Introduction
Gastric cancer is the second leading cause of
cancer-related mortality worldwide (1) and the predominant cause of
cancer-related mortality in numerous Asian countries, including
South Korea and China (2). Gastric
cancer is a multifactorial disease involving genetic and
environmental factors (3).
Furthermore, the prognosis of advanced gastric cancer patients
remains poor due to the high rate of metastatic recurrence
(4,5).
Ursolic acid is a pentacyclic triterpenoid compound
that is derived from a range of medicinal herbs, including
Rosemarinus officinalis, Eribotrya japonica,
Calluna vulgaris and Oldenlandia diffusa (6–8).
Ursolic acid has been demonstrated to exert a number of anticancer
activities, including the inhibition of tumorigenesis, tumor
promotion and angiogenesis (7), as
well as the induction of apoptosis in various cancer cell lines,
including melanoma, breast, gastric and hepatocellular carcinoma,
human non-small cell lung cancer and liver cancer cell lines
(7–12). In addition, ursolic acid has been
reported to inhibit in vivo tumor growth in various animal
models (13–15), and to suppress invasion and
migration in human lung, breast and ovarian cancer cells (10,16,17).
However, to the best of our knowledge, the anti-invasive activity
of ursolic acid in gastric cancer cells has yet to be reported.
Therefore, the present study aimed to investigate the inhibitory
effect of ursolic acid on the growth and invasive phenotype of
SNU-484 human gastric cancer cells.
The process of tumor metastasis occurs by tumor
cells disseminating from the primary tumor to distant secondary
organs or tissues. Metastasis involves multiple steps, including
the invasion, migration and dissemination of malignant tumor cells
(18). Furthermore, cancer cell
invasion has been extensively associated with the increased
expression of matrix-degrading matrix metalloproteinase (MMP)
enzymes (19,20).
Apoptosis involves a series of cellular events that
results in the activation of apoptosis-associated gene products,
such as B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax)
and the caspases (21,22). The Bcl-2 and Bax proteins are
functionally opposed: Whereas Bcl-2 acts to inhibit apoptosis, Bax
promotes apoptosis (23,24). Caspase-3 is an apoptosis-associated
cysteine peptidase that interacts with caspase-8 and -9, and is key
in mammalian cell apoptosis (25).
In addition, a number of other molecules are
involved in the process of apoptosis. The mitogen-activated protein
kinase (MAPK) signaling pathways modulate gene expression,
proliferation, motility and apoptosis (26–29).
Activation of c-Jun N-terminal kinase (JNK) and p38 MAPK have been
demonstrated to trigger apoptosis (27,30,31),
and extracellular signal-regulated kinase (ERK) activity promotes
apoptotic pathways via the induction of mitochondrial cytochrome
c release, caspase-8 activation or permanent cell cycle
arrest (32).
In order to examine the chemopreventive potential of
ursolic acid in gastric cancer cells in vitro, the present
study evaluated the effects of ursolic acid on proliferation,
apoptosis and the invasive phenotype of SNU-484 human gastric
cancer cells.
Materials and methods
Reagents
Ursolic acid (3β-hydroxy-urs-12-en-28-oic acid),
dimethyl sulfoxide (DMSO) and MTT were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) was
purchased from Cellgro Mediatech (Manassas, VA, USA), and fetal
bovine serum and penicillin-streptomycin were purchased from
Invitrogen Life Technologies (Grand Island, NY, USA). The annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit I was
purchased from BD Biosciences (San Diego, CA, USA).
Cell lines
The SNU-484 gastric cancer cell line was provided by
Dr H.D. Um (Korean Institute of Radiological and Medical Science,
Seoul, South Korea). The SNU-484 cells were cultured in DMEM
supplemented with 10% heat-inactivated fetal bovine serum and 100
U/ml penicillin-streptomycin. The cells were maintained in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
MTT assay
SNU-484 cells cultured in a 96-well plate were
treated with various concentrations of ursolic acid (0, 1, 5, 10
and 25 μM) for 24 h. Control cells were treated with DMSO equal to
the highest percentage of solvent used under experimental
conditions. Briefly, 25 mg/ml 0.5% MTT was added to the media and
the cells were incubated for an additional 4 h. Following removal
of 100 μl supernatant, which was replaced with an equal volume of
DMSO, the absorbance was measured at a wavelength of 540 nm using
an enzyme-linked immunosorbent assay (ELISA) plate reader (ELx800™;
BioTek Instruments, Inc., Winooski, VT, USA). The percentage of
surviving cells was defined as the relative absorbance of treated
versus untreated cells.
