Introduction
Ovarian cancer (OVCA) is the fifth leading cause of
cancer-related mortality among women in the United States and
Western Europe, with the highest mortality rate of all gynecologic
malignancies. Approximately 75% of patients with OVCA are diagnosed
at an advanced stage (III/IV) with disseminated intraperitoneal
metastases (1). Although the
majority of patients initially experience a complete clinical
response to primary surgery followed by platinum/taxane-based
chemotherapy, most patients eventually develop
platinum/taxane-resistant persistent or recurrent disease. These
patients have a poor prognosis. Despite cytoreductive surgery and
aggressive chemotherapy, the majority of patients with OVCA succumb
to the disease within 5 years (2).
These dismal statistics highlight the need for research into the
cellular basis of platinum response, as well as the design of
targeted therapeutic strategies.
The hepatocyte growth factor (HGF) signaling pathway
may be a viable target for the development of directed therapeutic
regimens for the treatment of OVCA. HGF is the only known ligand of
the HGF receptor (c-Met), which is expressed in approximately 70%
of OVCAs (3,4). Overexpression of HGF and/or c-Met has
been associated with poor clinical outcome in OVCA (5–7),
whereas c-Met overexpression has further been associated with a
chemo-resistant subset of ovarian clear-cell adenocarcinomas
(8,9). The targeted inhibition of c-Met has
been shown to reduce c-Met expression, block OVCA cell
proliferation, and reduce tumor burden in pre-clinical mouse models
(10–12).
In this study, we analyzed the expression of the HGF
receptor, c-Met, in primary OVCA and OVCA cell lines, and tested
the activity of the HGF/c-Met signaling inhibitor, MK8033, alone
and in combination with standard of care therapy (carboplatin plus
paclitaxel) in a panel of OVCA cells. We then identified the genes
associated with sensitivity to MK8033 plus carboplatin-paclitaxel
and used the principal component analysis (PCA) to define a gene
expression signature of OVCA cell response. Finally, this PCA
response signature was tested in an independent survival dataset of
primary OVCA for which response to chemotherapy and overall
survival were known.
Materials and methods
Cell culture
OVCA cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA) (CAOV3, OV90, OVCAR3 and
SKOV3); from the European Collection of Cell Cultures (Salisbury,
England) (A2780CP and A2780S); from Kyoto University (Japan) (CHI,
CHIcisR, M41, M41CSR, Tyknu and TyknuCisR); or were kind gifts from
Dr Patricia Kruk, Department of Pathology, College of Medicine,
University of South Florida, Tampa, FL, and Professor Susan Murphy,
Department of OBGYN/Division of Gynecologic Oncology, Duke
University, Durham, NC, USA (A2008, C13, CAOV2, HeyA8, IGR-OV1,
IMCC3, IMCC5, MCAS, OV2008, OVCA420, OVCA429, OVCA432, OVCA433,
FUOV1, PEO1, PEO4, SK-OV-6, T8, TOV-112D, TOV-21-G, Dov13, BG1,
Ovary1847, OVCAR10, OVCAR8, OVCAR5, OVCAR4, OVCAR2 and SK-OV-4).
Cell lines were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (Fisher Scientific,
Pittsburg, PA, USA), 1% sodium pyruvate, 1% penicillin/streptomycin
(Cellgro, Manassas, VA, USA), and 1% nonessential amino acids
(HyClone, Hudson, NH, USA). Mycoplasma testing was performed every
6 months in accordance with the manufacturer's protocol (Lonza,
Rockland, ME, USA).
Staining and scoring of c-Met expression
in OVCA
Slides were stained using a Ventana Discovery XT
automated system as per the manufacturer's protocol with
proprietary reagents (Ventana Medical Systems, Tucson, AZ, USA).
Briefly, slides were deparaffinized on the automated system with EZ
Prep solution (Ventana). The heat-induced antigen retrieval method
was used in Cell Conditioning 1 (Ventana). The mouse monoclonal
antibody that reacts to c-Met (#18-7366; Invitrogen) was used at a
1:2,000 concentration in PSS antibody diluent (Ventana) and
incubated for 60 min. The Ventana OmniMap anti-mouse secondary
antibody was used for 16 min. The detection system used was the
Ventana ChromoMap kit, and slides were counterstained with
hematoxylin. Slides were then dehydrated and coverslipped as per
normal laboratory protocol. The c-Met expression score was defined
as the product of intensity and cellularity, where an intensity of
1 was weak; 2, moderate; 3, strong, and a cellularity of 1 ≤33%, 2
=34–65% and 3 ≥66%.
RNA extraction and microarray expression
analysis
RNA extraction and microarray expression analyses
were performed as previously described (13). Briefly, RNA was extracted using the
RNeasy kit following the manufacturer's recommendations (Qiagen,
Valencia, CA, USA). Quality of the RNA was measured using an
Agilent 2100 Bioanalyzer. The targets for Affymetrix DNA microarray
analysis were prepared according to the manufacturer's
instructions. For OVCA cell lines (n=41), targets were hybridized
to customized Human Affymetrix HuRSTA gene chips (HuRSTA-2a520709)
[GEO accession number GSE34615 (14)].
Statistical analyses
Expression data were subjected to background
correction and normalization using the Robust Multichip Average
algorithm in the Affymetrix Expression Console (http://www.affymetrix.com/products/software/specific/expression_console_software.affx).
Pearson's correlation test was performed on individual gene
expression and IC50 values. Probe sets with P<0.001
were considered to have significant correlations with
IC50 values.
