Introduction
Head and neck cancers are a group of relatively
common malignant diseases worldwide, which occupies ~5% human
malignant carcinomas. In China, the morbidities of lip, oropharynx,
hypopharynx and larynx carcinoma were evaluated as 0.04–0.14, 1–2,
0.15–0.8 and 3–5/100000, respectively. Roughly 85–90% of oral and
oropharyngeal cancers are histologically squamous cell carcinomas
(SCC). SCC most commonly affects men from 50 to 70 years of age and
is associated with alcohol and tobacco consumption. The
distributions of head and neck cancers in China show clearly
geography difference, that oral and larynx cancers are commonly
identified in the northern part of China and nasopharyngeal cancer,
thyroid cancer, malignant lymphoma and skin cancer are predominant
in the southern part of China (1).
Human papillomavirus (HPV) is a small,
epitheliotropic, double-stranded DNA virus, whose whole genome is
~8 kb long. Up to now, ~150 HPV genetic subtypes have been
described. HPVs possess great importance in human carcinogenesis
since the 1970s when it was discovered to be the causative agent of
cervical cancer. Based on the propensity to immortalize human
keratinocyte cell lines and override cell cycle control mechanisms,
HPVs are classified into low, mediate and high-risk categories.
Besides the direct evidence that infections of high-risk HPVs are
etiologically associated with the carcinogenesis of cervical
cancer, more and more data have supported that HPV infection is
also closely related with other human SCCs, especially with head
and neck SCCs, among them HPV16 is associated with more than 90% of
HPV-related head and neck cancers (2). Other types less frequently detected in
oropharyngeal cancers include HPV6, 18, 31, 33, 35, 45, 52 and 58
(3). Recently, an Australian group
has reported an increasing incidence of potentially HPV-associated
oropharyngeal cancers in Australia between 1982 and 2005 (4).
During the past few decades, HPV DNA has been
detected in ~25% of head and neck SCCs overall. More importantly,
45–100% oral SCC cases were reported to be HPV-positive. The
variations of HPV distributions in SCCs may relate with different
factors, such as the location of cancers, the type of specimens
available, the techniques used for testing and the time period and
country or region the SCC specimens obtained (5). The prevalence of HPVs in Chinese
patients with head and neck SCCs has been also described. Using
various techniques, including PCR, hybridization in situ and
immunohistochemistry (IHC), HPV-positive rates vary from 33.3 to
72.6% among various SCCs, such lip, oral cavity, orophyrynx,
hypophrynx and larynx cancers (6).
In addition to the large variations of HPV-positive rates from
independent groups, repeatedly identified HPV DNA sequences and
their encoding proteins in the tissues of Chinese head and neck
SCCs propose an obvious tendency that HPV infection plays also
essential role in the pathogenesis of head and neck SCCs in
China.
To get more information on the HPV prevalence in
Chinese head and neck SCCs, 93 head and neck SCCs were screened
with HPV specific Luminex technique, PCR and IHC assays, 44.1%
(41/93) tested samples showed HPV-positive. Further analyses
revealed that the HPV-positive rates had no correlation with the
pathological and clinical grades of cancers, but showed significant
relation with the anatomic sites of tumors. Additionally, the
distribution of HPVs in various age groups of the patients with
head and neck SCCs was similar.
Patients and methods
Patients and specimens
The specimens from the 93 patients with malignant
squamous tumors, including 64 laryngocarcinoma, 5 oropharyngeal
carcinoma, 15 hypopharynx carcinoma and 9 lip carcinoma were
included in this study. All cases were hospitalized in the Head and
Neck Surgery Department, Peking University Cancer Hospital and
Institute, from 2006 to 2011. Among them, 84 were male and 9 were
female. The ages of the patients ranged from 27 to 84 years, with
the median of 57 years, 87.1% (81/93) and 57.0% (53/93) of the
patients had histories of tobacco or alcohol use. The professions
of the patients varied largely, and the permanent residences of the
patients were distributed widely in mainland of China. All samples
were surgically removed and conventionally fixed in 10% formalin
and paraffin embedded. Pathological assays verified that all
cancers were squamous cancers. The pathological grades of the
cancers were assessed by clinical pathologists in the Peking
University Cancer Hospital and Institute. The clinical grades of
patients with tumors were finally determined by surgeons in the
Peking University Cancer Hospital and Institute.
