Introduction
Pancreatic cancer is the fourth leading cause of
cancer death in the United States, with an estimated 43,140 new
cases and 36,800 deaths in 2010 (1). Patients diagnosed with pancreatic
cancer typically have a poor prognosis due to lack of early
diagnostic symptoms and effective treatments (2,3).
Therefore, efforts to treat pancreatic cancer have been directed
towards developing alternative modalities of therapy.
5-fluorouracil (5-FU), as a chemotherapy agent, is
widely used for the treatment of solid tumors such as pancreatic
cancer. It can also induce apoptosis in some sensitive cancer cell
lines by blocking the activity of thymidylate synthase (4). However, 5-FU presents the
disappointing chemotherapeutic effectiveness due to side effects
and multiple drug resistance (5,6).
Attempts to solve these problems have taken various approaches,
including the combined use of cancer drugs. It is recently shown
that combined treatment with various anticancer drugs increases the
efficiency and reduces the cytotoxicity of some cancer drugs
(7–12).
Natural products have been a successful source of
therapeutic agents and drug leads. Oleanolic acid (OA) belongs to
the group of pentacyclic triterpenes, which is widely distributed
in fruits and some medicinal herbs (13). It has been reported that OA has
antioxidant (14), antiinflammatory
(15), antidiabetic (16), antimutagenicity (17) and antitumor (18) properties. Recently, the antitumor
activity of OA has attracted more attention due to its marked
antitumor effects and pharmacological safety. Our recent study
reveals that OA induces cell death significantly via ROS-mediated
mitochondrial pathway in human Panc-28 cells (19). However, the combined effects of OA
and 5-FU in inhibiting cancer cell proliferation and inducing
apoptosis have not been investigated.
In the present study, the effects of OA combined
with 5-FU on cellular proliferation and apoptosis in human
pancreatic cancer Panc-28 cells were studied and the underlined
mechanism is also presented.
Materials and methods
Materials
Antibodies against caspase-3, -9, Bcl-2, Bax,
survivin, cytochrome C, cathepsin D, p53 and NF-κB were purchased
from Santa Cruz Biotechnology, Inc. Anti-mouse or anti-rabbit
antibodies were purchased from Beijing Solarbio Science &
Technology Co., Ltd.
Cell line and cell culture
Panc-28, a human pancreatic cancer cell line, was
cultured in DMEM/F12 supplemented with 10% FBS plus antibiotics of
penicillin and streptomycin (Sigma) at 37°C in 5% CO2 in
air. Cells were grown in 96-, 24- and 6-well plates.
Oleanolic acid and 5-fluorouracil
treatment
OA (Sigma) and 5-FU (Sigma) were dissolved in
dimethylsulfoxide (DMSO). Cells were pretreated with OA for 12 h.
Then, the supernatant fluid containing OA was removed and the fresh
medium containing 5-FU was added, and were incubated at 37°C for
another 24 h and harvested for the following examinations.
MTT assay
Inhibition of cell proliferation by OA was measured
by MTT assay (20). Briefly, cells
were grown in a 96-well plate (5×103/200 μl/well)
overnight, and were treated with OA (20, 30 and 40 μg/ml) for 12 h,
and then the supernatant fluid was removed. The fresh medium with
5-FU (10 μg/ml) was added. After incubation for another 24 h, 40 μl
of MTT solution (5 mg/ml) was added to each well. After incubation
for additional 4 h, the formazan precipitate was dissolved in 150
μl DMSO, and then the absorbance at 490 nm was measured using
ELx808™ Absorbance Microplate Reader (BioTek, USA). The nature of
the interactions between studied drugs was estimated by using
published methods (21,22), based on the principles described by
Chou and Talalay (23,24). Briefly, the expected value of
combination effect between treatment with OA and treatment with
5-FU is calculated by the following formula: [(observed treatment
with OA value)/(control value)] × [(observed treatment with 5-FU
value)/(control value)] × (control value), and the coefficient of
drug interaction (CDI) is calculated as (observed value)/(expected
value). CDI of <1 indicates a synergistic effect and CDI of
>1 indicates a less than additive or an antagonistic effect.
Flow cytometry analysis
Flow cytometry analysis was performed as described
by Luo et al (25). Briefly,
apoptosis was assessed by the binding of annexin V-FITC to
phosphotidylserine, which was externalized to the outer leaflet of
the plasma membrane. Cells treated with OA (30 μg/ml) and 5-FU (10
μg/ml) were resuspended in the binding buffer (Nanjing KeyGen
Biotech Co., Ltd., China) and reacted with 5 μl of annexin V-FITC
reagent and 5 μl of PI for 30 min at room temperature in the dark.
Stained cells were analyzed by flow cytometry.
DNA fragmentation analysis
DNA fragmentation was analyzed by agarose gel
electrophoresis (26). Cells
untreated or treated with 5-FU, OA or their combination were
collected by centrifugation and washed with PBS. Total DNA was
purified with a universal genomic DNA extraction kit (Takara
Biotechnology Co., Ltd, China) according to the manufacturer’s
instructions. The DNA was resolved in 2% agarose gel and visualized
by ultraviolet illumination (the Bio-Rad Chemi Doc XRS imaging
system, USA) after staining with ethidium bromide.
Measurement of mitochondrial membrane
potential (MMP)
The uptake of the cationic fluorescent dye rhodamine
123 was used for the estimation of mitochondrial membrane potential
(27). Cells untreated or treated
with reagents as described above were harvested and washed twice
with PBS. The cell pellets were then resuspended in 2 ml fresh
medium containing rhodamine 123 (2.0 μM) and incubated at 37°C for
10 min with gentle shaking. Cells were collected by centrifugation
and washed twice with PBS, then analyzed by fluorescence microscopy
and fluorescence spectrophotometer (excitation 507 nm, emission 529
nm).
