Introduction
Oral squamous cell carcinoma (OSCC) is the most
common type of cancer among head and neck neoplasms, and affects
275,000 individuals annually worldwide. In Brazil, the South
American nation with the highest OSCC incidence, OSCC is the
seventh most common type of cancer in the general population
(1–3). Approximately 80% of OSCC cases are
associated with tobacco and alcohol consumption; however, several
other factors may also favor its development, including human
papilloma virus infection and poor oral hygiene (1,4,5).
Although the oral cavity may be easily examined,
OSCC is often diagnosed late, which contributes to poor overall
survival (1,2). However, the identification of
molecular biomarkers may improve existing clinical parameters for
the development of novel diagnostic tools and treatment protocols,
as well as aid in assessing prognosis (6). The identification of these biomarkers
in the serum and saliva of OSCC patients is a promising and less
invasive approach for the diagnosis, prognosis and assessment of
disease status following therapy (7,8).
It is known that cancer can have a significant
inflammatory component and, in certain cases, inflammation itself
may trigger a malignant transformation. In other cases, such as in
OSCC, inflammation is caused and modulated by genetic and
epigenetic alterations induced by carcinogens, including tobacco
and alcohol. The modulation of the inflammatory response
contributes to tumor progression by increasing malignant cell
proliferation and survival, stimulating neoangiogenesis and
reducing antitumor immunity and tumor responses to therapies
(9–12).
Cytokines are important components of the
inflammatory cancer-associated process. Additionally, tumor cells
often overexpress cytokines in order to modulate their
microenvironment, which therefore indicates a potential role for
cytokines as biomarkers and therapeutic targets (12). Macrophage migration inhibitory
factor (MIF) is a pro-inflammatory cytokine that regulates the
innate immune response and has been shown to be important in
various autoimmune diseases, in addition to being involved in cell
proliferation, cell survival, migration and metastasis in cancer
(13–19). In addition, previous studies have
revealed that high serum MIF concentrations are observed in
patients with colorectal, prostate, colon and gastric cancers, when
compared with healthy subjects (20–23).
High serum MIF concentrations in patients with prostate, gastric
and hepatocellular cancer were found to be associated with a poor
prognosis (24–26).
In oral cancer, Kindt et al (27) demonstrated that, compared with
normal tissues, MIF is overexpressed in tumors, indicating that
this cytokine may contribute to tumor progression and the emergence
of second primary tumors (27).
The aim of the present study was to assess the serum
and saliva MIF concentrations in OSCC patients, prior to and
following surgical treatment, and their correlation with
clinicopathological characteristics. Serum and saliva MIF
concentrations were also investigated as potential markers for
disease control and recurrence in these patients.
Materials and methods
Study population
The study included 50 prospectively enrolled
patients with primary OSCC who were treated at Heliópolis Hospital
(Sao Paulo, Brazil) or the Padre Anchieta Teaching Hospital (Sao
Paulo, Brazil) between 2011 and 2013. This study was approved by
the ethics committees of Heliópolis Hospital, ABC Medical School
(São Bernardo do Campo, Brazil) and the Medical School of the
University of São Paulo (São Paulo, Brazil) and included only male
patients with no history of autoimmune disease or prior cancer
affecting any other anatomic areas, and to whom surgical treatment
with or without postoperative radiotherapy and/or chemotherapy had
been proposed. All patients provided written informed consent to
participate. The clinicopathological characteristics of the
patients are shown in Table I.
 | Table IClinicopathological characteristics of
patients with OSCC (n=50). |
Table I
Clinicopathological characteristics of
patients with OSCC (n=50).
| Clinicopathological
characteristics | OSCC frequency
(%) |
|---|
| Age (years) |
| Range | 40–88 |
| Median | 56.5 |
| Mean (standard
deviation) | 56.2 (8.3) |
| Ethnicity |
| Caucasian | 31 (62.0) |
| Non-caucasian | 16 (32.0) |
| Not available | 3 (6.0) |
| Smoking status |
| Current smoker | 42 (84.0) |
| Ex-smoker | 8 (16.0) |
| Alcohol
consumption |
| Current drinker | 29 (58.0) |
| Ex-drinker | 20 (40.0) |
| Never | 1 (2.0) |
| Postoperative RT |
| Yes | 22 (44.0) |
| No | 28 (56.0) |
| Postoperative CT |
| Yes | 8 (16.0) |
| No | 42 (84.0) |
| pT stage |
| pT1–2 | 25 (50.0) |
| pT3–4 | 25 (50.0) |
| pN stage |
| pN0 | 30 (60.0) |
| pN1–3 | 20 (40.0) |
| Pathological
staging |
| I/II | 17 (34.0) |
| III/IV | 33 (66.0) |
Patient samples
Prior to (0–30 days) and following (20 days to 3
months) surgery, 8-ml whole blood samples and 5 ml of total,
non-stimulated saliva were collected from each patient. The
collections were performed between 9 and 10am. The patients were
advised not to eat, drink, smoke or use oral hygiene products for
at least 2 h prior to collection. Blood samples and saliva were
cooled during transportation and were immediately centrifuged for
15 and 20 min, respectively, at 5031 × g and 4°C. Next, 0.8 μl of
protease inhibitor cocktail (cat. no. P8340; Sigma-Aldrich, St.
Louis, MO, USA) was added to each 400-μl aliquot of serum or
saliva. The samples were then stored at −80°C until enzyme-linked
immunosorbent assay (ELISA) analysis was performed. When required
for the study, the samples were thawed at room temperature
(18–25°C) and used immediately.
ELISA
The MIF concentrations in the serum and saliva
samples were assessed using an ELISA kit (Quantikine, cat. no.
DMF00B; R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions.
