Introduction
Lung cancer is a malignant tumor originating from
normal bronchial epithelial cells. Non-small cell lung cancer
(NSCLC) comprises the majority of lung cancer cases, with high
occurrence and a low five-year survival rate of ~15%. Accumulating
evidence has been previously documented concerning the molecular
mechanisms underlying lung cancer initiation and progression,
highlighting new targets for therapy. Defects in programmed cell
death or apoptosis are hallmark features of cancer and have been
implicated in lung tumorigenesis and drug resistance (1). Thus, inhibition of apoptosis offers a
novel strategy for cancer treatment.
Oxidative stress is a major apoptotic stimulus in
cancer cells, which have particularly high energy metabolism due to
their rapid growth and proliferation. Therefore, reactive oxygen
species (ROS) are excessively generated from a mitochondria source
and lead to lipid peroxidation, DNA damage and, consequently,
apoptosis in cells (2,3). By contrast, inhibition of oxidative
stress also shows anticancer effects. Antioxidants, such as
polyphenols, exhibit a wide variety of biological functions,
including apoptosis induction, growth arrest and inhibition of DNA
synthesis (4,5). Therefore, targeting the oxidative
stress pathways through induction or inhibition, the generation of
ROS may enhance the proapoptotic machinery of cancer cells and
offer a novel strategy for treatment.
Rhodiola rosea is a traditional Chinese
medicine and has long been used as an adaptogen for enhancing the
body’s resistance to fatigue, stimulating the nervous system and
preventing high altitude sickness (6). Salidroside, a phenol glycoside
compound extracted from Rhodiola rosea, is a potent
antioxidant. Salidroside has been reported to exert antidiabetic,
neuroprotective and hepatoprotective effects (7–9). It
has been hypothesized that salidroside may alleviate
mitochondrial-generated ROS and manipulate mitochondrial-related
apoptosis in a variety of cells (10). Moreover, salidroside has been found
to exert an antiproliferation effect on a number of various cancer
cells (11,12), and induce cell-cycle arrest and
apoptosis in breast cancer (13).
The aim of the current study was to investigate the
effects of salidroside on cell proliferation, the cell cycle,
apoptosis, invasion and epithelial-mesenchymal transition (EMT) in
the NSCLC A549 cell line. In addition, intracellular ROS levels and
phospho-p38 expression were detected, and their association with
A549 cells treated with salidroside was explored.
Materials and methods
Materials
Salidroside (purity, >99%) was purchased from the
National Institute of Pharmaceutical and Biological Products
(Beijing, China). Recombinant human transforming growth factor-β
(TGF-β) was purchased from R&D Systems (Minneapolis, MN, USA).
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum
(FBS) were obtained from Invitrogen Life Technologies (Carlsbad,
CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), vitamin C and 2′,7′-dichlorodihydrofluorescein
diacetate (DCFH-DA) were purchased from Sigma-Aldrich (Sigma, St.
Louis, MO, USA). Anti-Snail, -phospho-p38 and -β-actin antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Cell culture
The human alveolar adenocarcinoma cell line, A549,
was purchased from the Institute of Biochemistry and Cell Biology,
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). Cells were cultured in DMEM media and
supplemented with 10% FBS, at 37°C in a humidified incubator with
5% CO2.
Cell viability assay
Cell viability was determined by MTT assay. Briefly,
A549 cells at the logarithmic growth phase were randomly seeded
into 96-well culture plates at a density of 1×103
cells/ml and were cultured with 100 μl DMEM media (supplemented
with 10% FBS) in each well. Cell adhesion was achieved and the
cells were incubated with various concentrations of salidroside (0,
1, 5, 10 and 20 μg/ml) for 12, 24, 48 and 72 h. For cell viability
assay, 10 μl MTT solution (5 mg/ml) was added to each well and
incubated at 37°C for 4 h. Following centrifugation at 3,000 rpm
for 10 min, the supernatant was removed to obtain the formazan
pellet. Next, the pellet was dissolved completely with 100 μl DMSO.
An ELISA plate reader (Ricso RK201, Shenzhen Ricso Technology Co.,
Ltd, Shenzhen, China)was applied to measure the absorbance at a
wavelength of 570 nm, to determine the amount of pellet.
Cell cycle analysis
A549 cells at the logarithmic growth phase were
randomly seeded in 60-mm culture dishes. After reaching 50%
confluence, cells were cultured in serum-free medium for 24 h to
induce cell quiescence. Subsequently, cells were incubated with
various concentrations of salidroside (0, 1, 5, 10 and 20 μg/ml) in
complete medium. After 24 h, the cells were harvested by
trypsinization followed by centrifugation at 2,000 rpm for 5 min.
Next, cold 70% ethanol was added to cells for resuspension.
Finally, 1 ml propidium iodide (PI) stain solution (PI, 20 μg/ml
and DNase free RNase A, 100 μg/ml) was added to samples, which were
analyzed on a FACScan (Becton-Dickinson, Franklin Lakes, NJ, USA)
within 30 min. Data were acquired from 10,000 cells and processed
using Lysis II software (Becton-Dickinson).
