Introduction
Head and neck squamous cell carcinoma (HNSCC) which
includes cancers of the oral cavity, oropharynx, larynx and
hypopharynx is the sixth most common cancer worldwide and has an
incidence of approximately 600,000 cases per year (1). The current management and treatment of
HNSCC involves multi-modality approaches of surgery, chemotherapy
and radiotherapy (2). Despite
recent advances in early detection, diagnosis and treatment, the
5-year survival for patients with HNSCC has remained at 50% for the
past 30 years (3).
Molecular-targeted therapy, based on molecular findings of the last
50 years, is one of the most promising gateways to the development
of new strategies in oncology (4).
Cetuximab, a monoclonal antibody to the epidermal growth factor
receptor (EGFR), is the only molecular-targeted therapy to be
routinely used in clinical practice for the treatment of recurrent
and metastatic HNSCC (5). Based on
a limited number of phase II and III trials that have investigated
the efficacy of cetuximab in addition to cisplatin in patients who
were refractory to platinum-based therapy, the combination appears
to confer further benefit over anti-EGFR agents alone (6,7).
The first generation of immunotoxins developed 35
years ago, which heralded targeted therapy, employed chemical
conjugations of antibodies and either intact toxins or toxins with
attenuated cell-binding properties. We previously reported that
recombinant fusion protein IL4 (38–37)-PE38KDEL (also termed
IL4-PE), consisting of circularly permuted interleukin (IL)-4 and a
mutated form of Pseudomonas exotoxin (PE), induced
significant regression of established biliary tract tumors and
significantly improved the survival of animals with disseminated
tumors (8,9). In addition, IL4-PE was reported to be
highly and specifically cytotoxic to glioma cell lines in
vitro, and caused partial or complete regression of established
human glioblastoma multiforme tumors in nude mice (10). IL-4 receptor α (IL-4Rα)-targeted
protein-based immunotoxin was tested in the clinic for the
treatment of human solid tumors (11,12).
However, its clinical application faced many challenges, including
non-specific toxicities and immunogenicity (13).
To overcome these issues, we previously developed a
‘hybrid peptide’, composed of target-binding and cytotoxic
sequences containing cationic-rich D- and L-amino acids to form
amphipathic partial α-helices that disrupt the cancer cell membrane
selectively, and are stable when combined with a cancer-targeting
moiety (14). It is known that
peptide drugs are relatively easily synthesized using either
recombinant or solid-phase chemical synthesis techniques and the
production costs are generally affordable when compared to
antibody-based therapeutics (14).
IL-4Rα has been previously reported to have high expression on the
surface of a variety of human solid tumors such as renal cell
carcinoma, malignant melanoma and glioblastoma (15). Although the biological function of
IL-4Rα expression on solid tumors remains unclear, this receptor
may be an effective candidate for a novel molecular-targeted
therapy.
In the present study, we examined the expression
levels of IL-4Rα in both patient samples and HNSCC cell lines, and
then explored the antitumor activity of the IL-4Rα-lytic hybrid
peptide against HNSCC.
Materials and methods
Patient samples
HNSCC specimens were obtained from 5 patients (4
males, 1 female; mean age, 56.6 years), who underwent radical
surgery at the Department of Oral and Maxillofacial Surgery,
Tsukuba University Hospital, Japan from 2010 to 2011. Primary tumor
sites were the tongue and the gingiva. For immunoblot analysis, we
obtained HNSCC tissue from the cancerous lesion (cancer) and normal
tissue from the normal area (normal) in other specimens from 1
patient. For immunohistochemistry, tissue was fixed in 10% formalin
and paraffin embedded. The study protocol was approved in
accordance with the ethics guidelines of the Tsukuba University
(H23-61). All patients provided written informed consent for use of
specimens.
Peptides
The following IL-4Rα-lytic hybrid peptide, the lytic
peptide and IL-4 binding peptide were purchased from Invitrogen:
the IL-4Rα-lytic hybrid peptide, KQLIRFLK RLDRNGGGKLLLKLLKKLLKLLKKK (underlined letters are
D-amino acids); the lytic peptide, KLLLKLLKKLLKLL KKK; and the IL-4 binding
peptide, KQLIRFLKRLDRN. All peptides were synthesized by the use of
solid-phase chemistry, purified to homogeneity by reverse-phase
high-pressure liquid chromatography and assessed by mass
spectrometry. All peptides were dissolved in water and buffered to
pH 7.4.