The optical densities were determined using a
microplate reader (ELX800; BioTek Instruments, Inc., Winooski, VT,
USA) at a wavelength of 450 nm. To determine the standard curve,
the original concentrations of various dilutions of recombinant
human MIF were correlated with the corresponding optical
densities.
The dilutions used for serum and saliva samples were
1:10 and 1:100, respectively. The samples exhibiting absorbances
that were not included in the standard curve were diluted as
required. The MIF concentrations in the samples were calculated
according to the standard curve and presented as the mean of
triplicates (ng/ml).
Statistical analysis
The Shapiro-Wilk test was used to assess the
normally distributed MIF concentrations in the serum and saliva
samples. MIF concentrations in the samples prior to and following
surgery were compared using the Wilcoxon matched-pairs signed-ranks
test. The nonparametric Mann-Whitney U test was used to assess the
associations between variables with two categories relative to the
MIF concentrations in the serum or saliva. The nonparametric
Kruskal-Wallis test was used for variables with three categories.
P<0.05 was considered to indicate a statistically significant
difference. STATA software, version 7.0 (StataCorp., College
Station, TX, USA) was used to perform all statistical tests.
Novel indices were proposed for correlations with
the quantity of tumor tissue. The lymph node index (LNI) was
calculated as the sum of the number of metastatic lymph nodes and
diameter (cm) of the largest positive lymph node. The general index
(GNI) was defined as the sum of the LNI and the largest diameter
(cm) of the primary tumor.
Results
MIF concentrations in serum and saliva
samples prior to and following surgery
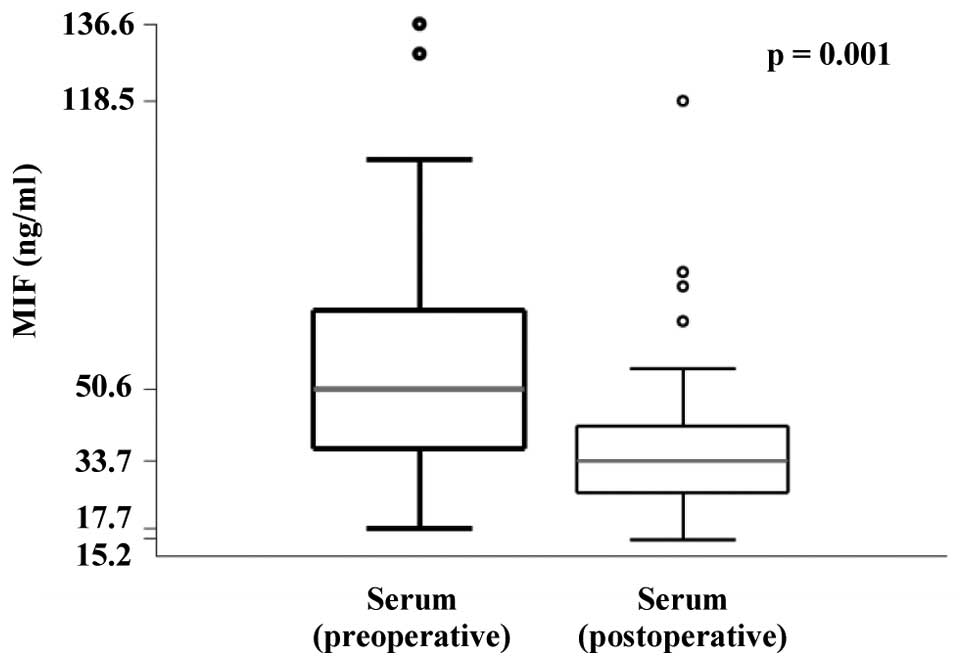
The results revealed that the MIF concentration was
significantly decreased in the postoperative serum samples
(Fig. 1). Confirming this result, a
statistically significant decrease in serum MIF concentrations was
identified following surgery when the data were stratified by
disease status following treatment (Table II). However, no significant
differences in MIF concentration were identified between the saliva
samples collected prior to and following surgery (data not
shown).
 | Table IIConcentration of MIF in the pre- and
postoperative serum and saliva of oral squamous cell carcinoma
patients and its association with disease outcome. |
Table II
Concentration of MIF in the pre- and
postoperative serum and saliva of oral squamous cell carcinoma
patients and its association with disease outcome.