Measurement of lysosomal membrane
permeabilization (LMP)
LMP was measured by the lysosomotropic base and
metachromatic fluorochrome acridine orange as described previously
(28). Briefly, cells untreated or
treated with reagents as described above were stained with acridine
orange (0.005 μg/ml, Sigma) for 6 min at 37°C without
CO2. Immediately after staining, cells were analyzed by
fluorescence microscopy.
Western blot analysis
Western blot analysis was performed as previously
described (29) to determine the
effect of OA and 5-FU on anti-/pro-apoptotic proteins, cells
untreated or treated with OA, 5-FU or their combination were
harvested by centrifugation at 3000 g for 10 min, washed twice with
ice-cold PBS, and lysed with RIPA buffer containing fresh protease
inhibitor mixture (50 μg/ml aprotinin, 0.5 mM phenylmethanesulfonyl
fluoride (PMSF), 1 mM sodium orthovanadate, 10 mM sodium fluoride
and 10 mM β-glycerolphosphate). Proteins were quantified using the
BCA protein assay (Biocolor BioScience & Technology, China).
Protein samples were resolved on 10–15% SDS-polyacrylamide gels
transferred to nitrocellulose membranes and probed with protein
specific antibodies followed by treatment with HRP-conjugated
secondary antibody. The relative quantity of protein were analyzed
by Gel-Pro Analyzer software.
Statistical analysis
All of the experiments were performed at least three
independent experiments, and the data are presented as the mean ±
SD. The statistical significance of the mean difference between the
control and treated groups was determined by a paired t-test.
P<0.05 was considered statistically significant.
Results
OA potentiates the 5-FU effect on
proliferation inhibition in Panc-28 cells
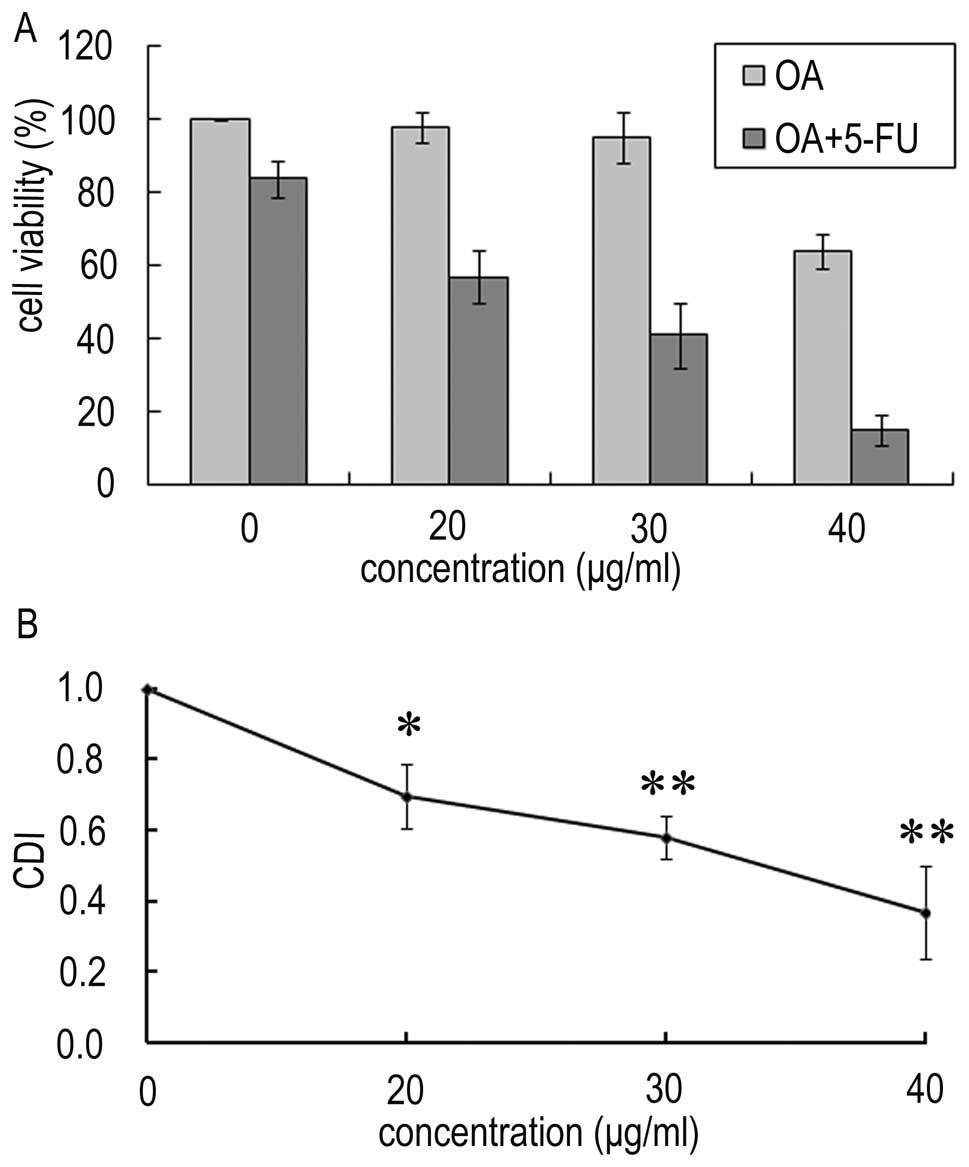
In order to determine the effect of OA and 5-FU on
Panc-28 cells, the respective cell viability of OA and 5-FU were
measured by MTT assay. As shown in Fig.
1A, the proliferation was decreased to 98, 95 and 64% in
Panc-28 cells treated with 20, 30 and 40 μg/ml OA, respectively,
while the viability of Panc-28 cells treated with 10 μg/ml 5-FU was
~84%. However, in the present of OA (20, 30 and 40 μg/ml), the cell
viability was decreased to 57, 41, and 15%, respectively. The CDIs
were 0.69, 0.58 and 0.37 respectively (Fig. 1B). This result indicates that the
combined treatment increases the inhibition effect on Panc-28
cells, and OA synergistically potentiates the effect of 5-FU on the
growth inhibition of human pancreatic Panc-28 cells.