Cell viability assays
For cells cultured in the presence of cisplatin,
resistance was quantified using MTT proliferation assay, in
accordance with manufacturer's instructions (Promega, Madison, WI,
USA). Cells (5,000/well) were seeded in 96-well plates, allowed to
adhere, and incubated with increasing doses of cisplatin for 48 h
at 37°C. Following drug incubation, plates were read at 570 nm
using a SpectraMax 190 microplate reader (Molecular Devices, USA).
For drug combination experiments, CellTiter-Blue cell viability
assays were performed using 384-well plates and an automated
pipetting station. Briefly, for these assays, 1.2×103
cells (24-μl volume) were plated in each well and allowed to adhere
overnight at 37°C and 5% CO2. Drug dilutions were then
added using the same pipetting station, and the plate was incubated
for 72 h. Following drug incubation, fluorescence was measured
using a Synergy 4 microplate reader (Bio-Tek Instruments, Inc.).
The fluorescence data were transferred to a spreadsheet program to
calculate the percent viability relative to untreated cells.
Experiments were repeated a minimum of three times.
Synergism analyses
Drug combination experiments were analyzed for
synergistic, additive, or antagonistic effects using the
combination index method developed by Chou and Talalay. For the
application of this method, the drug concentration dilutions of the
drugs were used at fixed dose ratios based on the IC50
values of each drug obtained from preliminary experiments (e.g.,
50:1, 2:5, 1:250). Briefly, the dose-effect curve for each drug
alone is determined based on experimental observations using the
median-effect principle and is compared to the effect achieved with
a combination of the two drugs to derive a combination index value.
This method involves plotting dose-effect curves, for each agent
and their combination, using the following median-effect equation:
fa/fu = (D/Dm)m, where D is the dose of the drug, Dm is the dose
required for a 50% effect (equivalent to IC50), fa and
fu are the affected and unaffected fractions, respectively
(fa=1-fu), and m is the exponent signifying the sigmoidicity of the
dose-effect curve. The computer software program XLfit was used to
calculate the values of Dm and m. The combination index values used
for the analysis of the drug combinations were determined by the
isobologram equation for mutually nonexclusive drugs that have
different modes of action: combination index = (D)1/(Dx)1 +
(D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (Dx)1 and (Dx)2 in the
denominators are the doses (or concentrations) for D1 (drug 1) and
D2 (drug 2) alone that gives x% inhibition, whereas (D)1 and (D)2
in the numerators are the doses of drugs 1 and 2 in combination
that also inhibited x% (i.e., isoeffective). Combination indices
<1, equal to 1, and >1 indicate synergism, additive effects
and antagonism, respectively.
Building signatures
The PCA methodology was used to derive a gene
expression signature with a corresponding score. First, the data
were reduced into a small set of uncorrelated principal components.
This set of principal components was generated based on its ability
to account for variation. The first principal component (1st PCA)
is used to represent the overall variability in the data. The gene
expression score is equal to
∑wixi, a weighted
average expression among the differentially expressed genes, where
xi represents gene i expression
level, wi is the corresponding weight
(loading coefficient) with
∑w2i = 1, and the
wi values maximize the variance of
∑wixi.
Directional signs of PCA scores are recognized to be arbitrary and
can vary between software and the algorithm used to calculate the
PCA model (15); however, this does
not affect the interpretation of the PCA model and can be easily
solved by multiplying both scores and loadings by −1, a 180°
rotation. Details of this methodology have been reported by our
group previously (13).
Associations with overall survival from
OVCA
PCA models were then explored for associations with
overall survival from OVCA (using median PC1 score as the threshold
to define high vs. low pathway score) using a dataset for which
both gene expression and overall survival data were available, the
publicly available Australian (Aus) Dataset (Affymetrix U133Plus
GeneChips, n=218).
Results
c-Met is significantly expressed in
OVCA
Since overexpression of HGF has been associated with
poor clinical outcome in OVCA (5–7), we
evaluated the expression of c-Met, the only known HGF receptor, in
OVCA cells and primary OVCA tumor samples. Pathological scoring of
c-Met immunostains (cellularity × intensity) in 79 primary OVCA
samples and 41 OVCA cell lines indicated the presence of c-Met
expression in 92% (73/79) (Table I)
and 83% (34/41) (Table II) of
samples, respectively. c-Met expression in primary OVCA did not
appear to be associated with stage, response to primary platinum
therapy, or CA125 levels (Table
I).
 | Table Ic-Met expression in ovarian cancer
samples and available clinical data. |
Table I
c-Met expression in ovarian cancer
samples and available clinical data.