Cell apoptosis assay
A549 cells were incubated with various
concentrations of salidroside (0, 1, 5, 10 and 20 μg/ml) for 24 h.
Subsequently, ≥2×105 cells were harvested from each
group for apoptosis assay by Annexin V-fluorescein isothiocyanate
(FITC) and PI double-staining. Following centrifugation at 2,000
rpm for 5 min, the pellet was resuspended in 100 μl 1X binding
buffer with 2.5 μl Annexin V and 5 μl PI (final concentration, 10
μg/ml). After incubation for 15 min in the dark, samples were
subjected to apoptosis assay by flow cytometry, followed by data
analysis using Lysis software. In total, ≥10,000 events were
analyzed for each sample.
Cell migration assay
The Boyden chamber invasion assay was performed to
determine the in vitro migration capability of A549 cells.
This experiment was performed in 24-well tissue culture plates with
Transwell filter membrane. The lower side of the filters were
coated with type I collagen (0.5 mg/ml) and the lower part of the
filter contained low-serum media. In the upper part of the
Transwell plate, 5×104 cells were resuspended in 100 μl
DMEM media, plated and incubated with salidroside (10 μg/ml) and/or
TGF-β (100 ng/ml). After 24 h, cells on the upper surface of the
filter were removed and cells that had migrated to the lower part
were considered invasive cells. These cells were stained with
hematoxylin and eosin (Sigma-Aldrich) and counted under an inverted
light microscope (IX70, Olympus, Tokyo, Japan; magnification, ×200)
as the number of migrated cells (invasion index). Each sample was
assayed in triplicate and repeated twice.
Measurement of ROS generation
Intracellular ROS levels were determined by a
fluorescence plate reader using DCFH-DA. The cells on 24-well
plates were treated with various concentrations of salidroside (0,
1, 5, 10 and 20 μg/ml) for 1, 3 and 6 h, and then incubated with
DCFH-DA at 37°C for 30 min. Following the removal of DCFH-DA, the
cells were washed with phosphate buffered saline. The fluorescence
plate reader (FACScan, Tecan Deutschland GmbH, Crailsheim, Germany)
was used to detect DCFH-DA-loaded cells. In order to determine
whether apoptois in A549 cells by Salidroside is dependent on
oxidative stress, a prominent water-soluble antioxidant, vitamin C
(100 μM), was pretreated to scavenge ROS.
Western blot analysis
Proteins of A549 cells were isolated and their
concentrations were determined by bicinchoninic acid protein
concentration assay kit (Beijing Biosea Biotechnology Co. Ltd.,
Beijing, China). Proteins (50 μg) were separated on sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels (polyacrylamide
concentration, 100 g/l) and electrophoretically transferred to a
polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was
blocked with 3% bovine serum albumin at 37°C for 1 h, and probed
with the mouse monoclonal antibodies against human Snail (1:1,000)
and phospho-p38 (1:1,000). The horseradish peroxidase-conjugated
rabbit anti-mouse IgG was used as secondary antibody at 1:1,000
dilution for 2 h at room temperature. The density of the targeted
bands was visualized using the enhanced chemiluminescence method
(Pierce® ECL Plus Western Blotting Substrate, Pierce
Biotechnology, Inc., Rockford, IL, USA) where Salidroside induces
G1 phase cell cycle arrest in A549 cells. β-actin was used as an
internal control.
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. Statistical analysis was performed using
commercially available software (SPSS, version 14.0; SPSS, Inc.,
Chicago, IL, USA). An unpaired, two-tailed Student’s t-test was
performed to compare the means of two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Salidroside inhibits the proliferation of
A549 cells
To evaluate the effect of salidroside on the cell
viability of A549 cells, cells were simultaneously treated with
various concentrations of salidroside (0, 1, 5, 10 and 20 μg/ml)
for different time periods (12, 24, 48 and 72 h). A549 cells
treated with DMEM media served as a normal control. The MTT assay
revealed that salidroside treatment could inhibit A549 cell
proliferation and decrease viable cells in a concentration- and
time-dependent manner, which was demonstrated by lower OD values at
570 nm. Salidroside showed the most potent effect on cell viability
at a 20-μg/ml concentration for all time points (Fig. 1A).
