Introduction
The majority of therapies for malignant tumours are
based on chemotherapeutic drugs with cytotoxic effects, which cause
death of tumour cells by direct damage to DNA or by inhibition of
cell division. Unfortunately, these drugs are mostly unspecific,
therefore, their administration often causes extended tissue
toxicity (1).
Cisplatin, or
cis-diamminedichloroplatinum(II) (CDDP) was the first
platinum-based anticancer drug developed for clinical purposes. It
is employed in children for treatment of haematological tumours and
in adults for treatment of solid tumours such as testicular
(2), prostatic and ovarian
(3,4), uterine, cervix, breast, bladder,
stomach (5,6) colon, brain, head-neck (7,8) and
both non-small and small-cell lung cancer (9,10).
The intravenous administration of cisplatin causes
an increase in initial tissue accumulation and in plasma levels for
an extended time. Cisplatin levels are high in plasma as the drug
reversibly binds to >90% of proteins; traces of the drug are
often detected years after chemotherapy (11,12).
Brouwers and co-researchers (13),
during a 6-year follow-up screening, demonstrated that plasma
levels of cisplatin have a half life (t/2) of 28.5 months (14,15).
Moreover, the elimination of the drug is not the same in all
tissues; elimination is faster in more rapidly regenerating tissues
in comparison to slower ones and each tissue has its own t/2
(16). The plasma levels of
cisplatin depend upon several factors: cumulative dose, follow-up
time, age of patient, glomerular filtration speed (GFR) during
chemotherapy, use of sodium thiosulphate (STS) during cisplatin
chemotherapy and method of administration (11,13). A
higher initial dose of the drug causes a higher tissue
concentration and a longer time for excretion, yet STS binding to
cisplatin lowers its initial tissue accumulation (16).
Cisplatin has both biliary (~10%) and urinary (90%)
clearance. The urinary clearance of cisplatin is characterised by
an initial fast excretion phase (20 min), followed by a second
slower phase (60–70 min) and a third very slow and incomplete phase
(24 h) (17,18).
The activity of cisplatin and the appearance of the
side effects depend on pharmacological parameters such as the
dosage (single and cumulative) and administration (schedule and
means), but also on systemic and individual conditions such as skin
pigmentation, age, diet, blood pH and interactions with
radiotherapy (19–21). Certain effects are dose-dependent;
thus, they can be controlled but not prevented (22–24).
Among the adverse effects that may develop, the most
frequent ones are gastrointestinal symptoms. More than 90% of
patients experience nausea and vomiting; these symptoms are
counteracted by the administration of antiemetic drugs such as the
antagonist of serotonin receptor 3 (5-HT3) and
dexamethasone. In a smaller number of cases, general symptoms are
detected, such as fever, hyposthenia, altered sleep-wake cycle,
myelosuppression and alteration in the liver, skin and respiratory
apparatus. Among the negative side effects with a more or less
severe involvement of tissues include neurotoxicity, nephrotoxicity
and ototoxicity (2,13,25–27).
The different responses to cisplatin treatment
depend on individual factors and on resistance mechanisms.
Resistance mechanisms include: reduction in intracellular drug
accumulation, drug inactivation by the cytosol, changes in DNA
repair mechanisms and alterations in proteins involved in apoptosis
(18,28–31).
Resistance may be induced by only one or several of the above
mentioned mechanisms (31). The
reduction in intracellular accumulation may depend upon an
increased drug outflow or a reduced inflow through the cell
membrane. The inflow by membrane channels may be modified by
several compounds including amphotericin B, aldehydes, inhibitors
of Na-K adenosine triphosphatase and ouabain (18). Molecules such as glutathione and
metallothioneins may react with cisplatin at the intracellular
level, inactivating it and preventing its binding to DNA. Others
may reduce its chemotherapeutic efficacy by modifying the DNA
repair mechanisms (32); among
these there are the DNA polymerase inhibitors (zidovudine and
ganciclovir), the inhibitors of topoisomerase II (etoposide and
novobiocin), the methylxanthines (caffeine and pentoxifylline) and
specific chemotherapeutic drugs (5-fluorouracil, cytarabine and
hydroxyurea) (33,34).
The different compounds studied for interference
with cisplatin toxicity inlcude sodium thiosulphate, D-methionine,
vitamins C and E, Gingko biloba extract and dexamethasone
(25,35–38).
To date, most clinical literature data report that
the efficacy and side effects of cisplatin treatment are associated
with other compounds in patients belonging to homogeneous groups or
with tumours in specific body sites. The purpose of the present
research was to evaluate the incidence of side effects in patients
with different types of tumours, undergoing chemotherapy with
cisplatin. Records of the patients retrospectively examined were
heterogeneous, in order to verify i) whether various chemotherapy
combinations increase the sensitivity of the organism to the toxic
effects of the drug; ii) whether a direct correlation could be
detected between the tumour site and a specific side effect; and
finally iii) whether the side effects were reciprocally related.
For this purpose, we examined the medical records of 123 patients
treated with cisplatin in the same hospital (St. Anna University
Hospital, Ferrara, Italy) during 2007 and 2008, with special
attention to the dosages and side effects reported.
Materials and methods
Study population
The medical records of 123 patients (81 males and 42
females), undergoing chemotherapy during 2007 and 2008 at the
Clinical Oncology Unit, St. Anna University Hospital in Ferrara
(Italy), were retrospectively examined in agreement with Italian
privacy and sensitive data laws (D.Lgs 196/03) and according to the
institutional guidelines of the St. Anna University Hospital.
Tumour distribution
All malignant tumours were classified according to
the Italian Association of Cancer Registries (AIRTUM, Associazione
Italiana Registri Tumori) and the International Classification of
Diseases. The cancers were recognised as follows: lung, head and
neck, gynaecological, melanoma, thymoma, gastric, occult,
neuroendocrine, urothelial, hepatic and thyroid.