| Sample | Diagnosis | Score | CR vs. IR | Debulking | Age (years) | Stage | CA125 |
|---|
| 1 | Focal
adenocarcinoma | 6 | CR | O | 57 | IIIC | 541 |
| 2 | Papillary serous
adenocarcinoma | 1 | CR | O | 84 | IIIC | 7350 |
| 3 | Adenocarcinoma | 2 | CR | O | 53 | IIIC | |
| 4 | Papillary serous
adenocarcinoma | 4 | CR | O | 61 | IIIC | |
| 5 | Papillary serous
adenocarcinoma | 6 | CR | O | 57 | IIIC | |
| 6 | Papillary serous
adenocarcinoma | 1 | CR | O | 55 | IV | |
| 7 | Papillary serous
adenocarcinoma | 2 | CR | O | 49 | IIIC | 305 |
| 8 | Papillary serous
adenocarcinoma | 1 | CR | O | 65 | IIIC | |
| 9 | Papillary serous
adenocarcinoma | 4 | CR | O | 60 | IIIC | 925 |
| 10 | Papillary serous
adenocarcinoma | 2 | CR | O | 51 | IIIB | |
| 11 | Papillary serous
adenocarcinoma | 4 | CR | O | 56 | IIIC | 316 |
| 12 | Papillary serous
adenocarcinoma | 2 | CR | O | 66 | IIIC | 224 |
| 13 | Papillary serous
adenocarcinoma | 2 | CR | S | 56 | IIIC | |
| 14 | Papillary serous
adenocarcinoma | 4 | CR | O | 73 | IIIC | |
| 15 | Papillary serous
adenocarcinoma | 1 | CR | O | 62 | IIIC | 1717 |
| 16 | Papillary serous
adenocarcinoma | 2 | CR | O | 78 | IIIC | |
| 17 | Papillary serous
adenocarcinoma | 6 | CR | O | 43 | IIIC | 64 |
| 18 | Papillary serous
adenocarcinoma | 1 | CR | O | 45 | IIIC | |
| 19 | Papillary serous
adenocarcinoma | 1 | CR | S | 74 | IIIC | 1800 |
| 20 | Papillary serous
adenocarcinoma | 2 | CR | O | 76 | IIIC | |
| 21 | Papillary serous
adenocarcinoma | 2 | IR | S | 79 | IV | |
| 22 | Papillary serous
adenocarcinoma | 2 | IR | S | 71 | IV | 1636 |
| 23 | Papillary serous
adenocarcinoma | 2 | CR | O | 56 | IIIC | |
| 24 | Papillary serous
adenocarcinoma | 1 | CR | O | 81 | IIIC | |
| 25 | Papillary serous
adenocarcinoma | 2 | CR | O | 56 | IIIC | 260 |
| 26 | papillary serous
adenocarcinoma | 3 | CR | O | 35 | IV | 47 |
| 27 | Papillary serous
adenocarcinoma | 2 | CR | O | 53 | IIIA | |
| 28 | Papillary serous
adenocarcinoma | 1 | CR | S | 77 | IV | >600 |
| 29 | Papillary serous
adenocarcinoma | 2 | CR | O | 65 | IIIC | 1118 |
| 30 | Papillary serous
adenocarcinoma | 1 | CR | S | 47 | IIIC | 712 |
| 31 | Papillary serous
adenocarcinoma | 2 | CR | S | 76 | IIIC | 1848 |
| 32 | Papillary serous
adenocarcinoma | 2 | CR | O | 70 | IIIC | |
| 33 | Papillary serous
adenocarcinoma | 3 | CR | S | 57 | IIIC | 266 |
| 34 | Adenocarcinoma
metastatic | 2 | CR | O | 57 | IIIC | 175 |
| 35 | Papillary serous
adenocarcinoma | 1 | CR | O | 65 | IV | 404 |
| 36 | Papillary serous
adenocarcinoma | 2 | CR | O | 76 | IV | |
| 37 | Papillary serous
adenocarcinoma | 1 | CR | O | 66 | IIIC | |
| 38 | Papillary serous
adenocarcinoma | 1 | CR | O | 68 | IIIC | |
| 39 | Papillary serous
adenocarcinoma | 3 | IR | O | 73 | IIIC | |
| 40 | Papillary serous
adenocarcinoma | 2 | CR | O | 63 | IV | |
| 41 | Papillary serous
adenocarcinoma | 2 | IR | S | 63 | IIIC | |
| 42 | Papillary serous
adenocarcinoma | 2 | IR | O | 47 | IIIC | |
| 43 | Papillary serous
adenocarcinoma | 2 | CR | O | 42 | IIIC | 110 |
| 44 | Papillary serous
adenocarcinoma | 1 | CR | O | 74 | IIIC | 4557 |
| 45 | Papillary serous
adenocarcinoma | 2 | CR | S | 64 | IIIC | |
| 46 | Papillary serous
adenocarcinoma | 2 | IR | S | 64 | IIIC | 456 |
| 47 | Papillary serous
adenocarcinoma | 1 | IR | O | 71 | IIIC | |
| 48 | Papillary serous
adenocarcinoma | 6 | IR | O | 69 | IIIC | |
| 49 | Papillary serous
adenocarcinoma | 3 | CR | O | 49 | IIIC | |
| 50 | Papillary serous
adenocarcinoma | 2 | CR | O | 62 | IV | |
| 51 | Papillary serous
adenocarcinoma | 2 | IR | | | | |
| 52 | Focal
adenocarcinoma | 3 | CR | S | 88 | IIIC | |
| 53 | Serous
adenocarcinoma | 1 | IR | O | 74 | IIIC | 101 |
| 54 | Papillary serous
adenocarcinoma | 2 | IR | O | 71 | IIIC | |
| 55 | Papillary serous
adenocarcinoma | 4 | IR | O | 69 | IIIC | 1606 |
| 56 | Papillary serous
adenocarcinoma | 1 | IR | O | 52 | IIIC | |
| 57 | Papillary serous
adenocarcinoma | 2 | CR | O | 67 | IIIC | |
| 58 | Papillary serous
adenocarcinoma | 1 | CR | O | 66 | IIIC | 824 |
| 59 | Papillary Serous
adenocarcinoma | 2 | IR | O | 52 | IIIC | |
| 60 | Papillary serous
adenocarcinoma | 1 | CR | S | 73 | IIIC | 2354 |
| 61 | Papillary serous
adenocarcinoma | 0 | CR | O | 75 | IIIC | |
| 62 | Papillary serous
adenocarcinoma | 2 | IR | O | 65 | IIIC | |
| 63 | Focal cellular
atypia | n/a | CR | O | 74 | IIIC | |
| 64 | Papillary serous
adenocarcinoma | 2 | IR | O | 79 | IIIC | 417 |
| 65 | Papillary serous
adenocarcinoma | 1 | CR | O | 73 | IIIC | 180 |
| 66 | Papillary serous
adenocarcinoma | 2 | IR | O | 53 | IV | 96 |
| 67 | Papillary serous
adenocarcinoma | 1 | CR | O | 60 | IIIC | |
| 68 | Papillary serous
adenocarcinoma | 2 | CR | | | | |
| 69 | Papillary serous
adenocarcinoma | 2 | IR | S | 53 | IIIC | |
| 70 | Papillary serous
adenocarcinoma | 1 | IR | O | 41 | IIIC | 2800 |
| 71 | Papillary serous
adenocarcinoma | 1 | CR | O | 80 | IIIC | |
| 72 | Papillary serous
adenocarcinoma | 2 | CR | O | 42 | IIIA | |
| 73 | Papillary serous
adenocarcinoma | 2 | IR | S | 66 | IIIC | 90 |
| 74 | Papillary serous
adenocarcinoma | 1 | CR | S | 60 | IIIC | 750 |
| 75 | Papillary serous
adenocarcinoma | 4 | CR | O | 77 | IIIC | 9814 |
| 76 | Papillary serous
adenocarcinoma | 0 | CR | O | 72 | III | |
| 77 | Papillary serous
adenocarcinoma | 1 | IR | O | 66 | IIIC | |
| 78 | Papillary serous
adenocarcinoma | 0 | CR | O | 54 | III | |
| 79 | Papillary serous
adenocarcinoma | 3 | CR | O | 38 | IIIC | |
 | Table IIc-Met expression in ovarian cancer
cell lines. |
Table II
c-Met expression in ovarian cancer
cell lines.