DNA extraction
Total DNAs from the tumor tissues were extracted
from the paraffin-embedded tissue blocks with a commercial genomic
DNA extraction FFPE kit (Qiagen). Briefly, 3–4 formalin-fixed,
paraffin-embedded 10 μm sections were soaked in xylene vortex
vigorously for at least 1 h. Then pellet was acquired and purified
under the kit protocol. Quality of the extracted DNAs was assessed
with a settled PCR protocol with a pair of actin-specific primers,
which produced a 240-bp long fragment.
Luminex technique for HPVs DNA
The presences of HPVs specific DNA sequences in the
extracted DNAs from the tested tumors were screened with a
Tellgenplex™ HPV DNA Test kit (Tellgen, China) using Luminex
technique, which allowed to detect 26 genotypes of HPVs including
19 high-risk HPV types (HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52,
53, 55, 56, 58, 59, 66, 68, 82 and 83) and 7 low-risk HPV types
(HPV6, 11, 40, 42, 44, 61 and 73). The experiments were performed
in Bio-Plex 200 system according to the manufacturer’s
instructions. Briefly, 2 μg of the extracted DNA was proceeded the
PCR protocol as following: 95°C 30 sec, 58°C 30 sec and 72°C 30 sec
for the first 5 circles, and 95°C 30 sec, 55°C 30 sec and 72°C 30
sec for another 35 circles. The PCR products were subjected into a
fast hybridization and the data were analyzed with the software
supplied by the manufacturer.
PCR protocols for HPV16 and HPV18
Two-rounded PCR techniques for HPV16 and HPV18 were
individually established. The primers were P16-1 (5′-GCAAGCAACAG
TTACTGCGA-3′) and P16-2 (5′-CAACAAGACATACATCG ACC-3′) for HPV16 E6
sequence and P18-1 (5′-CACTTCACT GCAAGACATAGA-3′) and P18-2
(5′-GTTGTGAAATCGT CGTTTTTCA-3′) for HPV18 E6 sequence. Extracted
DNAs (1.0 μg) were mixed with 1 μM of individual primers and 1.25 U
Pfu DNA polymerase in a 25 μl volume. PCR was performed with the
following conditions: 94°C 1 min, 57°C 1 min and 72°C 1 min, in
total 30 cycles, and another 10-min extension. For second round
PCR, 5 μl of the first round PCR product was mixed again with 10 μM
of individual primers and 1.25 U Pfu DNA polymerase in a 25 μl
volume. The PCR condition was 94°C 1 min, 52°C 1 min and 72°C 1
min, totally 40 cycles and another 10 min extension. The targeting
fragments of HPV16 E6 and HPV18 E6 were 333 and 322 bp,
respectively. All PCR assays were carefully carried out in the PCR
laboratory with four separating rooms to avoid of DNA
contamination. Actin-specific 240-bp long fragment was produced as
control.
Direct sequencing
The PCR products were analyzed in 2% agarose gel and
recovered from gel with QIAquick Gel Extraction kit (Qiagen).
Direct sequencing was performed using the same PCR primers and the
base sequences were read on ABI PRISMTM 3730XL DNA analyzer.
Immunohistochemistry (IHC)
Paraffin sections (5 μm) were deparaffinized in
xylene for 5 min twice and gradually rehydrated routinely. Sections
were quenched for endogenous peroxidases in 3%
H2O2 in methanol for 15 min, pretreated with
enzyme digestion antigen retrieval for 1 min. After blocking in 1%
normal goat serum, the sections were incubated overnight at 4°C
with 1:500-diluted mAb for HPV16/18 E6 (Abcam). The sections were
then incubated for 60 min with 1:1000-diluted HRP-conjugated goat
anti-mouse secondary antibody (Vector Labs, USA), and visualized by
incubation with 3,3-diaminobenzidine tetrahydrochloride (DAB). The
slices were dehydrated and mounted in Permount. Photomicrographs
were taken with a DP70 digital camera mounted on BX5 microscope
(Olympus Optical, Japan).
Statistical analyses
Statistical analyses were performed using SPSS
(Statistical Package for the Social Sciences, Chicago, IL) 11.5 for
windows. Prevalence of HPV infection in the head and neck squamous
carcinomas of different sites, pathological grades, clinical grades
and age groups were analyzed by the χ2 test. P<0.05
was considered statistically significant.
Ethical statement
Written consent for further investigation and
publication was obtained from the patients or the patients’
relatives, respectively. Usage of the stored human samples in this
study was approved by the Ethics Committees of Peking University
Cancer Hospital and Institute and National Institute for Viral
Disease Prevention and Control, China CDC.