Treatment
Doses and methods of cisplatin treatment were
modulated according to the drug therapeutic plan (alone, in
association with other chemotherapeutic agents or with
radiotherapy), depending on the tumour type and on the conditions
of the patients.
Cisplatin (cis-diamminedichloroplatinum(II),
CDDP) was administered alone or with gemcitabine (GEM), epirubicin
(EPI), etoposide (VP-16), 5-fluorouracil (5-FU), dacarbazine
(DTIC), vinorelbine (VNR) or in a combination called EDOC (EPI +
CDDP + vincristine + cyclophosphamide). In all cases, the drug
treatment was preceded by hydration and by antiemetic treatment
with dexamethasone and serotonin (5-hydroxytriptamine 3,
5-HT3) (from 30 min to 1 h and 30 min before
chemotherapy). The pretreatment was recommended by the American
Society of Clinical Oncology (ASCO, 2006) since cisplatin is one of
the chemotherapeutic agents with the most severe emetic side
effects (incidence >90%). Although the daily standard dose of
dexamethasone is 20 mg, in most cases the prescribed daily dose was
8 mg in 100 ml of saline solution, administered intravenously. The
5-HT3 drugs are a group of antagonists of the
5-HT3 serotonin receptor (ondansetron, granisetron or
dolasetron). The method of administration (oral or intravenous)
does not influence their efficacy in controlling symptoms. The
administration of the chemotherapeutic drugs was also preceded by
administration of two diuretics (furosemide and mannitol). The
hydration of the patients undergoing chemotherapy with cisplatin is
necessary to reduce dehydration and the relevant nephrotoxic
effects of the drug. When cisplatin is administered with GEM, 5-FU
or VNR, additional administration of dexamethasone is required (a
total of 16 mg), and when the therapy follows the EDOC scheme, the
amount of serotonin is doubled as well. Among the 123 patients
studied, 63 were also treated by radiotherapy, particularly when
affected by head-neck tumours.
Classification of adverse effects
The side effects observed, following the Common
Terminology Criteria for Adverse Events (CTCAE) v3.0 (National
Cancer Institute, 2006) were respectively categorised as follows:
auditory/ear (ototoxicity), blood/bone marrow (haematological
toxicity), constitutional symptoms, dermatology/skin
(dermatological disorders), gastrointestinal (gastrointestinal
disorders), hepatobiliary/pancreas (hepatic toxicity), neurology
(neurotoxicity), pulmonary/upper respiratory (respiratory
disorders), renal/genitourinary (nephrotoxicity) and
sexual/reproductive function (genital apparatus disorders). Changes
in sleep-wake cycle were classified in a separate category, termed
sleep-wake disorders, as they are not clearly categorised by
CTCAE.
Statistical analysis
The collected data represent cancer prevalence in
2008 among oncological patients of the St. Anna University Hospital
of Ferrara, undergoing therapy for a maximum of 6 years.
For all data, the average values and standard
deviations were calculated for dosages and the frequency of side
effects detected in all patients and for patients grouped by tumour
type. Concerning the possible association between the examined
variables, we calculated the Spearman non-parametric correlation.
The data were verified by r-Pearson and were plotted in dendrograms
by the unweighted pair-group method using arithmetic averages.
Statistica 7 (StatSoft srl., Italy, 2005) software was used.
Results
Patient characteristics
The patients had a mean age of 60.0±9.9 (SD) years
(calculated when they received the first treatment with cisplatin)
and an age range of 35–81 years. An overview of the data is shown
in Table I, which reports the
number of patients by gender, years since diagnosis and type of
cancer.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Gender | Years since
diagnosis | Age ± SD |
Tumour
classes |
|---|
|
|---|
| L | HN | GN | M | TM | GS | O | N | U | HE | TY |
|---|
| Male |
| 1 | 61±9.9 | 14 | 7 | - | 1 | - | 2 | 1 | 1 | 1 | - | - |
| 2 | 62±8.2 | 23 | 9 | - | 1 | 2 | 1 | - | - | 1 | - | - |
| 3 | 60±9.2 | 6 | 5 | - | 1 | - | - | - | 1 | - | 1 | - |
| 4 | 66±13.4 | 2 | - | - | - | - | - | - | - | - | - | - |
| 6 | 39 | - | 1 | - | - | - | - | - | - | - | - | - |
| Female |
| 1 | 56±11.5 | 1 | 1 | 5 | 1 | - | - | - | 1 | - | - | - |
| 2 | 60±13.4 | 3 | 4 | 5 | 1 | - | - | 1 | - | - | - | 2 |
| 3 | 58±8.3 | 4 | 1 | 7 | - | 1 | - | - | - | 1 | 1 | - |
| 4 | 53±5.7 | 1 | - | 1 | - | - | - | - | - | - | - | - |
Concerning the incidence of tumours in relation to
gender, we calculated the relative percentage for each tumour
typology. Thyroid and obviously gynaecological tumours were
restricted to females, while gastric carcinoma was detected only in
males. A frequency of 50–60% was detected in occult carcinoma,
hepatic carcinoma and melanoma (one male patient had hepatic
carcinoma and one female had biliary tract cancer). In all other
tumours examined the incidence was higher in males in comparison to
females.
As shown in Table I,
the tumours with the highest prevalence were those of the lung and
head and neck, with a 4:1 male:female ratio. The third most
frequent tumours in women were gynaecological tumours. In all other
cases, the number of affected individuals ranged from 2 (occult,
hepatic and thyroid cancers) to a maximum of 5 (melanoma). In
Table I different tumour histotypes
were grouped together. For example, all female tumours (5 cases of
cervical cancer, 4 of ovarian cancer, 4 of breast cancer, 3 of
endometrial cancer, one of breast-endometrial cancer and one of
vulvar cancer) were grouped under the GN (gynaecological) class.