Cell lines and culture conditions
The HNSCC cell lines (HSC-2, HSC-3, HSC-4, Ca9-22
and OSC-19) were purchased from the Japanese Collection of Research
Bioresources (Osaka, Japan). The human normal keratinocyte cell
line (HaCaT) was purchased from the American Type Culture
Collection (Manassas, VA, USA). These cell lines were maintained in
Dulbecco’s modified Eagle’s medium or RPMI-1640 containing 10%
heat-inactivated fetal calf serum (Nichirei Biosciences Inc.,
Tokyo, Japan) and 1% penicillin-streptomycin in a humidified
atmosphere with 5% CO2 at 37°C.
Immunoblot analysis
Immunoblot analysis was carried out as previously
described (9). Briefly, whole-cell
extracts were obtained using buffer containing 1% (v/v) Triton
X-100, 0.1% (w/v) SDS, and 0.5% (w/v) sodium deoxycholate,
separated by SDS-PAGE, and transferred onto a PVDF membrane. IL-4Rα
antibody was used at dilution 1:200 (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) and actin as the internal control
(Sigma-Aldrich, St. Louis, MO, USA). Proteins were visualized on
Hyperfirm using an enhanced chemiluminescence/western blotting
system (GE Healthcare, Piscataway, NJ, USA).
Quantitative real-time PCR
Total RNA of cells was isolated using High Pure RNA
Tissue kit (Roche, Basel, Switzerland). For the reverse
transcriptase reaction, 400 ng of the RNA sample was used. The
reaction was carried out in a final volume of 10 μl of reaction
mixture with Takara Kit II (Takara, Shiga, Japan). Aliquots (2 μl)
of the cDNA samples were amplified in a final volume of 20 μl of
PCR mixture containing SYBR Premix Ex Taq II (Takara). Quantitative
real-time PCR was carried out using PRISM 7000 (Applied Biosystems,
Carlsbad, CA, USA). The following primers were used: IL-4Rα
forward, 5′-CTGACCTGGAGCAACCCGTATC-3′ and IL-4Rα reverse,
5′-GCAGACGGACAACACGATACAG-3′; GAPDH forward,
5′-GTCTTCACCACCATGGAGAAGGCT-3′ and GAPDH reverse,
5′-CATGCCAGTGAGCTTCCCGTTCA-3′.
Cell viability assay
Cell viability assay was performed as previously
described (14). Briefly, cells
were seeded into 96-well plates at 3×103 cells/well in
90 μl of medium and incubated at 37°C for 24 h. Each peptide
(IL-4R-lytic hybrid peptide, lytic peptide or IL-4 binding peptide)
diluted in 10 μl culture medium was added to the cells. After a
72-h incubation, the cell viability assay using WST-8 solution
(Nacalai Tesque, Kyoto, Japan) was performed.
Immunohistochemistry
For immunostaining of IL-4Rα, 2-μm sections from
patient samples were stained using the Vectastain kit according to
the manufacturer’s instructions with the anti-IL-4Rα antibody
(R&D Systems, Minneapolis, MN, USA).
Binding assay
The IL-4 binding peptide labeled with fluorescein
isothiocyanate (FITC) was incubated with HSC-2 and HaCaT cells.
Quantification of the binding activity of this peptide to HSC-2 and
HaCaT cells treated with various concentrations for 30 min was
carried out. Cells were washed twice with phosphate-buffered
saline, and the peptides were detected using a flow cytometer
(FACSCalibur, BD Biosciences, San Jose, CA, USA).
Antitumor activity of the IL-4Rα-lytic
hybrid peptide in a human tumor xenograft mouse model in vivo
Animal experiments were carried out in accordance
with the guidelines of Tsukuba University. HSC-2 cells
(5×106) resuspended in 150 μl of phosphate-buffered
saline were inoculated subcutaneously into the flank region of 4-
to 6-week-old athymic female nude mice weighing 17–20 g. When the
tumors reached 20–60 mm3 in volume, animals were
assigned randomly to four groups. Saline (control), the
IL-4Rα-lytic hybrid peptide (5 or 10 mg/kg), or the lytic peptide
alone (5 mg/kg) was injected intratumorally (50 μl/injection) three
times a week for a total of 9 times. Tumors were measured with a
caliper, and the tumor volume (in mm3) was calculated
using the following formula: Length × width2 × 0.5. All
values are expressed as means ± SD.