Flow cytometry analysis
The cells were grown in six-well plates and
incubated for 24 h prior to treatment with ursolic acid. After 24
h, the cells were harvested and washed twice with
phosphate-buffered saline (PBS). The cells (5×105) were
subsequently double-stained with FITC-conjugated annexin V and
propidium iodide for 15 min at room temperature in 1X binding
buffer, followed by analysis using a Vision CBA Image Cytometry
system (Nexcelom Bioscience LLC, Lawrence, MA, USA).
Immunoblot analysis
The cells were cultured to 70% confluency and
incubated in serum-free media containing various concentrations of
ursolic acid (0.0, 1.0, 2.5 and 5.0 μM) for 24 h. To prepare
whole-cell extracts, the cells were washed with PBS and lysed by
adding SDS-lysis buffer containing protease inhibitor cocktail
(Roche Diagnostics GmbH, Mannheim, Germany). Equal amounts of
protein were subjected to 12% SDS-PAGE analysis and
electrophoretically transferred to a polyvinylidene fluoride
membrane (Bio-Rad Laboratories, Hercules, CA, USA). Subsequently,
the membranes were blocked with 5% w/v skimmed dried milk in PBS
with Tween-20 (PBST) and incubated with primary (dilution, 1:1,000
in PBST) and secondary (dilution, 1:4,000 in PBST) antibodies, and
detected using an enhanced chemiluminescence western blotting
detection system (GE Healthcare Life Sciences, Chalfont, UK).
Rabbit polyclonal anti-human p38 MAPK, rabbit polyclonal anti-human
phosphorylated (phospho) p38 MAPK, rabit polyclonal anti-human
phospho ERK1/2, mouse monoclonal anti-human ERK1/2, rabbit
polyclonal anti-human JNK, mouse monoclonal anti-human phospho JNK
and rabbit polyclonal anti-human poly(ADP-ribose) polymerase (PARP)
antibodies were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Mouse monoclonal anti-human Bcl-2, mouse
monoclonal anti-human caspase-3 and rabbit polyclonal anti-human
Bax antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
In vitro invasion assays
An in vitro invasion assay was performed
using a 24-well Transwell® unit with polycarbonate
filters (Corning Costar, Inc., Cambridge, MA, USA). The lower side
of the filter was coated with type I collagen, the upper side was
coated with Matrigel (Collaborative Research, Lexington, KY, USA),
the upper compartment of the Transwell® unit was filled
with serum-free medium and the lower compartment was filled with
the culture medium. Ursolic acid (0.0, 1.0, 2.5 and 5.0 μM) was
also added to each compartment. The cells were placed on the upper
side of the Transwell® plate and incubated for 24 h. The
cells that remained on the upper surface of the filter were removed
with a cotton swab, while the cells that had migrated through the
filter to the lower compartment were fixed with methanol and
stained with crystal violet for 20 min. The crystal violet dye
retained on the filters was extracted using 30% acetic acid and
cell invasion was measured by reading the absorbance at 595 nm
using an ELISA plate reader (ELx800; Bio-Tek Instruments).
Gelatin zymography assay
The cells were cultured to 70% confluency and
incubated in serum-free media containing various concentrations of
ursolic acid (0.0, 1.0, 2.5 and 5.0 μM) for 48 h. The conditioned
medium was collected and centrifuged at 13,000 × g for 10 min to
remove cell debris. Subsequently, the protein concentration was
measured using bicinchoninic acid assay reagents (Pierce
Biotechnology, Inc., Rockford, IL, USA), and equal amounts of
protein from the conditioned media were electrophoresed on 10%
SDS-PAGE gels containing 1 mg/ml gelatin. Following
electrophoresis, the gels were washed with renaturation buffer
(2.5% Triton X-100) three times for 30 min, rinsed for 15 min with
developing buffer [50 mM Tris-HCl buffer (pH 7.6) containing 5 mM
CaCl2, 0.02% Brij-35 and 0.2% sodium azide], and
incubated overnight at 37°C. The gels were stained with staining
buffer (0.5% Coomassie Brilliant Blue R-250 solution containing 10%
acetic acid and 20% methanol) for 30 min and destained with 10%
acetic acid solution. Areas of gelatinase activity were detected as
clear bands against the blue-stained gelatin background, and
relative band intensities were determined by the quantification of
each band using the Gel Doc™ XR+ imaging system (Bio-Rad
Laboratories).