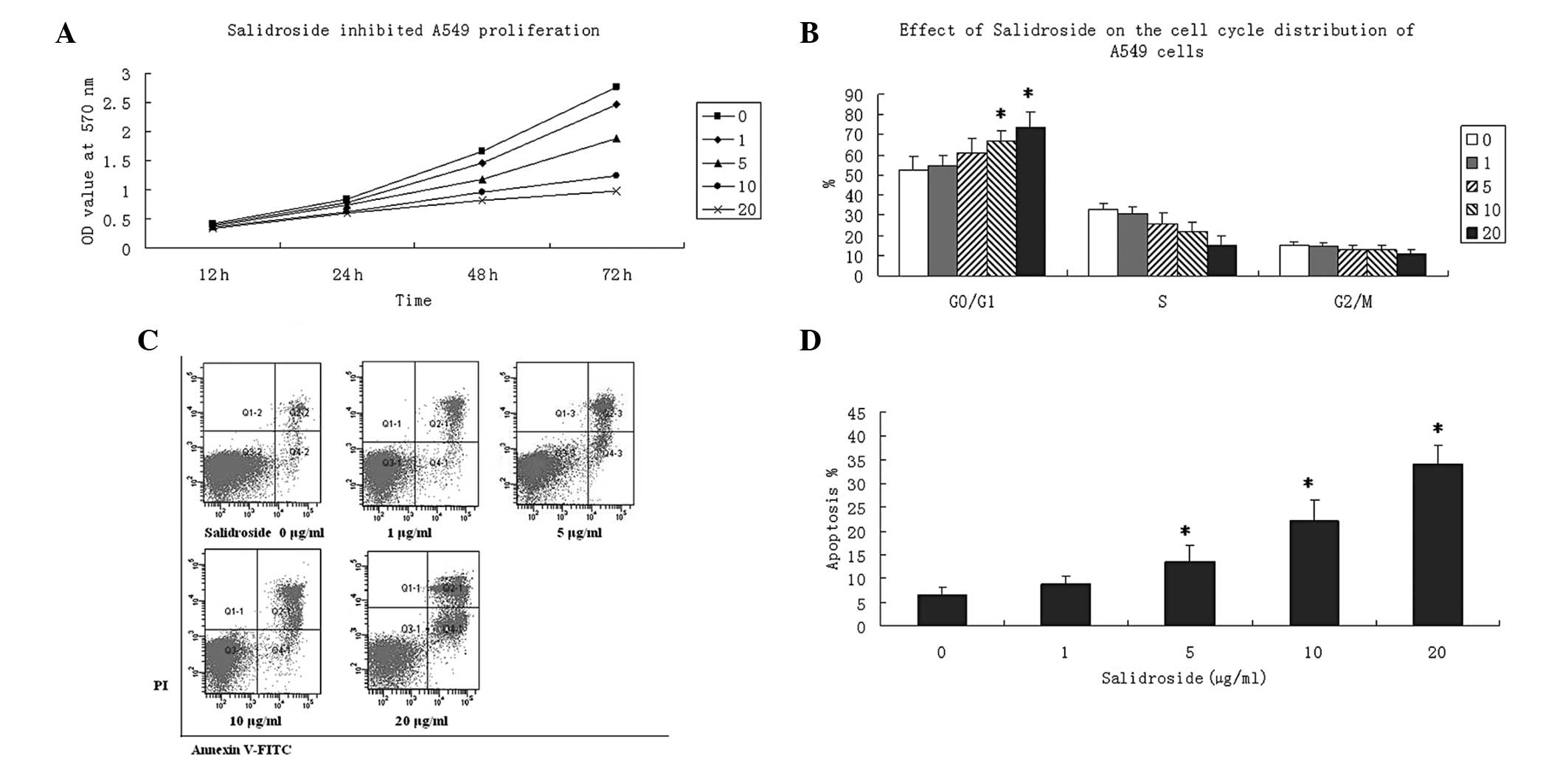 | Figure 1Effect of salidroside on the cell
viability of A549 cells. (A) Cells were seeded onto 96-well culture
plates and incubated with various concentrations of salidroside (0,
1, 5, 10 and 20 μg/ml) for 24 h. Cell proliferation was detected by
MTT assay. Data are presented as the OD values at 570 nm wavelength
and were obtained from at least three independent experiments. (B)
Cells were seeded and incubated with various concentrations of
salidroside (0, 1, 5, 10 and 20 μg/ml) for 24 h. PI (20 μg/ml)
staining was performed to determine the percentages of cells in the
G0/G1, S and G2/M phases. (C) Cell apoptosis was determined using
Annexin V-FITC and PI double-staining. Salidroside treatment
increased the apoptotic rate in A549 cells in a
concentration-dependent manner. Images from three experiments are
shown. (D) Apoptotic rates were analyzed in A549 cells treated with
various concentrations of salidroside (0, 1, 5, 10 and 20 μg/ml)
for 24 h. Annexin V+/PI− and Annexin
V+/PI+ populations were considered as
apoptotic cells. Data are presented as the mean ± SD and were
compared using a two-tailed, unpaired t-test.
*P<0.05, vs. the control group. PI, propidium iodide;
FITC, fluorescein isothiocyanate; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
Salidroside induces G0/G1 phase cell
cycle arrest in A549 cells
To investigate the detailed mechanism of the
underlying antiproliferative activity of salidroside, flow
cytometry was used to determine cell cycle distribution. Serum
starvation was performed on A549 cells to induce cell quiescence,
followed by treatment with various concentrations of salidroside
(0, 1, 5, 10 and 20 μg/ml) for 24 h. Salidroside significantly
increased the percentage of cells in the G0/G1 phase at
concentrations of 10 and 20 μg/ml (P<0.05). However, the
percentage of cells in the S and G2/M phases remained unchanged
following salidroside treatment (Fig.
1B). This assay indicated that NaHS inhibited the proliferation
of A549 cells by inducing G0/G1 phase arrest.
Salidroside increases apoptosis in A549
cells
To investigate whether decreased viability was
caused by increased apoptosis by salidroside treatment, A549 cells
were cultivated in the presence of salidroside (0, 1, 5, 10 and 20
μg/ml) for 24 h and double-stained with Annexin V-FITC and PI.