Lung cancers [42 non-small cell lung cancer (NSCLC), 9 small cell
lung cancer (SCLC), one sarcomatoid carcinoma and two unspecified
cases] were grouped under the L (lung) class.
Recording of adverse effects
The majority of patients had gastrointestinal
disorders, such as nausea and vomiting, diarrhoea, constipation,
epigastralgia, pyrosis, dysphasia, postprandial abdominal bloating
sensation, white tongue, dysgeusia and taste impairment. Among the
other adverse effects, constitutional symptoms included hyposthenia
and asthenia, fever, weight loss and appetite loss. Cisplatin
myelosuppression caused haematological toxic effects, such as
anaemia, leukopenia, neutropenia and thrombocytopenia. The
dermatological disorders included alopecia, itchiness, skin rash,
edema, arm phlebitis and mucositis. Neurotoxicity mainly involved
the peripheral system in comparison to the central nervous system.
The most common symptoms were paraesthesia, followed by
cephalalgia, speech impairments, aphasia, agnosia, lipothymia
(near-fainting syndrome), convulsions, panic and transient ischemic
attacks (mini-strokes), visual failure, sensory-motor deficits and
motor coordination impairments. Nephrotoxicity included electrolyte
alterations (hyperK+, hypoK+,
hyperCa++, hypoNa+, hypoCl-,
hypoMg+), an increase in blood nitrogen, creatinine and
urea, pollakiuria (abnormally frequent urination), hematuria,
oliguria, polyuria, urinary tract infections, and kidney spasms to
renal insufficiency. Hepatic toxicity was characterised by
hepatomegaly and a rise in hepatic enzymes (transaminases,
bilirubin, γ-glutamyl transpeptidases). Respiratory disorders
mostly involved cough, dyspnea, polypnea and chest pain. Symptoms
of ototoxicity mainly included vertigo, in a few subjects tinnitus
or hypoacousia. Genital apparatus disorders included female
gynaecological symptoms such as vaginal discharge.
Chemotherapy protocols and adverse
effects
Analysis of the average daily doses administered
revealed that the most intensive therapy (the highest dosage) was
applied for lung sarcoma tumours (treated with 148 mg/m2
of the chemotherapeutic drugs), followed by SCLC (143.75
mg/m2) and neuroendocrine tumours (136.6
mg/m2). The lowest daily dosage was administered to
patients affected by thyroid tumours (45 mg/m2),
followed by patients affected by uterine and vulvar tumours (54
mg/m2). The patients receiving a higher CDDP dosage were
affected by neuroendocrine tumours (cumulative dose 635.19
mg/m2), followed by those affected by urothelial tumours
(621.81 mg/m2) and SCLC (503.125 mg/m2). The
patients receiving the lowest cumulative doses (69
mg/m2) were those affected by ovarian tumours. Finally,
doses <100 mg/m2 were administered to the patients
with uterine, gastric, vulvar and thyroid tumours.
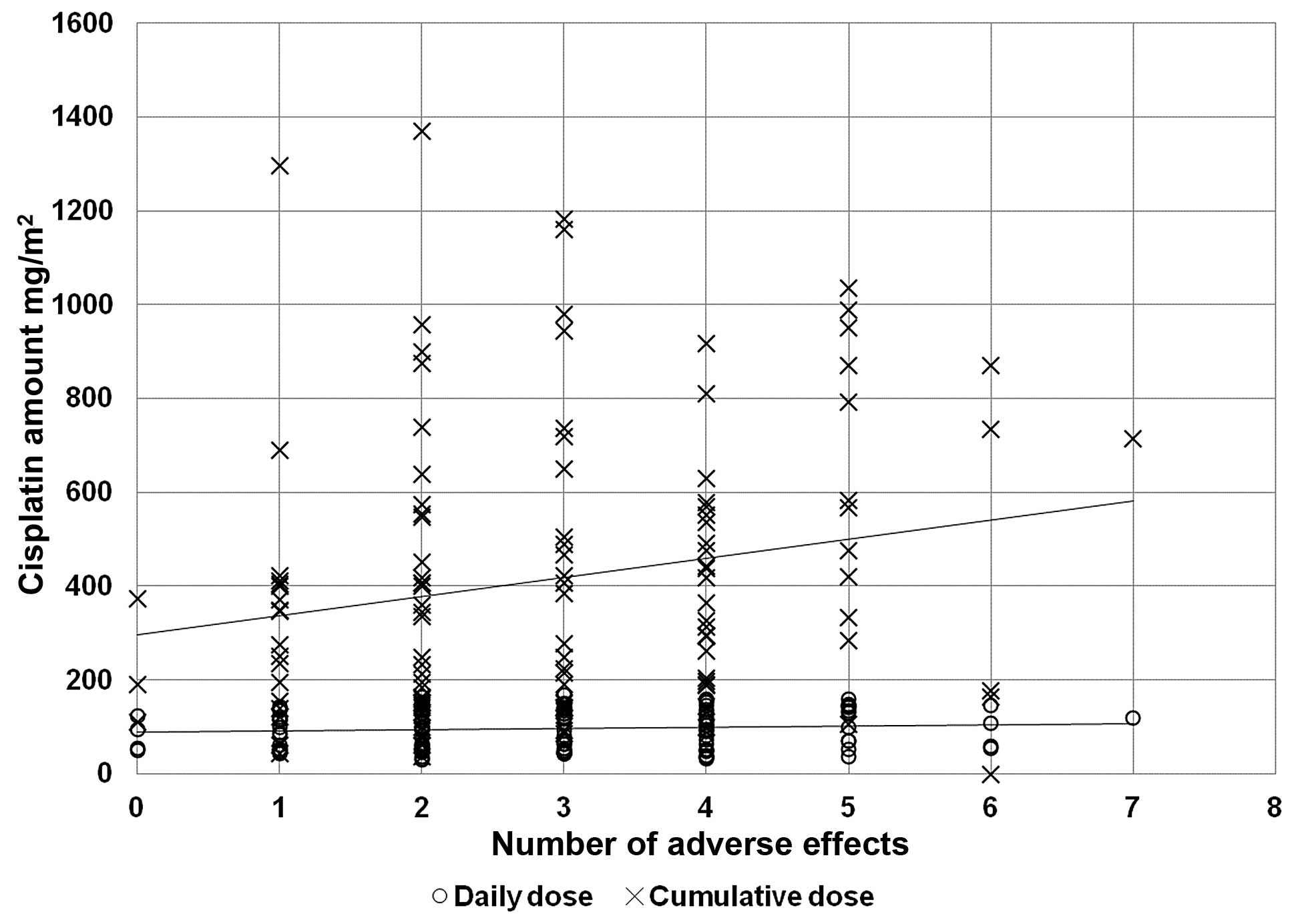
In order to verify the correlation between the
chemotherapy dosage and the incidence of adverse effects, we
plotted the number of toxic effects against cumulative and daily
doses of cisplatin administered (Fig.