Statistical analysis
The results are presented as the mean ± standard
deviation of three independent experiments run in triplicate and
analyzed by Student’s t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Ursolic acid inhibits cell growth in
SNU-484 cells
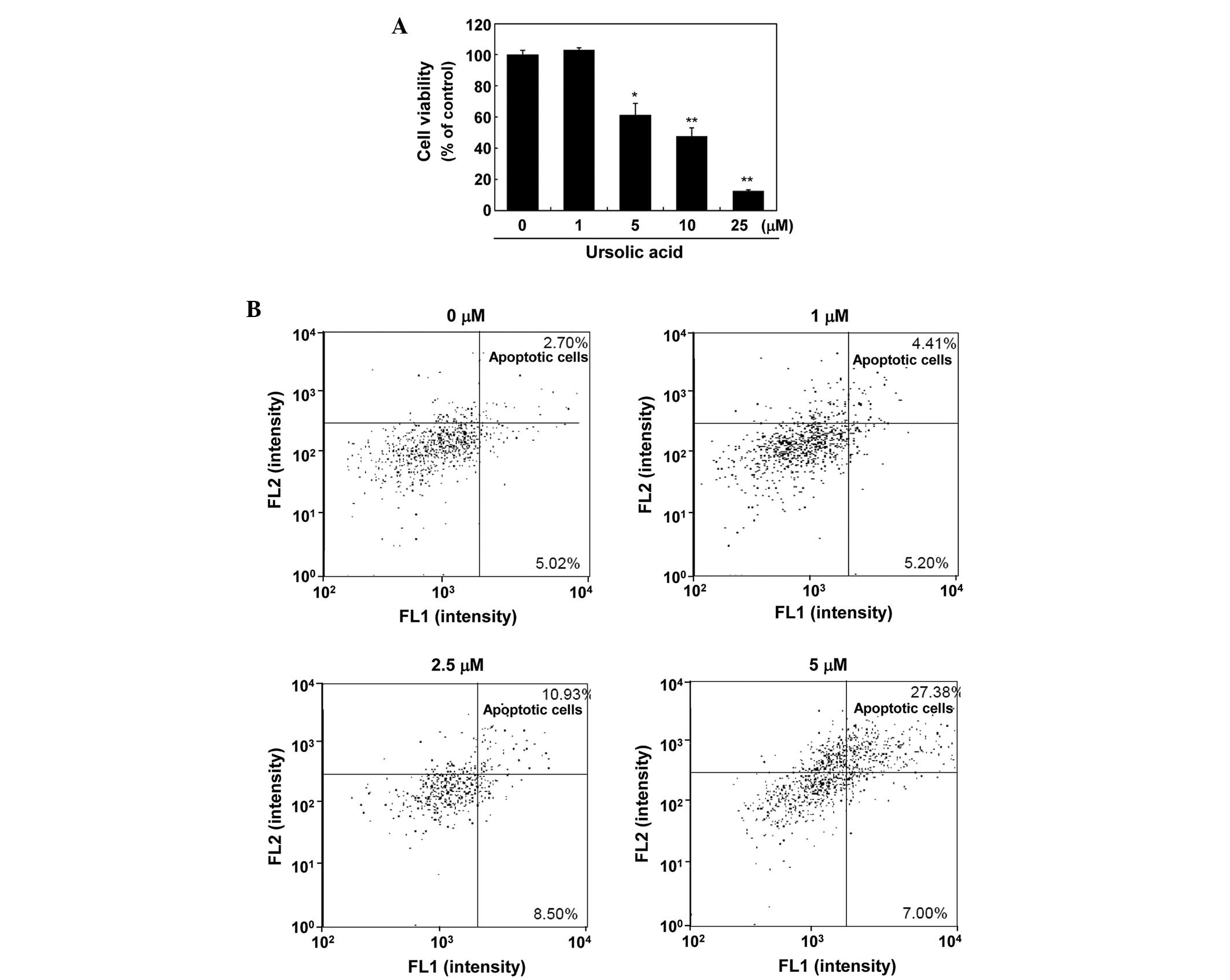
To investigate the effect of ursolic acid on the
growth of SNU-484 gastric cancer cells, an MTT assay was performed
on cells treated with various concentrations of ursolic acid. As
demonstrated in Fig. 1A, treatment
of the SNU-484 cells with ursolic acid for 24 h inhibited growth in
a dose-dependent manner (P<0.01), with a half maximal inhibitory
concentration of 9 μM.
Ursolic acid induces apoptosis and
regulates the expression of apoptosis-associated proteins
To investigate whether ursolic acid-induced growth
inhibition involves apoptosis, a flow cytometric analysis was
conducted. As demonstrated in Fig.
1B, ursolic acid induced the apoptosis of the SNU-484 cells in
a concentration-dependent manner. Treatment of the SNU-484 cells
with 5 μM ursolic acid for 24 h resulted in a significant increase
in annexin V- and propidium iodide -positive apoptotic cells
(27.38%) compared with the control cells (2.70%). These results
indicate that the observed growth inhibitory effect of ursolic acid
may be due to the induction of apoptotic cell death.