Salidroside was found to increase the apoptotic rate of A549 cells
in a concentration-dependent manner, and to significantly increase
the apoptotic rate at concentrations of 10 and 20 μg/ml (Fig. 1C and D).
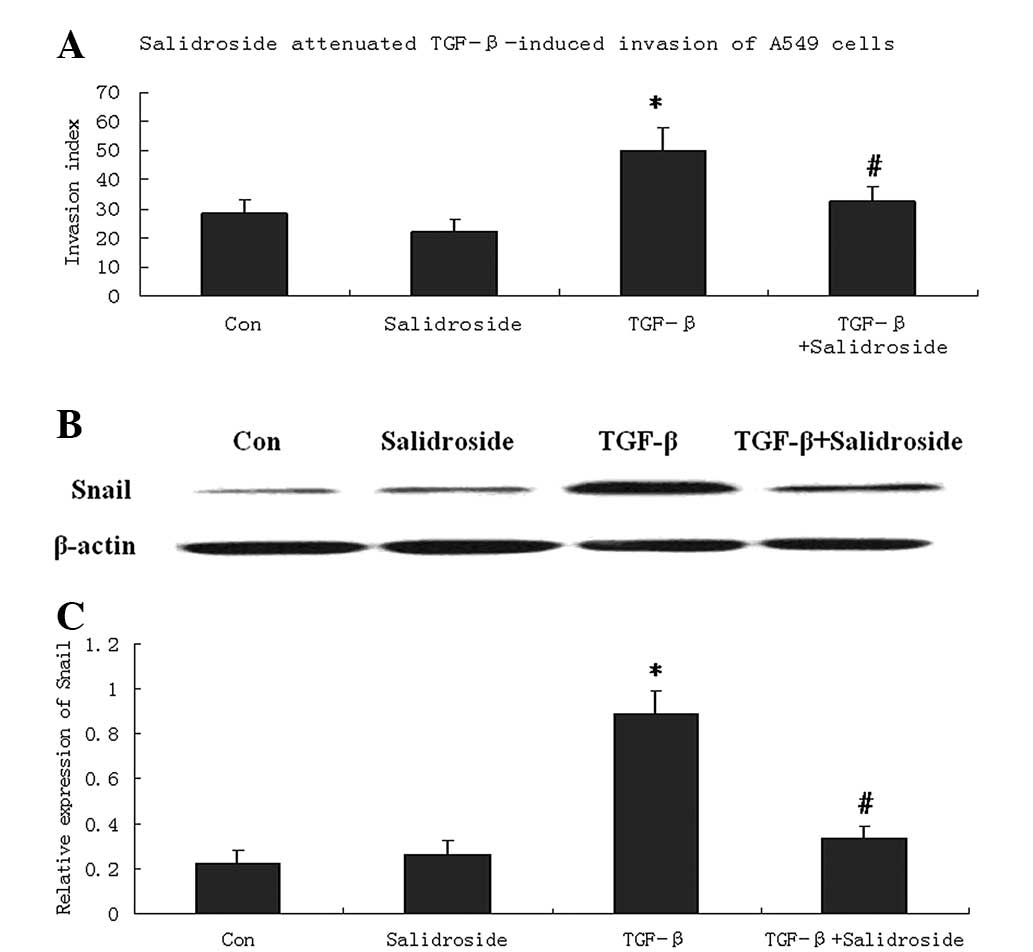
Salidroside inhibits the invasion and
expression of EMT marker protein, Snail
To investigate whether salidroside inhibits the
migration of tumor cells, the invasion capability of A549 cells was
determined by Boyden chamber invasion assay. A549 cells were
incubated with TGF-β to induce invasion. The results showed that
TGF-β significantly increased the invasion index of A549 cells.
Salidroside treatment significantly decreased the invasion index
compared with cells treated with TGF-β (Fig. 2A). However, compared with the
control cells, salidroside treatment alone only slightly decreased
the invasion index, with no significant difference.
To investigate whether EMT is involved in the
anti-invasive effect of salidroside, western blot analysis was
performed to determine the expression of Snail, an EMT marker
protein (14). In cells treated
with TGF-β, Snail protein levels were significantly decreased by
salidroside treatment. However, compared with control A549 cells,
the levels of Snail protein remained unchanged following
salidroside treatment (Fig.
2B).
Salidroside decreases ROS generation in
A549 cells
To investigate whether salidroside is involved in
ROS generation and ROS-related apoptosis signaling in A549 cells,
the fluorescence probe, DCFH-DA, was used to measure the
intracellular ROS levels. The results showed that ROS levels were
decreased by salidroside in a concentration- and time-dependent
manner. Salidroside at 10 and 20 μg/ml significantly decreased the
ROS levels in A549 cells after 1, 3 and 6 h (P<0.05; Fig. 3A).
