] than in the
AdC cases [42.0% (21/50); P=0.018]
Trop-2 expression associations with
clinicopathological variables and prognosis
Trop-2 overexpression in SCC did not differ
significantly with regard to patient gender, age, lymph node
metastasis, TNM stage or ECOG-PS. However, Trop-2 overexpression
was significantly correlated with the histological grade (P=0.035;
Table I). Trop-2 overexpression in
AdC did not differ significantly with regard to patient gender, age
or ECOG-PS. However, Trop-2 overexpression was significantly
correlated with the histological grade, lymph node metastasis and
TNM stage (P= 0.01, 0.024 and 0.015, respectively; Table II).
 | Table I.Trop-2 expression in association with
clinicopathological factors in SCC. |
Table I.
Trop-2 expression in association with
clinicopathological factors in SCC.
| Characteristics | Number | Trop-2 overexpression
| Positive rate
(%) | χ2 | P-value |
|---|
| No | Yes |
|---|
| Gender | | | | | | |
| Male | 25 | 7 | 18 | 72.0 | 0.6911 | 0.406 |
| Female | 12 | 5 | 7 | 58.3 | | |
| Age (years) | | | | | | |
| ≥60 | 19 | 5 | 14 | 73.7 | 0.6668 | 0.414 |
| <60 | 18 | 7 | 11 | 61.1 | | |
| Degree of
differentiation | | | | | | |
| Low-middle | 16 | 2 | 14 | 87.5 | 5.1110 | 0.035 |
| High | 21 | 10 | 11 | 52.4 | | |
| Lymph node
metastasis | | | | | | |