1), and we correlated these doses with the associated compound
to the adverse effects (Table II).
As expected, the cumulative amount of cisplatin was directly
related to the number of adverse effects (r2=0.3826,
P<0.001). The daily dose correlated with gastrointestinal and
respiratory disorders, while the cumulative dose also affected the
hepatic and haematological systems.
 | Table IIChemotherapy protocols and adverse
effects. |
Table II
Chemotherapy protocols and adverse
effects.
| Cisplatin dose |
Tumour
classes | | | |
|---|
|
|
| | | |
|---|
| Adverse
effects | Day | Cum | L | GN | U | GS | HE | N | TM | HN | M | TY | O | Total | AE (%) | S (%) |
|---|
| Constitutional
symptoms | 0.274 | 0.130 | 47 | 10 | 4 | 2 | 1 | 2 | 3 | 14 | 4 | 0 | 1 | 88 | 23 | 72 |
| Dermatological
disorders | 0.500 | 0.860 | 15 | 3 | 2 | 2 | 1 | 2 | 0 | 15 | 3 | 0 | 0 | 43 | 11 | 35 |
| Gastrointestinal
disorders | 0.009 | 0.000 | 45 | 14 | 4 | 2 | 1 | 3 | 2 | 13 | 4 | 0 | 1 | 89 | 24 | 72 |
| Genital apparatus
disorders | 0.596 | 0.365 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 2 |
| Haematological
toxicity | 0.338 | 0.001 | 29 | 7 | 2 | 2 | 0 | 2 | 3 | 14 | 3 | 2 | 2 | 66 | 18 | 54 |
| Hepatic
toxicity | 0.151 | 0.009 | 9 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 13 | 3 | 11 |
| Nephrotoxicity | 0.264 | 0.950 | 10 | 3 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | 21 | 6 | 17 |
| Neurotoxicity | 0.126 | 0.269 | 14 | 3 | 4 | 1 | 0 | 2 | 0 | 4 | 2 | 1 | 1 | 32 | 9 | 26 |
| Ototoxicity | 0.651 | 0.211 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 11 | 3 | 9 |
| Respiratory
disorders | 0.021 | 0.038 | 5 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 10 | 3 | 8 |
| Sleep-wake
disorders | 0.488 | 0.915 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 7 | 2 | 6 |
Tumour site and adverse effects
To study in more detail the side effects detected in
the different tumour classes, the number of subjects were
determined exhibiting each type of side effect ordered according to
tumour class (Table II). No side
effect was common to all tumour classes, and the most frequent side
effects were gastrointestinal toxicity, constitutional symptoms and
haematological toxicity. The constitutional symptoms and gastric
toxicity were consistently detected, except in patients with
thyroid tumours. Apart from systemic and gastric symptoms, the
patient with hepatic tumours also showed dermatological
alterations, while patients with lung cancer presented all side
effects together. The less common symptoms detected were sleep-wake
disorders and alterations of the reproductive, respiratory and
auditive tract. The less frequent side effect was genital apparatus
toxicity. Following analysis of the number of side effects detected
for each tumour class, it was possible to note that the toxicity
range was higher in individuals affected by lung cancer, while in
those affected by thyroid and hepatic cancer the number of side
effects was lower. The effects of chemotherapeutic associations
were analysed by Spearman non-parametric correlation. The
statistically significant results are reported by tumour and by
year of diagnosis in Table III.
No correlations were found among chemotherapeutic protocols and
adverse effects in thymoma, gastric, occult, neuroendocrine,
urothelial, hepatic and thyroid tumours.
 | Table IIITumour site and adverse effects. |
Table III
Tumour site and adverse effects.