Combined treatment with OA and 5-FU
potentiates apoptosis induction in Panc-28 cells
As have been confirmed earlier, OA or 5-FU induced
cell death in apoptotic pathway (19,30,31).
We next determine if the combination of the two compounds increases
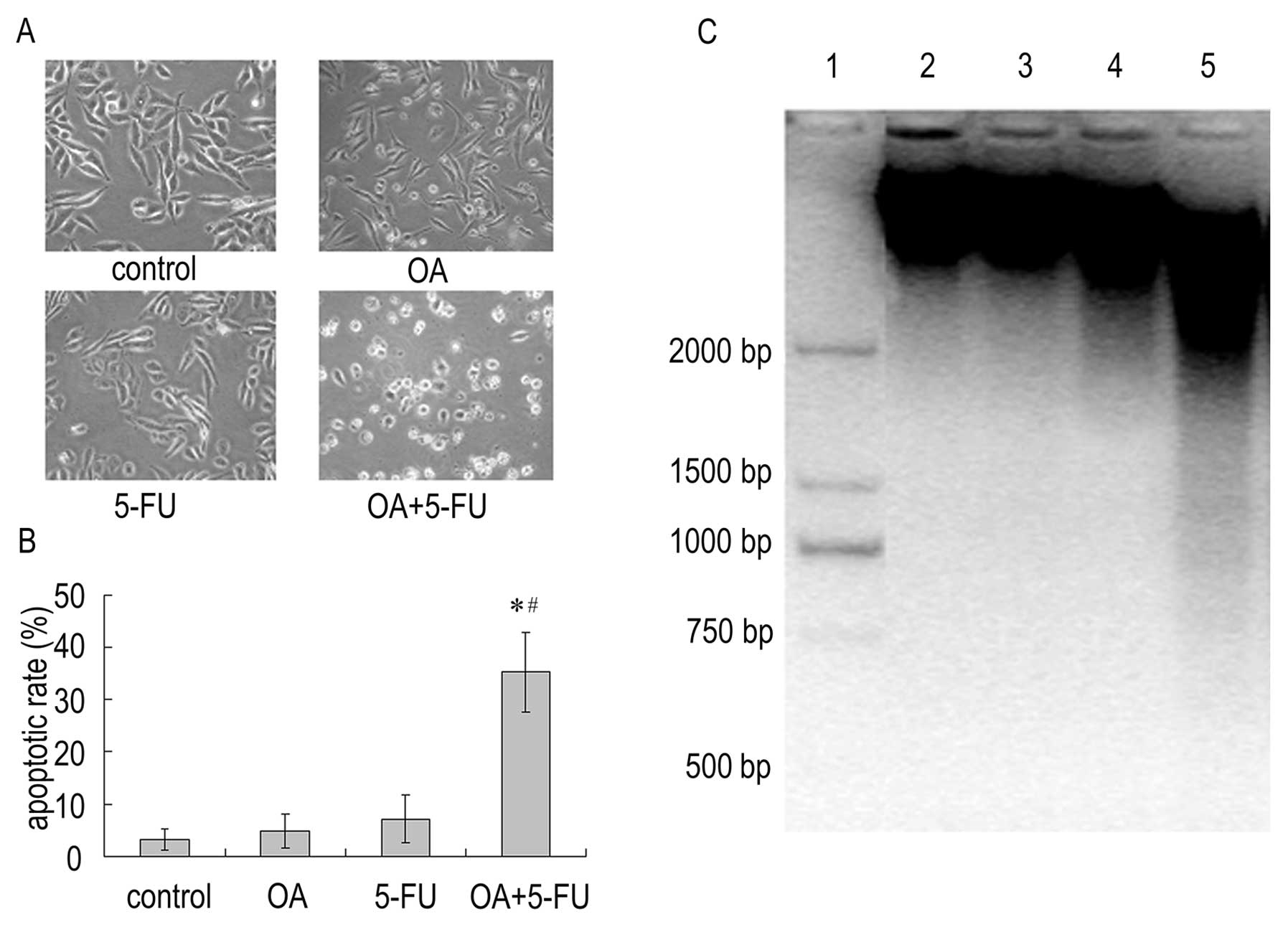
the apoptotic effect. As shown in Fig.
2, the average percentage of apoptotic cells increased
significantly when treating the cells with either OA or 5-FU; the
apoptotic rate was 4.78 and 7.14% in cells treated with OA (30
μg/ml) or 5-FU (10 μg/ml) respectively. However, combinations of
the two reagents produced significant increase in apoptotic cells;
the apoptotic rate induced by combination treatment increased to
35.33% (Fig. 2A and B). In
addition, fragments of degraded DNA were visible in Panc-28 cells
after treatment with the combination of OA (30 μg/ml) and 5-FU (10
μg/ml), while OA or 5-FU alone did not induce DNA fragmentation
(Fig. 2C). The result reveals that
the combined treatment with OA and 5-FU could enhance cell death
via apoptotic pathway in Panc-28 cells.
