Introduction
Many cancer immunotherapies developed in
experimental animals have been tested in clinical trials. Although
some have shown modest clinical effects, most have not been
effective (1,2). Recent studies have identified
myeloid-origin cells that are potent suppressors of tumor immunity
and therefore a significant impediment to cancer immunotherapy.
Suppressive myeloid cells were described three decades ago in
patients with cancer (3,4), but their functional importance in the
immune system has only recently been appreciated (5–8).
Indeed, accumulating evidence has now shown that a population of
cells with suppressive activity [known as myeloid-derived
suppressor cells (MDSCs)] contributes to the negative regulation of
immune responses during cancer and other diseases.
MDSCs have been identified in most patients and
experimental mice with tumors based upon their ability to suppress
T cell activation. In mice, MDSCs are uniformly characterized by
the expression of the cell surface molecule detected by antibodies
to Gr1 and CD11b (9). The variation
in MDSC phenotype is consistence with the concept that MDSCs are a
diverse family of cells that are in various intermediate stages of
myeloid cell differentiation (9).
In humans, MDSCs are most commonly defined as
CD14−CD11b+ cells or, more narrowly, as cells
that express the common myeloid marker CD33 but lack the expression
of markers of mature myeloid and lymphoid cells, and of the MHC
class II molecule HLA-DR. In this study,
CD11b+CD14−CD33+ cells were
analysed as MDSC (10). An
accumulation of MDSCs was associated with the decreased number of
dendritic cells in the peripheral blood of patients with several
types of cancer. Pathophysiology of MDSC in patients has not been
clarified well in contrast to studies in mice.
Tumor development and growth occurs as a result of
interactions between the tumor and host immune/inflammatory cells
with chronic inflammation having an important role in cancer
development and progression (11).
Many laboratory markers of systemic inflammatory response, which is
closely related to patient’s nutritional status, including
neutrophil/lymphocyte ratio (NLR) have been investigated as
prognostic markers with best evidence for their use demonstrated in
surgical patients.
In this report, we show the status of MDSCs in
normal volunteers and patients with various types of cancer, and
the correlation to laboratory data were analysed.
Materials and methods
Samples
Blood samples were taken from 53 patients with
various types of cancer and 18 normal volunteers with similar age
and gender distributions. The patients who received treatment
including surgery, chemotherapy, palliative care and follow-up in
the Department of Organ-Regulatory Surgery in Fukushima Medical
University from January to June, 2011, 45–89 years of age with
histologically confirmed cancer were enrolled in the study. Of
these 53 patients, 29 had breast cancer, and 21 had cancer of the
digestive system including 10 with colorectal, 6 with gastric, 1
with esophageal and 1 with pancreatic cancer, 1 with
cholangiocarcinoma, 1 with hepatocellular carcinoma and 1 with
GIST, and 3 had thyroid cancer (Table
I). The patients were newly diagnosed as advanced diseases and
blood samples were taken before the treatments including surgery
and chemotherapy except patients with breast cancer. Peripheral
blood mononuclear cells (PBMC) were separated on Ficoll-Hypaque
(Pharmacia-Biotech, Uppsala, Sweden). The isolated PBMC were washed
twice with RPMI-1640 (Wako Pure Chemical Industries Ltd., Osaka,
Japan) and were kept frozen at −80°C until use in freezing media
(BLC-1, Juji-Field Co. Ltd., Tokyo, Japan). This study was approved
by the ethics committee of Fukushima Medical University (2010-204)
and written informed consent was obtained from the patients and
normal donors who entered in this study.
 | Table IPatients. |
Table I
Patients.