| Cell line | Cellularity | Intensity | Score |
|---|
| A2008 | 2 | 2 | 4 |
| A2780CP | 0 | 0 | 0 |
| A2780S | 1 | 1 | 1 |
| BGI | 0 | 0 | 0 |
| C13 | 3 | 1 | 3 |
| CAOV3 | 3 | 1 | 3 |
| CHI | 0 | 0 | 0 |
| CHI cisR | 1 | 1 | 1 |
| CAOV2 | 3 | 2 | 6 |
| Dov 13 | 3 | 2 | 6 |
| HeyA8 | 3 | 1 | 3 |
| IGR-OV1 | 3 | 2 | 6 |
| IMCC3 | 2 | 1 | 2 |
| IMCC5 | 1 | 1 | 1 |
| M41 | 1 | 1 | 1 |
| M41CSR | 2 | 1 | 2 |
| MCAS | 3 | 1 | 3 |
| OV2008 | 1 | 1 | 1 |
| OV90 | 1 | 1 | 1 |
| Ovary1847 | 1 | 1 | 1 |
| OVCA 429 | 2 | 1 | 2 |
| OVCA 432 | Acellular | n/a | n/a |
| OVCA 433 | 3 | 1 | 3 |
| OVCA420 | 1 | 2 | 2 |
| OVCAR10 | 0 | 0 | 0 |
| OVCAR2 | 3 | 2 | 6 |
| OVCAR3 | 2 | 1 | 2 |
| OVCAR4 | 3 | 2 | 6 |
| OVCAR5 | 3 | 2 | 6 |
| OVCAR8 | 3 | 1 | 3 |
| PEO1 | 3 | 2 | 6 |
| PEO4 | 2 | 2 | 4 |
| SKOV8 | 2 | 1 | 2 |
| SKOV3 | 3 | 2 | 6 |
| SKOV4 | 0 | 0 | 0 |
| SKOV6 | 2 | 1 | 2 |
| T8 | 3 | 2 | 6 |
| Tov-112D | 2 | 1 | 2 |
| Tov-21-G | 1 | 1 | 1 |
| Tyknu | 0 | 0 | 0 |
| Tyknu CisR | 0 | 0 | 0 |
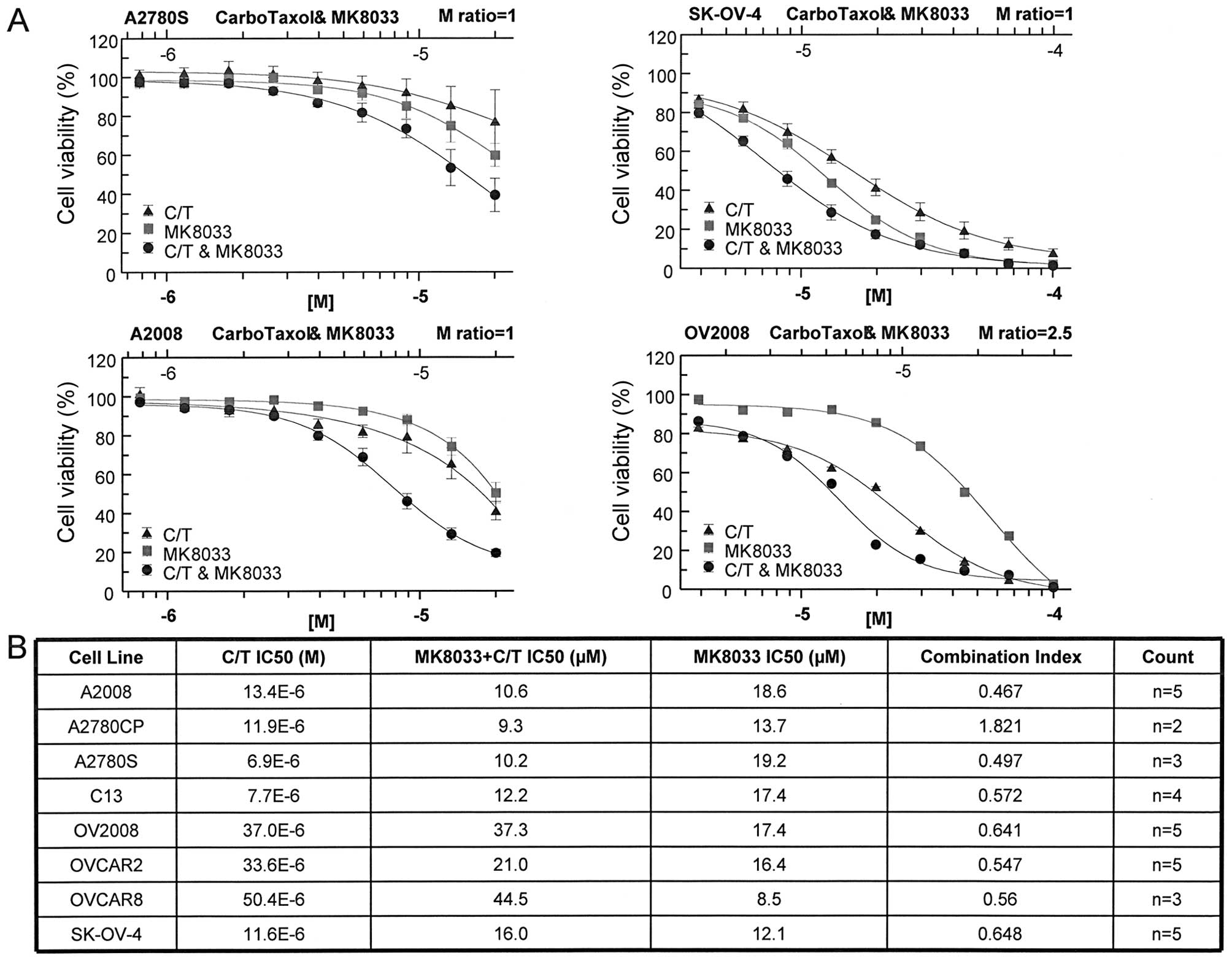
Inhibition of HGF/c-Met signaling has in
vitro anti-proliferative effects on OVCA cells and synergizes with
combination carboplatin plus paclitaxel
To determine whether HGF signaling influenced OVCA