Effect of combined treatment with OA and
5-FU on MMP in Panc-28 cells
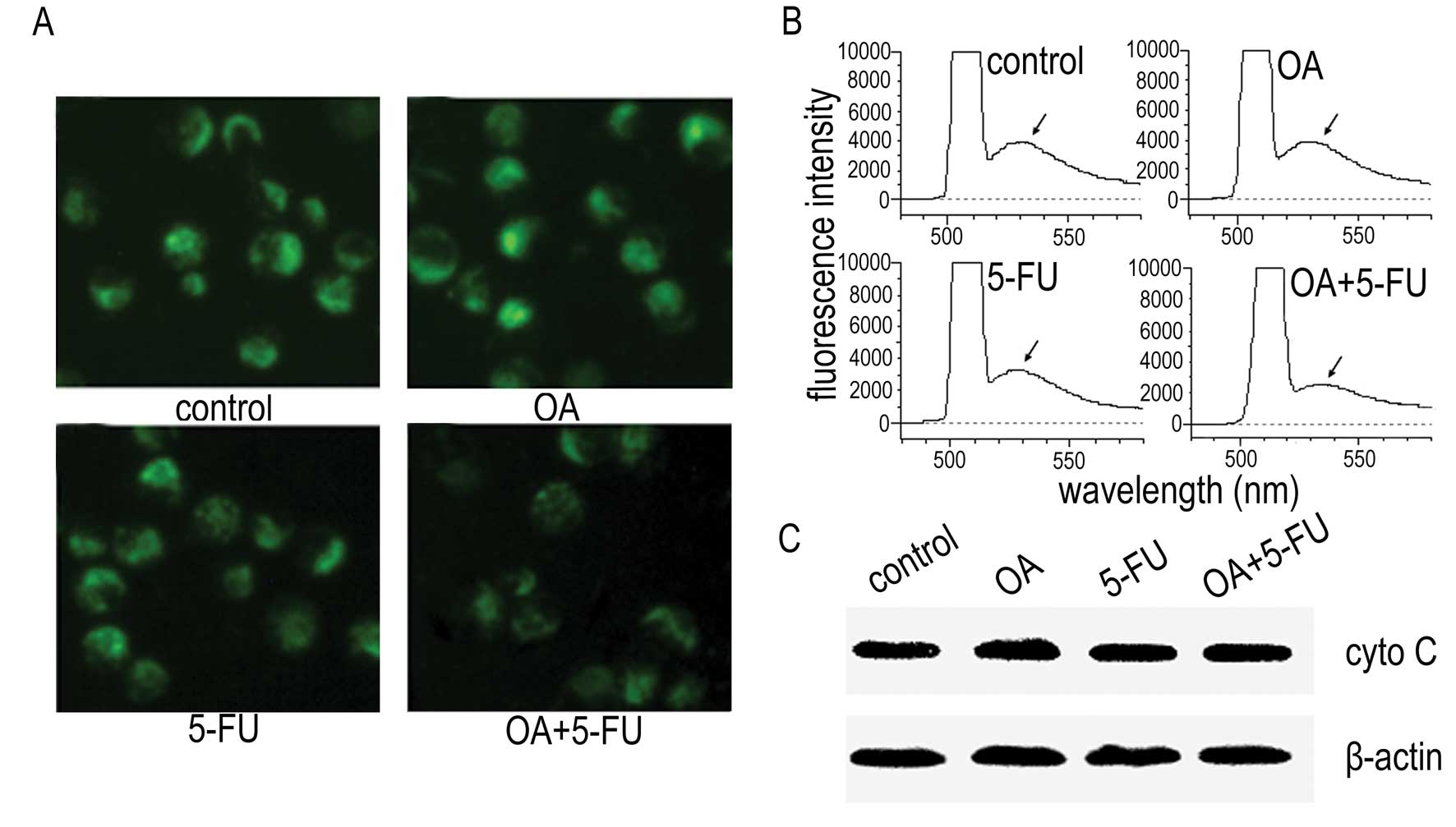
To determine the underlining mechanism of apoptosis
induced by the combination of OA and 5-FU, we measured the MMP
change and the level of the cytochrome C in cytosol. As shown in
Fig. 3A and B, treatment of the
cells with OA at 30 μg/ml or 5-FU at 10 μg/ml did not affect the
fluorescence intensity significantly, and the fluorescence
intensity of the cells treated with the combination of OA and 5-FU
resulted in slight decrease of the fluorescence intensity as
determined by fluorescence microscopy (Fig. 3A) and fluorescence spectrophotometer
(Fig. 3B). We then checked the
expression of cytochrome C and the results showed that the release
of cytochrome C from mitochondria to cytosol was not changed in
Panc-28 cells either treated with OA, 5-FU or treated with the
combination of OA and 5-FU (Fig.
3C). The results suggested that the enhancement of apoptosis
induced by the combination of OA and 5-FU might not be mediated by
the mitochondrial pathway.
Effect of combined treatment with OA and
5-FU on lysosomal membrane permeabilization (LMP) in Panc-28
cells
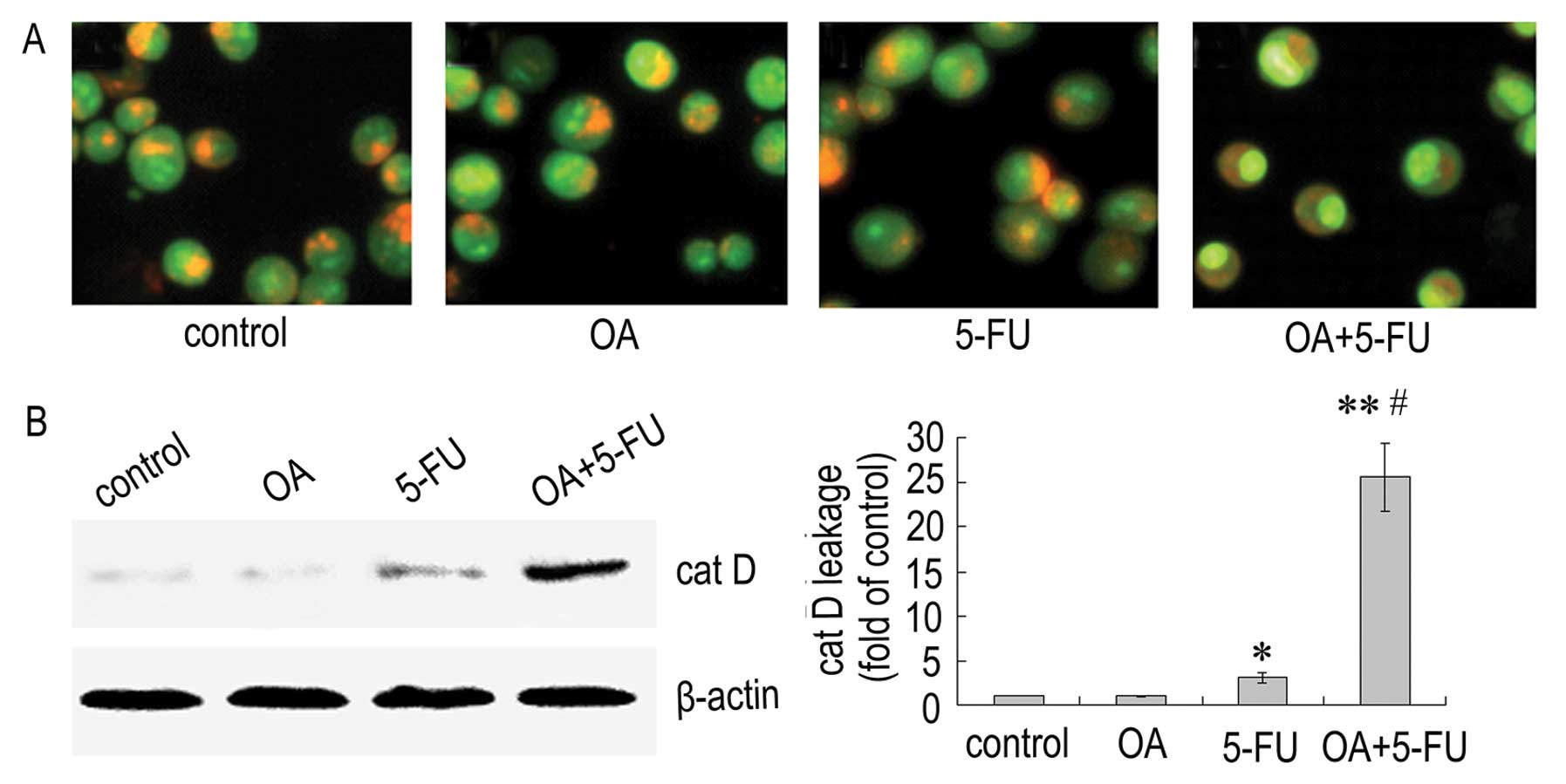
To determine if LMP is affected by the combination
of OA and 5-FU, we analyzed the alterations in Panc-28 cells using