| Adverse
effects | Treatment | SpR | t(N-2) | P-value |
|---|
| Gynaecological
tumours |
| Respiratory
disorders | EPI | 0.686 | 3.771 | 0.002 |
| Ototoxicity | VP-16 | 0.542 | 2.582 | 0.020 |
| Dermatological
disorders | VP-16 | 0.478 | 2.177 | 0.045 |
|
Nephrotoxicity | 5-FU | 0.686 | 3.771 | 0.002 |
| Neurotoxicity | Taxol | 0.542 | 2.582 | 0.020 |
|
Nephrotoxicity | Taxol | 0.686 | 3.771 | 0.002 |
| Neurotoxicity | Paclitaxel | 0.542 | 2.582 | 0.020 |
|
Nephrotoxicity | Paclitaxel | 0.686 | 3.771 | 0.002 |
| Constitutional
symptoms | Radiotherapy | −0.620 | −3.162 | 0.006 |
| Head and neck
tumours |
| Neurotoxicity | Daily dose
cisplatin | 0.379 | 2.089 | 0.047 |
| Neurotoxicity | Dexamethasone | 0.556 | 3.407 | 0.002 |
| Constitutional
symptoms | Radiotherapy | −0.466 | −2.687 | 0.012 |
| Lung tumours |
| Dermatological
disorders | Daily dose
cisplatin | 0.282 | 2.120 | 0.039 |
| Neurotoxicity | Dexamethasone | 0.312 | 2.368 | 0.022 |
|
Nephrotoxicity | Dexamethasone | −0.316 | −2.404 | 0.020 |
|
Nephrotoxicity | Radiotherapy | 0.437 | 3.504 | 0.001 |
| Gastrointestinal
disorders | Cumulative dose
cisplatin | 0.483 | 3.982 | 0.000 |
| Haematological
toxicity | Cumulative dose
cisplatin | 0.487 | 4.024 | 0.000 |
| Number of adverse
effects | Cumulative dose
cisplatin | 0.484 | 3.990 | 0.000 |
| Melanoma |
| Constitutional
symptoms | Daily dose
cisplatin | −0.889 | −3.354 | 0.044 |
| Number of adverse
effects | Radiotherapy | 0.913 | 3.873 | 0.030 |
Side effects are reciprocally
related
Based on the differences in side effects among the
tumour classes, we also examined the possible association of side
effects together or to any tumour class by Spearman non-parametric
correlation. The results, summarised in Table IV, support the hypothesis that
dermatological disorders were associated with sleep-wake disorders
and haematological toxicity, gastrointestinal disorders were
associated with respiratory disorders and ototoxicity, and genital
apparatus disorders were associated with ototoxicity and hepatic
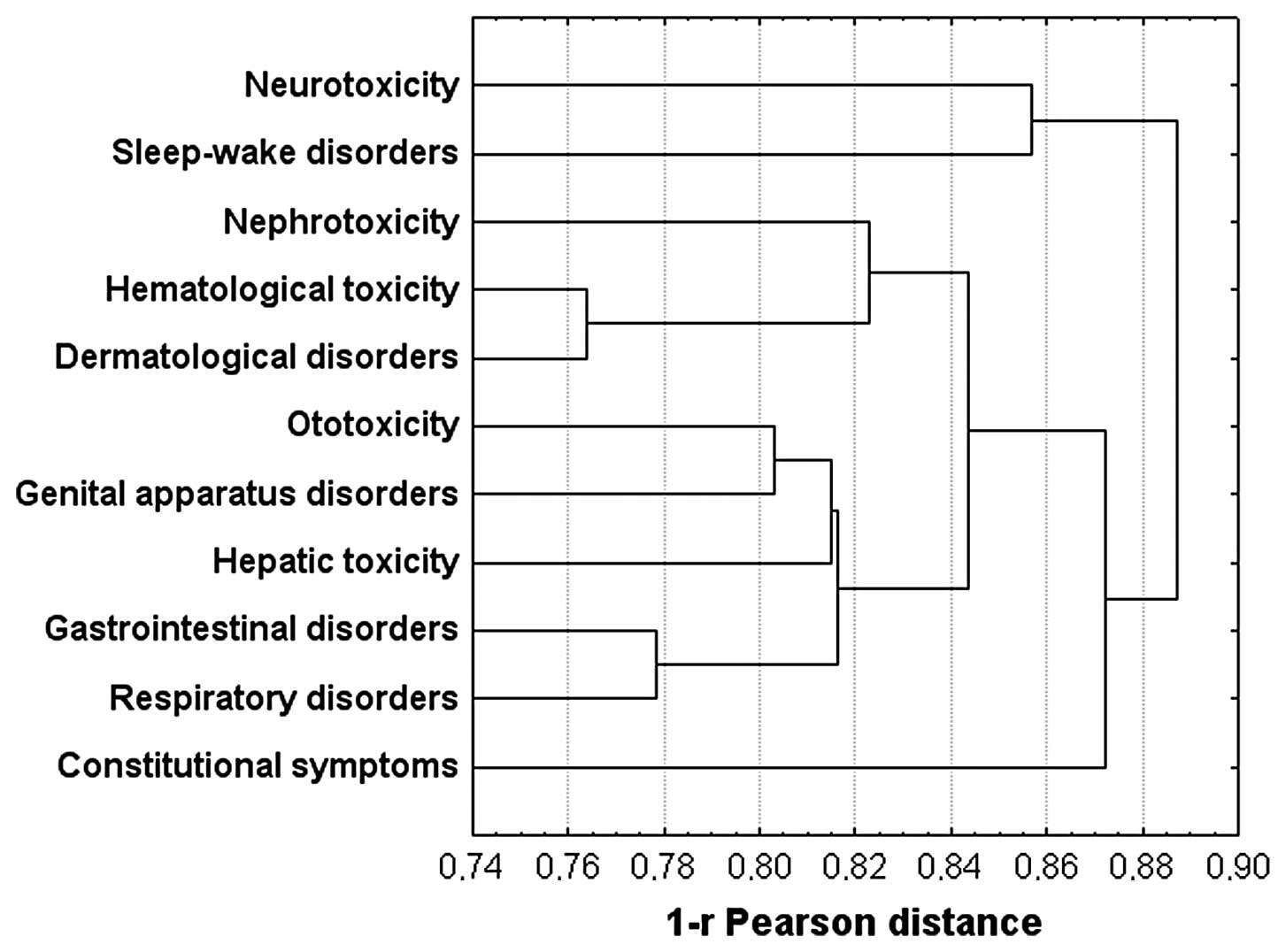
toxicity. The last result was also found in the dendrogram of the
data plotted using the hierarchical union method (Fig. 2). Notably, neurotoxicity was
associated with sleep-wake disorders and, together with
constitutional symptoms, stand out from all other groups.