| | Preoperative
serum
MIF (ng/ml) | | Postoperative
serum
MIF (ng/ml) | | | Preoperative
saliva
MIF (ng/ml) | | Postoperative
saliva
MIF (ng/ml) | |
|---|
| |
| |
| | |
| |
| |
|---|
| Variable | n | Range | Median | n | Range | Median | P-valuea | n | Range | Median | n | Range | Median | P-valuea |
|---|
| Local recurrence |
| No | 34 | 17.7–136.6 | 53.2 | 28 | 16.2–118.5 | 35.2 | 0.014 | 33 | 42.7–1059.1 | 248.7 | 27 | 79.7–1015.5 | 244.0 | 0.732 |
| Yes | 12 | 17.7–95.0 | 46.1 | 10 | 15.1–55.3 | 28.8 | 0.012 | 12 | 32.5–1770.6 | 468.0 | 8 | 64.5–2046.8 | 342.9 | 0.575 |
| P-value | | | 0.423 | | | 0.208 | | | | 0.072 | | | 0.239 | |
| Regional
recurrence |
| No | 39 | 17.7–136.6 | 51.1 | 32 | 15.1–118.5 | 34.9 | 0.004 | 38 | 42.7–1770.6 | 320.8 | 30 | 79.7–2046.8 | 256.1 | 0.854 |
| Yes | 7 | 20.3–95.5 | 48.6 | 6 | 23.2–35.3 | 27.8 | 0.046 | 7 | 32.5–1013.2 | 499.0 | 5 | 64.5–543.5 | 261.6 | 0.500 |
| P-value | | | 0.963 | | | 0.149 | | | | 0.900 | | | 0.741 | |
| Distant
metastasis |
| No | 44 | 17.7–136.6 | 49.8 | 36 | 15.1–118.5 | 33.7 | 0.002 | 43 | 32.5–1770.6 | 282.4 | 33 | 64.5–2046.8 | 250.9 | 0.896 |
| Yes | 2 | 59.6–95.5 | 77.6 | 2 | 30.7–35.3 | 33.0 | NE | 2 | 461.8–764.3 | 613.0 | 2 | 278.4–512.1 | 395.2 | NE |
| P-value | | | 0.161 | | | 0.999 | | | | 0.247 | | | 0.286 | |
| Local or regional
recurrence |
| No | 31 | 17.7–136.6 | 52.3 | 26 | 16.2–118.5 | 35.7 | 0.021 | 30 | 42.7–1059.1 | 228.8 | 25 | 79.7–1015.5 | 244.0 | 0.257 |
| Yes | 15 | 17.7–95.5 | 48.3 | 12 | 15.1–55.3 | 28.8 | 0.010 | 15 | 32.5–1770.6 | 474.2 | 10 | 64.5–2046.8 | 279.5 | 0.799 |
| P-value | | | 0.582 | | | 0.149 | | | | 0.060 | | | 0.342 | |
| Second primary
tumor |
| No | 40 | 17.7–136.6 | 49.8 | 32 | 15.1–118.5 | 34.4 | 0.006 | 39 | 42.7–1770.6 | 332.2 | 29 | 79.7–1015.5 | 261.4 | 0.699 |
| Yes | 6 | 17.7–104.6 | 59.5 | 6 | 23.2–41.5 | 31.4 | 0.075 | 6 | 32.5–960.3 | 300.8 | 6 | 64.5–2046.8 | 261.2 | 0.249 |
| P-value | | | 0.514 | | | 0.603 | | | | 0.664 | | | 0.965 | |
| Mortality from
cancer |
| No | 37 | 17.7–136.6 | 50.1 | 32 | 15.1–118.5 | 34.9 | 0.004 | 36 | 42.7–1490.2 | 228.8 | 31 | 79.7–1015.5 | 250.9 | 0.861 |
| Yes | 9 | 17.7–95.5 | 59.6 | 6 | 23.2–35.3 | 28.8 | 0.075 | 9 | 32.5–1770.6 | 764.3 | 4 | 64.5–2046.8 | 395.2 | 0.465 |
| P-value | | | 0.589 | | | 0.200 | | | 0.023 | | | | 0.437 | |
Associations between serum and saliva MIF
concentrations and clinicopathological data
Correlations between the concentrations of MIF in
serum and saliva, collected prior to and following surgical
treatment, and clinicopathological data are shown in Table III.
 | Table IIIConcentration of MIF in pre- and
postoperative serum and saliva in patients with oral squamous cell
carcinoma and its association with clinicopathological
variables. |
Table III
Concentration of MIF in pre- and
postoperative serum and saliva in patients with oral squamous cell
carcinoma and its association with clinicopathological
variables.
| | Preoperative
serum
MIF (ng/ml) | | | Postoperative
serum
MIF (ng/ml) | | | Preoperative
saliva
MIF (ng/ml) | | | Postoperative
saliva
MIF (ng/ml) | |
|---|
| |
| | |
| | |
| | |
| |
|---|
| Variable | n | Range | Median | P-value | n | Range | Median | P-value | n | Range | Median | P-value | n | Range | Median | P-value |
|---|
| Age (years) | | | | 0.051 | | | | 0.826 | | | | 0.992 | | | | 0.594 |
| ≤56.5 | 25 | 17.7–95.5 | 44.0 | | 21 | 15.2–78.1 | 33.2 | | 25 | 32.5–1013.2 | 348.5 | | 20 | 64.5–2046.8 | 270.0 | |
| >56.5 | 25 | 20.9–136.6 | 59.1 | | 17 | 16.2–118.5 | 34.2 | | 23 | 67.6–1770.6 | 309.4 | | 15 | 122.1–1015.5 | 244.0 | |
| Smoking status | | | | 0.711 | | | | 0.216 | | | | 0.072 | | | | 0.040 |
| Current | 42 | 17.7–136.6 | 51.7 | | 34 | 15.1–118.5 | 32.7 | | 40 | 32.5–1770.6 | 228.8 | | 29 | 64.5–2046.8 | 244.0 | |