| Normal
volunteers | 18 |
| Cancer | 53 |
| Digestive system
(n=21) |
| Cholangiocellular
carcinoma | 1 |
| Hepatocellular
carcinoma | 1 |
| Pancreatic | 1 |
| Esophageal | 1 |
| Gastric | 6 |
| Colorectal | 10 |
| GIST | 1 |
| Breast | 29 |
| Thyroid | 3 |
| Total | 71 |
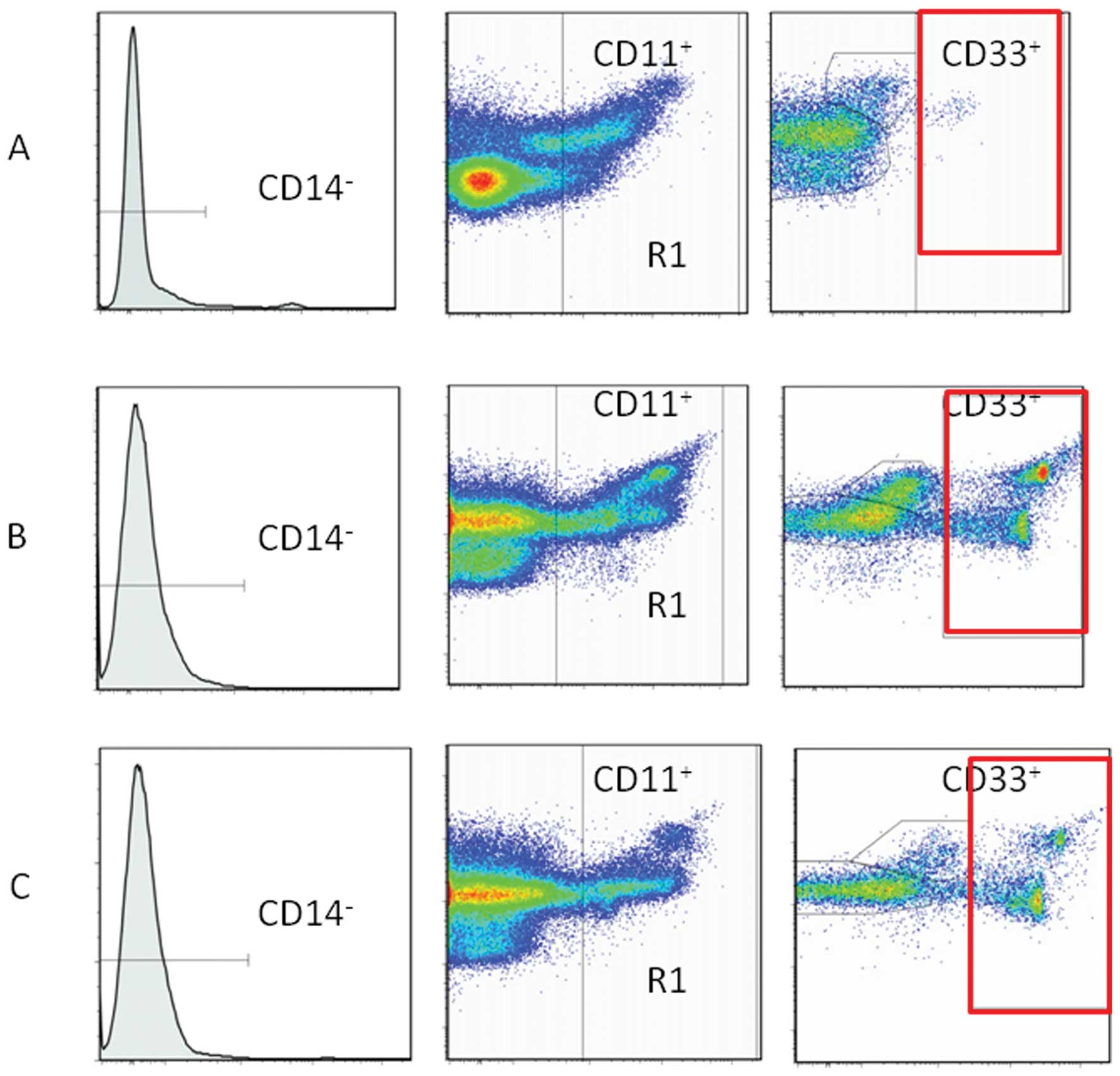
Flow cytometry
Cells were labeled for immunofluorescence and
analyzed by flow cytometry for cell surface. Cells were labeled
with fluorescent isothiocyanate (FITC), phycoerythrin (PE),
Phycoerythrin Cyanin 5.1 (PC5). Antibodies were used at 10, 10 and
50 μg/ml were diluted each in PBS. The cells were incubated with
the antibodies for 20 min at 4°C and washed with PBS. These
included FITC-conjugated CD14 (Abcam Cambridge, UK), PE-conjugated
CD11b (Beckman Coulter, Marseille, France), PC5-conjugated CD33
(Beckman Coulter). Data acquisition and analysis were performed on
a FACSAriaII flow cytometer (BD Bioscience, Mountain View, CA,
Fig. 1) using FlowJo software (Tree
Star Inc. Ashland, OR).
Proliferation assay
Lymphocyte proliferation assay were carried out with
using PBMC suspended in RPMI-1640 (Wako Pure Chemical Industries,
Osaka, Japan) and 10% fetal calf serum (Sigma, St. Louis, MO).
Phytohemmaglutinin (PHA) mitogenesis was observed for 80 h at 10
μg/ml of PHA into PBMC. The cultures were undertaken at 37°C in a
5% CO2 atmosphere with the addition of
3H-thymidine (Japan Radioisotope Association, Tokyo,
Japan) for the last 8 h of incubation. Cells were harvested and
3H-thymidine incorporation was counted using a liquid
scintillation counter (Perkin-Elmer Inc., Waltham, MA) and
expressed as count per minute (cpm). Stimulation index (SI) was
obtained by calculating total CPM/control cpm in which PHA was not
added to PBMC.