acridine orange staining. The results showed that the fluorescence
intensity of the cells treated with OA or 5-FU alone did not have a
significant change, while the cells treated with the combination of
OA and 5-FU displayed less intense red fluorescence and more
intense green fluorescence (Fig.
4A). We next detected the level of cathepin D in Panc-28 cells,
and the result showed that the level of cathepsin D in cytosol was
elevated significantly in cells treated with the combination of OA
and 5-FU compared to OA or 5-FU alone (Fig. 4B). The results indicated that the
combined use of OA and 5-FU could enhance the LMP following the
increase of cathepin D leakage and that LMP pathway may play an
important role in the apoptosis induced by the combination of OA
and 5-FU.
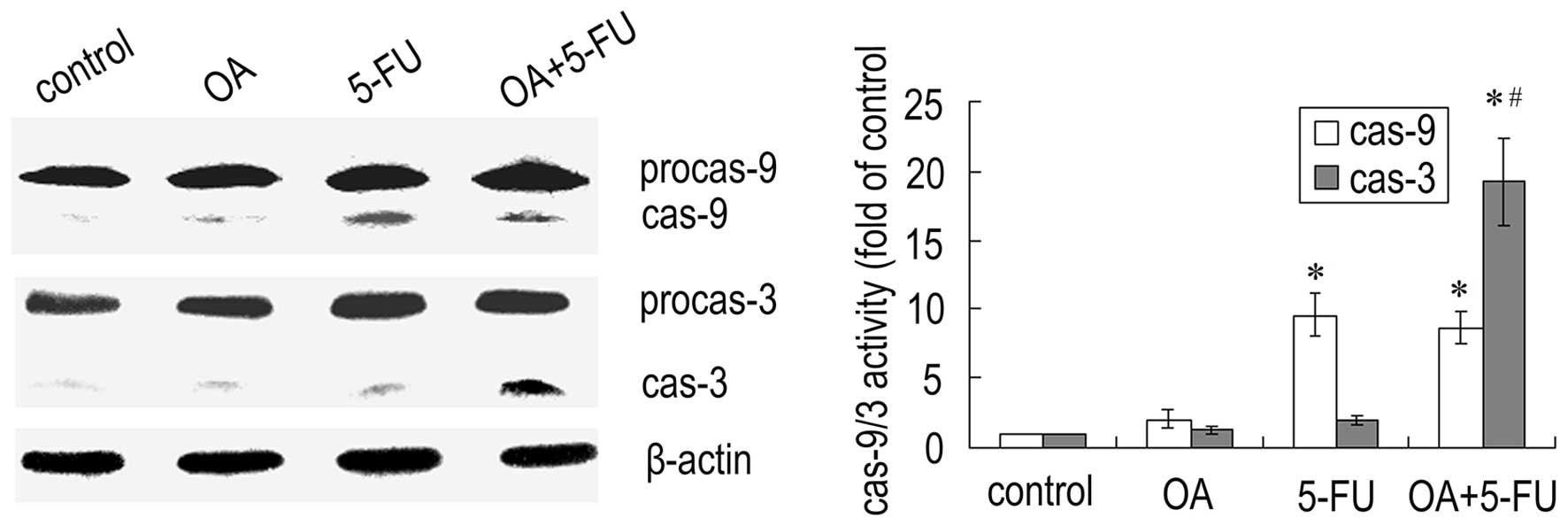
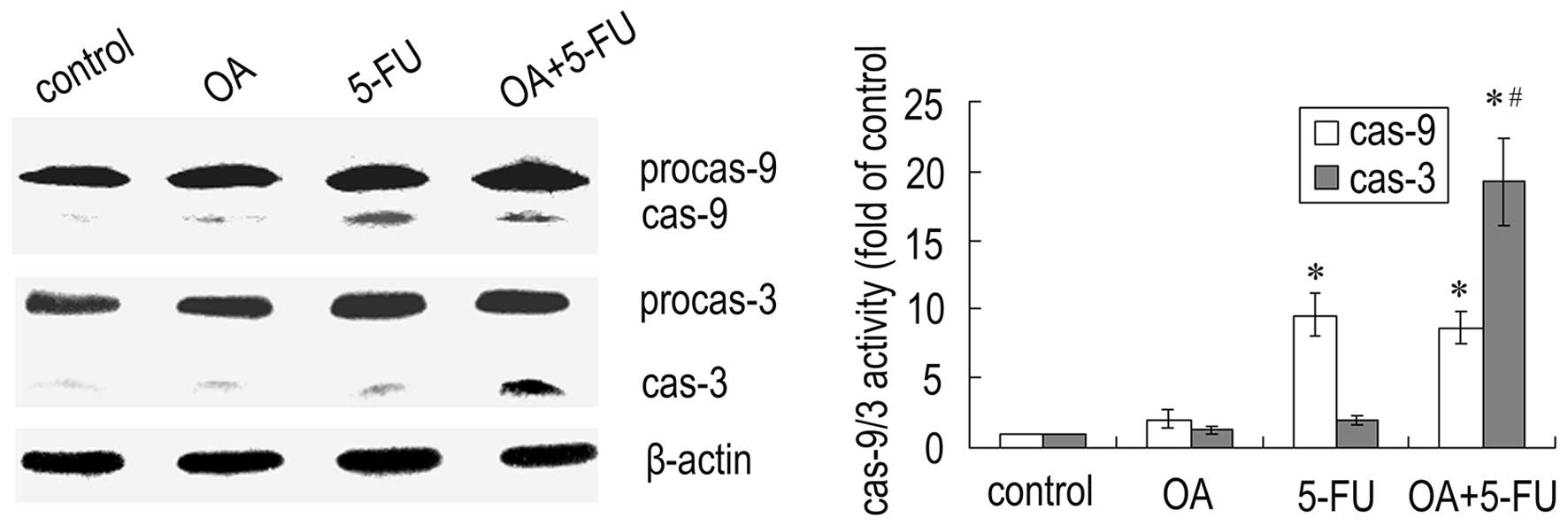
Combined treatment with OA and 5-FU
affects the activation of caspases in Panc-28 cells
Since caspases are the important mediators of
apoptosis, we next checked the expression of caspases. As shown in
Fig. 5, treatment the cells with
the combination of OA and 5-FU resulted in a significant increase
in caspase-3 activity compared to that treated with OA or 5-FU
alone, while the activity of caspase-9 did not have a significant
change (Fig. 5). The results