cell line chemosensitivity, we evaluated the activity of MK8033, an
inhibitor of HGF/c-Met signaling. MK8033 exhibited strong
anti-proliferative effects against a panel of OVCA cell lines
(Fig. 1). Furthermore, when
combined in constant molar ratio of 1 with carboplatin plus
paclitaxel (molar ratio of 20,000:1), MK8033 significantly lowered
the carboplatin-paclitaxel IC50 in the majority of cell
lines tested (Fig. 1). Combination
index values calculated using the Chou-Talalay isobologram equation
indicated synergistic activity between MK8033 and
carboplatin-paclitaxel for almost all OVCA cell lines tested, as
indicated by combination index values of <0.7 (Fig. 1).
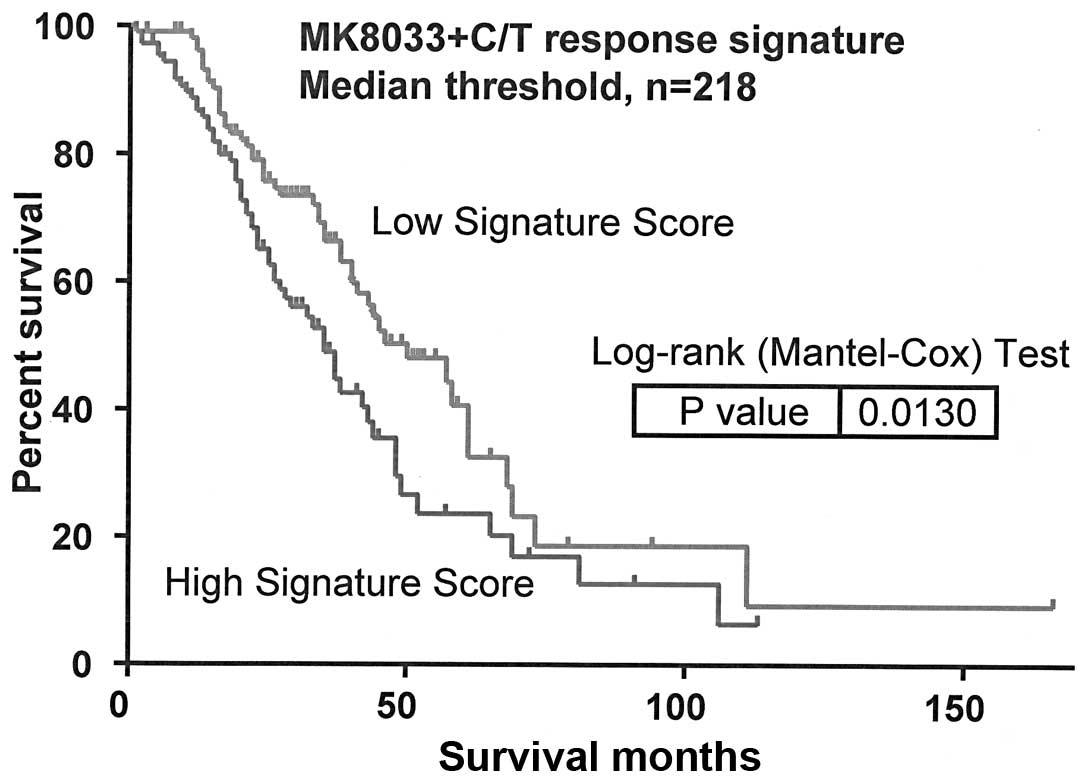
Molecular determinants of
MK8033-carboplatin-paclitaxel sensitivity influence overall
survival from OVCA
Pearson's correlation test of IC50 values
and Affymetrix HuRSTA genechip expression data identified 47 genes
to be correlated with MK8033 plus carboplatin-paclitaxel
sensitivity [false discovery rate (FDR) of <0.2] (Table III). PCA modeling of these genes
indicated that the expression signature was associated with overall
survival from OVCA (AUS dataset, n=218, P=0.013) (Fig. 2).