Statistical analysis
Differences between the groups were determined by
Student’s t-test. Relationships between two variables were
quantified by Spearman’s rank correlation coefficient. Significance
was assumed at p<0.05.
Results and Discussion
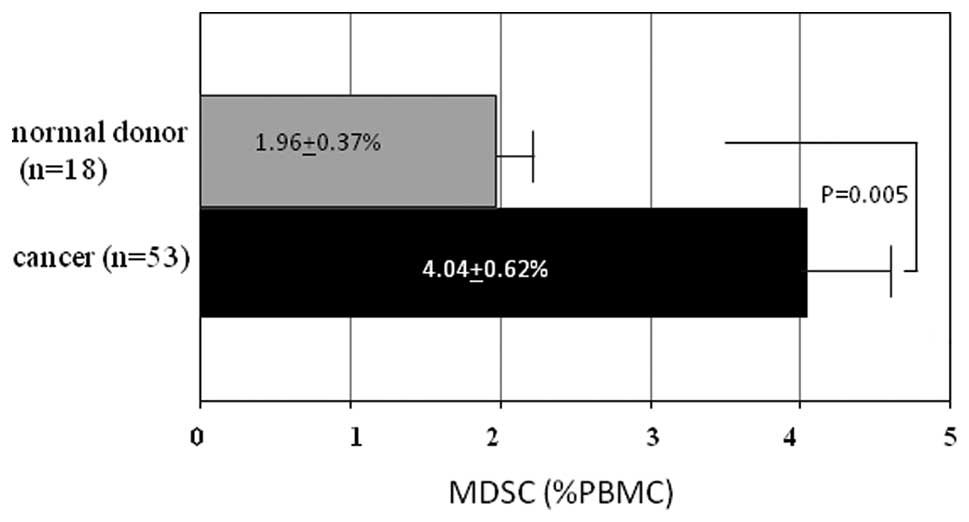
We have tested PBMCs from 53 patients with various
types of cancer and GIST, and those from 18 normal volunteers. A
highly significant increase was seen in percentages of DSC in PBMCs
from patients (4.04±0.624%, p<0.005, Fig. 2) compared with normal volunteers
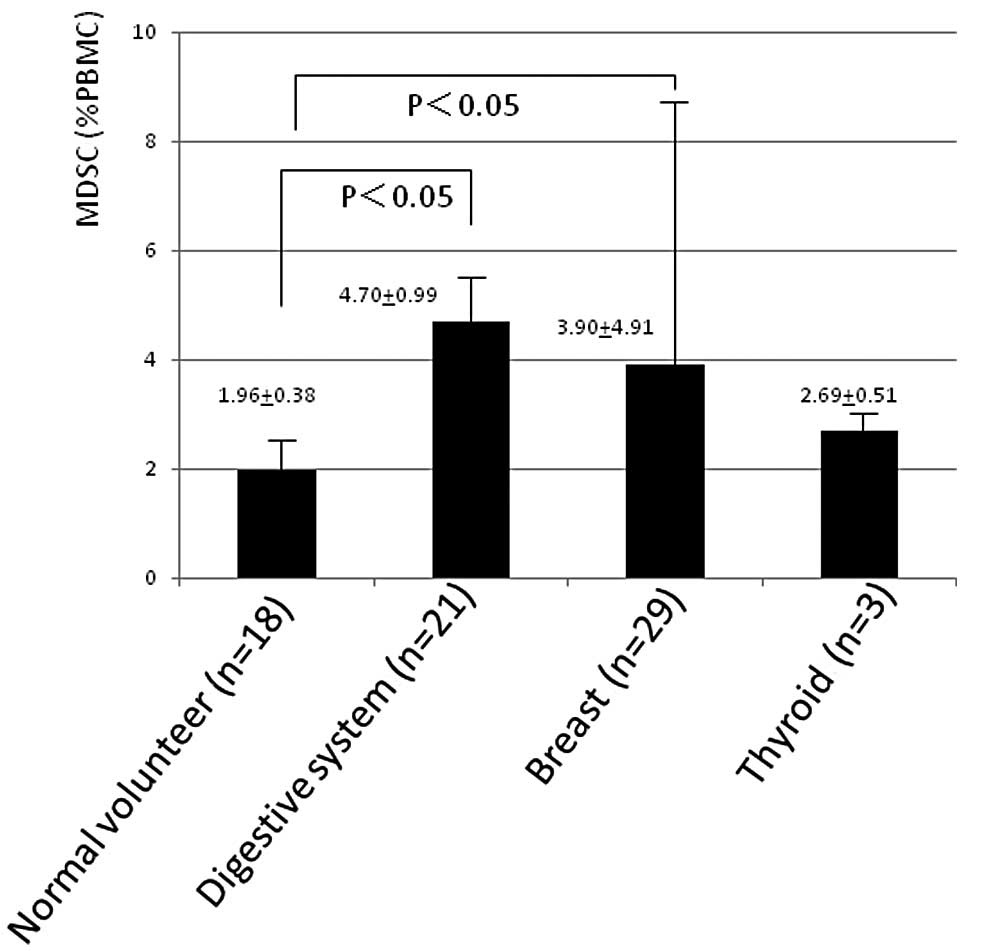
(1.96±0.37%). Among these patients, MDSC (%) was higher in 21
patients with cancer of digestive system including hepatocellular,
cholangiocellular and pancreatic carcinoma, and esophageal, gastric
and colorectal cancer, and GIST (4.70±0.99, p<0.05, Fig. 3) and in 29 patients with breast
cancer (3.90±4.91, p<0.05) compared with normal volunteers. MDSC
was 2.69±0.51% in 3 patients with thyroid carcinoma. These data of
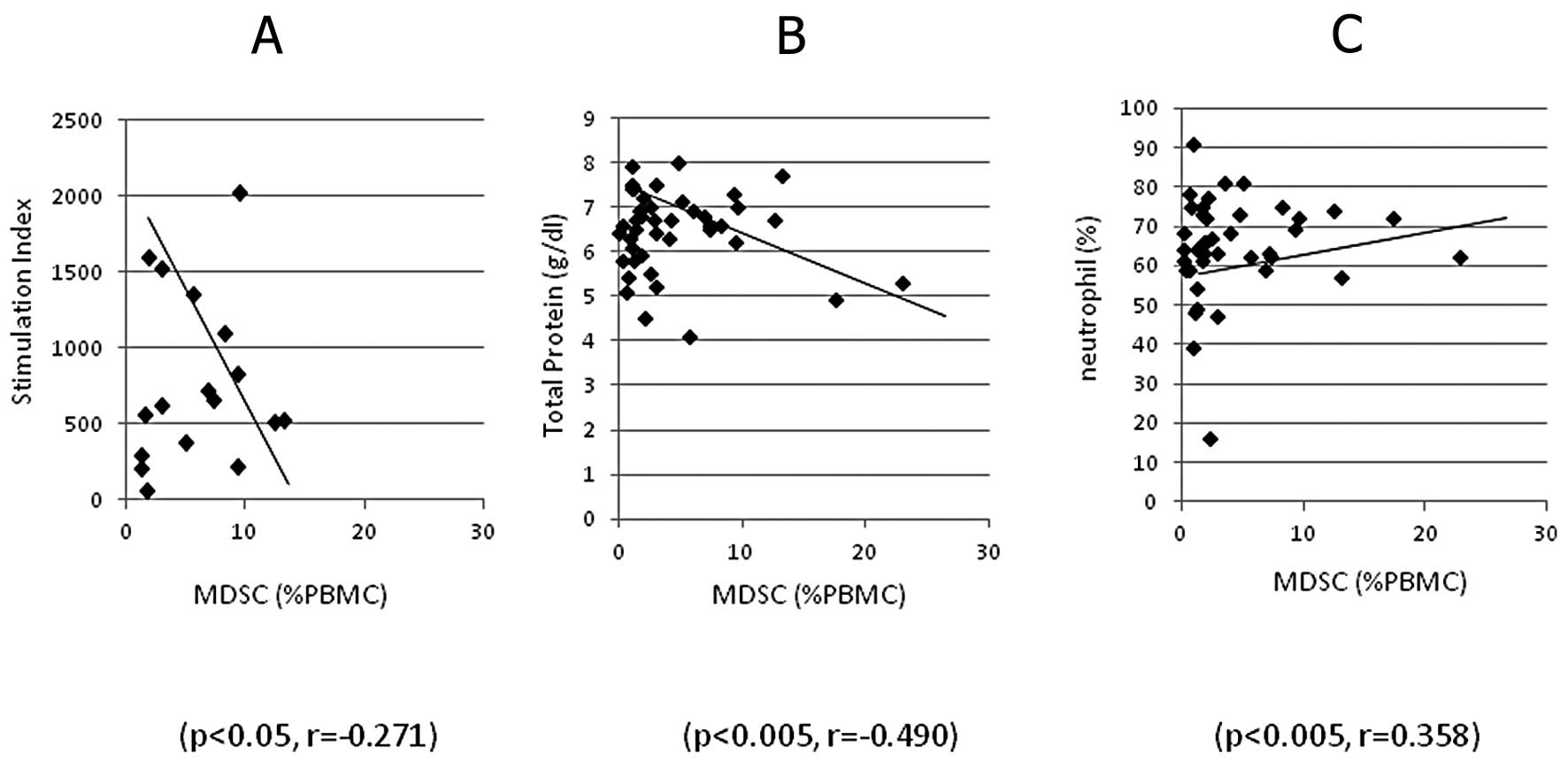
patients was analysed in correlation to clinical laboratory data