| Ex-smoker | 8 | 25.8–76.2 | 49.8 | | 4 | 32.4–45.6 | 40.9 | | 8 | 82.6–1059.1 | 466.5 | | 6 | 221.8–1015.5 | 373.6 | |
| Alcohol
consumption | | | | 0.630 | | | | 0.263 | | | | 0.587 | | | | 0.919 |
| Current | 29 | 17.7–104.6 | 50.1 | | 24 | 15.1–118.5 | 31.6 | | 28 | 32.5–1490.2 | 216.2 | | 21 | 64.5–1015.5 | 261.4 | |
| Never or
ex-drinker | 21 | 17.7–136.6 | 56.9 | | 14 | 20.5–78.1 | 35.2 | | 20 | 42.7–1770.6 | 383.6 | | 14 | 106.5–2046.8 | 238.6 | |
| pT stage | | | | 0.184 | | | | 0.619 | | | | 0.001 | | | | 0.091 |
| pT1 + pT2 | 25 | 17.7–136.6 | 57.0 | | 18 | 16.2–118.5 | 35.8 | | 24 | 45.6–867.6 | 165.9 | | 19 | 79.7–878.6 | 215.8 | |
| pT3 + pT4 | 25 | 17.7–95.0 | 48.3 | | 20 | 15.1–78.1 | 32.8 | | 24 | 32.5–1770.6 | 445.8 | | 16 | 64.5–2046.8 | 314.7 | |
| Lymph node status
(pN) | | | | 0.018 | | | | 0.362 | | | | 0.317 | | | | 0.570 |
| pN0 | 30 | 20.9–136.6 | 56.9 | | 23 | 16.2–118.5 | 36.3 | | 29 | 42.7–1490.2 | 255.5 | | 24 | 79.7–1015.5 | 261.4 | |
| pN+ | 20 | 17.7–104.6 | 37.8 | | 15 | 15.1–74.8 | 32.3 | | 19 | 32.5–1770.6 | 474.2 | | 11 | 64.5–2046.8 | 250.9 | |
| Pathological
staging | | | | 0.040 | | | | 0.380 | | | | 0.032 | | | | 0.479 |
| GI + GII | 17 | 20.9–136.6 | 59.6 | | 13 | 16.2–118.5 | 36.9 | | 17 | 45.6–461.8 | 172.6 | | 14 | 79.7–878.6 | 229.9 | |
| GIII + GIV | 33 | 17.7–104.6 | 48.3 | | 25 | 15.1–78.1 | 32.4 | | 31 | 32.5–1770.6 | 417.4 | | 21 | 64.5–2046.8 | 261.6 | |
| Differentiation
grade | | | | 0.618a | | | | 0.997a | | | | 0.938a | | | | 0.883a |
| Poor | 2 | 17.7–104.6 | 61.2 | | 2 | 28.1–41.5 | 34.8 | | 2 | 67.6–960.3 | 514.0 | | 2 | 122.1–2046.8 | 1084.5 | |
| Moderate | 38 | 20.3–136.6 | 51.7 | | 31 | 15.1–118.5 | 34.2 | | 36 | 32.5–1059.1 | 320.8 | | 27 | 64.5–1015.5 | 261.4 | |
| Well | 10 | 17.7–79.1 | 44.3 | | 5 | 18.3–51.0 | 32.3 | | 10 | 82.6–1770.6 | 254.9 | | 6 | 106.5–832.8 | 261.6 | |
| Blood vessel
invasion | | | | 0.562 | | | | NE | | | | 0.700 | | | | 0.815 |
| Not detected | 45 | 17.7–129.5 | 48.6 | | 34 | 15.1–118.5 | 32.3 | | 43 | 32.5–1770.6 | 255.5 | | 30 | 64.5–2046.8 | 261.5 | |
| Detected | 2 | 56.9–59.6 | 58.2 | | 1 | 78.1 | 78.1 | | 2 | 348.5–406.2 | 377.4 | | 2 | 221.8–261.4 | 241.6 | |
| Lymphatic
invasion | | | | 0.960 | | | | 0.724 | | | | 0.653 | | | | 0.079 |
| Not detected | 36 | 17.7–129.5 | 49.8 | | 27 | 15.1–118.5 | 32.4 | | 34 | 42.7–1490.2 | 252.1 | | 25 | 79.7–2046.8 | 272.8 | |
| Detected | 11 | 22.8–104.6 | 48.6 | | 8 | 23.2–78.1 | 33.4 | | 11 | 32.5–1770.6 | 406.2 | | 7 | 64.5–278.4 | 221.8 | |
| Perineural
invasion | | | | 0.714 | | | | 0.711 | | | | 0.818 | | | | 0.258 |
| Not detected | 27 | 17.7–129.5 | 49.5 | | 21 | 15.1–118.5 | 32.4 | | 26 | 49.1–1059.1 | 252.1 | | 21 | 112.8–2046.8 | 261.4 | |
| Detected | 20 | 22.8–95.0 | 49.9 | | 14 | 19.9–78.1 | 33.4 | | 19 | 32.5–1770.6 | 406.2 | | 11 | 64.5–832.8 | 261.4 | |
| Inflammatory
infiltrate | | | | 0.510 | | | | 0.338 | | | | 0.988 | | | | 0.872 |
| Poor | 12 | 17.7–95.0 | 44.0 | | 8 | 15.1–55.3 | 31.2 | | 11 | 42.7–1770.6 | 176.9 | | 6 | 79.7–2046.8 | 275.4 | |
| Moderate +
intense | 29 | 20.3–129.5 | 49.5 | | 24 | 16.2–118.5 | 33.9 | | 29 | 32.5–1490.2 | 332.2 | | 23 | 64.5–1011.9 | 261.4 | |
| Surgical margin
status | | | | 0.355 | | | | 0.645 | | | | 0.045 | | | | 0.887 |
| Tumor-free | 44 | 17.7–136.6 | 50.6 | | 35 | 15.1–118.5 | 34.2 | | 43 | 32.5–1490.2 | 248.7 | | 33 | 64.5–2046.8 | 261.4 | |
| Positive | 6 | 39.0–95.0 | 58.2 | | 3 | 29.5–55.3 | 33.2 | | 5 | 309.4–1770.6 | 499.0 | | 2 | 150.0–543.5 | 346.7 | |
The MIF concentrations in the preoperative saliva of
patients was associated with tumor size; saliva concentrations were
higher in patients with pT3 and pT4 stage tumors (P=0.001; Table III) and in patients with tumors
>2.5 cm (P=0.020; Table IV).