Results
Identification of HPV-related sequences
in the cancer tissues
The presence of HPV sequences in the fixed tumor
tissues was firstly screened by a commercial Luminex technique for
HPV genotyping. Out of 93 tumor samples, 7 cases showed positive
HPV signals, in which 4 were HPV18-positive, 2 were HPV16-positive
and 1 was HPV52-positive. To obtain more detailed evidence, the
presence of HPVs sequences in tumor samples were further assayed
with the established PCR protocols specific for HPV16, 18 and 52.
Among the tested samples, 6 were HPV18-positive, 6 were
HPV16-positive and 1 was HPV52-positive. Sequencing assays of the
positive PCR products were verified to be E6 sequences of the
relative HPVs. Three out of 4 cases showing HPV18-positive in
Luminex and the case showing HPV52-positive in Luminex were
positive in the PCR assays, whereas 2 cases showing HPV16-positive
in Luninex were PCR-negative. Collectively, 7 samples contained
HPV18 specific sequences, 8 contained HPV16 sequences and one
contained HPV52 sequences.
Identification of HPV-related proteins in
the cancer tissues
To assess the HPV-related proteins in tumor tissues,
the tissue sections of various cancers were immunohistochemically
stained with mAb against HPV16/18 E6 oncoprotein. From 93 cancer
cases, 29 samples (31.2%) showed positive signals while none of the
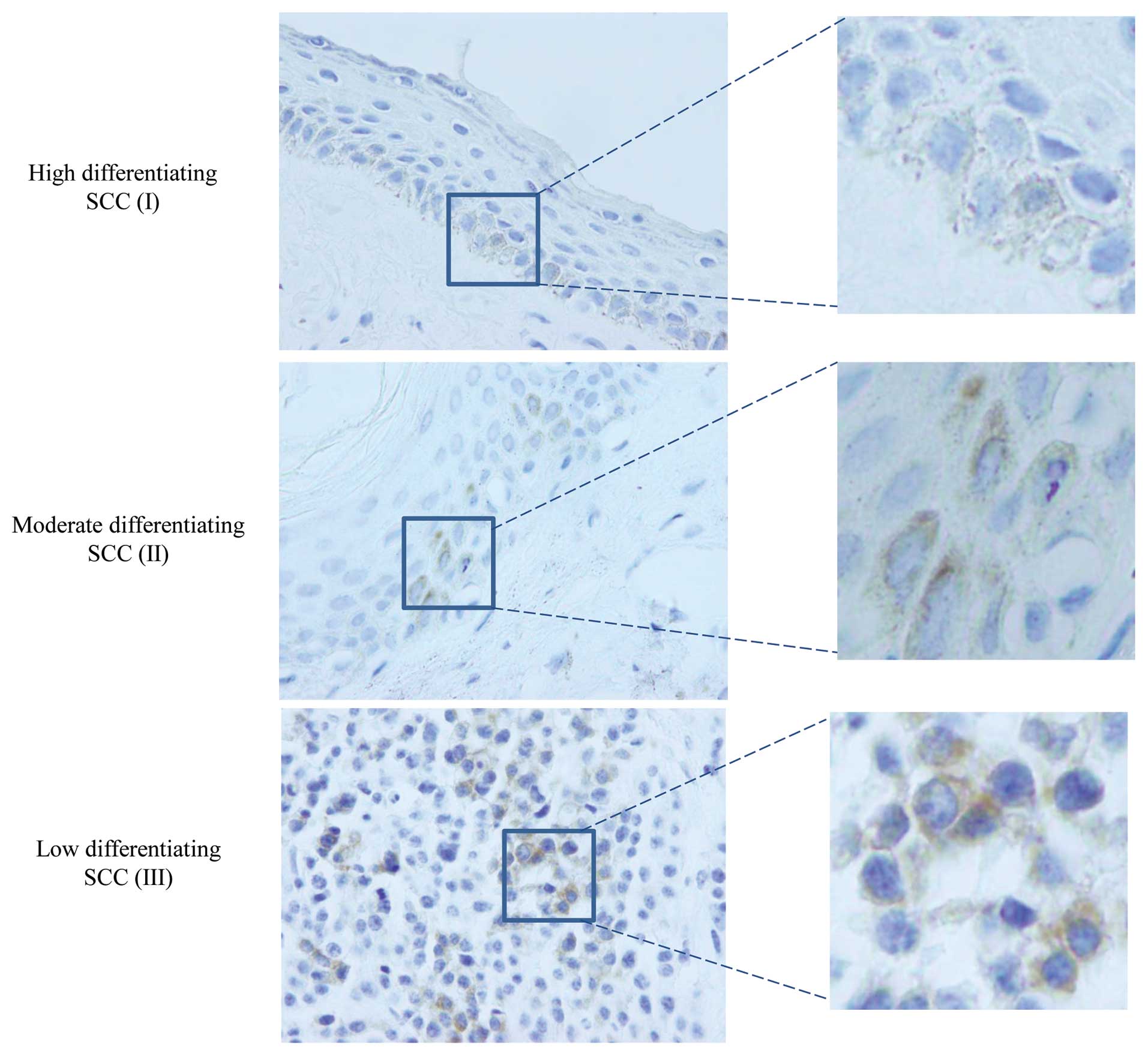
five polyps of larynx revealed positive signal. The brown-stained
signals were present and restricted to cell nuclei and/or cytoplasm
of the cancer cells, without detectable morphological difference
among various pathological grades according to cell differentiation
(Fig. 1). Additionally, among 16
cancer samples containing HPV16 or 18 sequences, 4 were positive in
IHC assay. This highlights a relatively high distribution of HPV
associated proteins in the cancer tissues. However, the presence of
HPV proteins and HPV gene sequences did not correlate with each
other. The total positive rate of HPV genomes and its encoding
products in the tested samples was 44.1% (41/93).
Correlations of HPV infections with tumor
sites
Among the 93 tested cancers, 64 were
laryngocarcinoma, 5 were oropharyngeal carcinoma, 15 were
hypopharynx carcinoma and 9 were lip carcinoma. Table I summarized the results of HPV
genomes and proteins in various cancers. The presences of HPV
sequences and/or proteins in the cancer tissues seemed to differ