 | Table IVCorrelation of side effects. |
Table IV
Correlation of side effects.
| | SpR | t(N-2) | P-value |
|---|
| Sleep-wake
disorders | Dermatological
disorders | −0.193 | −2.168 | 0.032 |
| Haematological
toxicity | Dermatological
disorders | 0.236 | 2.676 | 0.008 |
| Respiratory
disorders | Gastrointestinal
disorders | 0.222 | 2.499 | 0.014 |
| Ototoxicity | Gastrointestinal
disorders | 0.184 | 2.058 | 0.042 |
| Ototoxicity | Genital apparatus
disorders | 0.197 | 2.210 | 0.029 |
| Hepatic
toxicity | Genital apparatus
disorders | 0.185 | 2.070 | 0.041 |
Discussion
The present study describes the frequency of side
effects in 123 patients affected by solid tumours and treated with
cisplatin at the St. Anna Hospital of Ferrara, Italy during 2007
and 2008. This is the first study considering heterogeneous
populations as previous literature data concern studies limited to
specific tumours (4,39–43).
The highest tumour incidence was in males (63%), and
the most frequent tumour was lung tumour (43.4%, with a male/female
4:1 ratio and average age of 62 years), as supported by data from
Regione Emilia-Romagna (Italy) reported in 2006 (44).
The most frequent adverse effects detected (~72%)
involved the gastrointestinal apparatus and constitutional symptoms
(fever, hyposthenia, asthenia, weight loss and sleep-wake rhythm
alterations) while the least frequent effects involved genital
apparatus disorders (2%). The highest number of side effects was
detected in patients with lung cancer; this could be due to the
fact that this was the most frequent tumour class. To attenuate the
adverse effects of cisplatin treatment, it is necessary to evaluate
individual variations due to age, duration of therapy, range of
doses and synergism with other compounds causing the toxicity.
Considering the therapeutical protocols associated with different
tumours, we examined the cisplatin dosages administered. According
to this analysis, no direct correlation was noted between the
administered daily doses of cisplatin and the other
chemotherapeutic drugs involved. During chemotherapy, dexamethasone
is applied as an antiemetic (45)
and only recently its otoprotective qualities have been recognised
in animal models (37). Correlation
analyses showed that dexamethasone protects against nephrotoxicity
in lung tumour patients. However, a positive correlation between
the drug dosage and neurotoxicity was detected in both lung and
head-neck tumour patients. In esophageal tumours, the rate of
successful cisplatin treatment is 25–35% for metastatic carcinomas
and 50–60% for local tumours at an advanced stage (18). For head and neck tumours, cisplatin
is usually administered in association with 5-fluorouracil and
radiotherapy (7,18). In our data set, radiotherapy was
inversely correlated to constitutional symptoms, thus, showing a
protective effect. In lung tumour patients, the cisplatin dosage
was the main cause of adverse effects. However, in NSCLC,
cisplatin-based chemotherapy shows a complete response only in ~30%
of cases; therefore, it represents only a palliative care treatment
(40). Cisplatin is usually
administered in association with GEM at the suggested weekly dose
of 51 mg/m2 (with gemcitabine 1500 mg/m2)
(40). In SCLC, cisplatin is
usually administered with VP-16; if the tumour is locally advanced,
the positive response to chemotherapy is ~50–60%, with an average
survival time of 7–11 months. In more advanced tumours, paclitaxel
is administered with cisplatin, with a positive response rate of
~34–41%. After chemotherapy, the relapse of tumours occurs in 95%
of cases (9,31). For melanoma treatment, radiotherapy
is correlated with the increasing number of adverse effects and
decreased cisplatin dosages are associated with constitutional
symptoms. The literature data report that cisplatin is administered
alone or with VNR and DTIC, at doses ~100–200 mg/m2. The
positive response is ~16% (46). In
patients with gynaecological tumours, the cisplatin dosage was not
correlated with any adverse effect; in cervical cancer the
chemotherapy with cisplatin (not associated with other drugs)
usually follows surgical and radiotherapy treatment. Among patients
with ovarian cancer, 70% show an initial positive response to
cisplatin but only <25% survive up to 5 years. In these tumours,
cisplatin is employed in association with paclitaxel or topotecan
(3). In the present study group,
paclitaxel was correlated with both neurotoxic and nephrotoxic
effects. Concerning the other tumour classes, no correlations with
adverse effects were detected. In urothelial (or transitional cell)
carcinomas, locally advanced or metastatic, and particularly in
bladder cancer, cisplatin has been recommended for the last 20
years in association with methotrexate, vinblastine and doxorubicin
(M-VAC). This drug combination yielded positive results in 50–70%
of cases, with a 10–20% complete response and an average survival
time of ~1 year (18). However, the
high toxicity of this drug combination promoted successful research
on new combinations of compounds, such as gemcitabine, taxane and
paclitaxel. Cisplatin combined with gemcitabine and paclitaxel
yielded a positive response in 78% of cases, with an average
survival time of 24 month (47).
Thymus tumours (including thymoma) at stage III or IV are routinely
treated by polychemotherapy mainly involving cisplatin, adriamycin,
etoposide, cyclophosphamide or ifosfamide (48,49).