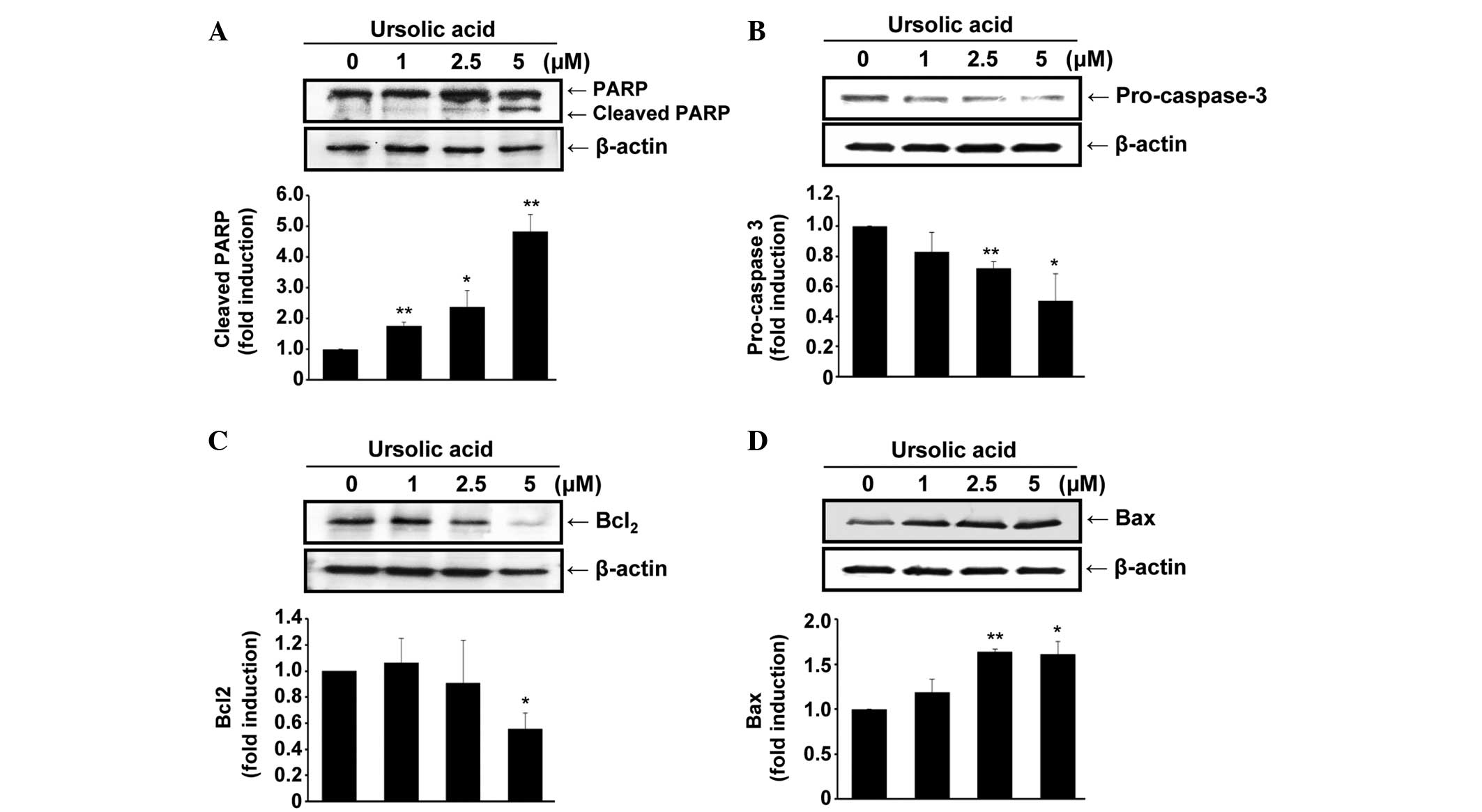
To evaluate the molecular mechanisms underlying
ursolic acid-induced apoptosis, the protein expression levels of
PARP, pro-caspase-3, Bcl-2 and Bax were determined in cells treated
with ursolic acid for 24 h. PARP, a well-established substrate for
caspase-3 and thus an indicator of apoptosis (33), appeared to be cleaved by ursolic
acid (P<0.01; Fig. 2A), and the
expression of pro-caspase-3 was decreased by ursolic acid
(P<0.05; Fig. 2B), indicating
that ursolic acid may induce the activation of caspase-3 in the
SNU-484 cells.
In addition, the expression levels of Bcl-2 and Bax
were examined in the ursolic acid-treated cells. Ursolic acid
dose-dependently inhibited the expression of Bcl-2 (P<0.05),
whereas Bax expression levels were increased by the administration
of ursolic acid (P<0.05; Fig. 2C and
D). These data demonstrate that ursolic acid induces apoptosis
in SNU-484 cells, possibly by downregulating the anti-apoptotic
Bcl-2 protein and upregulating the pro-apoptotic Bax protein.