| No | 10 | 5 | 5 | 50.0 | 1.9299 | 0.165 |
| Yes | 27 | 7 | 20 | 74.1 | | |
| TNM stage | | | | | | |
| IIIb | 20 | 8 | 12 | 60.0 | 1.1376 | 0.319 |
| IV | 17 | 4 | 13 | 76.5 | | |
| PS score | | | | | | |
| 0–1 | 14 | 6 | 8 | 57.1 | 1.1169 | 0.291 |
| 2 | 23 | 6 | 17 | 73.9 | | |
 | Table II.Trop-2 expression in association with
clinicopathological factors in AdC. |
Table II.
Trop-2 expression in association with
clinicopathological factors in AdC.
| Characteristics | Number | Trop-2 overexpression
| Positive rate
(%) | χ2 | P-value |
|---|
| No | Yes |
|---|
| Gender | | | | | | |
| Male | 36 | 22 | 14 | 38.9 | 0.5109 | 0.475 |
| Female | 14 | 7 | 7 | 50.0 | | |
| Age (years) | | | | | | |
| ≥60 | 28 | 17 | 11 | 39.3 | 0.1925 | 0.661 |
| <60 | 22 | 12 | 10 | 45.5 | | |
| Degree of
differentiation | | | | | | |
| Low-middle | 25 | 10 | 15 | 60.0 | 6.6502 | 0.010 |
| High | 25 | 19 | 6 | 24.0 | | |
| Lymph node
metastasis | | | | | | |
| No | 14 | 12 | 2 | 14.3 | 6.1309 | 0.024 |
| Yes | 36 | 17 | 19 | 52.8 | | |
| TNM stage | | | | | | |
| IIIb | 29 | 21 | 8 | 27.6 | 5.8888 | 0.015 |
| IV | 21 | 8 | 13 | 61.9 | | |
| PS score | | | | | | |
| 0–1 | 17 | 12 | 5 | 29.4 | 1.6756 | 0.196 |
| 2 | 33 | 17 | 16 | 48.5 | | |
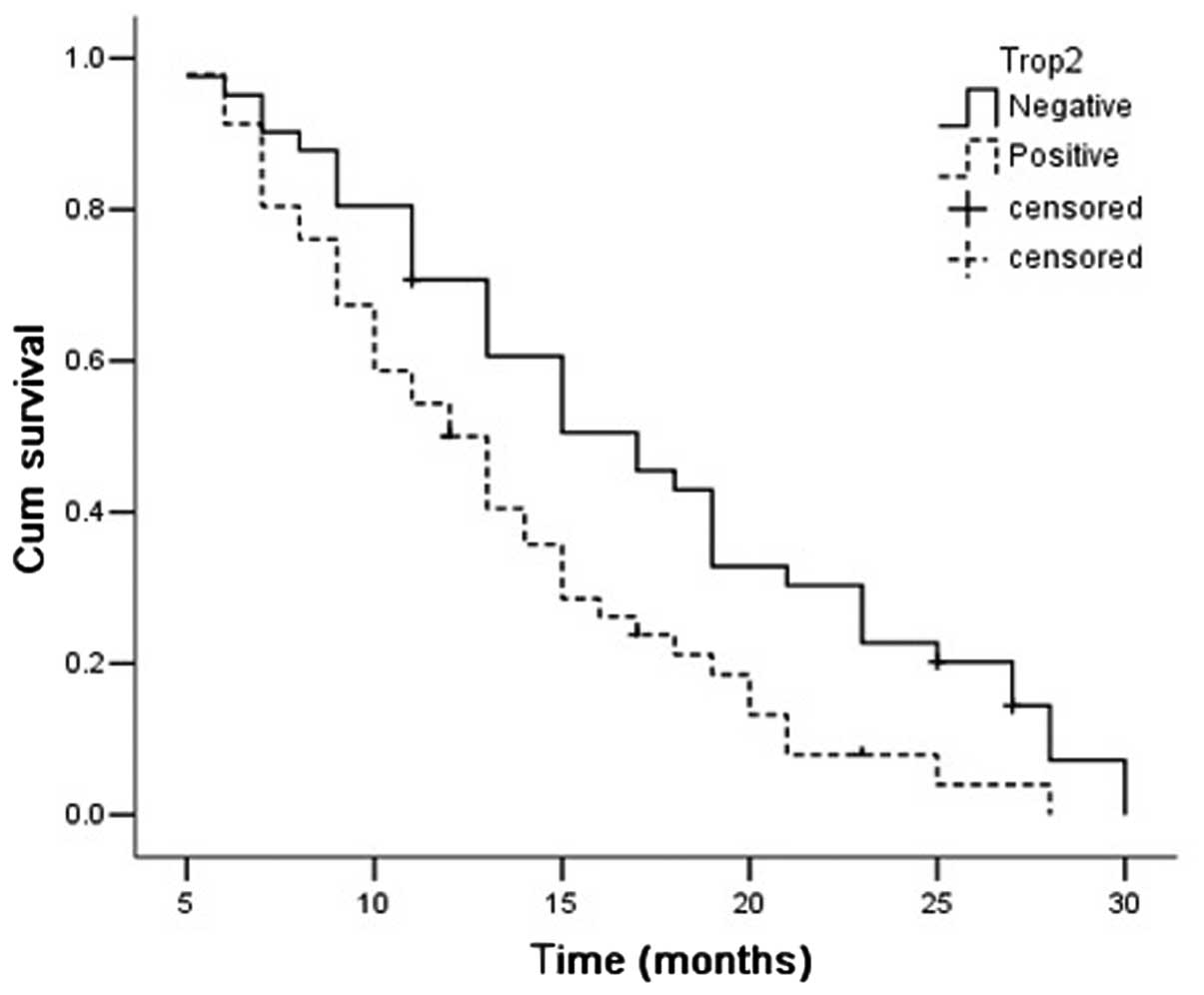
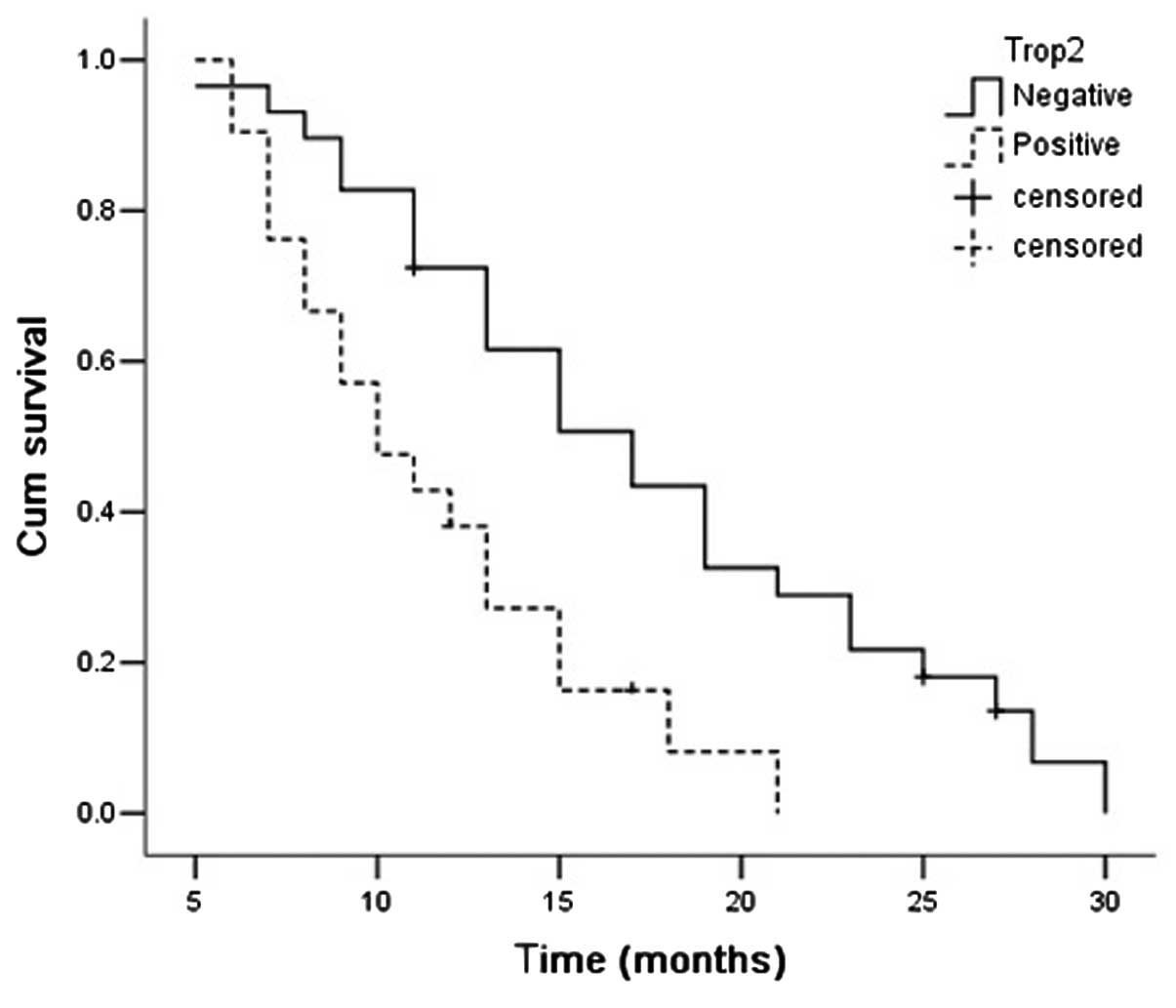