and, MDSC (%) was significantly inversely correlated to stimulation
indices of PHA-blastogenesis of lymphocytes (p<0.05, r=−0.271,
Fig. 4A) and serum concentration of
total protein (p<0.005, r=−0.490, Fig. 4B), and positively correlated to
neutrophil count (p<0.005, r=0.358, Fig. 4C). MDSC (%) in detailed digestive
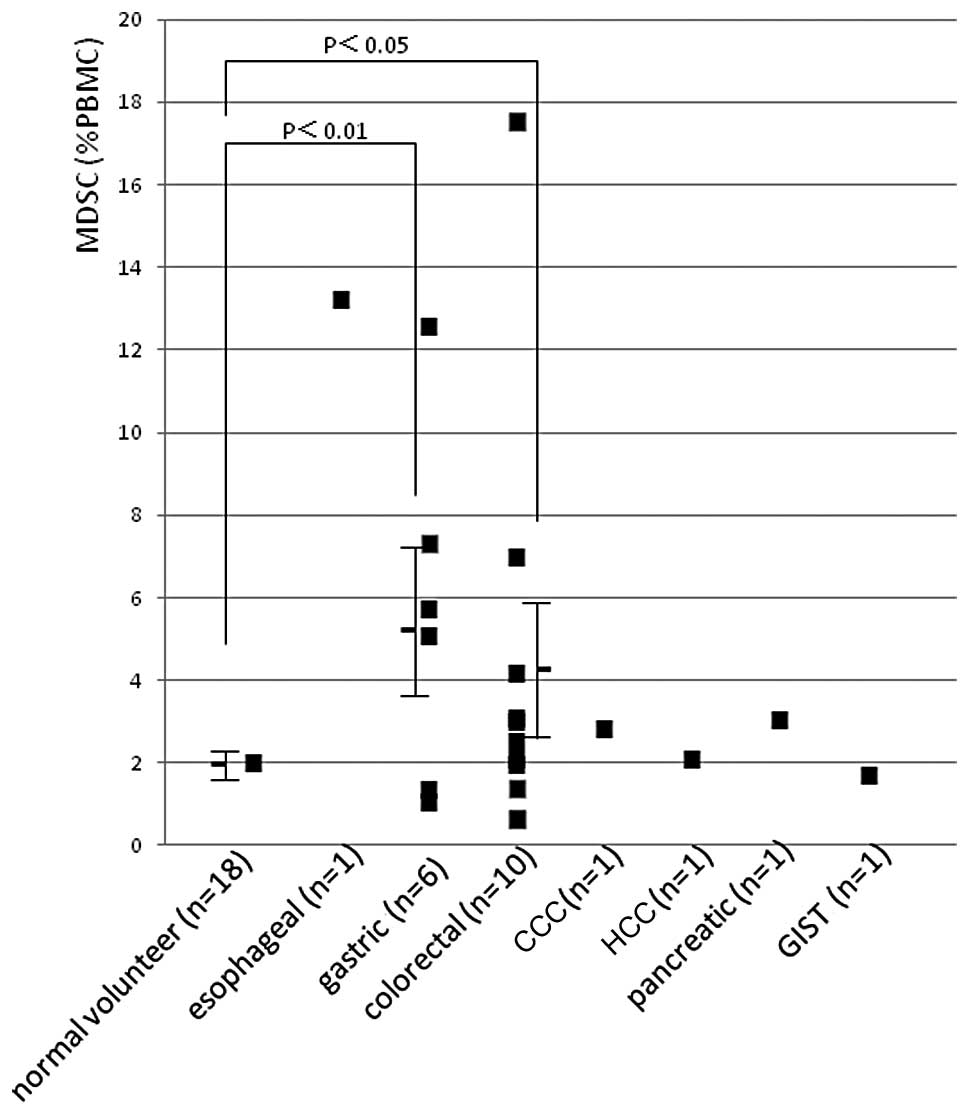
system diseases is shown in Fig. 5
and those of 6 patients with gastric cancer and of 10 with
colorectal cancer were higher (p<0.01 and <0.05,
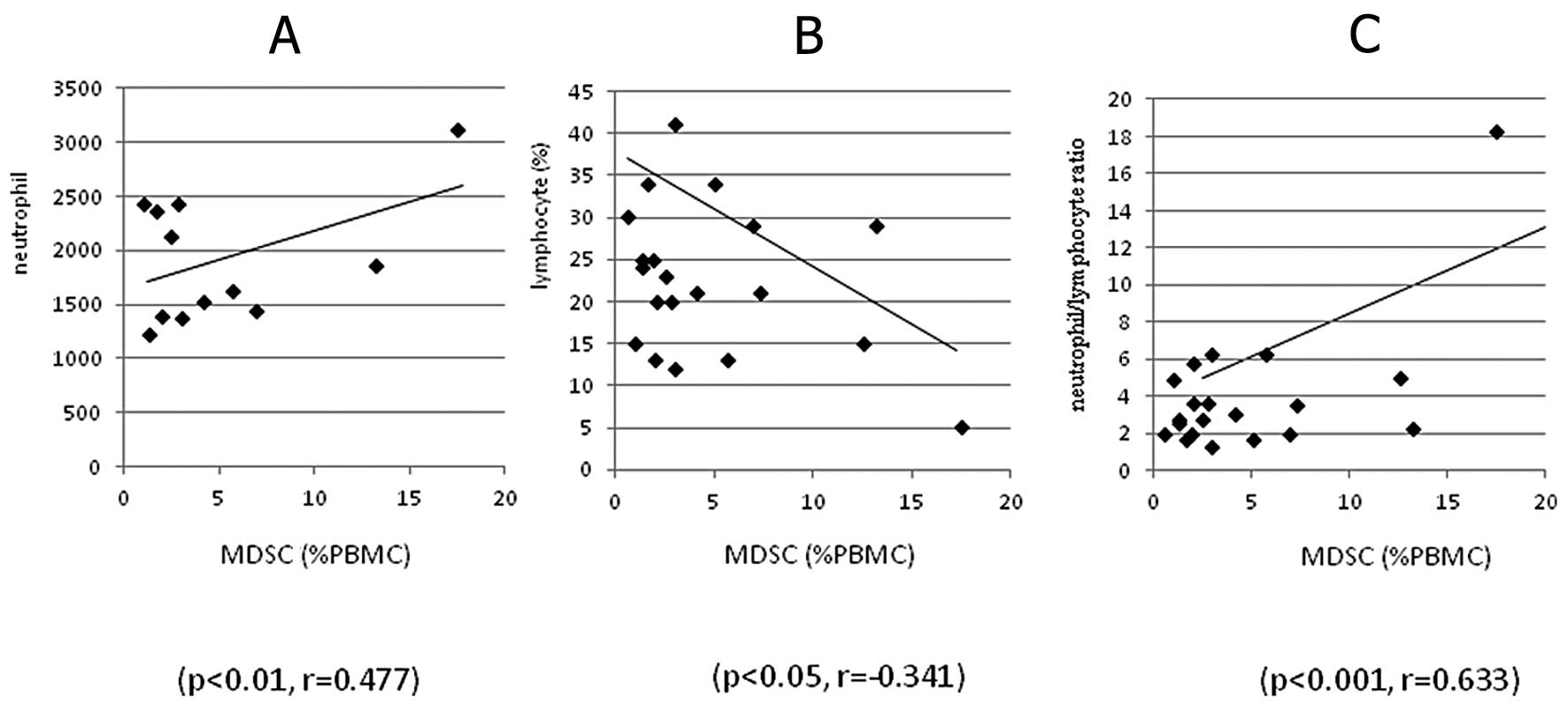
respectively) than in normal volunteers. MDSC (%) in 16 patients
with gastric and colorectal cancer was also significantly
correlated to neutrophil count (p<0.01, r=0.477, Fig. 6A) and inversely with lymphocyte
count (p<0.05, r=−0.341, Fig.
6B), and showed highly significant correlation to
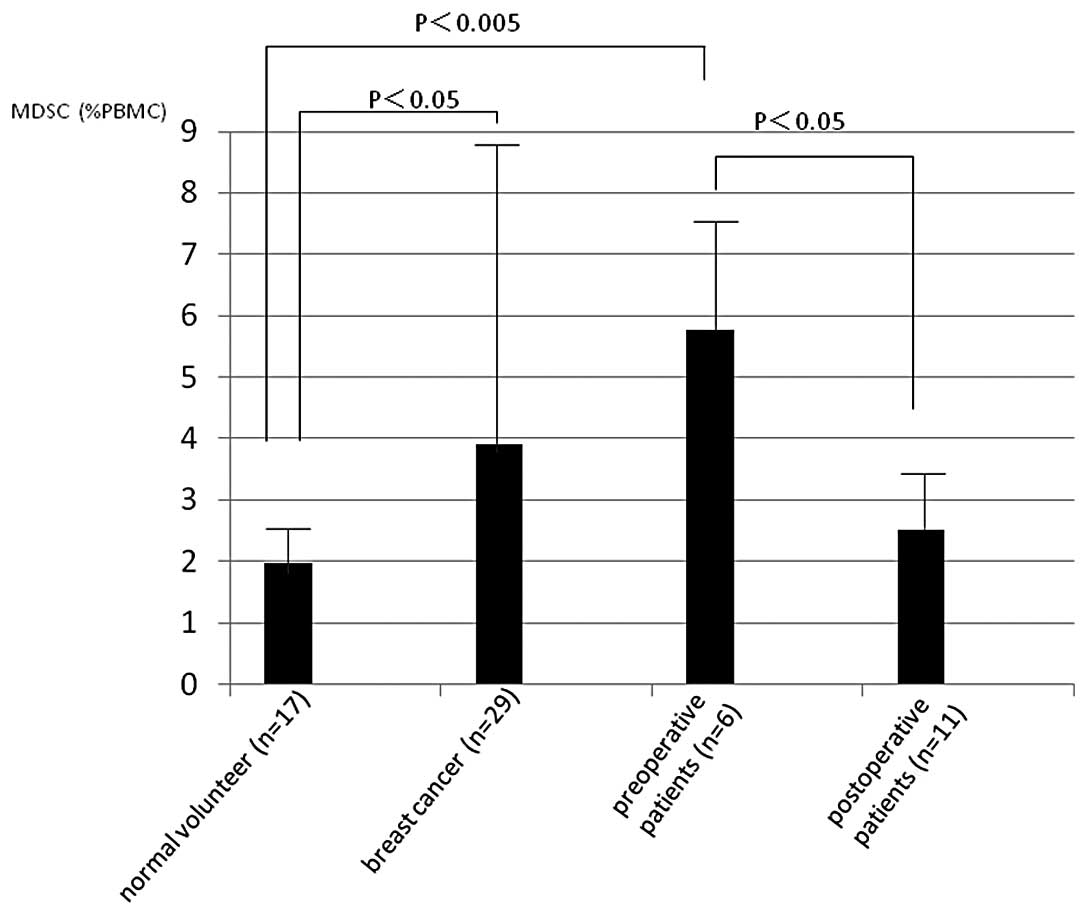
neutrophil/lymphocyte rate (p<0.001, r=0.633, Fig. 6C). Of the 29 patients with breast
cancer, 6 were preoperative and 11 postoperative, MDSC (%) in
preoperative patients was significantly increased compared to