Consequently, more advanced disease stage, stages III and IV
(P=0.032; Table III), and a high
GNI (0.025; Table IV) were
associated with increased MIF concentrations in the saliva samples
collected prior to tumor resection. Salivary MIF concentrations
prior to surgery varied according to surgical margin involvement
(P=0.045; Table III).
 | Table IVCorrleation between the concentration
of MIF in pre- and postoperative serum and saliva samples of oral
squamous cell carcinoma and tumor characteristics. |
Table IV
Corrleation between the concentration
of MIF in pre- and postoperative serum and saliva samples of oral
squamous cell carcinoma and tumor characteristics.
| | Preoperative
serum
MIF (ng/ml) | | | Postoperative
serum
MIF (ng/ml) | | | Preoperative
saliva
MIF (ng/ml) | | | Postoperative
saliva
MIF (ng/ml) | |
|---|
| |
| | |
| | |
| | |
| |
|---|
| Variable | n | Range | Median | P-value | n | Range | Median | P-value | n | Range | Median | P-value | n | Range | Median | P-value |
|---|
| Lymph node
involvement | | | | 0.023 | | | | 0.922 | | | | 0.468 | | | | 0.347 |
| No | 28 | 20.9–129.5 | 55.5 | | 22 | 15.1–118.5 | 32.8 | | 27 | 42.7–1490.2 | 255.5 | | 23 | 79.7–1015.5 | 261.4 | |
| Yes | 20 | 17.7–104.6 | 37.8 | | 14 | 23.2–74.8 | 33.2 | | 19 | 32.5–1770.6 | 474.2 | | 10 | 64.5–2046.8 | 236.3 | |
| Largest diameter of
metastatic lymph node (cm) | | | | 0.545 | | | | 0.072 | | | | 0.327 | | | | 0.425 |
| ≤1.5 | 10 | 17.7–59.6 | 35.8 | | 5 | 29.5–74.8 | 35.2 | | 10 | 76.4–1770.6 | 426.2 | | 3 | 221.8–388.9 | 250.9 | |
| >1.5 | 10 | 17.7–104.6 | 40.1 | | 9 | 23.2–41.5 | 28.2 | | 9 | 32.5–960.3 | 474.2 | | 7 | 64.5–2046.8 | 162.3 | |
| Lymph node
index | | | | 0.025 | | | | 0.338 | | | | 0.447 | | | | 0.174 |
| ≤3.5 | 12 | 17.7–59.6 | 26.7 | | 7 | 24.0–74.8 | 34.6 | | 12 | 49.1–1770.6 | 426.2 | | 5 | 162.3–2046.8 | 250.9 | |
| >3.5 | 8 | 23.1–104.6 | 56.7 | | 7 | 23.2–41.5 | 29.5 | | 7 | 32.5–1013.2 | 474.2 | | 5 | 64.5–278.4 | 122.1 | |
| Largest diameter of
primary tumor (cm) | | | | 0.754 | | | | 0.724 | | | | 0.020 | | | | 0.028 |
| ≤2.5 | 13 | 17.7–104.6 | 54.2 | | 10 | 18.3–118.5 | 33.8 | | 13 | 49.1–867.6 | 125.0 | | 10 | 79.7–512.1 | 166.5 | |
| >2.5 | 35 | 17.7–129.5 | 48.6 | | 26 | 15.2–78.1 | 32.8 | | 33 | 32.5–1770.6 | 406.2 | | 23 | 64.5–2046.8 | 278.4 | |
| General index | | | | 0.071 | | | | 0.648 | | | | 0.025 | | | | 0.120 |
| ≤2.9 | 10 | 20.9–99.9 | 64.4 | | 8 | 18.3–118.5 | 33.8 | | 10 | 45.6–461.8 | 142.0 | | 8 | 79.7–512.1 | 193.2 | |
| >2.9 | 38 | 17.7–129.5 | 46.1 | | 28 | 15.1–78.1 | 32.8 | | 36 | 32.5–1770.6 | 395.5 | | 25 | 64.5–2046.8 | 261.6 | |
In preoperative serum samples, the MIF
concentrations were found to be significantly lower in patients
with lymph node involvement (P=0.018; Table III). However, when comparing the
LNI, patients with an LNI >3.5 exhibited higher MIF
concentrations in preoperative serum samples (P=0.025; Table IV).
Table II shows that
MIF concentrations in the preoperative saliva samples were higher
in patients who succumbed to the disease than in surviving patients
(P=0.023).
No significant associations were identified between
the MIF concentrations in serum and saliva collected prior to
surgery and perineural invasion, or tumoral inflammatory cell
infiltration (Table III).
Discussion
MIF has been considered to present an important link
between inflammation and cancer due to its pro-inflammatory role,
overexpression in various tumor tissues and interactions with
pathways that aid tumor progression. Its molecular mechanisms
involve, among others, the inhibition of p53,
extracellular-signal-regulated kinase/mitogen-activated protein
kinase and AKT/protein kinase B activation, and sustained
hypoxia-inducible factor 1-α activation, all of which promote tumor
cell proliferation, cell survival and tumor-associated
neoangiogenesis (16,28–32).
Under normal conditions, a variety of immune cells, as well as the
pituitary gland and endothelial and epithelial cells of different
organs, express MIF (15,33). Several studies have identified MIF
overexpression in tumors when compared with healthy tissues, and
high MIF concentrations were detected in the serum of cancer
patients when compared with healthy controls. Therefore, these data
indicate a potential function for this protein as a biomarker of
neoplastic diseases (20–22,34–40).
In the present study, in order to evaluate MIF as a
serological and salivary biomarker of OSCC, MIF concentration in
pre- and postoperative serum and saliva samples of patients with
OSCC was investigated. To avoid interference of uncontrolled
variables when comparing the MIF concentrations in different
individuals, we used samples collected from the same patient
following tumor resection as controls. Since MIF overexpression is
also associated with autoimmune diseases (41–43),
as well as with previously mentioned polymorphisms in the gene
promoter of mif (44,45), a
comparison of samples of the same individual prior to and following
tumor resection allowed control of these variables.
In this study, the serological MIF concentrations
following tumor resection were significantly lower than those prior
to tumor resection. This is consistent with the hypothesis that,
since MIF is often overexpressed in tumors, it may also be detected
at high levels in the serum of patients with OSCC. Considering the
non-significant differences in MIF serological concentrations
according to tumor size, this result indicates a possible function
for MIF as a serological marker for OSCC detection, regardless of
tumor extension. The serum MIF concentration was found to inversely
correlate with lymph node involvement, in contrast to previous
studies, which have reported that MIF induces the migration and
invasion of tumor cells (38,46–49).