Results
Expression of IL-4Rα in HNSCC tissue
specimens
We first analyzed the expression levels of IL-4Rα in
HNSCC tissue specimens. Patient characteristics including age,
gender, primary site of tumor, TNM classification and
differentiation are shown in Table
I. Immunoblot analysis and immunohistochemistry were performed
to investigate the expression levels of IL-4Rα in tissue specimens
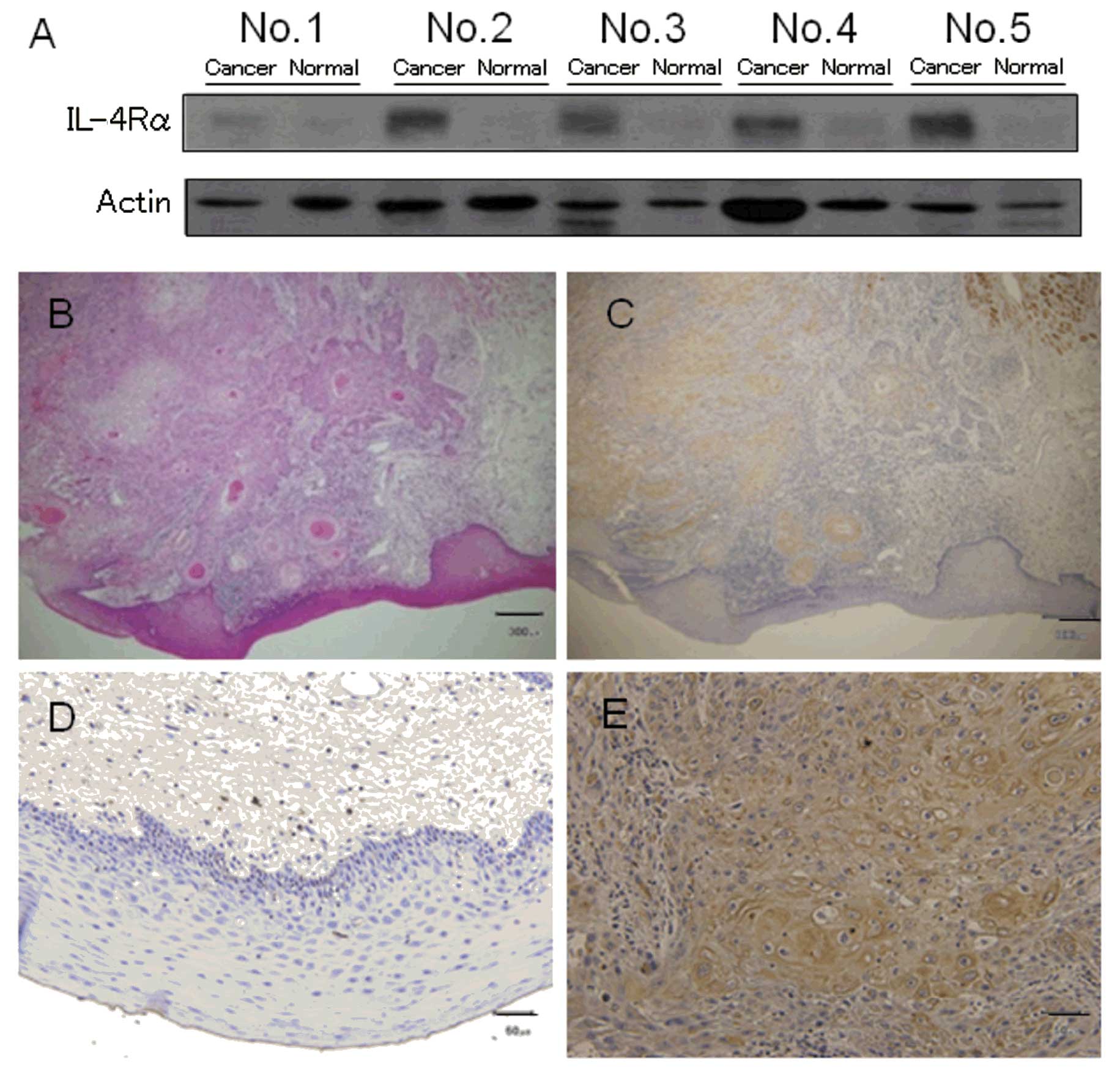
from HNSCC patients. Immunoblot analysis showed that IL-4Rα was
expressed in all HNSCC specimens (tongue and gingival carcinomas)
but not in the normal tissue specimens from the same patients
(Fig. 1A). Similarly,
immunohistochemical analysis using anti-IL-4Rα antibody showed
IL-4Rα immunopositivity in the HNSCC cancerous epithelium but not
in the normal epithelium in all patients. Fig. 1B-E shows the staining pattern for
patient no. 2.