Ursolic acid activates p38 MAPK and
JNK
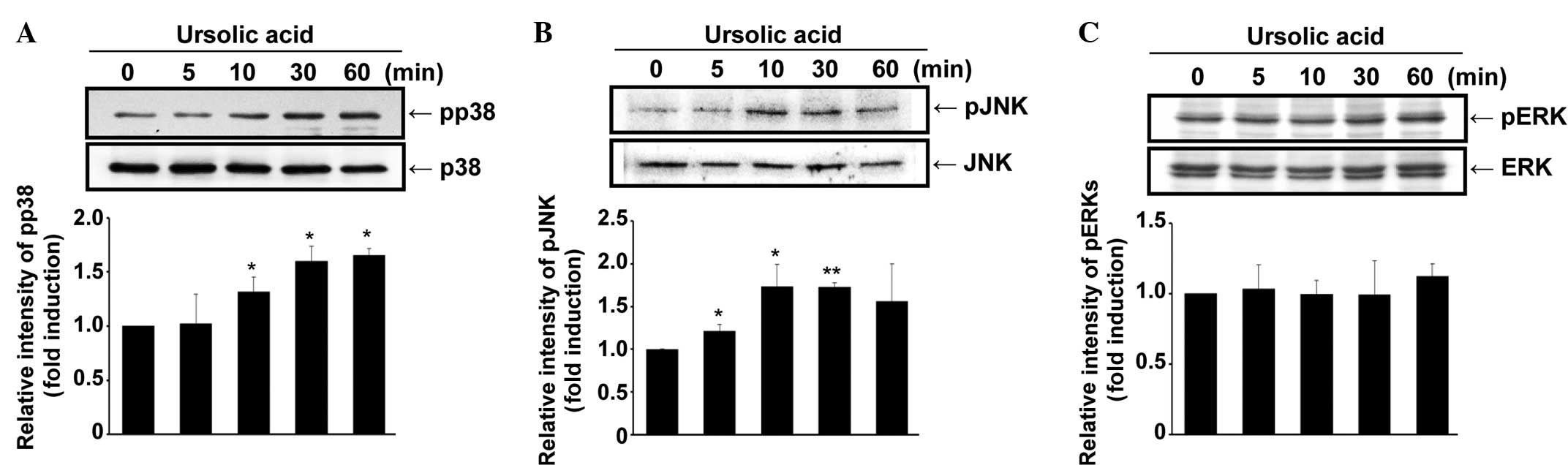
A kinetic study was performed to examine the effect
of ursolic acid on the activation of MAPKs. As demonstrated in
Fig. 3A, phospho-p38 MAPK
expression was increased in the SNU-484 gastric cancer cells by the
administration of ursolic acid in a time-dependent manner
(P<0.05). In addition, the phosphorylation level of JNK
increased up to 10 min after ursolic acid treatment (P<0.01) and
then slightly decreased up to 60 min after (Fig. 3B). By contrast, the activation of
ERK and phospho-ERK was not significantly altered by the
administration of ursolic acid (Fig.
3C). These results demonstrate that ursolic acid activates p38
MAPK and JNK, indicating the possible involvement of these
signaling pathways in the ursolic acid-induced apoptosis of SNU-484
gastric cancer cells.
Ursolic acid inhibits the invasive
phenotype of SNU484 cells
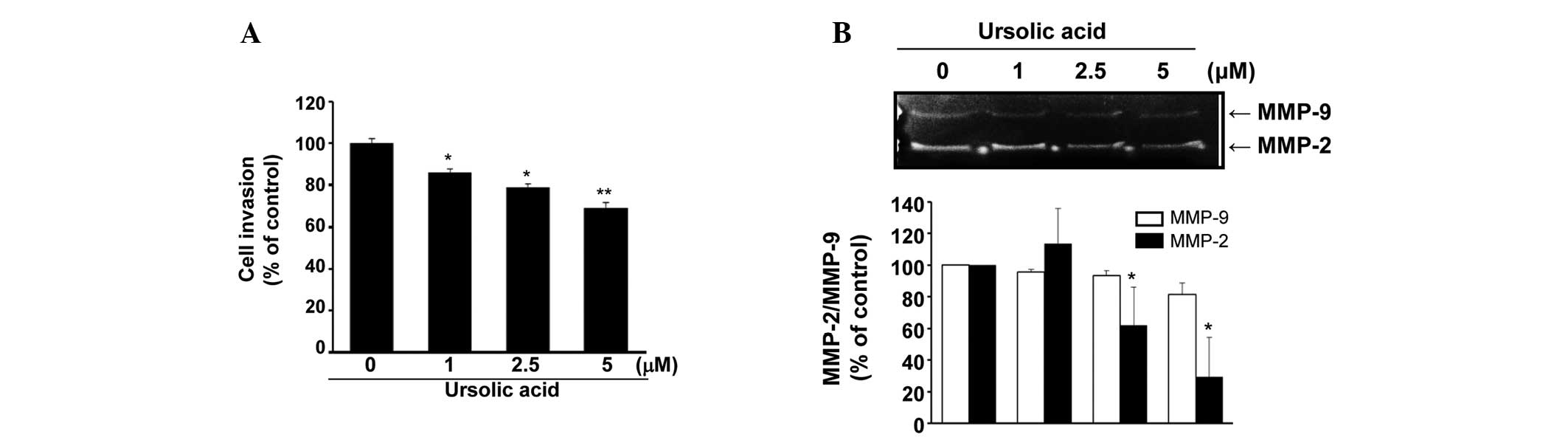
To examine the effect of ursolic acid on the
invasive phenotype of the SNU-484 cells, an in vitro
invasion assay was performed. As demonstrated in Fig. 4A, the invasive ability of the
SNU-484 cells was significantly inhibited by ursolic acid
administration in a dose-dependent manner (P<0.01). In addition,
the effect of ursolic acid on MMP-2 and/or MMP-9 in the SNU-484
cells was examined. As demonstrated in Fig. 4B, the expression level of MMP-2 was
dose-dependently decreased by the administration of ursolic acid
(P<0.05), while the expression of MMP-9 was not significantly
reduced. These data indicate that ursolic acid may inhibit the
invasiveness of SNU-484 cells via the downregulation of MMP-2.
Discussion
Gastric cancer is one of the predominant human
malignancies, and chemoprevention using natural products has
previously been considered as a promising approach for the control
and management of cancer (34,35).
The present study demonstrated that ursolic acid efficiently
induces apoptosis and inhibits the invasive phenotype of SNU-484
human gastric cancer cells. These findings indicate a potential
application of ursolic acid in regulating the aggressiveness of
human gastric cancer.
Cancer cells often escape apoptosis by increasing
the expression levels of anti-apoptotic genes or by decreasing the
expression of pro-apoptotic genes (22). Ursolic acid has previously been
demonstrated to induce apoptosis by causing the release of
cytosolic cytochrome c, activating caspase-3, reducing the
expression of Bcl-2 and increasing the expression of Bax in HeLa
(36) and MDA-MB-231 breast cancer
cells (37). Similarly, the results
of the present study indicate that ursolic acid may induce