largely based on the sites of the malignant tumors, 35.9% (23/64)
laryngocarcinoma, 46.7% (7/15) hypopharynx carcinoma, 60.0% (3/5)
oropharyngeal carcinoma and 88.9% (8/9) lip carcinoma were
HPV-positive either in the assays of viral nucleosides (Luminex and
PCR) or in that of viral proteins (IHC). Statistical analysis
revealed a significant difference (P=0.022) in the anatomic sites
of tumors. It illustrates a clear declining manner of HPV infection
in the head and neck tumors from outside (lip) to inside (larynx).
Moreover, the positive rates of viral sequences and proteins were
different among various tumors. The detection rates of HPV16/18
oncoprotein was significantly higher than that of HPVs DNA in lip
cancers (77.8 vs 11.1%, P=0.008) and higher in larynx cancers (28.1
vs 10.9%, P=0.015) having significant difference, while the
detection rate of HPV sequences was higher than that of viral
protein in hypopharynx cancers (40.0 vs 13.3%, P=0.107) having no
significant difference (Table I).
Only a few cases (4/93) showed positive both in HPV sequences and
proteins.
 | Table IPrevalence of HPV infection in the
head and neck squamous carcinomas of different sites. |
Table I
Prevalence of HPV infection in the
head and neck squamous carcinomas of different sites.
| HPV-positive | |
|---|
|
| |
|---|
| Site | HPV sequence (%) | HPV protein (%) | HPV sequence +
protein (%) | Total (%) |
|---|
| Larynx | 7/64 (10.9) | 18/64 (28.1) | 2/64 (3.1) | 23/64 (35.9) |
| Hypopharynx | 6/15 (40.0) | 2/15 (13.3) | 1/15 (6.7) | 7/15 (46.7) |
| Oropharynx | 2/5 (40) | 2/5 (40) | 1/5 (20) | 3/5 (60.0) |
| Lip | 1/9 (11.1) | 7/9 (77.8) | 0/9 (0.0) | 8/9 (88.9) |
| Total | 16/93 (17.2) | 29/93 (31.2) | 4/93 (4.3) | 41/93 (44.1)a |
Correlations of HPV infections with tumor
pathological and clinical grades
To address the possible correlation of HPV infection
with tumor pathological classification, the prevalence of HPV was
sub-grouped according to the tumor pathological grades. Based on
the tumor cell differentiation pathologically, the enrolled cancers
were divided into the groups of high, moderate and low
differentiation. On the whole, the HPV positive rates were higher
in the groups of high- (46.6%, 13/28) and moderate-differentiation
cancers (46.0%, 23/51) than that in the group of
low-differentiation cancers (27.8%, 5/18) (Table II), but statistical analysis of
χ2 did not reveal difference (P=0.513).
