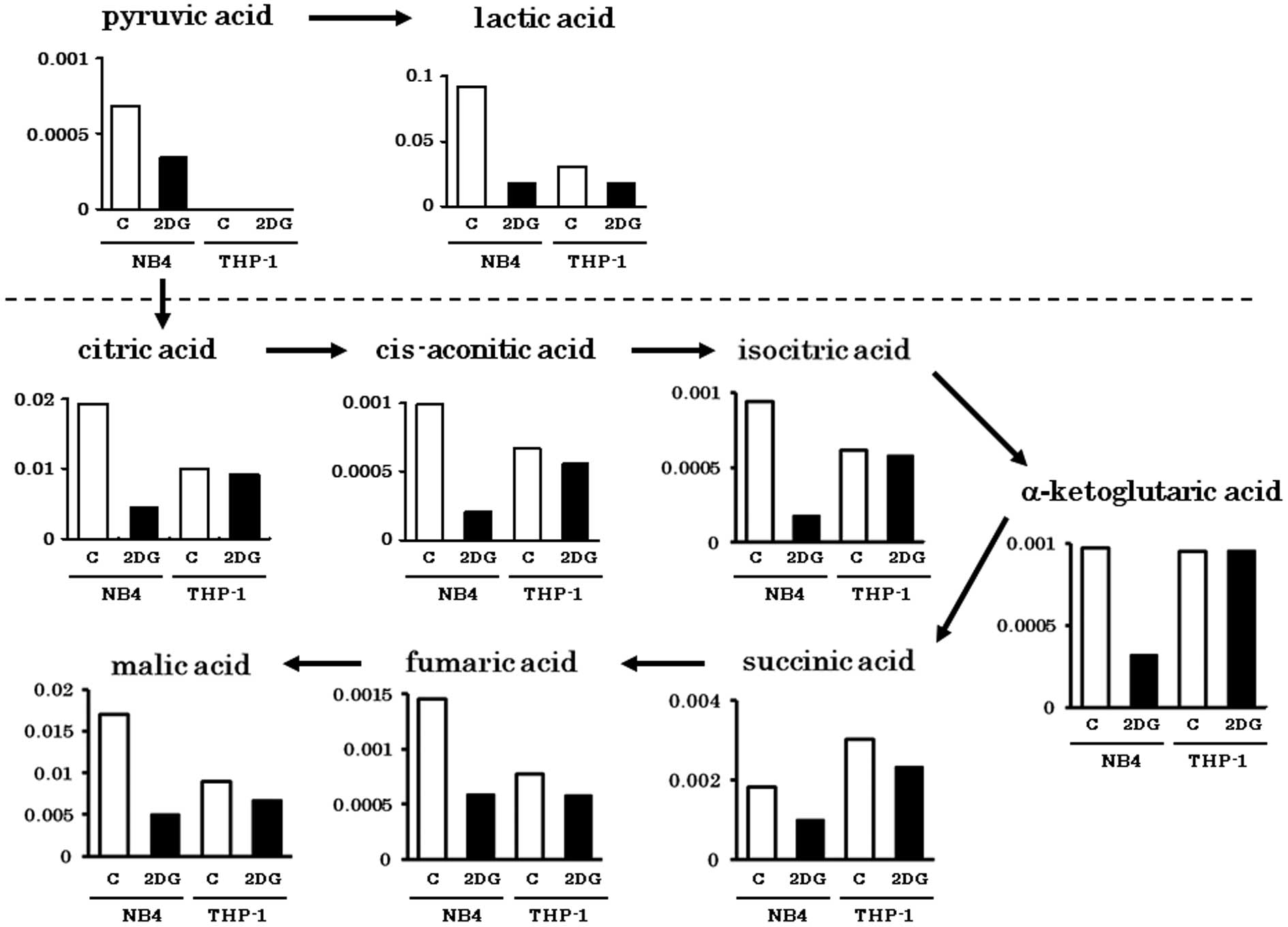
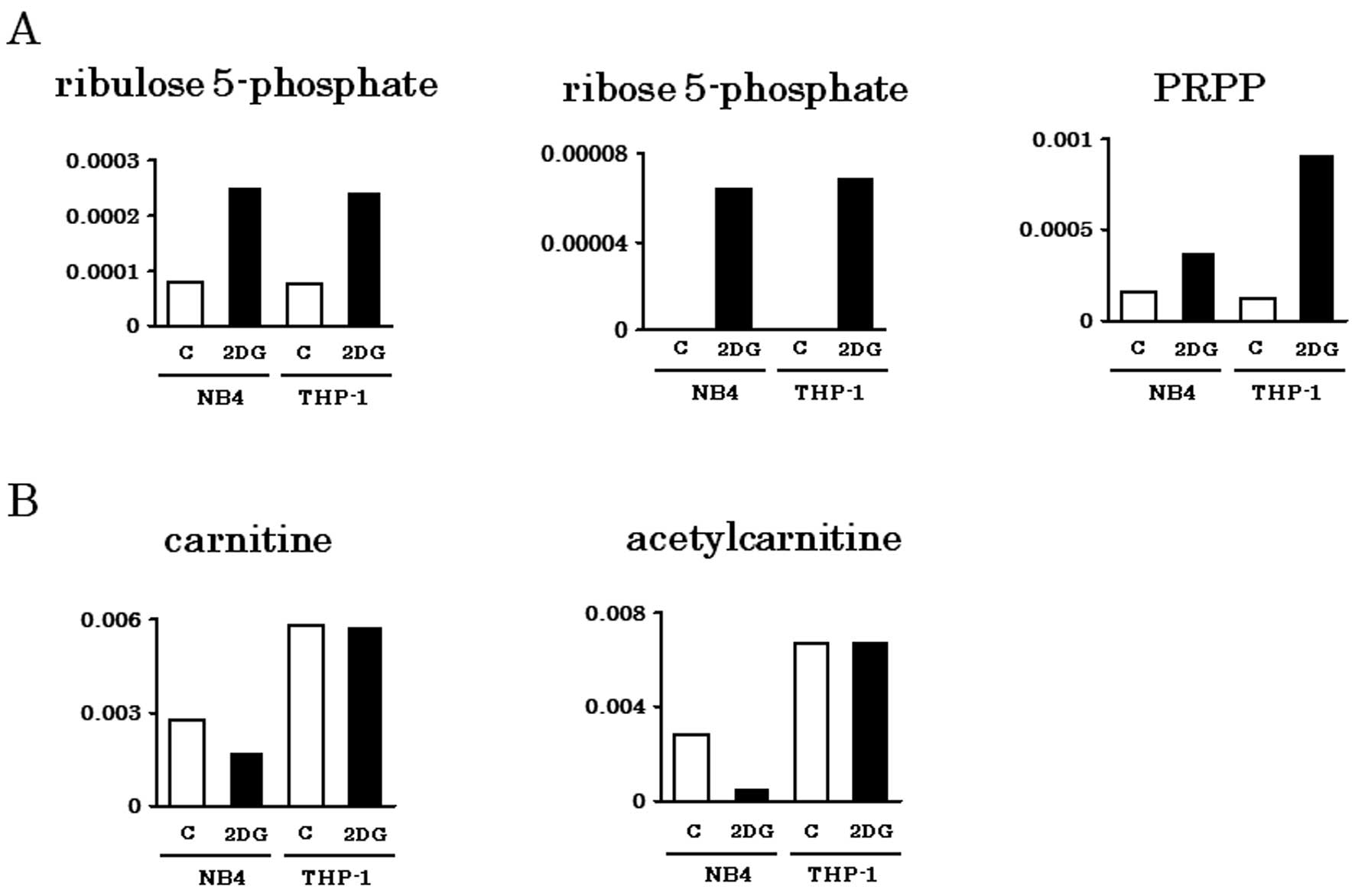
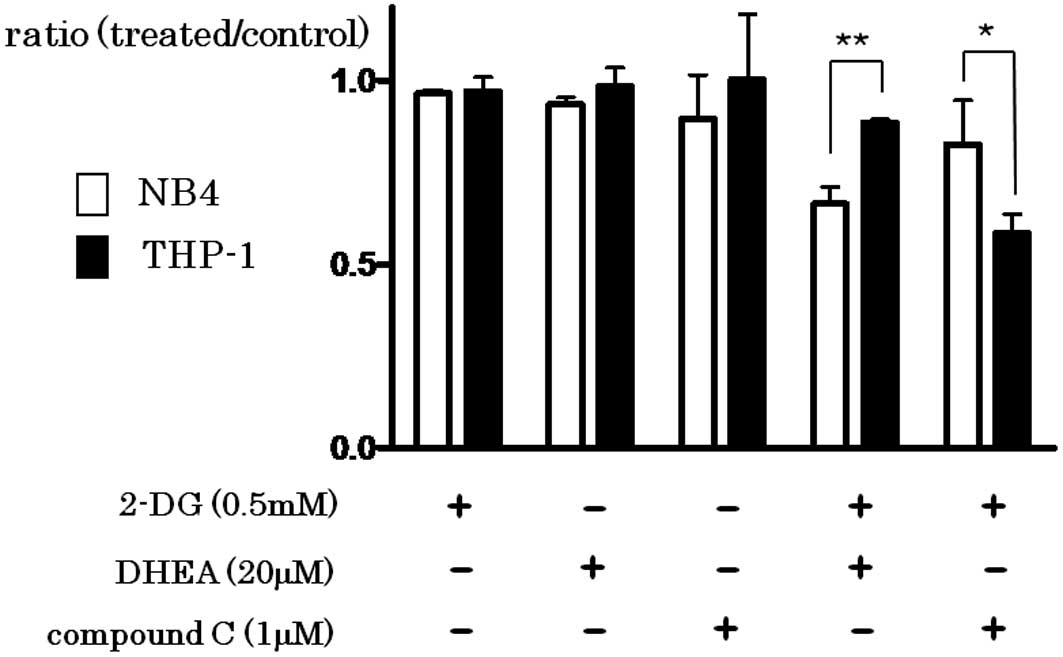
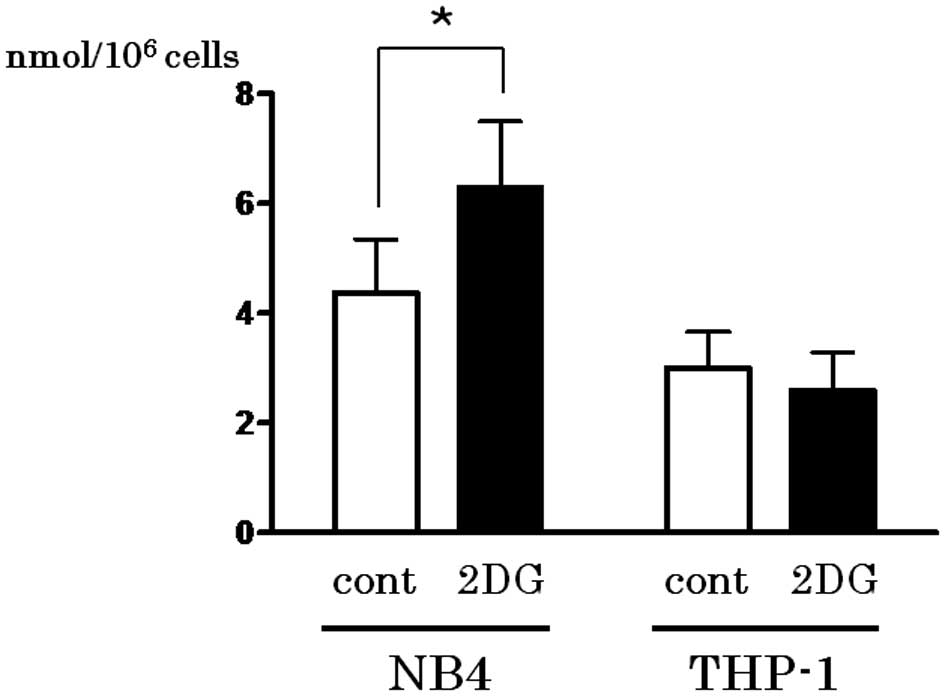
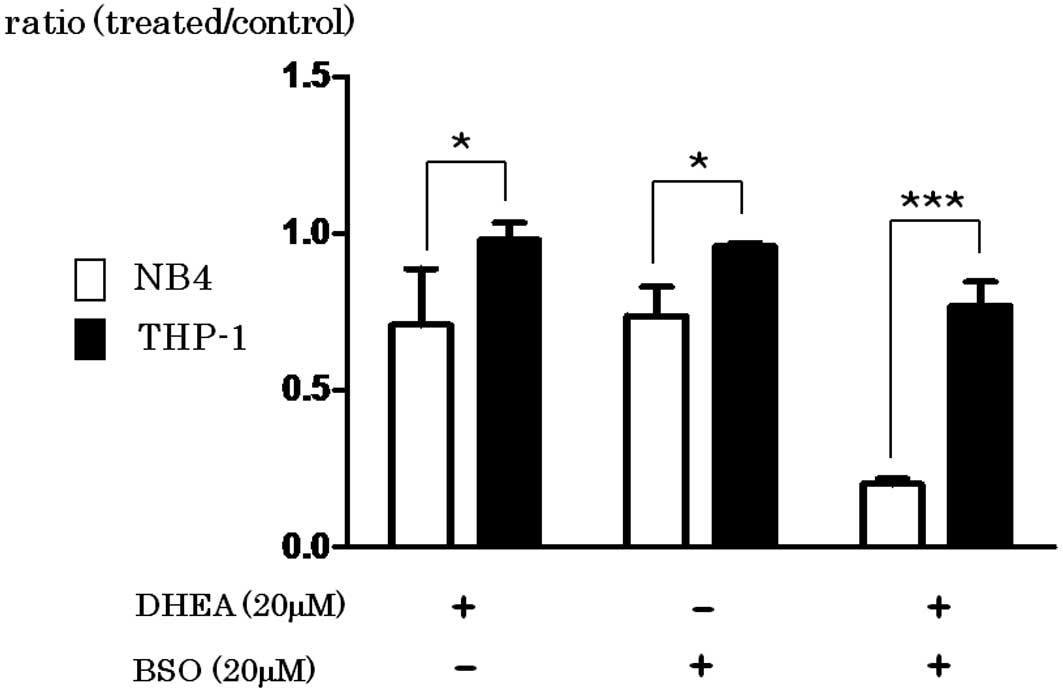
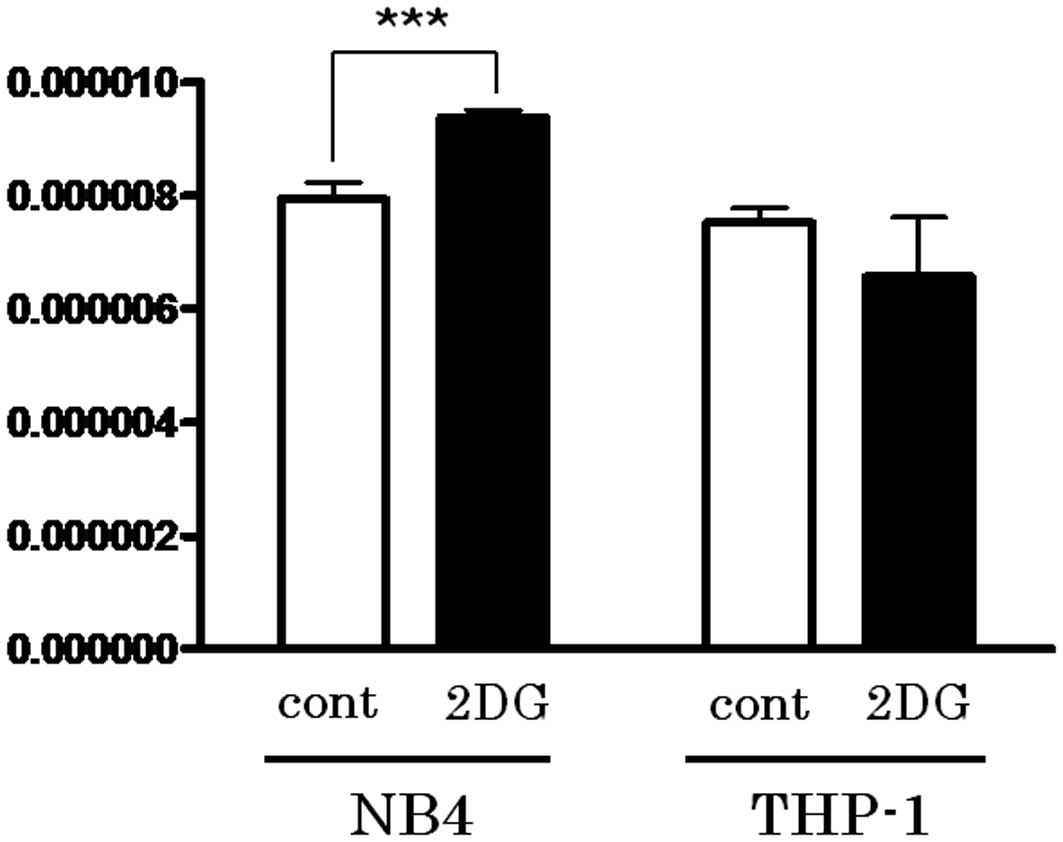
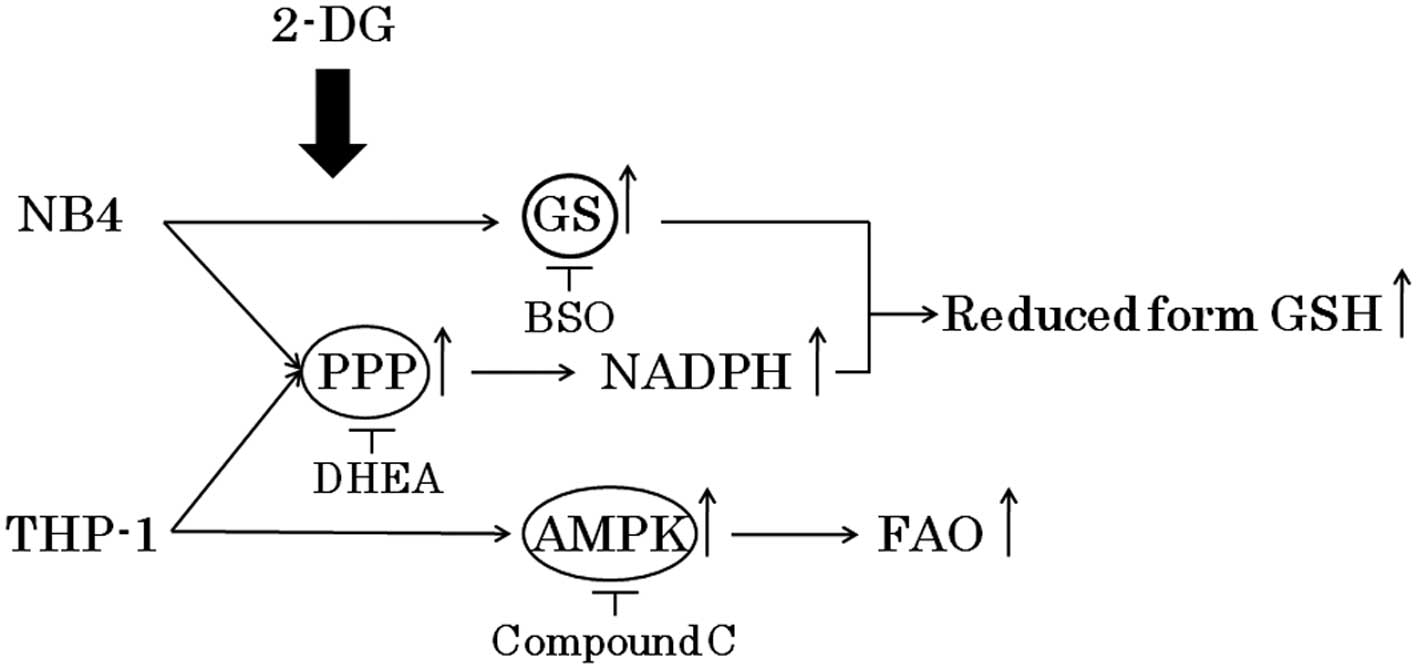
Spandidos Publications style