After verifying the most common side effects and
which tumour class showed the highest frequency, we also examined
the possible associations among the different side effects. The
results of these analyses, performed using the Spearman
non-parametric correlation test, showed a close relationship
between dermatological disorders and either haematological toxicity
or sleep-wake disorders, and between respiratory and
gastrointestinal disorders, as previously shown in the literature
(50).
Other correlations were found between genital
apparatus disorders and either ototoxicity or hepatic toxicity, and
between ototoxicity and gastrointestinal disorders. These
relationships may be due to metabolic effects, but they are
difficult to explain as the patient group was highly heterogeneous.
Moreover, gastrointestinal disorders are the most common side
effects in patients undergoing chemotherapy (50). Ototoxicity is mostly detected in
patients with lung or head and neck tumours treated with a high
dosage of cisplatin.
In conclusion, through analysis of this
heterogeneous group of patients, we confirmed that the main factor
influencing the occurrence and severity of adverse effects is the
dosage of cisplatin administered, both for single and cumulative
doses.
Acknowledgements
The authors wish to thank all of the personnel
working at the Clinical Oncology Unit, St. Anna University Hospital
(Ferrara, Italy) for their technical assistance and valuable
assistance in collecting the data.
References
|
1
|
Blanchard EM: Cisplatin and solid tumours:
still working, after all these years. J Solid Tumors. 2:26–33.
2012.
|
|
2
|
Chaudhary UB and Haldas JR: Long-term
complications of chemotherapy for germ cell tumours. Drugs.
63:1565–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tewari KS and Monk BJ: Gynecologic
Oncology Group trials of chemotherapy for metastatic and recurrent
cervical cancer. Curr Oncol Rep. 7:419–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong DK, Bookman MA, McGuire W,
Bristow RE, Schilder JM and Group GO: A phase I study of
paclitaxel, topotecan, cisplatin and filgrastim in patients with
newly diagnosed advanced ovarian epithelial malignancies: a
Gynecologic Oncology Group study. Gynecol Oncol. 105:667–671. 2007.
View Article : Google Scholar
|
|
5
|
Ridwelski K, Gebauer T, Fahlke J, et al:
Combination chemotherapy with docetaxel and cisplatin for locally
advanced and metastatic gastric cancer. Ann Oncol. 12:47–51. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KW, Kim JH, Yun T, et al: Phase II
study of low-dose paclitaxel and cisplatin as a second-line therapy
after 5-fluorouracil/platinum chemotherapy in gastric cancer. J
Korean Med Sci. 22(Suppl): S115–S121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cullen KJ, Yang Z, Schumaker L and Guo Z:
Mitochondria as a critical target of the chemotheraputic agent
cisplatin in head and neck cancer. J Bioenerg Biomembr. 39:43–50.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vokes EE: Induction chemotherapy for head
and neck cancer: recent data. Oncologist. 15(Suppl 3): 3–7. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niell HB, Herndon JE, Miller AA, et al:
Randomized phase III intergroup trial of etoposide and cisplatin
with or without paclitaxel and granulocyte colony-stimulating
factor in patients with extensive-stage small-cell lung cancer:
Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 23:3752–3759.
2005. View Article : Google Scholar
|
|
10
|
Bosch ME, Sánchez AJ, Rojas FS and Ojeda
CB: Analytical methodologies for the determination of cisplatin. J
Pharm Biomed Anal. 47:451–459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hohnloser JH, Schierl R, Hasford B and
Emmerich B: Cisplatin based chemotherapy in testicular cancer
patients: long term platinum excretion and clinical effects. Eur J
Med Res. 1:509–514. 1996.PubMed/NCBI
|
|
12
|
Gietema JA, Meinardi MT, Messerschmidt J,
et al: Circulating plasma platinum more than 10 years after
cisplatin treatment for testicular cancer. Lancet. 355:1075–1076.
2000.PubMed/NCBI
|
|
13
|
Brouwers EE, Huitema AD, Beijnen JH and
Schellens JH: Long-term platinum retention after treatment with
cisplatin and oxaliplatin. BMC Clin Pharmacol. 8:72008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tothill P, Klys HS, Matheson LM, McKay K
and Smyth JF: The long-term retention of platinum in human tissues
following the administration of cisplatin or carboplatin for cancer
chemotherapy. Eur J Cancer. 28A:1358–1361. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerl A and Schierl R: Urinary excretion of
platinum in chemotherapy-treated long-term survivors of testicular
cancer. Acta Oncol. 39:519–522. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gregg RW, Molepo JM, Monpetit VJ, et al:
Cisplatin neurotoxicity: the relationship between dosage, time, and
platinum concentration in neurologic tissues, and morphologic
evidence of toxicity. J Clin Oncol. 10:795–803. 1992.PubMed/NCBI
|
|
17
|
DeConti RC, Toftness BR, Lange RC and
Creasey WA: Clinical and pharmacological studies with
cis-diamminedichloroplatinum (II). Cancer Res. 33:1310–1315.
1973.PubMed/NCBI
|
|
18
|
Go RS and Adjei AA: Review of the
comparative pharmacology and clinical activity of cisplatin and
carboplatin. J Clin Oncol. 17:409–422. 1999.PubMed/NCBI
|
|
19
|
Schweitzer VG: Cisplatin-induced
ototoxicity: the effect of pigmentation and inhibitory agents.
Laryngoscope. 103:1–52. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lautermann J, Song B, McLaren J and
Schacht J: Diet is a risk factor in cisplatin ototoxicity. Hear
Res. 88:47–53. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka F, Whitworth CA and Rybak LP:
Influence of pH on the ototoxicity of cisplatin: a round window
application study. Hear Res. 177:21–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertolaso L, Martini A, Bindini D, et al:
Apoptosis in the OC-k3 immortalized cell line treated with
different agents. Audiology. 40:327–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hyppolito MA, de Oliveira JA and Rossato
M: Cisplatin ototoxicity and otoprotection with sodium salicylate.