apoptosis by decreasing the expression of Bcl-2 and increasing the
expression of Bax in SNU-484 gastric cancer cells.
The MAPK family of proteins regulate various stress
responses, and thus are crucial for the maintenance of cells
(38). Among the various MAPK
pathways, the JNK and p38 MAPK pathways have been indicated to be
key regulators of stress-induced apoptosis (39). There has previously been controversy
regarding the role of p38 MAPK signaling in cell death; depending
on the cell system used, p38 MAPK signaling has been shown to
promote cell death (40,41), and to enhance cell survival and
growth (42,43). Furthermore, ursolic acid has been
demonstrated to induce apoptosis via the activation of JNK, but not
p38 MAPK, in pituitary adenoma, human leukemia K562 and prostate
cancer cells (42–46). The results of the present study
demonstrated that ursolic acid activates JNK and p38 MAPK in human
gastric cancer cells, indicating that the role of MAPK signaling in
the induction of apoptosis may be cell-type specific.
Tumor cell invasion is an early step of the
metastatic cascade, representing the onset of the transition from
the benign stage to malignant progression (47). Numerous studies have identified an
association between cell invasion and the inhibition of MMPs
(48–50). Furthermore, our previous study and
another study have demonstrated that among the MMPs, MMP-2
expression is associated with the invasive phenotype of gastric
cancer cells (51,52). Consistent with these previous
studies, the present study demonstrated that ursolic acid inhibits
the invasive phenotype of SNU-484 gastric cancer cells by
downregulating MMP-2 expression.
In conclusion, the present study clearly
demonstrates that ursolic acid induces apoptosis and inhibits cell
invasion in SNU-484 human gastric cancer cells. Considering that
gastric cancer is one of the most common types of cancer and that
metastasis is the major cause of mortality in gastric cancer
patients, the findings of the present study may provide insights
into the potential application of ursolic acid as an agent for the
prevention and treatment of human gastric cancer.
Acknowledgements
The present study was supported by the Duksung
Women’s University Research Grant 2012. The authors thank Dr Choon
Sik Jeong of Duksung Women’s University (Seoul, South Korea) for
partaking in useful discussions on the study.
References
|
1
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: a global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh
KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al; Asia
Pacific Working Group of Gastric Cancer. Screening for gastric
cancer in Asia: current evidence and practice. Lancet Oncol.