 | Table IIIGenes associated with ovarian cancer
cell sensitivity to MK8033 plus carboplatin-paclitaxel. |
Table III
Genes associated with ovarian cancer
cell sensitivity to MK8033 plus carboplatin-paclitaxel.
| Signature gene
symbols |
|---|
| SETD6 | UBQLN1 | HDAC3 | LOC653441 |
| RNF207 | NDOR1 | CLTCL1 | PHC1 |
| ABCC8 | IGHM | PPIL4 | MATR3 |
| DNASE1L1 | FBXO4 | REST | CLYBL |
| ACD | PRKAA1 | LPHN3 | MLLT4 |
| NUP155 | RIPK4 | DKFZp667 | EFCBP2 |
| FAM91A2 | TASP1 | ACTN2 | KIAA0528 |
| FAM91A1 | ADRBK2 | OSBPL6 | PPM1J |
| MGC5566 | FAM46C | STAR | TMEFF1 |
| MRPL30 | ITIH4 | FUNDC2 | SGK269 |
| MAGIX | C1orf164 | RAB15 | LOC389634 |
| HFE | IL17RD | EDA | |
Discussion
HGF is a paracrine growth factor originally found to
be associated with liver regeneration (16–18).
HGF signaling orchestrates a multitude of biological functions
during embryogenesis, including cellular migration, invasion,
proliferation, angiogenesis, wound healing, and tissue repair
(19–29), and has been further associated with
the development or progression of several different types of
cancer, including OVCA (6,7,12,30–45).
In OVCA, increased levels of HGF or overexpression of the HGF
receptor c-Met has been associated with poor clinical outcome
(5–7,16–18).
We found a near ubiquitous expression of c-Met in
both primary OVCA and OVCA cell lines. To better understand the
importance of HGF signaling in OVCA, we evaluated the activity of
the novel inhibitor of HGF/c-Met signaling, MK8033, alone and in
combination with carboplatin plus paclitaxel in a panel of OVCA
cell lines (n=8). Although MK8033 showed anti-proliferative effects
as a single agent, when used in a constant ratio to
carboplatin-paclitaxel, the isobologram analysis indicated
synergistic activity in the inhibition of OVCA cell proliferation.
Pearson's correlation of cell line gene expression data and
IC50 values identified the expression of 47 genes to be
correlated with sensitivity to MK8033 plus carboplatin-paclitaxel.
As shown by PCA modeling, genes correlated with MK8033 plus
carboplatin-paclitaxel response were associated with overall
survival from OVCA, suggesting that these genes may have a
biological influence on OVCA response to therapy. Further analysis
of the MK8033 plus carboplatin-paclitaxel response signature
identified 6 of 47 genes to be significantly correlated with OVCA
survival, including i) HFE (+ correlation, P=0.02); ii) UBQLN1 (+
correlation, P=0.04); iii) CLTCL1 (- correlation, P=0.04); iv)
LPHN3 (- correlation, P=0.04); v) OSBPL6 (- correlation, P=0.03);
and vi) SGK269 (- correlation, P=0.04).
The products of several of these genes have
previously been implicated in the development and/or progression of
cancer. For example, the product of the HFE gene, hemochromatosis
protein, is involved in the regulation of iron absorption (46). Mutations in HFE and deregulation of
iron absorption have been linked to liver cirrhosis and colon
cancer risk (47,48). Ubiquilin 1, encoded by the UBQLN1
gene, has been shown to be associated with lung adenocarcinoma
(49) and may affect OVCA response
to metallodrugs (50). CLTCL1
encodes the heavy chain of clathrin, and the expression and/or
activity of clathrin has been found to be associated with bladder
cancer (51), TRAIL-resistant
breast cancer (52), and uptake of
some chemotherapeutic agents (53).
Furthermore, the oxysterol binding protein (OSBP)-related protein
6, encoded by the OSBPL6 gene, belongs to a family of proteins
recently identified as receptors for several natural products,
including cephalostatin-1, ritterazine B, schweinfurthin A and
OSW-1 (54).
In summary, we showed that targeted inhibition of
HGF/c-Met signaling using the c-Met specific inhibitor, MK8033,
worked synergistically with combinations of carboplatin plus
paclitaxel to induce OVCA cell growth arrest. Furthermore, we
demonstrated that genes associated with response to MK8033 plus
carboplatin-paclitaxel may influence overall survival from OVCA,
based on PCA modeling. The results of this study suggest that the
inhibition of HGF/c-Met signaling may be a beneficial addition to
the OVCA standard of care regimen of carboplatin plus paclitaxel
therapy.
Acknowledgements
The authors thank Rasa Hamilton (Moffitt Cancer
Center) for editorial assistance. We would also like to thank Merck
Pharmaceuticals for their contributions. This study was supported
in part by the Hearing the Ovarian Cancer Whisper, Jacquie Liggett
Foundation, the Ocala Royal Dames for Cancer Research Inc., and the
Phi Beta Psi Sorority.