 | Table IIPrevalence of HPV infection in the
squamous head and neck carcinomas according to their pathological
grades. |
Table II
Prevalence of HPV infection in the
squamous head and neck carcinomas according to their pathological
grades.
| Cell
differentiation | High (I) (%) | Moderate (II)
(%) | Low (III) (%) | P-value |
|---|
| Larynx | 6/19 (31.6) | 14/35 (40.0) | 3/10 (33.3) | 0.755 |
| Hypopharynx | 2/4 (50.0) | 5/7 (71.4) | 0/4 (0.0) | 0.073 |
| Oropharynx | 2/2 (100.0) | 0/2 ((0.0) | 1/1 (100.0) | 0.082 |
| Lip | 4/4 (100.0) | 3/4 (75.0) | 1/1 (100.0) | 0.495 |
| Total | 14/29 (48.3) | 22/48 (45.8) | 5/16 (31.3) | 0.513 |
To see the correlation of HPV infection with the
clinical progression, all enrolled cancers were classified
according to their clinical grades. Generally, the HPV-positive
rates in the groups of the clinical phase I, II, III and IV were
51.9 (14/27), 37.1 (13/35), 46.2 (12/26) and 40.0% (2/5),
respectively (Table III).
Although the HPV-positive rate in the group of phase I was higher
than those of the rest, statistic analysis of χ2 did not
reveal significant difference. Furthermore, the HPV infections in
the patients with or without metastasis were analyzed. The
HPV-positive rates in the patients with metastasis (53.3%, 8/15)
and without metastasis (42.3%, 33/78) were comparable, without
statistic difference. It seems that HPV infection in the tumor
tissues does not obviously affect the clinical progressions of the
oral and throat carcinomas.
 | Table IIIPrevalence of HPV infection in the
squamous head and neck carcinomas according to their clinical
grades. |
Table III
Prevalence of HPV infection in the
squamous head and neck carcinomas according to their clinical
grades.
| Clinic | Phase I (%) | Phase II (%) | Phase III (%) | Phase IV (%) | P-value |
|---|
| Larynx | 7/20 (35.0) | 6/22 (24.0) | 10/19 (47.6) | 0/3 (0.0) | 0.194 |
| Hypopharynx | 1/1 (100.0) | 3/7 (42.9) | 2/6 (33.3) | 1/1 (100.0) | 0.431 |
| Oropharynx | 2/2 (100.0) | 0/1 (0.0) | 0/1 (0.0) | 1/1 (100.0) | 0.172 |
| Lip | 4/4 (100.0) | 4/5 (80.0) | 0/0 (0.0) | 0/0 (0.0) | 1.0 |
| Total | 14/27 (51.9) | 13/35 (37.1) | 12/26 (46.2) | 2/5 (40.0) | 0.7 |
Correlations of HPV infection with the
gender, age and histories of tobacco and alcohol use of the
patients
Among the enrolled 84 male patients, 40.5% (34/84)
cases were HPV-positive, while predominantly more female patients
(77.8%, 7/9) showed HPV-positive, revealing an obvious
gender-tendency. Among 7 HPV-positive female patients, 4 were lip
carcinoma, 2 were larynx carcinoma and 1 was hypopharynx carcinoma.
The median age of HPV-positive patients was 58 years, which was
slightly older than that of HPV-negative patients (56.5 years old).
The HPV-positive data among the different age groups are summarized
in Table IV. Most cases were in
the age groups of 40–50, 50–60, 60–70 and >70 years and
HPV-positive rates in those four groups were 21.4, 48.6, 37.0 and
53.8%, respectively. There were only four patients <40 years,
but all were HPV-positive. Statistical analysis did not reveal
difference in HPV-positive rates among the age groups of 40–50,
50–60, 60–70 and >70 years. Furthermore, the HPV infections
among the patients with or without history of tobacco and alcohol
use were analyzed. HPV detection rates in the groups of tobacco and
alcohol use were 41.9 (34/81) and 39.6% (21/53), respectively,
which were lower than that in the groups without the history of
tobacco (58.3%, 7/12) and alcohol (50.0%, 20/40) use, but there is
no significant difference between the HPV-positive in the groups
using tobacco or alcohol and not using tobacco or alcohol (P=0.289
and 0.321).