Eur Arch Otorhinolaryngol. 263:798–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Previati M, Lanzoni I, Corbacella E, et
al: RNA expression induced by cisplatin in an organ of
Corti-derived immortalized cell line. Hear Res. 196:8–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arafa HM, Abdel-Hamid MA, El-Khouly AA,
Elmazar MM and Osman AM: Enhancement by dexamethasone of the
therapeutic benefits of cisplatin via regulation of tumor
angiogenesis and cell cycle kinetics in a murine tumor paradigm.
Toxicology. 222:103–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drottar M, Liberman MC, Ratan RR and
Roberson DW: The histone deacetylase inhibitor sodium butyrate
protects against cisplatin-induced hearing loss in guinea pigs.
Laryngoscope. 116:292–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hatzopoulos S, Di Stefano M, Albertin A
and Martini A: Evaluation of cisplatin ototoxicity in a rat animal
model. Ann NY Acad Sci. 884:211–225. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Previati M, Lanzoni I, Astolfi L, et al:
Cisplatin cytotoxicity in organ of Corti-derived immortalized
cells. J Cell Biochem. 101:1185–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Previati M, Lanzoni I, Corbacella E, et
al: Cisplatin-induced apoptosis in human promyelocytic leukemia
cells. Int J Mol Med. 18:511–516. 2006.PubMed/NCBI
|
|
31
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Timmer-Bosscha H, Mulder NH and de Vries
EG: Modulation of cis-diamminedichloroplatinum(II) resistance: a
review. Br J Cancer. 66:227–238. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gosland M, Lum B, Schimmelpfennig J, Baker
J and Doukas M: Insights into mechanisms of cisplatin resistance
and potential for its clinical reversal. Pharmacotherapy. 16:16–39.
1996.PubMed/NCBI
|
|
34
|
Adjei AA, Budihardjo II, Rowinsky EK, et
al: Cytotoxic synergy between pyrazoloacridine (NSC 366140) and
cisplatin in vitro: inhibition of platinum-DNA adduct removal. Clin
Cancer Res. 3:761–770. 1997.PubMed/NCBI
|
|
35
|
Rybak LP and Whitworth CA: Ototoxicity:
therapeutic opportunities. Drug Discov Today. 10:1313–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van den Berg JH, Beijnen JH, Balm AJ and
Schellens JH: Future opportunities in preventing cisplatin induced
ototoxicity. Cancer Treat Rev. 32:390–397. 2006.PubMed/NCBI
|
|
37
|
Daldal A, Odabasi O and Serbetcioglu B:
The protective effect of intratympanic dexamethasone on
cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck
Surg. 137:747–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Astolfi L, Simoni E, Ciorba A and Martini
A: In vitro protective effects of Ginkgo biloba against cisplatin
toxicity in mouse cell line OCk3. Audiol Med. 6:251–258. 2008.
View Article : Google Scholar
|
|
39
|
Chen WC, Jackson A, Budnick AS, et al:
Sensorineural hearing loss in combined modality treatment of
nasopharyngeal carcinoma. Cancer. 106:820–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rademaker-Lakhai JM, Crul M, Pluim D, et
al: Phase I clinical and pharmacologic study of a 2-weekly
administration of cisplatin and gemcitabine in patients with
advanced non-small cell lung cancer. Anticancer Drugs.
16:1029–1036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rivera F, Vega-Villegas ME, López-Brea MF,
et al: Long term results of a phase II trial of induction
chemotherapy with uracil-ftegafur (UFT), vinorelbine and cisplatin
(UFTVP) followed by radiotherapy concomitant with UFT and
carboplatin (RT/UFTJ) in non-resectable locally advanced (stage
IV-B) squamous cell head and neck carcinoma and peripheral blood
stem cell support (PBSCS) with febrile neutropenia. Clin Transl
Oncol. 9:40–47. 2007.
|
|
42
|
Uhm JE, Lim HY, Kim WS, et al: Paclitaxel
with cisplatin as salvage treatment for patients with previously
treated advanced transitional cell carcinoma of the urothelial
tract. Neoplasia. 9:18–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zuur CL, Simis YJ, Verkaik RS, et al:
Hearing loss due to concurrent daily low-dose cisplatin
chemoradiation for locally advanced head and neck cancer. Radiother
Oncol. 89:38–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Regione Emilia-Romagna certified
(Internet). Reportistica predefinita REM anno. 2006, (updated 2007
Oct 30). Available from: http://www.regione.emilia-romagna.it/sas/rem/report_predef/2006/REM05_2.htm.
|
|
45
|
Kris MG, Hesketh PJ, Somerfield MR, et al:
American Society of Clinical Oncology guideline for antiemetics in
oncology: update 2006. J Clin Oncol. 24:2932–2947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Atallah E and Flaherty L: Treatment of
metastatic malignant melanoma. Curr Treat Options Oncol. 6:185–193.
2005. View Article : Google Scholar
|
|
47
|
von der Maase H: Current and future
perspectives in advanced bladder cancer: is there a new standard?
Semin Oncol. 29:3–14. 2002.PubMed/NCBI
|
|
48
|
Tiseo M and Ardizzoni A: Chemotherapy in
the treatment of thymic tumours. Oncol Rev. 2:95–101. 2008.
View Article : Google Scholar
|
|
49
|
Girard N, Mornex F, Van Houtte P, Cordier
JF and van Schil P: Thymoma: a focus on current therapeutic
management. J Thorac Oncol. 4:119–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
National Cancer Institute.
Gastrointestinal Complications PDQ®. 2012, National
Cancer Institute; Bethesda, MD: (Updated 07/18/2012). Available
from: http://cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/HealthProfessional.
|