Miwa H, Shikami M, Goto M, Mizuno S, Takahashi M, Tsunekawa-Imai N, Ishikawa T, Mizutani M, Horio T, Gotou M, Gotou M, et al: Leukemia cells demonstrate a different metabolic perturbation provoked by 2-deoxyglucose. Oncol Rep 29: 2053-2057, 2013.
APA
Miwa, H., Shikami, M., Goto, M., Mizuno, S., Takahashi, M., Tsunekawa-Imai, N. ... Nitta, M. (2013). Leukemia cells demonstrate a different metabolic perturbation provoked by 2-deoxyglucose. Oncology Reports, 29, 2053-2057. https://doi.org/10.3892/or.2013.2299
MLA
Miwa, H., Shikami, M., Goto, M., Mizuno, S., Takahashi, M., Tsunekawa-Imai, N., Ishikawa, T., Mizutani, M., Horio, T., Gotou, M., Yamamoto, H., Wakabayashi, M., Watarai, M., Hanamura, I., Imamura, A., Mihara, H., Nitta, M."Leukemia cells demonstrate a different metabolic perturbation provoked by 2-deoxyglucose". Oncology Reports 29.5 (2013): 2053-2057.
Chicago
Miwa, H., Shikami, M., Goto, M., Mizuno, S., Takahashi, M., Tsunekawa-Imai, N., Ishikawa, T., Mizutani, M., Horio, T., Gotou, M., Yamamoto, H., Wakabayashi, M., Watarai, M., Hanamura, I., Imamura, A., Mihara, H., Nitta, M."Leukemia cells demonstrate a different metabolic perturbation provoked by 2-deoxyglucose". Oncology Reports 29, no. 5 (2013): 2053-2057. https://doi.org/10.3892/or.2013.2299