References
|
1
|
Baker VV: Salvage therapy for recurrent
epithelial ovarian cancer. Hematol Oncol Clin North Am. 17:977–988.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omura GA, Brady MF, Homesley HD, et al:
Long-term follow-up and prognostic factor analysis in advanced
ovarian carcinoma: the Gynecologic Oncology Group experience. J
Clin Oncol. 9:1138–1150. 1991.PubMed/NCBI
|
|
3
|
Di Renzo MF, Narsimhan RP, Olivero M, et
al: Expression of the Met/HGF receptor in normal and neoplastic
human tissues. Oncogene. 6:1997–2003. 1991.
|
|
4
|
Huntsman D, Resau JH, Klineberg E and
Auersperg N: Comparison of c-met expression in ovarian epithelial
tumors and normal epithelia of the female reproductive tract by
quantitative laser scan microscopy. Am J Pathol. 155:343–348. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aune G, Lian AM, Tingulstad S, et al:
Increased circulating hepatocyte growth factor (HGF): a marker of
epithelial ovarian cancer and an indicator of poor prognosis.
Gynecol Oncol. 121:402–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goode EL, Chenevix-Trench G, Hartmann LC,
et al: Assessment of hepatocyte growth factor in ovarian cancer
mortality. Cancer Epidemiol Biomarkers Prev. 20:1638–1648. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawada K, Radjabi AR, Shinomiya N, et al:
c-Met overexpression is a prognostic factor in ovarian cancer and
an effective target for inhibition of peritoneal dissemination and
invasion. Cancer Res. 67:1670–1679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Gene amplification and protein
overexpression of MET are common events in ovarian clear-cell
adenocarcinoma: their roles in tumor progression and
prognostication of the patient. Mod Pathol. 24:1146–1155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Accumulative copy number increase of MET
drives tumor development and histological progression in a subset
of ovarian clear-cell adenocarcinomas. Mod Pathol. 25:122–130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basha R, Ingersoll SB, Sankpal UT, et al:
Tolfenamic acid inhibits ovarian cancer cell growth and decreases
the expression of c-Met and survivin through suppressing
specificity protein transcription factors. Gynecol Oncol.
122:163–170. 2011. View Article : Google Scholar
|
|
11
|
Zillhardt M, Christensen JG and Lengyel E:
An orally available small-molecule inhibitor of c-Met, PF-2341066,
reduces tumor burden and metastasis in a preclinical model of
ovarian cancer metastasis. Neoplasia. 12:1–10. 2010.
|
|
12
|
Zillhardt M, Park SM, Romero IL, et al:
Foretinib (GSK1363089), an orally available multikinase inhibitor
of c-Met and VEGFR-2, blocks proliferation, induces anoikis, and
impairs ovarian cancer metastasis. Clin Cancer Res. 17:4042–4051.
2011. View Article : Google Scholar
|
|
13
|
Marchion DC, Cottrill HM, Xiong Y, et al:
BAD phosphorylation determines ovarian cancer chemosensitivity and
patient survival. Clin Cancer Res. 17:6356–6366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bicaku E, Xiong Y, Marchion DC, et al: In
vitro analysis of ovarian cancer response to cisplatin,
carboplatin, and paclitaxel identifies common pathways that are
also associated with overall patient survival. Br J Cancer.
106:1967–1975. 2012. View Article : Google Scholar
|
|
15
|
Jolliffe IT: Principal Component Analysis.
2nd edition. Springer-Verlag; New York: 2002
|
|
16
|
Nakamura T, Nawa K and Ichihara A: Partial
purification and characterization of hepatocyte growth factor from
serum of hepatectomized rats. Biochem Biophys Res Commun.
122:1450–1459. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura T, Teramoto H and Ichihara A:
Purification and characterization of a growth factor from rat
platelets for mature parenchymal hepatocytes in primary cultures.
Proc Natl Acad Sci USA. 83:6489–6493. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura T, Nawa K, Ichihara A, Kaise N
and Nishino T: Purification and subunit structure of hepatocyte
growth factor from rat platelets. FEBS Lett. 224:311–316. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeffers M, Rao MS, Rulong S, et al:
Hepatocyte growth factor/scatter factor-Met signaling induces
proliferation, migration, and morphogenesis of pancreatic oval
cells. Cell Growth Differ. 7:1805–1813. 1996.PubMed/NCBI
|
|
20
|
Montesano R, Matsumoto K, Nakamura T and
Orci L: Identification of a fibroblast-derived epithelial morphogen
as hepatocyte growth factor. Cell. 67:901–908. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidner KM, Di Cesare S, Sachs M,
Brinkmann V, Behrens J and Birchmeier W: Interaction between Gab1
and the c-Met receptor tyrosine kinase is responsible for
epithelial morphogenesis. Nature. 384:173–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bladt F, Riethmacher D, Isenmann S, Aguzzi
A and Birchmeier C: Essential role for the c-met receptor in the
migration of myogenic precursor cells into the limb bud. Nature.