 | Table IClinicopathological features of the
HNSCC patients. |
Table I
Clinicopathological features of the
HNSCC patients.
| No. | Age (years) | Gender | Primary site | TNM
classification | Differentiation of
SCC |
|---|
| 1 | 57 | Male | Tongue | T1N0M0 | Well |
| 2 | 40 | Male | Tongue | T2N0M0 | Moderate |
| 3 | 47 | Female | Tongue | T2N0M0 | Well |
| 4 | 78 | Male | Gingiva | T3N2bM0 | Well |
| 5 | 61 | Male | Gingiva | T4aN2bM0 | Moderate |
Expression of IL-4Rα in cultured HNSCC
cell lines
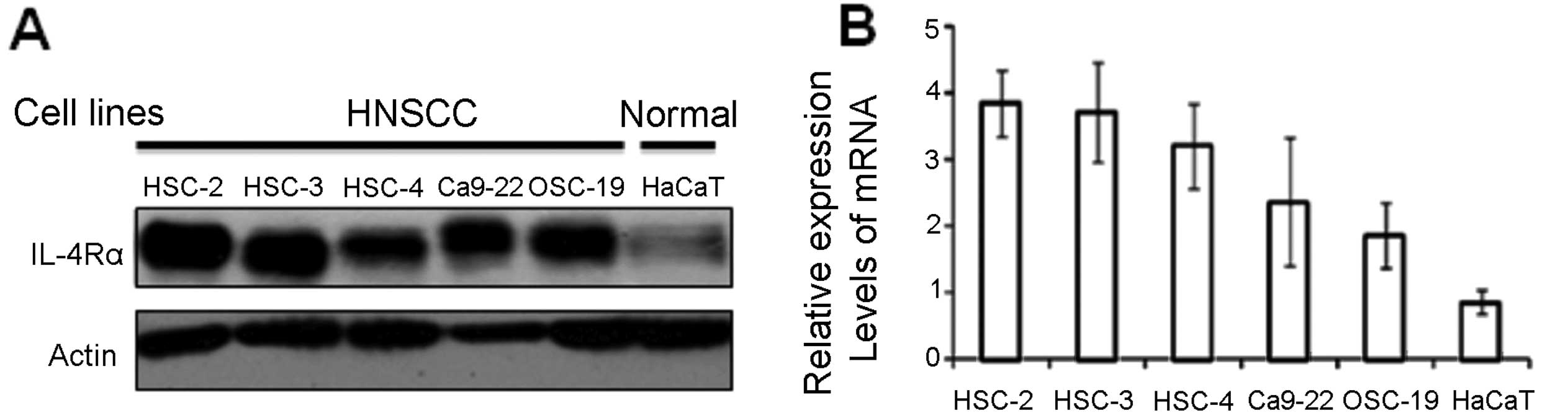
We next investigated the expression levels of IL-4Rα
in HNSCC cell lines by immunoblot and real-time PCR analyses.
Immunoblot and real-time PCR analyses demonstrated that all HNSCC
cell lines expressed IL-4Rα but HaCaT did not (Fig. 2A). We also examined mRNA expression
levels of IL-4Rα by real-time PCR analysis (Fig. 2B). Relative expression levels of
IL-4Rα in HSC-2 and HSC-3 cells were ~4-fold higher than that of
HaCaT cells. The lowest expression level of IL-4Rα in HNSCC cell
lines was found in OSC-19, however, this level was still 2-fold
higher than that of HaCaT (Fig.
2B).