 | Table IVPrevalence of HPV infection in the
squamous head and neck carcinomas based on the age groups. |
Table IV
Prevalence of HPV infection in the
squamous head and neck carcinomas based on the age groups.
| Age (years) | <30 (%) | 30–40 (%) | 40–50 (%) | 50–60 (%) | 60–70 (%) | >70 (%) | P-value |
|---|
| Larynx | - | 1/1 (100.0) | 2/11 (18.2) | 9/23 (39.1) | 7/22 (31.8) | 4/7 (57.1) | 0.296 |
| Hypopharynx | - | - | 0/1 (0.0) | 4/8 (50.0) | 2/3 (66.7) | 1/3 (33.3) | 0.658 |
| Oropharynx | - | - | 1/2 (50.0) | 2/2 (100.0) | 0/1 (0.0) | - | 0.233 |
| Lip | 1/1 (100.0) | 2/2 (100.0) | - | 2/2 (100.0) | 1/1 (100.0) | 2/3 (66.7) | 0.690 |
| Total | 1/1 (100.0) | 3/3 (100.0) | 3/14 (21.4) | 17/35 (48.6) | 10/27 (37.0) | 7/13 (53.8) | 0.097 |
Discussion
The head and neck cancers in human refer to the
malignant lesions at special anatomic sites, which include the lip,
oral cavity, nose and para-nasal sinuses, naso-pharynx,
oro-pharynx, hypopharynx and larynx. Although numerous studies have
confirmed the presence of HPV genome and oncoproteins in head and
neck SCCs, establishment of a stable linkage between HPV and a
subset of SCCs is still difficult because of the heterogeneity of
SCCs, and the fact that only a fraction of cases are
HPV-associated. In this study, we provide data of HPV involvement
in Chinese patients with SCCs at the sites of lip, oropharynx, and
larynx. Overall 44.1% (41/93) tested cases had HPV. These data are
generally coincidental with the results of some previous studies of
Chinese investigators (7), as well
as that of international groups (8). In line with the previous observation
(9), higher HPV detecting rates are
observed in the patients who do not use tobacco or alcohol.
In our study, the HPV detection rates show a tumor
site-related distribution, which decrease from outside (lip) to
inside (larynx). Although the enrolled case numbers of lip,
oropharynx and hypopharynx cancers in this study are limited
compared with that of larynx cancers, we still believe that this
site-related HPV distribution in head and neck SCCs is meaningful,
possibly reflecting the different exposure opportunity to HPVs. The
exact transmission of HPV in oral mucosa remains uncertain, but
direct skin to skin contact is believed to be essential, primarily
by means of vaginal, anal and oral sex and less commonly by
vertical transmission (10).
Numerous studies have elucidated that oral HPV infection is closely
linked with open mouthed kissing and oral sex (11–13). A
case control study of 100 newly diagnosed patients with OSCC and
200 control patients without cancer has illustrated that a high
lifetime number of vaginal-sex partners (26 or more) and a high
lifetime number of oral-sex partners (6 or more) were associated
with oropharyngeal cancer (14).
Those comprehensive studies demonstrate the importance of oral sex
in the HPV infection in head and neck SCCs. Although the oral sex
histories in our Chinese patients are difficult to be addressed,
the anatomic site-dependent HPV infections in the head and neck
SCCs may reflect the importance of the direct contact.
Our data suggest that the HPV infection seems not to
relate with either the pathological or clinical grades of head and
neck SCCs. However, higher HPV detection rates are observed in
patients with metastasis. Some earlier studies have reported that
HPV-positive patients tended to present larger tumors and at a
higher stage, while other have proposed opposite observation
(15). Recent reports suggest that
HPV-positive oropharyngeal cancers typically are detected at later
stages (involvement of regional lymph nodes and distant metastasis)
than HPV-negative cancers (16,17).
Patients with HPV-positive oropharyngeal cancers have consistently
higher survival rates and better response to radiation therapy and
chemotherapy and are less likely to experience progression and
recurrence of tumors (18–21). In a meta-analysis of 37 studies,
patients diagnosed with HPV-HNSCC had a lower risk of mortality and
of recurrence as compared with patients with HPV-negative tumors
(22). Whether HPV infection can be
used as a prognostic predictor in Chinese head and neck SCCs needs
further studies.