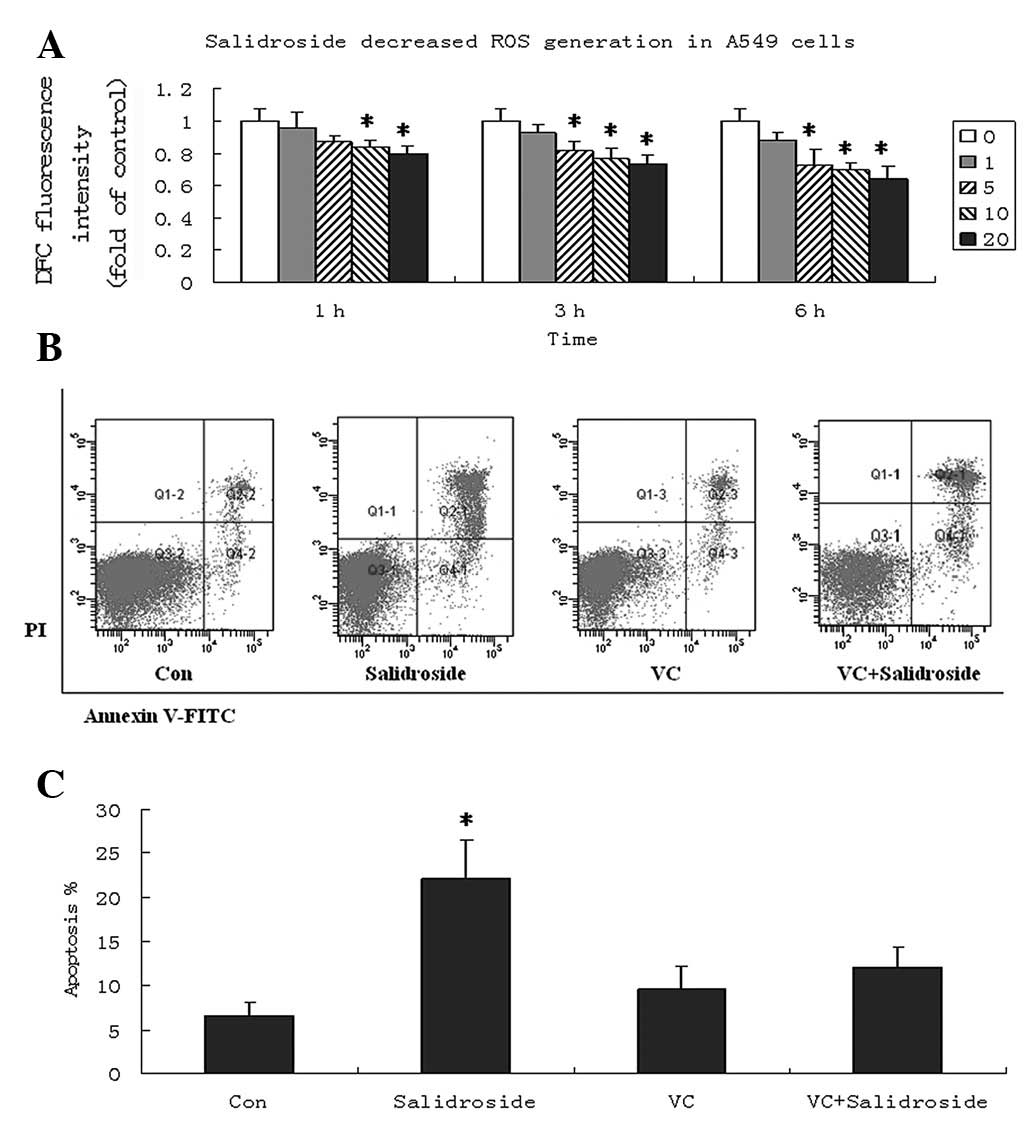 | Figure 3Salidroside decreases intracellular
ROS in A549 cells. (A) Cells were treated with various
concentrations of salidroside (0, 1, 5, 10 and 20 μg/ml) for 1, 3
and 6 h, followed by a 30-min incubation with
2′,7′-dichlorodihydrofluorescein diacetate at 37˚C for ROS
detection. Data are presented as the fold increase compared with
that of the control cells, and graphs present the mean ± SD. (B) VC
pretreatment decreased the apoptosis of A540 cells induced by
salidroside. VC (100 μM) was applied to A549 cells for 1 h.
Subsequently, A549 cells were treated with salidroside (10 μg/ml)
for 24 h and apoptosis was determined by Annexin V-FITC and PI
double-staining. Images from three experiments are shown. (C)
Apoptotic rates were analyzed in A549 cells. Annexin
V+/PI− and Annexin
V+/PI+ populations were considered to be
apoptotic cells. Data are presented as the mean ± SD and were
compared using a two-tailed, unpaired t-test.
*P<0.05, vs. the control group. ROS, reactive oxygen
species; VC, vitamin C; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
The effect of intracellular ROS levels on apoptosis
was further investigated following salidroside treatment. A549
cells were pretreated with 100 μM vitamin C (VC) for 1 h and
cultured with salidroside (10 μg/ml). Pretreatment of A549 cells
with VC significantly attenuated the apoptosis effect of
salidroside and the apoptosis rate remained at ~10%, even at a
10-μM concentration (Fig. 3B and
C). These results indicated that decreased intracellular ROS
may be a mechanism underlying the cell death of A549 cells by
salidroside.