However, with regard to LNI, which may present the total metastatic
lymph node mass, the serological MIF concentration was higher in
individuals with a higher LNI. The LNI was calculated to compare
the MIF concentrations in serum and saliva with more representative
data regarding the total quantity of regional tumor present in the
patient, since the number of metastatic lymph nodes or the diameter
of the largest metastatic lymph node alone was not sufficient.
The dual and complex role of MIF has been discussed
in detail. A study of patients with head and neck squamous cell
carcinoma revealed correlations between low and high tumoral
immunohistochemical expression of MIF and poor survival, and
between moderate expression and improved survival (50). In addition, in the same study, high
MIF expression in the tumor was positively associated with lymph
node involvement, whereas low and moderate expression was found to
correlate with no regional metastasis. Verjans et al
(51) revealed that cytoplasmic MIF
expression in tumor tissues was associated with improved survival
in breast cancer patients, indicating that intracellular MIF may
inhibit cell proliferation and indicate a favorable prognosis,
whereas extracellular tumor tissue-derived MIF may be
pro-inflammatory and may be associated with an unfavorable
prognosis (51). These results
demonstrate that the function of MIF in the progression and
prognosis of several malignancies remains controversial, and
further studies are required to investigate its different
mechanisms of action, particularly with regard to its origin (from
healthy tissues or tumors) and location within the cell.
Accordingly, the current study group is also investigating MIF
expression in tumor tissue and surgical margins.
To the best of our knowledge, the present study is
the first to investigate MIF levels in saliva samples. It was
observed that high levels of this protein are present in pre- and
postoperative saliva of OSCC patients. Considering that OSCC cells
secrete proteins that are eluted into the saliva, possibly via
direct contact, it was hypothesized that salivary MIF concentration
would decrease following tumor resection. However, this was not
observed. We hypothesized that this result may be due to the
constitutive expression of MIF by endocrine, immune, and
particularly epithelial cells that are in direct contact with the
external environment and regulate host responses to infections and
stress. Pathogen-associated molecular patterns and inflammatory
cytokines, including tumor necrosis factor-α and interferon-γ, are
potential inducers of MIF secretion by macrophages, and the vast
microbiota present in the oral cavity may facilitate this process,
thus maintaining a high MIF concentration in the saliva (15). In addition, the inflammation
triggered by the operative wound healing process may have increased
the MIF concentrations in the saliva samples. However, an interval
of 20–30 days following surgery was selected for sample collection,
and longer periods were not considered as following this period the
referred patients began radiotherapy and chemotherapy treatment,
which may have interfered with the analysis. The salivary MIF
concentrations were significantly higher in patients with larger
tumors and those at more advanced pathological stages, than in
patients with smaller tumors and those at initial pathological
stages. On the basis of these results, we hypothesize that salivary
MIF may not originate exclusively from OSCC cancer cells and,
therefore, may not present a reliable marker for tumor diagnosis.
MIF may also originate from endocrine, immune, and epithelial
cells, and this may contribute to tumor progression via the
aforementioned mechanisms. Regarding disease control status and
prognosis evaluation, higher salivary MIF concentrations were
identified in patients who succumbed to the disease than in those
who survived. However, the limited follow-up period of this study
was not long enough to comprehensively evaluate survival. This
result indicates a possible role for MIF in the prognosis of
patients with OSCC; however, further studies are required to
confirm this correlation.
As previously reported, MIF expression may be
induced by epidermal growth factor (EGF) in breast cancer cells and
also appears to be involved in the proliferative pathway activated
by EGF (52). To date, the EGF
receptor (EGFR) pathway is the most important pathway associated
with OSCC development, and the investigation of the association
between MIF and EGFR in OSCC may be extremely noteworthy. In
addition, OSCC is an extremely heterogeneous disease; using MIF as
a biomarker may be more useful when associated with other markers
with known importance in OSCC development and prognosis, including
other cytokines and proteins of the EGFR pathway.
In conclusion, the increased serological MIF
concentrations in these patients prior to treatment observed in
this study indicate a potential role for MIF as a biomarker for the
early detection of OSCC recurrence. However, a long-term validation
study is required, with a greater number of patients to evaluate
serological MIF concentration in different disease statuses and
during follow up or including MIF in a panel of markers.
Acknowledgements
The authors would like to thank the São Paulo
Research Foundation (grant nos. FAPESP 2010/20765-3; and
2011/03281-5) and the Coordination of Improvement of Higher Level
Personnel (CAPES) for financial support, the Head and Neck Genome
Project (GENCAPO; www.gencapo.famerp.br) for scientific support and
Miss. Karina Aparecida Meira da Silva and Mrs. Natalia Pita
Magalhães for their assistance with sample collection.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
|
|
2
|
de Camargo Cancela M, Voti L, Guerra-Yi M,
Chapuis F, Mazuir M and Curado MP: Oral cavity cancer in developed
and in developing countries: population-based incidence. Head Neck.
32:357–367. 2010.
|
|
3
|
INCA National Cancer Institute.
Estimate/2012-Cancer Incidence in Brazil. Ministry of Health;
Brasília, Brazil: pp. 982009
|
|
4
|
Gillison ML: Current topics in the
epidemiology of oral cavity and oropharyngeal cancers. Head Neck.
29:779–792. 2007.
|
|
5
|
Marques LA, Eluf-Neto J, Figueiredo RA,
Góis-Filho JF, Kowalski LP, Carvalho MB, Abrahão M and Wünsch-Filho
V: Oral health, hygiene practices and oral cancer. Rev Saude
Publica. 42:471–479. 2008.
|
|
6
|
Palka KT, Slebos RJ and Chung CH: Update
on molecular diagnostic tests in head and neck cancer. Semin Oncol.