HPV16 is the most prevalent genotype in cervical
carcinoma, and is also the most frequently detected HPV type in
head and neck SCCs, found in up to 90% of HPV-positive cases
(2,23). Using the HPV Luminex technique that
covers 26 HPV genotypes and HPV16- and 18-specific PCR, HPV16 and
18 DNAs were detected in 7 and 8 patients with head and neck SCC in
this study. A previous study also showed that 7 (23.3%) and 10
(33.3%) of 30 Japanese and 11 (36.7%) and 5 (16.7%) of 30 Chinese
samples contained HPV16 and HPV18, respectively (24). It highlights that HPV18 is another
prevalent genotype in head and neck SCC in Northeast Asia, besides
HPV16. Because of limited case numbers, HPV18 prevalence in head
and neck SCC in the region and its clinical implication need to be
investigated. Using HPV16/18 oncoprotein specific-IHC, more
HPV-positive cases have been identified with the formalin-fixed and
paraffin-embedded tissues from head and neck SCC cases. Except for
the possibility of relatively higher sensitivity of IHC assay, HPV
DNA is probably easier to be detected in the fresh or fresh-frozen
specimen (5). Our study also
reveals a low overlapping ratio of coexistence of both HPV DNA and
oncoprotein in the samples, in which only 4 out of 41 HPV-positive
samples show double-positive. Mismatch between persistence of viral
genome and its encoding protein is common event in many persistent
infectious viruses, especially in the integrated viruses with
truncated viral genome fragments and with multiple integrating
sites in host chromosome, such as high-risk HPVs (25,26).
Hence, the detection difference between HPV DNA and oncoproteins
may not only represent the diversity of the testing sensitivities,
but also reflect the complicated carcinogenesis of HPVs. Our data
show an obviously predominant HPV16/18 oncoprotein positivity in
lip cancers compared with SCC in other sites. Whether this
phenomenon is related with relatively low HPV DNA detection in lip
cancers in many other reports (27,28)
need further study. Although determination of p16 expression status
with IHC has served as a reasonable surrogate marker for
biologically relevant high-risk HPV infection (29), direct detection of high-risk E6/E7
mRNA or protein would be the ideal test for classifying a tumor as
truly HPV-associated.
Acknowledgements
This study was supported by China Mega-Project for
Infectious Disease (2011ZX10004-101 and 2008ZX10004-008) and the
SKLID Development Grant (2008SKLID102, 2011SKLID211 and
2012SKLID302). The Project Sponsored by the Young Scholar
Scientific Research Foundation of China CDC (2012A102).
References
|
1
|
Gao N, Li Y, Li LJ and Wen YM: Clinical
analysis of head and neck cancer cases in south-west China
1953–2002. J Int Med Res. 37:189–197. 2009.PubMed/NCBI
|
|
2
|
Marur S, D’Souza G, Westra WH and
Forastiere AA: HPV-associated head and neck cancer: a virus-related
cancer epidemic. Lancet Oncol. 11:781–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machado J, Reis PP, Zhang T, et al: Low
prevalence of human papillomavirus in oral cavity carcinomas. Head
Neck Oncol. 2:62010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hocking JS, Stein A, Conway EL, Regan D,
Grulich A, Law M and Brotherton JML: Head and neck cancer in
Australia between 1982 and 2005 show increasing incidence of
potentially HPV-associated oropharyngeal cancers. Br J Cancer.
104:886–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramqvist T and Dalianis T: Oropharyngeal
cancer epidemic and human papillomavirus. Emerg Infect Dis.
16:1671–1677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu B, Lu Z, Wang P, Basang Z and Rao X:
Prevalence of high-risk human papillomavirus types (HPV-16, HPV-18)
and their physical status in primary laryngeal squamous cell
carcinoma. Neoplasma. 57:594–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun P, Chen XP, Pei F, et al: Relationship
between nasal inverted papilloma and human papillomavirus subtypes.
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 45:310–313.
2010.PubMed/NCBI
|
|
8
|
Syrjänen S: Human papillomavirus (HPV) in
head and neck cancer. J Clin Virol. 32:59–66. 2005.
|
|
9
|
Cleveland JL, Junger ML, Saraiya M,
Markowitz LE, Dunne EF and Epstein JB: The connection between human
papillomavirus and oropharyngeal squamous cell carcinomas in the
United States: implications for dentistry. J Am Dent Assoc.