9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.PubMed/NCBI
|
|
4
|
Macdonald JS: Gastric cancer - new
therapeutic options. N Engl J Med. 355:76–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D and Chua YJ: East meets west
in the treatment of gastric cancer. N Engl J Med. 357:1863–1865.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ngo SN, Williams DB and Head RJ: Rosemary
and cancer prevention: preclinical perspectives. Crit Rev Food Sci
Nutr. 51:946–954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shishodia S, Majumdar S, Banerjee S and
Aggarwal BB: Ursolic acid inhibits nuclear factor-κB activation
induced by carcinogenic agents through suppression of IκBα kinase
and p65 phosphorylation: correlation with down-regulation of
cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer
Res. 63:4375–4383. 2003.PubMed/NCBI
|
|
8
|
Wang X, Zhang F, Yang L, Mei Y, Long H,
Zhang X, Zhang J, Qimuge-Suyila and Su X: Ursolic acid inhibits
proliferation and induces apoptosis of cancer cells in vitro and in
vivo. J Biomed Biotechnol. 2011:4193432011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CC, Huang CY, Mong MC, Chan CY and Yin
MC: Antiangiogenic potential of three triterpenic acids in human
liver cancer cells. J Agric Food Chem. 59:755–762. 2011. View Article : Google Scholar
|
|
10
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Xing D, Chen Q and Chen WR:
Enhancement of chemotherapeutic agent-induced apoptosis by
inhibition of NF-κB using ursolic acid. Int J Cancer. 127:462–473.
2010.
|
|
12
|
Ma CM, Cai SQ, Cui JR, Wang RQ, Tu PF,
Hattori M, Hattori M and Daneshtalab M: The cytotoxic activity of
ursolic acid derivatives. Eur J Med Chem. 40:582–589. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shanmugam MK, Manu KA, Ong TH,
Ramachandran L, Surana R, Bist P, Lim LH, Kumar AP, Hui KM and
Sethi G: Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid
leads to suppression of metastasis in transgenic adenocarcinoma of
mouse prostate model. Int J Cancer. 129:1552–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Angel RE, Smith SM, Glickman RD,
Perkins SN and Hursting SD: Antitumor effects of ursolic acid in a
mouse model of postmenopausal breast cancer. Nutr Cancer.
62:1074–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasad S, Yadav VR, Sung B, Reuter S,
Kannappan R, Deorukhkar A, Diagaradjane P, Wei C,
Baladandayuthapani V, Krishnan S, et al: Ursolic acid inhibits
growth and metastasis of human colorectal cancer in an orthotopic
nude mouse model by targeting multiple cell signaling pathways:
chemosensitization with capecitabine. Clin Cancer Res.
18:4942–4953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh CT, Wu CH and Yen GC: Ursolic acid, a
naturally occurring triterpenoid, suppresses migration and invasion
of human breast cancer cells by modulating c-Jun N-terminal kinase,
Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res.
54:1285–1295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu LB, Wang J, Ma BZ and Sun WZ:
Inhibitive effect of ursolic acid on the invasion and metastasis of
ovarian carcinoma cells HO-8910PM. Sichuan Da Xue Xue Bao Yi Xue
Ban. 41:986–988. 2010.(In Chinese).
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steller-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar
|
|
20
|
Stetler-Stevenson WG, Liotta LA and
Kleiner DE Jr: Extracellular matrix 6: role of matrix
metalloproteinases in tumor invasion and metastasis. FASEB J.
7:1434–1441. 1993.PubMed/NCBI
|
|
21
|
Chiarugi V, Magnelli L, Cinelli M and Basi
G: Apoptosis and the cell cycle. Cell Mol Biol Res. 40:603–612.
1994.PubMed/NCBI
|
|
22
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kroemer G: The proto-oncogene Bcl-2 and
its role in regulating apoptosis. Nat Med. 3:614–620. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blanc C, Deveraux QL, Krajewski S, Jänicke
RU, Porter AG, Reed JC, Jaggi R and Marti A: Caspase-3 is essential
for procaspase-9 processing and cisplatin-induced apoptosis of
MCF-7 breast cancer cells. Cancer Res. 60:4386–4390.
2000.PubMed/NCBI
|
|
26
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
27
|
Westwick JK, Bielawska AE, Dbaibo G,
Hannun YA and Brenner DA: Ceramide activates the stress-activated
protein kinases. J Biol Chem. 270:22689–22692. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tibbles LA and Woodgett JR: The
stress-activated protein kinase pathways. Cell Mol Life Sci.