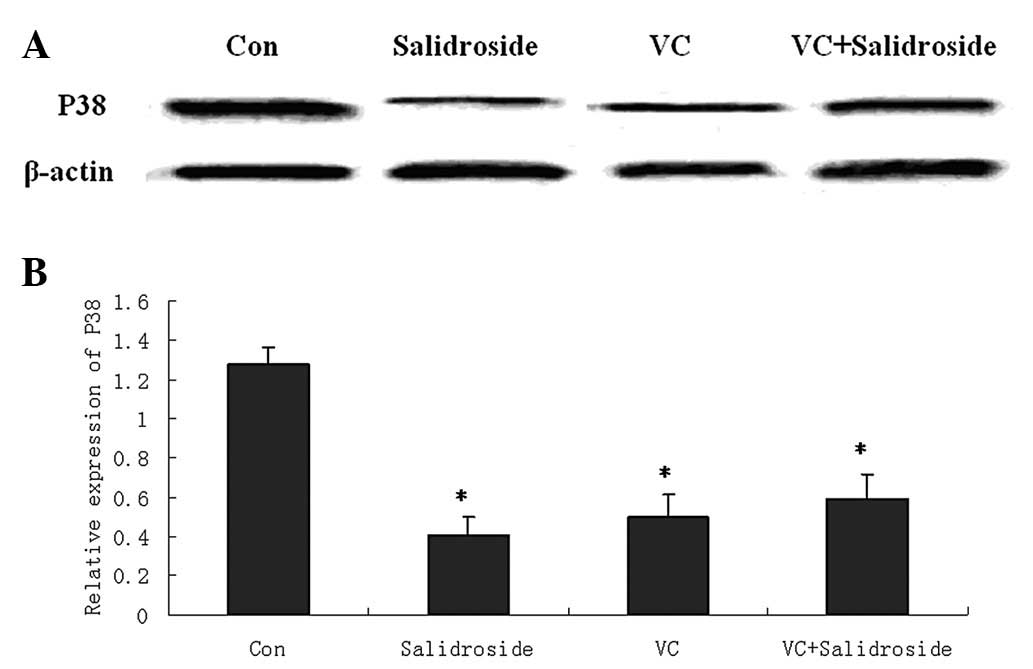
Salidroside decreases phospho-p38 MAPK
expression
To investigate the apoptosis signaling pathways
underlying salidroside-treated A549 cells, phospho-p38 MAPK [one of
the signaling proteins associated with oxidative stress (15)] was investigated for its protein
expression. A549 cells were pretreated with 100 μM VC followed by
salidroside treatment (10 μg/ml) for 24 h. Western blot analysis
showed that salidroside significantly decreased phospho-p38 protein
expression. VC pretreatment was found to also significantly
decrease the phospho-p38 protein levels. However, salidroside could
not further decrease phospho-p38 protein levels in VC-pretreated
A549 cells (Fig. 4).
Discussion
In the present study, salidroside, a phenol
glycoside compound extracted from Rhodiola rosea, was found
to show anticancer effects on in vitro cultured lung cancer
A549 cells. These effects were demonstrated by suppressed cell
proliferation, tumor invasion and EMT; arrested cell cycle; and
reduced apoptosis. The underlying mechanisms may be associated with
the inhibition of intracellular ROS generation and decreased
phospho-p38 expression by salidroside. Salidroside decreased the
intracellular ROS levels and phospho-p38 expression in A549 cells,
which may be important for the anticancer activity observed in lung
cancer cells.
The present study investigated the anticancer
effects of salidroside on lung cancer cells, indicating a novel
strategy for lung cancer treatment. Salidroside was found to reduce
viable cells in a dose-dependent manner and the detailed mechanism
lies in cell cycle arrest and induction of apoptosis. Following
salidroside treatment, the percentage of cells in the G0/G1 phase
was significantly increased. The results are consistent with those
of a previous study demonstrating that salidroside caused G1- or
G2-phase arrest in various cancer cell lines (11). Previously, salidroside has been
found to demonstrate potent antiapoptotic effects in a variety of
cells, including neurons (16),
cardiomyocytes (17) and endothelia
(18). However, a potent apoptotic
effect of salidroside has been identified on lung cancer cells.
Salidroside appears to exhibit antiapoptotic effects on non-tumor
cells and apoptotic effects on tumor cells. For example,
salidroside showed cytotoxic effects on breast cancer cells
(13). Moreover, polyphenols, as
antioxidants, also induce apoptosis in neutrophils (19), and liver (20) and breast (21) cancer cells. In this regard,
salidroside inhibits survival signals, such as the Akt
phosphorylation and mammalian target of the rapamycin pathway, and
destructs mitochondrial integrity (20,21).