normal volunteers (p<0.005) and it is significantly decreased in
postoperative patients (p<0.05, Fig.
7) compared to preoperative patients with breast cancer.
The evidence presented in this study shows that
percentage of MDSCs in peripheral circulating blood increased in
various types of cancer. These percentages inversely correlated to
stimulation indices of PHA-blastogenesis, lymphocyte count and
serum levels of total protein, and positively to neutrophil count
and neutrophil/lymphocyte ratios. Increase of neutrophil and
decrease of lymphocyte are sometimes seen in far advanced patients
with malignant diseases in the clinic and the ratios
neutrophil/lymphocyte has been used as one of the easiest and
effective markers of chronic inflammation and its related
immunosuppression in these patients. Thus it is clear that MDSCs
are increased in patients with cancer and closely related to
suppression of cell-mediated immune responses. These data also
suggested that it is increased further in the terminal stages of
the patients whose nutritional status is impaired as seen in
hypoproteinemia.
In patients with breast cancer, MDSCs (%) decreased
in postoperative condition compared to preoperative patients. MDSCs
have been reported to decrease after several kinds of chemotherapy
including gemcitabine, 5-FU plus cisplatin (8). On the other hand, it increased by
doxorubicin-cyclophosphamide (7)
and this may be the results of influence by certain
chemotherapeutic agent that induce inflammatory responses. Since
some of the postoperative patients received chemotherapy prior to
surgery, there may be a possibililty that the decrease of MDSCs
seen after the resection of the breast tumor might be a result
influenced by chemotherapy. But it seems mainly to be the systemic
effect by removal of the tumor since chemotherapy was done
approximately 6 weeks prior to the surgery. It is important now to
make a further evaluation of the individual effect of chemotherapy
or removal of the tumor on changes of MDSCs separately with
increased number of patients. If chemotherapy successfully decrease
MDSCs, it would be the strong tool as an adjuvant therapy for
antigen-specific cancer immunotherapy. The mechanisms of
tumor-induced T cell anergy in patients remains incompletely
understood. MDSCs found in the spleen of mice with colon cancer
block T cell function through nitric oxide and arginase production,
requiring cell-cell contact (3).
Recently the focus has been on highly suppressive myeloid cells
infiltrating mouse lung carcinomas, which had high arginase
activity and rapidly depleted arginine, blocking T cell
proliferation, cytokine production, and CD3ζ chain expression
(4). The exact mechanism of
increased production of immature myeloid cells in cancer patients
is not clear yet. However, it is known that tumor cells may produce
several growth factors and cytokines able to stimulate myelopoiesis
(12,13). In addition, vascular endothelial
growth factor produced by many tumors is able to affect
myelopoiesis (14). It is possible
that increased production of these growth factors may affect the
normal pathway of cell differentiation resulting in the
accumulation of immature myeloid cells. Systemic chronic
inflammation has been reported to play a role in developing and
growth of tumor and importantly in suppression of tumor immunity
(11,15,16).