The median OS time of all patients was 15.197 months
(95% CI, 13.688–16.706). The survival time was significantly better
in the patients with Trop-2-negative expression than those with
Trop-2-positive expression [17.25 months (95% CI, 14.922–19.577)
vs. 13.274 months (95% CI, 11.507–15.041); P= 0.008]. The survival
time was significantly longer in the Trop-2-negative AdC patients
[17.275 months (95% CI, 14.575–19.975) vs. 11.469 months (95% CI,
11.507–15.041); P=0.002], but not in the SCC patients [17.167
months (95% CI, 12.428–21.906) vs. 14.647 months (95% CI,
12.062–17.232); P=0.276; Figs.
4–6].
In the univariate survival tests of AdC that were
performed using clinicopathological factors, the statistically
significant parameters, other than Trop-2 expression (HR, 2.606; P=
0.004) were lymph node metastasis (HR, 2.258; P= 0.011), TNM stage
(HR, 2.478; P= 0.005) and ECOG-PS (HR, 2.586; P=0.005). Other
variables, including age, gender and tumor differentiation, were
not associated with a better survival outcome. In the multivariate
survival tests, Trop-2 expression (HR, 2.381; P= 0.038), TNM stage
(HR, 2.193; P= 0.03) and ECOG-PS (HR, 2.696; P= 0.007) were
identified as independent prognostic markers in advanced AdC
(Table III).
 | Table III.Multivariate analysis of survival in
advanced pulmonary adenocarcinoma. |
Table III.