376:768–771. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grant DS, Kleinman HK, Goldberg ID, et al:
Scatter factor induces blood vessel formation in vivo. Proc Natl
Acad Sci USA. 90:1937–1941. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar
|
|
26
|
Sonnenberg E, Meyer D, Weidner KM and
Birchmeier C: Scatter factor/hepatocyte growth factor and its
receptor, the c-met tyrosine kinase, can mediate a signal exchange
between mesenchyme and epithelia during mouse development. J Cell
Biol. 123:223–235. 1993. View Article : Google Scholar
|
|
27
|
Mizuno S and Nakamura T: Hepatocyte growth
factor: a regenerative drug for acute hepatitis and liver
cirrhosis. Regen Med. 2:161–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trusolino L, Bertotti A and Comoglio PM:
MET signalling: principles and functions in development, organ
regeneration and cancer. Nat Rev Mol Cell Biol. 11:834–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You WK and McDonald DM: The hepatocyte
growth factor/c-Met signaling pathway as a therapeutic target to
inhibit angiogenesis. BMB Rep. 41:833–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sattler M, Reddy MM, Hasina R, Gangadhar T
and Salgia R: The role of the c-Met pathway in lung cancer and the
potential for targeted therapy. Ther Adv Med Oncol. 3:171–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gumustekin M, Kargi A, Bulut G, et al:
HGF/c-Met overexpressions, but not met mutation, correlates with
progression of non-small cell lung cancer. Pathol Oncol Res.
18:209–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El-Attar HA and Sheta MI: Hepatocyte
growth factor profile with breast cancer. Indian J Pathol
Microbiol. 54:509–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Previdi S, Maroni P, Matteucci E, Broggini
M, Bendinelli P and Desiderio MA: Interaction between human-breast
cancer metastasis and bone microenvironment through activated
hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J
Cancer. 46:1679–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim EJ, Eom SJ, Hong JE, Lee JY, Choi MS
and Park JH: Benzyl isothiocyanate inhibits basal and hepatocyte
growth factor-stimulated migration of breast cancer cells. Mol Cell
Biochem. 369:431–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Derman MP, Chen JY, Spokes KC, Songyang Z
and Cantley LG: An 11-amino acid sequence from c-met initiates
epithelial chemotaxis via phosphatidylinositol 3-kinase and
phospholipase C. J Biol Chem. 271:4251–4255. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li C, Wu JJ, Hynes M, et al: c-Met is a
marker of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YJ, Kim DH, Lee SH, Kim DW, Nam HS and
Cho MK: Expression of the c-Met proteins in malignant skin cancers.
Ann Dermatol. 23:33–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syed ZA, Yin W, Hughes K, Gill JN, Shi R
and Clifford JL: HGF/c-met/Stat3 signaling during skin tumor cell
invasion: indications for a positive feedback loop. BMC Cancer.
11:1802011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshizawa Y, Yamada Y, Kanayama S, et al:
Signaling pathway involved in cyclooxygenase-2 up-regulation by
hepatocyte growth factor in endometrial cancer cells. Oncol Rep.
26:957–964. 2011.PubMed/NCBI
|
|
40
|
You H, Ding W, Dang H, Jiang Y and
Rountree CB: c-Met represents a potential therapeutic target for
personalized treatment in hepatocellular carcinoma. Hepatology.
54:879–889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Osada S, Kanematsu M, Imai H and Goshima
S: Clinical significance of serum HGF and c-Met expression in tumor
tissue for evaluation of properties and treatment of hepatocellular
carcinoma. Hepatogastroenterology. 55:544–549. 2008.PubMed/NCBI
|
|
42
|
Eksioglu-Demiralp E, Akdeniz T and Bayik
M: Aberrant expression of c-met and HGF/c-met pathway provides
survival advantage in B-chronic lymphocytic leukemia. Cytometry B
Clin Cytom. 80:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Que W and Chen J: Knockdown of c-Met
inhibits cell proliferation and invasion and increases
chemosensitivity to doxorubicin in human multiple myeloma U266
cells in vitro. Mol Med Rep. 4:343–349. 2011.PubMed/NCBI
|
|
44
|
Inno A, Salvatore MD, Cenci T, et al: Is
there a role for IGF1R and c-MET pathways in resistance to
cetuximab in metastatic colorectal cancer? Clin Colorectal Cancer.
10:325–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bu R, Uddin S, Bavi P, et al: HGF/c-Met
pathway has a prominent role in mediating antiapoptotic signals
through AKT in epithelial ovarian carcinoma. Lab Invest.
91:124–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou XY, Tomatsu S, Fleming RE, et al: HFE
gene knockout produces mouse model of hereditary hemochromatosis.
Proc Natl Acad Sci USA. 95:2492–2497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xue X, Taylor M, Anderson E, et al:
Hypoxia-inducible factor-2alpha activation promotes colorectal
cancer progression by dysregulating iron homeostasis. Cancer Res.
72:2285–2293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ba Q, Hao M, Huang H, et al: Iron
deprivation suppresses hepatocellular carcinoma growth in
experimental studies. Clin Cancer Res. 17:7625–7633. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang C and Saunders AJ: An emerging role
for Ubiquilin 1 in regulating protein quality control system and in
disease pathogenesis. Discov Med. 8:18–22. 2009.PubMed/NCBI
|
|
50
|
Guidi F, Puglia M, Gabbiani C, et al:
2D-DIGE analysis of ovarian cancer cell responses to cytotoxic gold
compounds. Mol Biosyst. 8:985–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Menashe I, Figueroa JD, Garcia-Closas M,
et al: Large-scale pathway-based analysis of bladder cancer
genome-wide association data from five studies of European
background. PLoS One. 7:e293962012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y and Zhang B: TRAIL resistance of
breast cancer cells is associated with constitutive endocytosis of
death receptors 4 and 5. Mol Cancer Res. 6:1861–1871. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bolden SW, Hambley JW, Johnston GA and
Rogers LJ: Neonatal stress and long-term modulation of GABA
receptors in rat brain. Neurosci Lett. 111:258–262. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Burgett AW, Poulsen TB, Wangkanont K, et
al: Natural products reveal cancer cell dependence on
oxysterol-binding proteins. Nat Chem Biol. 7:639–647. 2011.
View Article : Google Scholar : PubMed/NCBI
|