Tumor invasion is a multistage process that involves
enhanced cell adhesion to extracellular matrix proteins. TGF-β acts
as a tumor suppressor early in carcinogenesis, but in specific
types of late stage cancer it is a prometastatic factor. TGF-β
levels are elevated in cancer with more invasive phenotypes, and
promote tumor invasion and metastasis (22). In the current study, TGF-β was
incubated with A549 cells to induce invasion and significantly
increase the invasion index of A549 cells. Salidroside was found to
significantly decrease the invasion index of A549 cells induced by
TGF-β. The observations are consistent with previous studies
reporting that salidroside inhibits the migration and invasion of
fibrosarcoma HT1080 cells, which was demonstrated by upregulated
E-cadherin expression and downregulated β1-integrin expression
(23). EMT is a vital step in the
acquisition of epithelial cells with malignant phenotypes,
including migration, invasion and metastasis to a new location
(24). The results of the present
study showed that following TGF-β treatment in A549 cells,
salidroside significantly downregulated the expression of Snail, an
EMT marker gene. This indicates that salidroside may suppress
invasion through inhibition of the EMT process in A549 cells. It
was also found that in control A549 cells without TGF-β, Snail
protein levels remained unchanged following salidroside treatment.
This may be explained by previous observations that salidroside
suppresses TGF-β production and expression in high glucose-induced
mesangial cell and experimental hepatic fibrosis rats, respectively
(25,26).
The current study found that salidroside decreases
ROS generation in A549 cells in a dose- and time-dependent manner.
Pretreatment with antioxidant VC eliminates apoptosis induced by
salidroside. This indicated that the capability of apoptosis
induction by salidroside may rely on the high state of oxidative
stress. Therefore, depletion of ROS by VC pretreatment reduced the
sensitivity to salidroside. Salidroside was found to significantly
decrease the protein expression of phospho-p38, a signaling protein
associated with oxidative stress. However, in VC pretreated A549
cells, salidroside did not further decrease phospho-p38 protein
levels. This indicated that high phospho-p38 expression is
dependent on high levels of intracellular oxidative stress, which
yields a high sensitivity of A549 cells to salidroside-induced
apoptosis. Therefore, a decrease in phospho-p38 levels may be
involved in apoptosis due to reduced ROS levels by salidroside. In
a number of cell types, ROS-induced p38-MAPK activation is
associated with increased apoptosis (27,28),
which is contrary to the results of the current study. Salidroside
is a phenol glycoside compound and shares a similar structure to
polyphenols. As antioxidants, polyphenols have direct scavenging
activity toward ROS and indirect antioxidant activity, the latter
includes activation of antioxidant enzymes, such as glutathione
peroxidase, glutathione S-transferase, catalase and NAD(P)H:
quinone oxidoreductase-1 (4).
Furthermore, the various fates of cells treated with polyphenols
depend on their concentration, cell type, intracellular oxidative
stress levels and stage of the pathological process (29). Therefore, further investigation is
required to identify the detailed mechanism underlying the
intercorrelation between ROS-induced p38-MAPK activation and
apoptosis in lung cancer cells treated with salidroside,
particularly the expression analysis of antioxidant enzymes.
In tumor cells, p38 MAPK is important in successful
invasion and metastasis (30).
Previously, p38siRNA has exerted an inhibitory effect on high
glucose-induced EMT in tubular epithelial cells (31). In the present study, however, the
correlation between the decreased protein expression of phospho-p38
and reduced tumor invasion by salidroside remains unknown and
requires further study. The anticancer effects of salidroside must
be further validated by in vivo animal studies.
In conclusion, salidroside shows anticancer effects
in lung cancer cells. Decreased intracellular ROS and phospho-p38
may be the underlying mechanisms of salidroside activity. The
present study indicates that salidroside is a promising therapeutic
strategy for the treatment of lung cancer.
References
|
1
|
Han SW and Roman J: Targeting apoptotic
signaling pathways in human lung cancer. Curr Cancer Drug Targets.
10:566–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azad N, Iyer A, Vallyathan V, Wang L,
Castranova V, Stehlik C and Rojanasakul Y: Role of
oxidative/nitrosative stress-mediated Bcl-2 regulation in apoptosis
and malignant transformation. Ann N Y Acad Sci. 1203:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scatena R: Mitochondria and cancer: a
growing role in apoptosis, cancer cell metabolism and
dedifferentiation. Adv Exp Med Biol. 942:287–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu ML: Dietary polyphenols as antioxidants
and anticancer agents: more questions than answers. Chang Gung Med
J. 34:449–460. 2011.
|
|
5
|
Di Domenico F, Foppoli C, Coccia R and
Perluigi M: Antioxidants in cervical cancer: chemopreventive and
chemotherapeutic effects of polyphenols. Biochim Biophys Acta.
1822:737–747. 2012.PubMed/NCBI
|
|
6
|
Panossian A and Wagner H: Stimulating
effect of adaptogens: an overview with particular reference to
their efficacy following single dose administration. Phytother Res.
19:819–838. 2005. View
Article : Google Scholar
|
|
7
|
Yu S, Liu M, Gu X and Ding F:
Neuroprotective effects of salidroside in the PC12 cell model
exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol.
28:1067–1078. 2008. View Article : Google Scholar
|
|
8
|
Wu YL, Piao DM, Han XH and Nan JX:
Protective effects of salidroside against acetaminophen-induced
toxicity in mice. Biol Pharm Bull. 31:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HB, Ge YK, Zheng XX and Zhang L:
Salidroside stimulated glucose uptake in skeletal muscle cells by
activating AMP-activated protein kinase. Eur J Pharmacol.