Multivariate analysis of survival in
advanced pulmonary adenocarcinoma.
| Parameter | Regression
co-efficient | Standard error | Wald | HR (95% CI) | P-value |
|---|
| Trop-2
overexpression | 0.868 | 0.417 | 4.325 | 2.381
(1.051–5.394) | 0.038 |
| Age (≥60 vs.
<60) | −0.154 | 0.433 | 0.126 | 0.857
(0.367–2.004) | 0.722 |
| Gender (male vs.
female) | 0.285 | 0.410 | 0.484 | 1.330
(0.595–2.972) | 0.487 |
| TNM stage (IIIb vs.
IV) | 0.785 | 0.361 | 4.727 | 2.193
(1.080–4.453) | 0.030 |
| Degree of
differentiation (Low-middle vs. high) | −0.362 | 0.429 | 0.714 | 0.696
(0.300–1.614) | 0.398 |
| Lymph node
metastasis (Yes vs. no) | 0.477 | 0.388 | 1.510 | 1.611
(0.753–3.448) | 0.219 |
| PS score (0–1 vs.
2) | 0.992 | 0.368 | 7.254 | 2.696
(1.310–5.549) | 0.007 |
Discussion
The Trop-2 protein (also termed GA733-1, M1S1 and
EGP-1), is a human trophoblast cell-surface antigen encoded by the
TACSTD2 gene of human chromosome 1p32 (10). The TACSTD2 gene lacks introns and is
formed by exon shuffling and retroposition of the TACSTD1 gene via
an mRNA intermediate. TACSTD2 encodes a 35 kDa, type 1
transmembrane protein, which contains 323 amino acids and a single
transmembrane domain. Trop-2 is a calcium channel protein that is
associated with the regulation of intracellular calcium
concentration (11). Moreover,
Trop-2 plays a significant role in the regulation of tumor
proliferation by increasing the stability of cyclin D1 or
activating the signal pathway of ERKl-MAPK (12,13).
Early studies identified Trop-2 gene mutations to be
associated with Gelatinous Drop-like Corneal Dystrophy (GDLD), a
rare autosomal recessive genetic disease which leads to severe
vision disorders and even blindness (14–16).
More recently, studies have observed that Trop-2 is highly
expressed in a number of human epithelial tumors compared with the
restricted expression found in normal tissues, and that it is also
associated with poor overall patient survival (3–5). These
previous studies have revealed that Trop-2 may promote tumor
proliferation, aggressiveness and metastasis (6). Trop-2 expression has also been
detected in the early stage of NSCLC, but its clinical significance
in operative NSCLC remains controversial (7,8).
Kobayashi et al (7) revealed
that 87 of 130 patients (67%) with small-sized pulmonary AdC (<2
cm diameter) were immunopositive for Trop-2 expression and
therefore associated with a poor OS. The multivariate analysis
showed that Trop-2 overexpression was an independent, unfavorable
prognostic marker in AdC and NSCLC. Pak et al (8) reported that 64 of 164 patients (39%)
with stage II or III NSCLC were immunopositive for Trop-2
expression. The Trop-2 expression in patients with AdC [23/100
(23%)] was significantly lower than that in the SCC patients [41/64
(64%)]. Trop-2 overexpression showed a better OS in the AdC
patients. The inconsistent results between the two studies suggest
that the biological role of Trop-2 may vary between early and
advanced pulmonary AdC.
To the best of our knowledge, there are no studies
with regard to the correlation between Trop-2 expression and
advanced NSCLC in the English-language literature. The present
study detected that Trop-2 expression occurred in the membrane of
lung cancer cells and occasionally in the nucleus and cytoplasm.
Trop-2 expression was higher in advanced NSCLC than in the
tumor-adjacent normal tissues. These results revealed that the
Trop-2 gene may be associated with tumorigenesis and that the
progression of advanced NSCLC. Trop-2 expression in the present
study was significantly higher in NSCLC than in the tumor-adjacent
normal tissues. Additionally, Trop-2 was significantly
overexpressed in SCC compared with AdC, as observed in a previous
study (8). The present study also
detected that Trop-2 overexpression in SCC was only correlated with
the histological grade of the tumor. However, Trop-2 expression in
AdC was correlated with the histological grade, lymph node
metastasis and TNM stage. The multivariate analysis showed that
Trop-2 is an independent prognostic marker in advanced pulmonary
AdC and that it may play a more significant role in the
pathogenesis of this disease.