NLR have been reported to be one of the best markers for that and
also a good prognostic marker, and to be high in patients with
hypoalbuminemia (17,18). In our previous studies, the results
showed that a suppression of cell-mediated immune reactions was
closely related to the nutritional status, and that it seems to
play a role in developing cancer cachexia (19–21).
In conclusion, it was shown that MDSC correlated to
nutritional impairment and it may be involved in an immunological
mechanism to induce cancer cachexia. It is hoped that we can
control immune suppression and chronic inflammation through
modulating MDSCs by a selective inhibition by molecular targeting
or decreasing with chemotherapy in the near future.
Acknowledgements
We deeply thank Mr. Shunichi Saito for his technical
assistance in preparatory experiments.
References
|
1
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pardoll D and Allison J: Cancer
immunotherapy: breaking the barriers to harvest the crop. Nat Med.
10:887–902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gabrilovich DI and Nagaj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenberg SO and Sinha P: Myeloid-derived
suppressor cells: linking inflammation and cancer. J Immunol.
182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zea AH, Rodriguez PC, Atkins MB, Hernandez
C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A,
O’Neill A and Ochoa AC: Arginase-producing myeloid suppressor cells
in renal cell carcinoma patients: a mechanism of tumor evasion.
Cancer Res. 65:3044–3048. 2005.PubMed/NCBI
|
|
6
|
Ochoa AC, Zea AH, Hernandez C and
Rodriguez PC: Arginase, prostaglandins, and myeloid-derived
suppressor cells in renal cell carcinoma. Clin Cancer Res.
13:S721–S726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garett-Mayre E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar
|
|
8
|
Gabitass RF, Annels NE, Crawshaw J, Pandha
HS and Middleton GE: Use of gemcitabine-(GEM) and
fluoropyrimidine-based chemotherapy to reduce myeloid-derived
suppressor cells (MDSCs) in pancreatic (PC) and esophagogastric
cancer (EGC). J Clin Oncol. 29:25882011.
|
|
9
|
Kusmartsev S, Nefedova Y, Yoder D and
Gabrilovich DI: Antigen-specific inhibition of CD8+ T
cell response by immature myeloid cells in cancer is mediated by
reactive oxygen species. J Immunol. 172:989–999. 2004.PubMed/NCBI
|
|
10
|
Almand B, Clark JI, Nikitina E, van Beynen
J, English NR, Knight SC, Carbone DP and Gabrilovich DI: Increased
production of immature myeloid cells in cancer patients: a
mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blackwill F and Mantovani A: Cancer and
inflammation: implications for pharmacology and therapeutics. Clin
Pharmacol Ther. 87:401–406. 2010. View Article : Google Scholar
|
|
12
|
Gabrilovich DI, Chen HL, Girgis KR,
Cinninghan HT, Meny GM, Nadaf S, Kavanauch D and Carbone DP:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1099. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menetrier-Caux C, Montmain G, Dieu MC,
Bain C, Favrot MC, Caux C and Blay JY: Inhibition of the
differentiation of denderitic cells from CD34+
progenitors by tumor cells: role of interleukin-6 and
macrophage-colony-stimulating factor. Blood. 92:4778–4791.
1998.PubMed/NCBI
|
|
14
|
Breitman TR, Collins SJ and Keene BR:
Terminal differentiation of human promyelocytic leukemic cells in
primary culture in response to retinoic acid. Blood. 57:1000–1011.
1981.PubMed/NCBI
|
|
15
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chua W, Charles KA, Baracos VE and Clarke
SJ: Neurrophil/lymphocyte ratio predicts chemotherapy outcomes in
patients with advanced colorectal cancer. Br J Cancer.
104:1288–1295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim
YT and Lee K: Pretreatment neutrophil to lymphocyte ratio is
elevated in epithelial ovarian cancer and predicts survival after
treatment. Cancer Immunol Immunother. 58:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata M, Takekawa M and Amano S:
Increased serum concentrations of soluble tumor necrosis factor
receptor 1 in noncachectic and cachectic patients with advanced
gastric and colorectal cancer. Surg Today. 28:884–888. 1998.
View Article : Google Scholar
|
|
20
|
Shibata M and Takekawa M: Increased serum
concentration of circulating soluble receptor for interleukin-2 and
its effect as a prognostic indicator in cachectic patients with
gastric and colorectal cancer. Oncology. 56:54–58. 1999. View Article : Google Scholar
|
|
21
|
Shibata M, Nagata Y, Kimura T, Kanou H,
Nezu T and Fukuzawa M: Elevated serum concentration of
interleukin-1 receptor antagonist (IL-1ra) is correlated to
interleukin-6 and to hypoalbuminemia in cachectic patients with
colorectal cancer. Int J Clin Oncol. 5:116–120. 2000. View Article : Google Scholar
|