588:165–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schriner SE, Abrahamyan A, Avanessian A,
et al: Decreased mitochondrial superoxide levels and enhanced
protection against paraquat in Drosophila melanogaster supplemented
with Rhodiola rosea. Free Radic Res. 43:836–843. 2009.
View Article : Google Scholar
|
|
11
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: the anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Li X, Simoneau AR, Jafari M and Zi
X: Rhodiola rosea extracts and salidroside decrease the
growth of bladder cancer cell lines via inhibition of the mTOR
pathway and induction of autophagy. Mol Carcinog. 51:257–267. 2012.
View Article : Google Scholar
|
|
13
|
Hu X, Zhang X, Qiu S, Yu D and Lin S:
Salidroside induces cell-cycle arrest and apoptosis in human breast
cancer cells. Biochem Biophys Res Commun. 398:62–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carver EA, Jiang R, Lan Y, Oram KF and
Gridley T: The mouse snail gene encodes a key regulator of the
epithelial-mesenchymal transition. Mol Cell Biol. 21:8184–8188.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato A, Okada M, Shibuya K, et al: Pivotal
role for ROS activation of p38 MAPK in the control of
differentiation and tumor-initiating capacity of glioma-initiating
cells. Stem Cell Res. 12:119–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu ZQ, Zhou Y, Zeng YS, Lin YK, Li Y,
Zhong ZQ and Chan WY: Protective Effects of a Rhodiola Crenulata
Extract and Salidroside on Hippocampal Neurogenesis against
Streptozotocin-Induced Neural Injury in the Rat. PLoS One.
7:e296412012. View Article : Google Scholar
|
|
17
|
Zhong H, Xin H, Wu LX and Zhu YZ:
Salidroside attenuates apoptosis in ischemic cardiomyocytes: a
mechanism through a mitochondria-dependent pathway. J Pharmacol
Sci. 114:399–408. 2010. View Article : Google Scholar
|
|
18
|
Tan CB, Gao M, Xu WR, Yang XY, Zhu XM and
Du GH: Protective effects of salidroside on endothelial cell
apoptosis induced by cobalt chloride. Biol Pharm Bull.
32:1359–1363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jančinová V, Perečko T, Harmatha J, Nosál’
R and Drábiková K: Decreased activity and accelerated apoptosis of
neutrophils in the presence of natural polyphenols. Interdiscip
Toxicol. 5:59–64. 2012.PubMed/NCBI
|
|
20
|
Park HS, Park KI, Lee DH, et al:
Polyphenolic extract isolated from Korean Lonicera japonica
Thunb. induce G2/M cell cycle arrest and apoptosis in HepG2 cells:
involvements of PI3K/Akt and MAPKs. Food Chem Toxicol.
50:2407–2416. 2012.
|
|
21
|
Castillo-Pichardo L and Dharmawardhane SF:
Grape polyphenols inhibit Akt/mammalian target of rapamycin
signaling and potentiate the effects of gefitinib in breast cancer.
Nutr Cancer. 64:1058–1069. 2012. View Article : Google Scholar
|
|
22
|
Shang D, Liu Y, Yang P, Chen Y and Tian Y:
TGFBI-promoted adhesion, migration and invasion of human renal cell
carcfinoma depends on inactivation of von Hippel-Lindau tumor
suppressor. Urology. 79:966.e1–e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun C, Wang Z, Zheng Q and Zhang H:
Salidroside inhibits migration and invasion of human fibrosarcoma
HT1080 cells. Phytomedicine. 19:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar
|
|
25
|
Yin D, Yao W, Chen S, Hu R and Gao X:
Salidroside, the main active compound of Rhodiola plants, inhibits
high glucose-induced mesangial cell proliferation. Planta Med.
75:1191–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ouyang J, Gao Z, Ren Z, Hong D, Qiao H and
Chen Y: Synergistic effects of rMSCs and salidroside on the
experimental hepatic fibrosis. Pharmazie. 65:607–613.
2010.PubMed/NCBI
|
|
27
|
Yang LH, Ho YJ, Lin JF, Yeh CW, Kao SH and
Hsu LS: Butein inhibits the proliferation of breast cancer cells
through generation of reactive oxygen species and modulation of ERK
and p38 activities. Mol Med Rep. 6:1126–1132. 2012.PubMed/NCBI
|
|
28
|
Chye SM, Tiong YL, Yip WK, et al:
Apoptosis induced by para-phenylenediamine involves formation of
ROS and activation of p38 and JNK in chang liver cells. Environ
Toxicol. 2012 Nov 22;(Epub ahead of print).
|
|
29
|
Giovannini C and Masella R: Role of
polyphenols in cell death control. Nutr Neurosci. 15:134–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
del Barco Barrantes I and Nebreda AR:
Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans.
40:79–84. 2012.PubMed/NCBI
|
|
31
|
Lv ZM, Wang Q, Wan Q, Lin JG, Hu MS, Liu
YX and Wang R: The role of the p38 MAPK signaling pathway in high
glucose-induced epithelial-mesenchymal transition of cultured human
renal tubular epithelial cells. PLoS One. 6:e228062011. View Article : Google Scholar
|