35:198–210. 2008.
|
|
7
|
Hu S, Arellano M, Boontheung P, Wang J,
Zhou H, Jiang J, Elashoff D, et al: Salivary proteomics for oral
cancer biomarker discovery. Clin Cancer Res. 14:6246–6252.
2008.
|
|
8
|
Nagler RM: Saliva as a tool for oral
cancer diagnosis and prognosis. Oral Oncol. 45:1006–1010. 2009.
|
|
9
|
Douglas WG, Tracy E, Tan D, Yu J, Hicks WL
Jr, Rigual NR, Loree TR, et al: Development of head and neck
squamous cell carcinoma is associated with altered cytokine
responsiveness. Mol Cancer Res. 2:585–593. 2004.
|
|
10
|
Feller L, Altini M and Lemmer J:
Inflammation in the context of oral cancer. Oral Oncol. 49:887–892.
2013.
|
|
11
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33(Suppl 1): S79–S84. 2013.
|
|
12
|
Mantovani A, Garlanda C and Allavena P:
Molecular pathways and targets in cancer-related inflammation. Ann
Med. 42:161–170. 2010.
|
|
13
|
Bloom BR and Bennett B: Mechanism of a
reaction in vitro associated with delayed-type hypersensitivity.
Science. 153:80–82. 1966.
|
|
14
|
David JR: Delayed hypersensitivity in
vitro: its mediation by cell-free substances formed by lymphoid
cell-antigen interaction. Proc Natl Acad Sci USA. 56:72–77.
1966.
|
|
15
|
Calandra T and Roger T: Macrophage
migration inhibitory factor: a regulator of innate immunity. Nat
Rev Immunol. 3:791–800. 2003.
|
|
16
|
Bucala R and Donnelly SC: Macrophage
migration inhibitory factor: a probable link between inflammation
and cancer. Immunity. 26:281–285. 2007.
|
|
17
|
Mitchell RA and Bucala R: Tumor
growth-promoting properties of macrophage migration inhibitory
factor (MIF). Semin Cancer Biol. 10:359–366. 2000.
|
|
18
|
Mitchell RA: Mechanisms and effectors of
MIF-dependent promotion of tumourigenesis. Cell Signal. 16:13–19.
2004.
|
|
19
|
Grieb G, Merk M, Bernhagen J and Bucala R:
Macrophage migration inhibitory factor (MIF): a promising
biomarker. Drug News Perspect. 23:257–264. 2010.
|
|
20
|
Yasasever V, Camlica H, Duranyildiz D,
Oguz H, Tas F and Dalay N: Macrophage migration inhibitory factor
in cancer. Cancer Invest. 25:715–719. 2007.
|
|
21
|
Muramaki M, Miyake H, Yamada Y and Hara I:
Clinical utility of serum macrophage migration inhibitory factor in
men with prostate cancer as a novel biomarker of detection and
disease progression. Oncol Rep. 15:253–257. 2006.
|
|
22
|
Lee H, Rhee H, Kang HJ, et al: Macrophage
migration inhibitory factor may be used as an early diagnostic
marker in colorectal carcinomas. Am J Clin Pathol. 129:772–779.
2008.
|
|
23
|
He XX, Yang J, Ding YW, Liu W, Shen QY and
Xia HH: Increased epithelial and serum expression of macrophage
migration inhibitory factor (MIF) in gastric cancer: potential role
of MIF in gastric carcinogenesis. Gut. 55:797–802. 2006.
|
|
24
|
Meyer-Siegler KL, Bellino MA and
Tannenbaum M: Macrophage migration inhibitory factor evaluation
compared with prostate specific antigen as a biomarker in patients
with prostate carcinoma. Cancer. 94:1449–1456. 2002.
|
|
25
|
Hira E, Ono T, Dhar DK, El-Assal ON,
Hishikawa Y, Yamanoi A and Nagasue N: Overexpression of macrophage
migration inhibitory factor induces angiogenesis and deteriorates
prognosis after radical resection for hepatocellular carcinoma.
Cancer. 103:588–598. 2005.
|
|
26
|
Xia HH, Yang Y, Chu KM, Gu Q, Zhang YY, He
H, Wong WM, et al: Serum macrophage migration-inhibitory factor as
a diagnostic and prognostic biomarker for gastric cancer. Cancer.
115:5441–5449. 2009.
|
|
27
|
Kindt N, Lechien J, Decaestecker C,
Rodriguez A, Chantrain G, Remmelink M, Laurent G, et al: Expression
of macrophage migration-inhibitory factor is correlated with
progression in oral cavity carcinomas. Anticancer Res.
32:4499–4505. 2012.
|
|
28
|
Hudson JD, Shoaibi MA, Maestro R, Carnero
A, Hannon GJ and Beach DH: A proinflammatory cytokine inhibits p53
tumor suppressor activity. J Exp Med. 190:1375–1382. 1999.
|
|
29
|
Denz A, Pilarsky C, Muth D, Rückert F,
Saeger HD and Grützmann R: Inhibition of MIF leads to cell cycle
arrest and apoptosis in pancreatic cancer cells. J Surg Res.
160:29–34. 2010.
|
|
30
|
Li GQ, Xie J, Lei XY and Zhang L:
Macrophage migration inhibitory factor regulates proliferation of
gastric cancer cells via the PI3K/Akt pathway. World J
Gastroenterol. 15:5541–5548. 2009.
|
|
31
|
Schrader J, Deuster O, Rinn B, Schulz M,
Kautz A, Dodel R, Meyer B, et al: Restoration of contact inhibition
in human glioblastoma cell lines after MIF knockdown. BMC Cancer.