142:915–924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heck JE, Berthiller J, Vaccarella S, et
al: Sexual behaviours and the risk of head and neck cancers: a
pooled analysis in the International Head and Neck Cancer
Epidemiology (INHANCE) consortium. Int J Epidemiol. 39:166–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D’Souza G, Zhang HH, D’Souza WD, Meyer RR
and Gillison ML: Moderate predictive value of demographic and
behavioral characteristics for a diagnosis of HPV16-positive and
HPV16-negative head and neck cancer. Oral Oncol. 46:100–104.
2010.PubMed/NCBI
|
|
12
|
Kreimer AR: Oral sexual behaviors and the
prevalence of oral human papillomavirus infection. J Infect Dis.
199:1253–1254. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rintala M, Grénman S, Puranen M and
Syrjänen S: Natural history of oral papillomavirus infections in
spouses: a prospective Finnish HPV family study. J Clin Virol.
35:89–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D’Souza G, Kreimer AR, Viscidi R, et al:
Case-control study of human papillomavirus and oropharyngeal
cancer. N Engl J Med. 356:1944–1956. 2007.PubMed/NCBI
|
|
15
|
Fouret P, Martin F, Flahault A and
Saint-Guily JL: Human papillomavirus infection in themalignant and
premalignant head and neck epithelium. Diagn Mol Pathol. 4:122–127.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaturvedi AK, Engels EA, Anderson WF and
Gillison ML: Incidence trends for human papillomavirus-related and
-unrelated oral squamous cell carcinomas in the United States. J
Clin Oncol. 26:612–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watson M, Saraiya M, Ahmed F, et al: Using
population-based cancer registry data to assess the burden of human
papillomavirusassociated cancers in the United States: overview of
methods. Cancer. 113:2841–2854. 2008. View Article : Google Scholar
|
|
18
|
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman
SM and Tsao AS: Meta-analysis of the impact of human papillomavirus
(HPV) on cancer risk and overall survival in head and neck squamous
cell carcinomas (HNSCC). Head Neck Oncol. 2:152010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fakhry C, Westra WH, Li S, et al: Improved
survival of patients with human papillomavirus-positive head and
neck squamous cell carcinoma in a prospective clinical trial. J
Natl Cancer Inst. 100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ang KK, Harris J, Wheeler R, et al: Human
papillomavirus and survival of patients with oropharyngeal cancer.
N Engl J Med. 336:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Settle K, Posner MR, Schumaker LM, et al:
Racial survival disparity in head and neck cancer results from low
prevalence of human papillomavirus infection in black oropharyngeal
cancer patients. Cancer Prev Res. 2:776–781. 2009. View Article : Google Scholar
|
|
22
|
Ragin CC and Taioli E: Survival of
squamous cell carcinoma of the head and neck in relation to human
papillomavirus infection: review and meta analysis. Int J Cancer.
121:1813–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Psyrri A and DiMaio D: Human
papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract
Oncol. 15:24–31. 2008. View Article : Google Scholar
|
|
24
|
Tang X, Jia L, Ouyang J and Takagi M:
Comparative study of HPV prevalence in Japanese and North-east
Chinese oral carcinoma. J Oral Pathol Med. 32:393–398. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pett M and Coleman N: Integration of
high-risk human papillomavirus: a key event in cervical
carcinogenesis? J Pathol. 212:356–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kraus I, Driesch C, Vinokurova S, et al:
The majority of viral-cellular fusion transcripts in cervical
carcinomas cotranscribe cellular sequences of known or predicted
genes. Cancer Res. 68:2514–2522. 2008. View Article : Google Scholar
|
|
27
|
Paz IB, Cook N, Odom-Maryon T, Xie Y and
Wilczynski SP: Human papillomavirus (HPV) in head and neck cancer.
An association of HPV 16 with squamous cell carcinoma of Waldeyer’s
tonsillar ring. Cancer. 79:595–604. 1997.PubMed/NCBI
|
|
28
|
Shimizu M, Adachi A, Zheng S, et al:
Detection of various types of human papillomavirus DNA, mainly
belonging to the cutaneous-group, more frequently in normal tissue
than in squamous cell carcinomas of the lip. J Dermatol Sci.
36:33–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smeets SJ, Hesselink AT, Speel EJ, et al:
A novel algorithm for reliable detection of human papillomavirus in
paraffin embedded head and neck cancer specimen. Int J Cancer.
121:2465–2472. 2007. View Article : Google Scholar : PubMed/NCBI
|