further confirmed that the enhancement of apoptosis induced by the
combination of OA and 5-FU was not dependent on the caspase-9
mediated mitochondrial pathway.
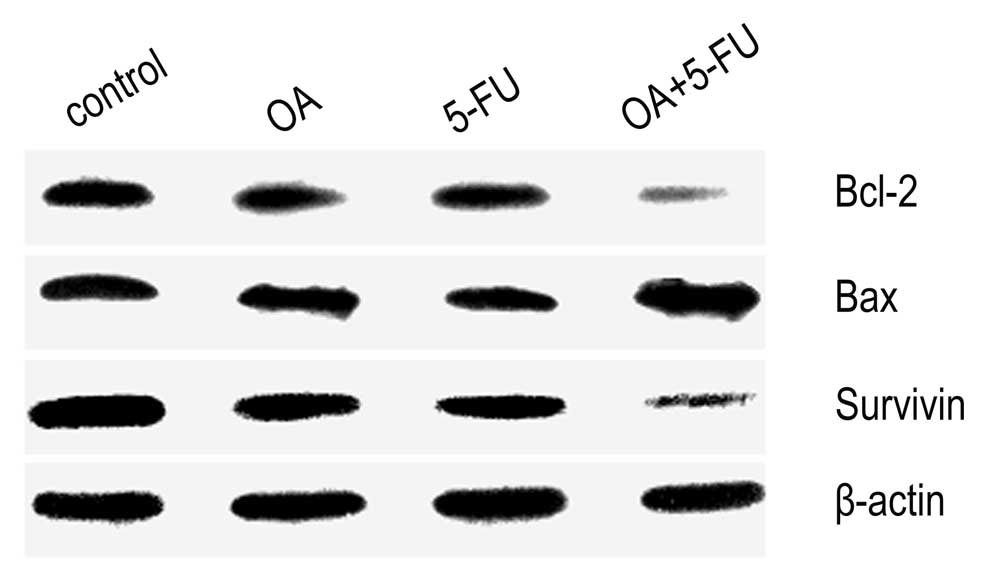
Combined treatment with OA and 5-FU
affects the expression of apoptosis-related proteins in Panc-28
cells
The expression of Bax, and Bcl-2 was determined by
western blot analysis. As shown in Fig.
6, the expression of Bax was increased, while the expression of
Bcl-2 was decreased significantly in cells treated by the
combination of OA and 5-FU compared to that treated with OA or 5-FU
alone (Fig. 6). Additionally, the
combined treatment with OA and 5-FU inhibited the level of survivin
in cytosol significantly (Fig. 6).
Thus, the data confirmed that combination of OA and 5-FU could
regulate the level of some apoptosis-related proteins.
The expression of p53 and NF-κB was also determined
by western blot analysis. Treatment of human pancreatic Panc-28
cells with OA alone did not affect the p53 expression (Fig. 7) and the p53 expression was
increased in cells treated with 5-FU either in the presence or
absence of OA (Fig. 7), suggesting
that OA did not affect 5-FU induced p53 expression. However, in the
presence of OA, the expression of NF-κB induced by 5-FU was
significantly increased (Fig. 7).