9:4642009.
|
|
32
|
Rendon BE, Willer SS, Zundel W and
Mitchell RA: Mechanisms of macrophage migration inhibitory factor
(MIF)-dependent tumor microenvironmental adaptation. Exp Mol
Pathol. 86:180–185. 2009.
|
|
33
|
Calandra T, Bernhagen JL, Mitchell RA and
Bucala R: The macrophage is an important and previously
unrecognized source of macrophage migration inhibitory factor. J
Exp Med. 179:1895–1902. 1994.
|
|
34
|
Camlica H, Duranyildiz D, Oguz H, Oral EN
and Yasasever V: The diagnostic value of macrophage migration
inhibitory factor (MIF) in gastric cancer. Pathol Oncol Res.
14:79–83. 2008.
|
|
35
|
Fang W, Li X, Jiang Q, Liu Z, Yang H, Wang
S, Xie S, et al: Transcriptional patterns, biomarkers and pathways
characterizing nasopharyngeal carcinoma of Southern China. J Transl
Med. 6:322008.
|
|
36
|
Kamimura A, Kamachi M, Nishihira J, Ogura
S, Isobe H, Dosaka-Akita H, Ogata A, et al: Intracellular
distribution of macrophage migration inhibitory factor predicts the
prognosis of patients with adenocarcinoma of the lung. Cancer.
89:334–341. 2000.
|
|
37
|
Mohri Y, Mohri T, Wei W, Qi YJ, Martin A,
Miki C, Kusunoki M, et al: Identification of macrophage migration
inhibitory factor and human neutrophil peptides 1–3 as potential
biomarkers for gastric cancer. Br J Cancer. 101:295–302. 2009.
|
|
38
|
Ren Y, Law S, Huang X, Lee PY, Bacher M,
Srivastava G and Wong J: Macrophage migration inhibitory factor
stimulates angiogenic factor expression and correlates with
differentiation and lymph node status in patients with esophageal
squamous cell carcinoma. Ann Surg. 242:55–63. 2005.
|
|
39
|
Tomiyasu M, Yoshino I, Suemitsu R, Okamoto
T and Sugimachi K: Quantification of macrophage migration
inhibitory factor mRNA expression in non-small cell lung cancer
tissues and its clinical significance. Clin Cancer Res.
8:3755–3760. 2002.
|
|
40
|
Xia HH, Zhang ST, Lam SK, Lin MC, Kung HF
and Wong BC: Expression of macrophage migration inhibitory factor
in esophageal squamous cell carcinoma and effects of bile acids and
NSAIDs. Carcinogenesis. 26:11–15. 2005.
|
|
41
|
Cooke G, Armstrong ME and Donnelly SC:
Macrophage migration inhibitory factor (MIF), enzymatic activity
and the inflammatory response. Biofactors. 35:165–168. 2009.
|
|
42
|
Sanchez-Zamora Y, Terrazas LI,
Vilches-Flores A, et al: Macrophage migration inhibitory factor is
a therapeutic target in treatment of non-insulin-dependent diabetes
mellitus. FASEB J. 24:2583–2590. 2010.
|
|
43
|
Bucala R: MIF Rediscovered. MIF, A Most
Interesting Factor. World Scientific Press; London: 2007
|
|
44
|
Baugh JA, Chitni S, Donnelly SC, et al: A
functional promoter polymorphism in the macrophage migration
inhibitory factor (MIF) gene associated with disease severity in
rheumatoid arthritis. Genes Immun. 3:170–176. 2002.
|
|
45
|
Donn RP, Shelley E, Ollier WE, et al: A
novel 5′-flanking region polymorphism of macrophage migration
inhibitory factor is associated with systemic-onset juvenile
idiopathic arthritis. Arthritis Rheum. 44:1782–1785. 2001.
|
|
46
|
Sun B, Nishihira J, Yoshiki T, et al:
Macrophage migration inhibitory factor promotes tumor Invasion and
metastasis via the Rho-dependent pathway. Clin Cancer Res.
11:1050–1058. 2005.
|
|
47
|
Funamizu N, Hu C, Lacy C, Schetter A,
Zhang G, He P, et al: Macrophage migration inhibitory factor (MIF)
induces epithelial to mesenchymal transition, enhances tumor
aggressiveness and predicts clinical outcome in resected pancreatic
ductal adenocarcinoma. Int J Cancer. Jul 23–2013.(Epub ahead of
print).
|
|
48
|
Han I, Lee MR, Nam KW, Oh JH, Moon KC and
Kim HS: Expression of macrophage migration inhibitory factor
relates to survival in high-grade osteosarcoma. Clin Orthop Relat
Res. 466:2107–2113. 2008.
|
|
49
|
Liu H, Chen G, Zhang W, et al:
Overexpression of macrophage migration inhibitory factor in adenoid
cystic carcinoma: correlation with enhanced metastatic potential. J
Cancer Res Clin Oncol. 139:287–295. 2013.
|
|
50
|
Suzuki F, Nakamaru Y, Oridate N, Homma A,
Nagahashi T, Yamaguchi S, Nishihira J, et al: Prognostic
significance of cytoplasmic macrophage migration inhibitory factor
expression in patients with squamous cell carcinoma of the head and
neck treated with concurrent chemoradiotherapy. Oncol Rep.
13:59–64. 2005.
|
|
51
|
Verjans E, Noetzel E, Bektas N, Schütz AK,
Lue H, Lennartz B, Hartmann A, et al: Dual role of macrophage
migration inhibitory factor (MIF) in human breast cancer. BMC
Cancer. 9:2302009.
|
|
52
|
Lim S, Choong LY, Kuan CP, Yunhao C and
Lim YP: Regulation of macrophage inhibitory factor (MIF) by
epidermal growth factor receptor (EGFR) in the MCF10AT model of
breast cancer progression. J Proteome Res. 8:4062–4076. 2009.
|