55:1230–1254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu R, Shtil AA, Tan TH, Roninson IB and
Kong AN: Adriamycin activates c-jun N-terminal kinase in human
leukemia cells: a relevance to apoptosis. Cancer Lett. 107:73–81.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen YR, Meyer CF and Tan TH: Persistent
activation of c-Jun N-terminal kinase 1 (JNK1) in gamma
radiation-induced apoptosis. J Biol Chem. 271:631–634. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death - apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar
|
|
33
|
Germain M, Affar EB, D’Amours D, Dixit VM,
Salvesen GS and Poirier GG: Cleavage of automodified
poly(ADP-ribose) polymerase during apoptosis. Evidence for
involvement of caspase-7. J Biol Chem. 274:28379–28384. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karikas GA: Anticancer and chemopreventing
natural products: some biochemical and therapeutic aspects. J BUON.
15:627–638. 2010.
|
|
35
|
Ou Y, Li Q, Wang J, Li K and Zhou S:
Antitumor and apoptosis induction effects of paeonol on mice
bearing EMT6 breast carcinoma. Biomol Ther (Seoul). 22:341–346.
2014. View Article : Google Scholar
|
|
36
|
Li Y, Lu X, Qi H, Li X, Xiao X and Gao J:
Ursolic acid induces apoptosis through mitochondrial intrinsic
pathway and suppression of ERK1/2 MAPK in HeLa cells. J Pharmacol
Sci. 125:202–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KH, Seo HS, Choi HS, Choi I, Shin YC
and Ko SG: Induction of apoptotic cell death by ursolic acid
through mitochondrial death pathway and extrinsic death receptor
pathway in MDA-MB-231 cells. Arch Pharm Res. 34:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raingeaud J, Gupta S, Rogers JS, Dickens
M, Han J, Ulevitch RJ and Davis RJ: Pro-inflammatory cytokines and
environmental stress cause p38 mitogen-activated protein kinase
activation by dual phosphorylation on tyrosine and threonine. J
Biol Chem. 270:7420–7426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sarkar D, Su ZZ, Lebedeva IV, Sauane M,
Gopalkrishnan RV, Valerie K, Dent P and Fisher PB: Mda-7 (IL-24)
mediates selective apoptosis in human melanoma cells by inducing
the coordinated overexpression of the GADD family of genes by means
of p38 MAPK. Proc Natl Acad Sci USA. 99:10054–10059. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Porras A, Zuluaga S, Black E, Valladares
A, Alvarez AM, Ambrosino C, Benito M and Nebreda AR: P38α
mitogen-activated protein kinase sensitizes cells to apoptosis
induced by different stimuli. Mol Biol Cell. 15:922–933. 2004.
View Article : Google Scholar :
|
|
42
|
Yosimichi G, Nakanishi T, Nishida T,
Hattori T, Takano-Yamamoto T and Takigawa M: CTGF/Hcs24 induces
chondrocyte differentiation through a p38 mitogen-activated protein
kinase (p38MAPK), and proliferation through a p44/42
MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem.
268:6058–6065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park JM, Greten FR, Li ZW and Karin M:
Macrophage apoptosis by anthrax lethal factor through p38 MAP
kinase inhibition. Science. 297:2048–2051. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gong YY, Liu YY, Yu S, Zhu XN, Cao XP and
Xiao HP: Ursolic acid suppresses growth and adrenocorticotrophic
hormone secretion in AtT20 cells as a potential agent targeting
adrenocorticotrophic hormone-producing pituitary adenoma. Mol Med
Rep. 9:2533–2539. 2014.PubMed/NCBI
|
|
45
|
Liu XS and Jiang J: Induction of apoptosis
and regulation of the MAPK pathway by ursolic acid in human
leukemia K562 cells. Planta Med. 73:1192–1194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang YX, Kong CZ, Wang HQ, Wang LH, Xu CL
and Sun YH: Phosphorylation of Bcl-2 and activation of caspase-3
via the c-Jun N-terminal kinase pathway in ursolic acid-induced
DU145 cells apoptosis. Biochimie. 91:1173–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lynch CC and Matrisian LM: Matrix
metalloproteinases in tumor-host cell communication.
Differentiation. 70:561–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement-membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee YD, Cui MN, Yoon HH, Kim HY, Oh IH and
Lee JH: Down-modulation of Bis reduces the invasive ability of
glioma cells induced by TPA, through NF-κB mediated activation of
MMP-9. BMB Rep. 47:262–267. 2014. View Article : Google Scholar :
|
|
50
|
Ham M and Moon A: Inflammatory and
microenvironmental factors involved in breast cancer progression.
Arch Pharm Res. 36:1419–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yong HY and Moon A: Roles of
calcium-binding proteins, S100A8 and S100A9, in invasive phenotype
of human gastric cancer cells. Arch Pharm Res. 30:75–81. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tsuchiya A, Kikuchi Y, Ando Y, Yoshida T
and Abe R: Lymph node metastases in gastric cancer invading the
submucosal layer. Eur J Surg Oncol. 21:248–250. 1995. View Article : Google Scholar : PubMed/NCBI
|