These results suggest that NF-κB plays an important role in the
apoptosis induced by the combination of OA and 5-FU in Panc-28
cells.
Discussion
In our previous study we confirmed that OA could
induce pancreatic cancer cell apoptosis via ROS-mediated
mitochondrial pathway and lysosomal pathway (19). In this study, we provide evidence
that OA enhanced the inhibition of proliferation and increased
apoptosis induced by 5-FU in Panc-28 cells, and the CDI was
associated with the concentration of OA. Therefore, the combination
of OA and 5-FU should be considered as a potential regimen for the
treatment of pancreatic cancer.
It is well documented that 5-FU could inhibit DNA
proliferation in cancer cells by inhibiting the activity of
thymidylate synthase, leading to apoptosis (32). OA induced cancer cell apoptosis via
the mitochondria pathway following mitochondrial membrane potential
loss and cytochrome C release (33,34).
In our previous study we confirmed that OA could induce pancreatic
cancer cells apoptosis via the ROS-mediated mitochondrial and
lysosomal pathways (19). The
present data showed that LMP and leakage of cathepsin D into
cytosol was elevated in pancreatic cancer cells treated with the
combination of OA and 5-FU. LMP caused lysosome damage and leakage
of lysosomal constituents such as cathepsins. The leakage of
cathepsin D could be sufficient to trigger apoptosis, since this
enzyme can directly activate procaspase-3 (35). Cathepsin D could also cleave Bid
following the start-up of mitochondrial pathway (36). However, our study revealed that
mitochondrial pathway was not involved in the combination of OA and
5-FU induced cell apoptosis, since cytochrome C was not released
and the activation of caspase-9 was not enhanced in the cells
treated with the combination regimen. Therefore, the lysosomal
induced pathway may play a key role in the apoptotic pathway. As
have been reported previously, some anticancer agents induce cell
death via lysosomal apoptotic pathway (37); anthrax lethal toxin induces
lysosomal membrane permeabilization and cathepsin release in RAW
264.7 cells (28).
NF-κB, as a nuclear transcription factor, plays
vital roles in immunity, inflammation, oxidative stress, cell
proliferation and apoptosis (38).
In this study, the results showed that treatment of the pancreatic
cells with the combination of OA and 5-FU results in a significant
increase of NF-κB expression, suggesting that NF-κB mediated cell
apoptosis plays a key role in the biological process induced by the
combination of OA and 5-FU in Panc-28 cells. It is well documented
that activation of NF-κB can affect the pro-apoptotic targets
including the death receptors Fas (CD95), TRAIL receptors DR4, DR5
and DR6, the death-inducing ligands FasL, TNFα and TRAIL, tumor
suppressor p53 and the Bcl-2 family member Bax and the proapoptotic
alternatively spliced form of Bcl-xL, Bcl-xS (39). In this study, the expression of Bax
increased and the Bcl-2 expression was decreased, when cells were
treated with the combination of OA and 5-FU. It is conceivable that
the increased expression of caspase-3 induced by the treatment of
combination of OA and 5-FU may be related with death receptor
apoptotic pathway. Additionally, NF-κB can also enhance cell death
via upregulation of the tumor suppressor CYLD, leading to
disassembly of the IKK complex (39). Further study is in progress in our
laboratory to address the more complicated mechanisms.
In conclusion, the present study provides solid
evidence that the combination of OA and 5-FU was able to increase
the growth inhibition of Panc-28 cells and the induction of
apoptosis via lysosomal-mediated leakage of cathepsin D. Some
apoptotic proteins such as caspase-3, NF-κB, and Bcl-2 play
important roles in the apoptotic pathway induced by the combination
regimen. In vivo studies using xenograft tumor mice are
needed to reveal whether the combination treatment would enhance
the growth inhibition of pancreatic tumors.
Acknowledgements
This study was supported by the State Innovative
Drugs Development Program of China (2009ZX09103-661 and
2009ZX09102).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
2
|
Chua YJ and Zalcberg JR: Pancreatic
cancer-is the wall crumbling? Ann Oncol. 119:1224–1230. 2008.
View Article : Google Scholar
|
|
3
|
Pierantoni C, Pagliacci A, Scartozzi M,
Berardi R, Bianconi M and Cascinu S: Pancreatic cancer: progress in
cancer therapy. Crit Rev Oncol Hematol. 67:27–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang PM, Bunz F, Yu J, Rago C, et al:
Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced
apoptosis in colorectal cancer cells. Nat Med. 7:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malet-Martino M and Martino R: Clinical
studies of three oral prodrugs of 5-fluorouracil (Capecitabine,
UFT, S-1): a review. Oncologist. 7:288–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
7
|
Borja-Cacho D, Yokoyama Y, Chugh RK, et
al: TRAIL and triptolide: an effective combination that induces
apoptosis in pancreatic cancer cells. J Gastrointest Surg.
14:252–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dizaji MZ, Malehmir M, Ghavamzadeh A,
Alimoghaddam K and Ghaffari SH: Synergistic effects of arsenic
trioxide and silibinin on apoptosis and invasion in human
glioblastoma U87MG cell line. Neurochem Res. 37:370–380. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Menéndez JA, Barbacid MM, Montero S, et
al: Effects of gamma-linolenic acid and oleic acid on paclitaxel
cytotoxicity in human breast cancer cells. Eur J Cancer.
37:402–413. 2001.PubMed/NCBI
|
|
10
|
Nakagawa T, Shimizu M, Shirakami Y, et al:
Synergistic effects of acyclic retinoid and gemcitabine on growth
inhibition in pancreatic cancer cells. Cancer Lett. 273:250–256.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yagi1 H, Yotsumoto F, Sonoda1 K, Kuroki M,
Mekada E and Miyamoto S: Synergistic anti-tumor effect of
paclitaxel with CRM197, an inhibitor of HB-EGF, in ovarian cancer.