In conclusion, Trop-2 is closely correlated with
unfavorable prognostic factors in advanced NSCLC. Trop-2 is also an
independent prognostic marker in advanced AdC. The present results
further indicate that Trop-2 may be a potential new therapeutic
target for advanced AdC. However, due to the limitations inherent
in retrospective analyses, the prognostic value of Trop-2
overexpression requires further validation in larger prospective
studies.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Jiang HF and Bo JJ: Trophoblastic cell
surface protein 2 and tumor. J Int Oncol. 39:96–98. 2012.(In
Chinese).
|
|
3.
|
Ohmachi T, Tanaka F, Mimori K, Inoue H,
Yanaga K and Mori M: Clinical significance of TROP2 expression in
colorectal cancer. Clin Cancer Res. 12:3057–3063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fong D, Spizzo G, Gostner JM, et al:
TROP2: a novel prognostic marker in squamous cell carcinoma of the
oral cavity. Mod Pathol. 21:186–191. 2008.PubMed/NCBI
|
|
5.
|
Fong D, Moser P, Krammel C, et al: High
expression of TROP2 correlates with poor prognosis in pancreatic
cancer. Br J Cancer. 99:1290–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang J, Day R, Dong Y, Weintraub SJ and
Michel L: Identification of Trop-2 as an oncogene and an attractive
therapeutic target in colon cancers. Mol Cancer Ther. 7:280–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kobayashi H, Minami Y, Anami Y, et al
IASLC International Staging Committee; Cancer Research and
Biostatistics; Observers to the Committee; Participating
Institutions: Expression of the GA733 gene family and its
relationship to prognosis in pulmonary adenocarcinoma. Virchows
Arch. 457:69–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pak MG, Shin DH, Lee CH and Lee MK:
Significance of EpCAM and TROP2 expression in non-small cell lung
cancer. World J Surg Oncol. 10:532012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Groome PA, Bolejack V, Crowley JJ, et al
IASLC International Staging Committee; Cancer Research and
Biostatistics; Observers to the Committee; Participating
Institutions: The IASLC Lung Cancer Staging Project: validation of
the proposals for revision of the T, N, and M descriptors and
consequent stage groupings in the forthcoming (seventh) edition of
the TNM classification of malignant tumors. J Thorac Oncol.
2:694–705. 2007. View Article : Google Scholar
|
|
10.
|
Calabrese G, Crescenzi C, Morizio E, Palka
G, Guerra E and Alberti S: Assignment of TACSTD1 (alias TROP1,
M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2
(alias TROP2, M1S1) to human chromosome 1p32 by in situ
hybridization. Cytogenet Cell Genet. 92:164–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Taylor JT, Zeng XB, Pottle JE, et al:
Calcium signaling and T-type calcium channels in cancer cell
cycling. World J Gastroenterol. 14:4984–4991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nelsen CJ, Kuriyama R, Hirsch B, et al:
Short term cyclin D1 overexpression induces centrosome
amplification, mitotic spindle abnormalities, and aneuploidy. J
Biol Chem. 280:768–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Trop2 expression contributes to tumor pathogenesis by activating
the ERK MAPK pathway. Mol Cancer. 9:2532010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhang B, Yao YF and Zhou P: Two novel
mutations identified in two Chinese gelatinous drop-like corneal
dystrophy families. Mol Vis. 13:988–992. 2007.PubMed/NCBI
|
|
15.
|
Markoff A, Bogdanova N, Uhlig CE, Groppe
M, Horst J and Kennerknecht I: A novel TACSTD2 gene mutation in a
Turkish family with a gelatinous drop-like corneal dystrophy. Mol
Vis. 12:1473–1476. 2006.PubMed/NCBI
|
|
16.
|
Alavi A, Elahi E, Tehrani MH, et al: Four
mutations (three novel, one founder) in TACSTD2 among Iranian GDLD
patients. Invest Ophthalmol Vis Sci. 48:4490–4497. 2007. View Article : Google Scholar : PubMed/NCBI
|