Int J Cancer. 124:1429–1439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sei S, Mussio JK, Yang Q, et al:
Synergistic antitumor activity of oncolytic reovirus and
chemotherapeutic agents in non-small cell lung cancer cells. Mol
Cancer. View Article : Google Scholar : 2009.PubMed/NCBI
|
|
13
|
Liu J: Oleanolic acid and ursolic acid:
research perspectives. J Ethnopharmacol. 100:92–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Ye X, Liu R, et al: Antioxidant
activities of oleanolic acid in vitro: possible role of Nrf2 and
MAP kinases. Chem Biol Interact. 184:328–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubén M, Juliana CT, Marita, Mercedes A,
Valentina RG and María LN: Beneficial actions of oleanolic acid in
an experimental model of multiple sclerosis: a potential
therapeutic role. Biochem Pharmacol. 79:198–208. 2010.PubMed/NCBI
|
|
16
|
Teodoro T, Zhang L, Alexander T, Yue J,
Vranic M and Volchuk A: Oleanolic acid enhances insulin secretion
in pancreatic β-cells. FEBS Lett. 582:1375–1380. 2008.PubMed/NCBI
|
|
17
|
Resende FA, Barcala CA, Faria MC, Kato FH,
Cunha WR and Tavares DC: Antimutagenicity of ursolic acid and
oleanolic acid against doxorubicin-induced clastogenesis in Balb/c
mice. Life Sci. 79:1268–1273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei J, Liu M, Liu H, et al: Oleanolic acid
arrests cell cycle and induces apoptosis via ROS-mediated
mitochondrial depolarization and lysosomal membrane
permeabilization in human pancreatic cancer cells. J Appl Toxicol.
(In press).
|
|
20
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mai Z, Blackburn GL and Zhou J: Soy
phytochemicals synergistically enhance the preventive effect of
tamoxifen on the growth of estrogen-dependent human breast
carcinoma in mice. Carcinogenesis. 28:1217–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao CY, Hua HJ, Chen P, Yu DC, Cao J and
Teng LS: Combined use of chemotherapeutics and oncolytic adenovirus
in treatment of AFP expressing hepatocellular carcinoma.
Hepatobiliary Pancreat Dis Int. 8:282–287. 2009.PubMed/NCBI
|
|
23
|
Chou TC and Talalay P: Analysis of
combined drug effects: a new look at a very old problem. Trends
Pharmacol Sci. 4:450–454. 1983. View Article : Google Scholar
|
|
24
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo M, Liu X, Zua Y, Fu Y, Zhang S, Yao L
and Efferth T: Cajanol, a novel anticancer agent from Pigeonpea
[Cajanus cajan (L. ) Millsp] roots, induces apoptosis in human
breast cancer cells through a ROS-mediated mitochondrial pathway.
Chem Biol Interact. 188:151–160. 2010.PubMed/NCBI
|
|
26
|
Ning X, Zhao J, Zhang Y, Cao S, Liu M,
Ling P and Lin X: A novel anti-tumor protein extracted from
Meretrix meretrix Linnaeus induces cell death by increasing cell
permeability and inhibiting tubulin polymerization. Int J Oncol.
35:805–812. 2009.PubMed/NCBI
|
|
27
|
Cao J, Liu Y, Jia L, et al: Curcumin
induces apoptosis through mitochondrial hyperpolarization and mtDNA
damage in human hepatoma G2 cells. Free Radic Biol Med. 43:968–975.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Averette KM, Pratt MR, Yang Y, et al:
Anthrax lethal toxin induced lysosomal membrane permeabilization
and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PLoS
One. 4:e79132009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Yang S, Liu M, et al: Interaction
between thymidylate synthase and its cognate mRNA in zebrafish
embryos. PLoS One. 5:e106182010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong D, Poot M, Hu D and Oda D:
5-fluorouracil-induced apoptosis in cultured oral cancer cells.
Oral Oncol. 36:236–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warr JR, Bamford A and Quinn DM: The
preferential induction of apoptosis in multidrug-resistant KB cells
by 5-fluorouracil. Cancer Lett. 175:39–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugamura K, Makino M, Shirai H, Kimura O,
Maeta M, Itoh H and Kaibara N: Enhanced induction of apoptosis of
human gastric carcinoma cells after preoperative treatment with
5-fluorouracil. Cancer. 79:12–17. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng L, Au-Yeung W, Xu YH, Wang SS, Zhu Q
and Xiang P: Oleanolic acid from Prunella Vulgaris L. induces
SPC-A-1 cell line apoptosis via regulation of Bax, Bad and Bcl-2
expression. Asian Pac J Cancer Prev. 12:403–408. 2011.PubMed/NCBI
|
|
34
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hishita T, Tada-Oikawa S, Tohyama K, et
al: Caspase-3 activation by lysosomal enzymes in cytochrome
c-independent apoptosis in myelodysplastic syndrome-derived cell
line P39. Cancer Res. 61:2878–2884. 2001.PubMed/NCBI
|
|
36
|
Reiners JJ, Caruso JA, Mathieu P,
Chelladurai B, Yin XM and Kessel D: Release of cytochrome c and
activation of pro-caspase-9 following lysosomal photodamage
involves Bid cleavage. Cell Death Differ. 9:934–944. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Servais H, van Der Smissen P, Thirion G,
van der Essen G, van Bambeke F, Tulkens PM and Mingeot-Leclercq MP:
Gentamicin-induced apoptosis in LLC-PK1 cells: involvement of
lysosomes and mitochondria. Toxicol Appl Pharm. 206:321–333. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bian X, McAllister-Lucas LM, Shao F, et
al: NF-kappa B activation mediates doxorubicin-induced cell death
in N-type neuroblastoma cells. J Biol Chem. 276:48921–48929. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dutta J, Fan Y, Gupta N, Fan G and Gélinas
C: Current insights into the regulation of programmed cell death by
NF-κB. Oncogene. 25:6800–6816. 2006.
|