Cytotoxic activity of the IL-4Rα-lytic
hybrid peptide in HNSCC cell lines
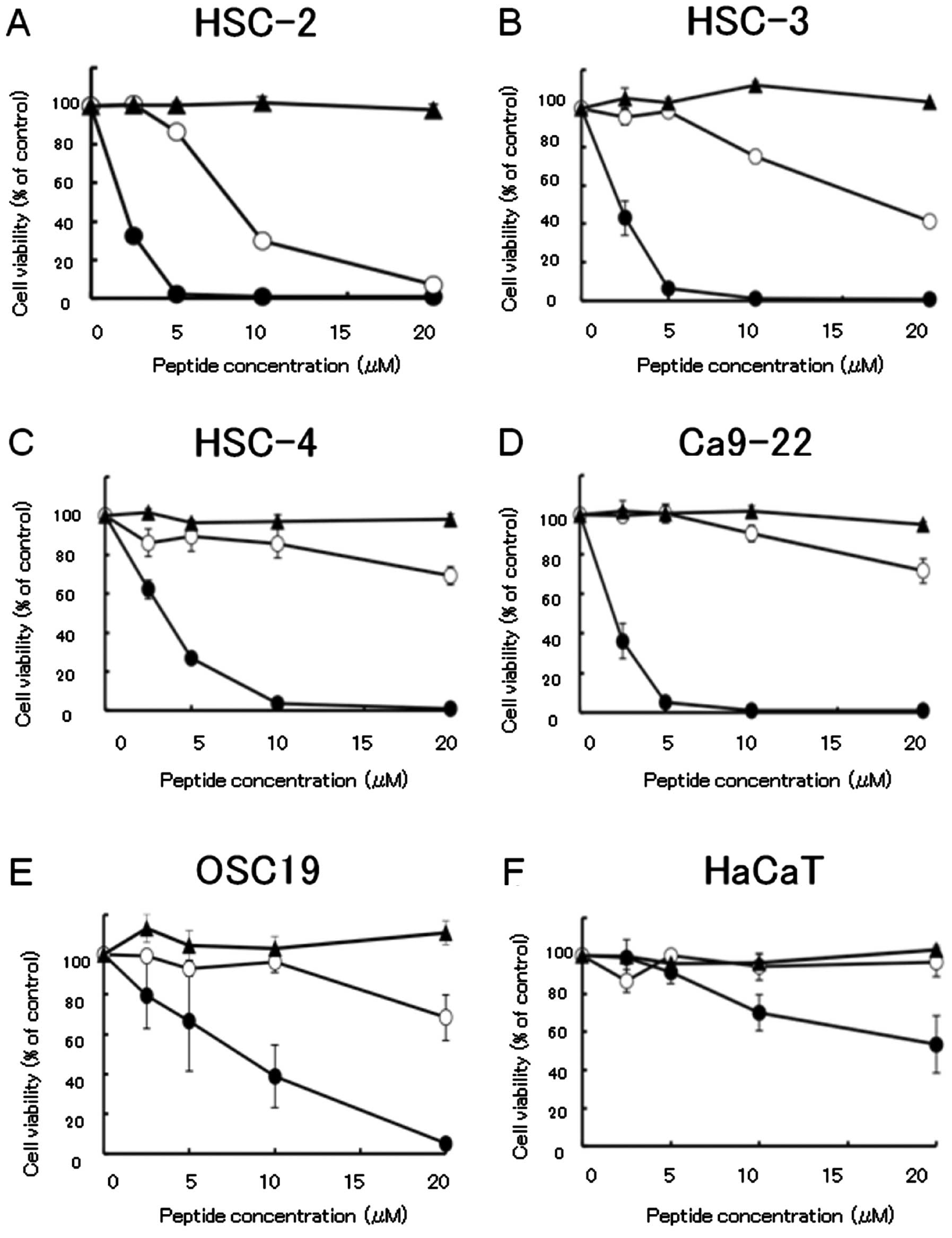
To assess the in vitro cytotoxic activity of
the IL-4Rα-lytic hybrid peptide in HNSCC and HaCaT cells, the WST
assay was performed using HNSCC cell lines treated with the
IL-4Rα-lytic hybrid peptide, lytic peptide or IL-4 binding peptide.
HSC-2, HSC-3, HSC-4 and Ca9-22 cells were sensitive to the
IL-4Rα-lytic hybrid peptide; the concentration that killed 50% of
all cells (IC50) was <5 μM. The OSC-19 cell line was
also sensitive to the IL-4Rα-lytic hybrid peptide with an
IC50 of <10 μM. In contrast, optimal cell killing was
not induced in HaCaT cells by either the lytic peptide or IL-4
binding peptide or IL-4Rα-lytic hybrid peptide (Fig. 3). The cytotoxic activity of the
hybrid peptide was strongly enhanced when compared with that of the
lytic peptide. The cytotoxic activity of the IL-4Rα-lytic hybrid
peptide increased 4.0- to 13.2-fold when compared with that of the
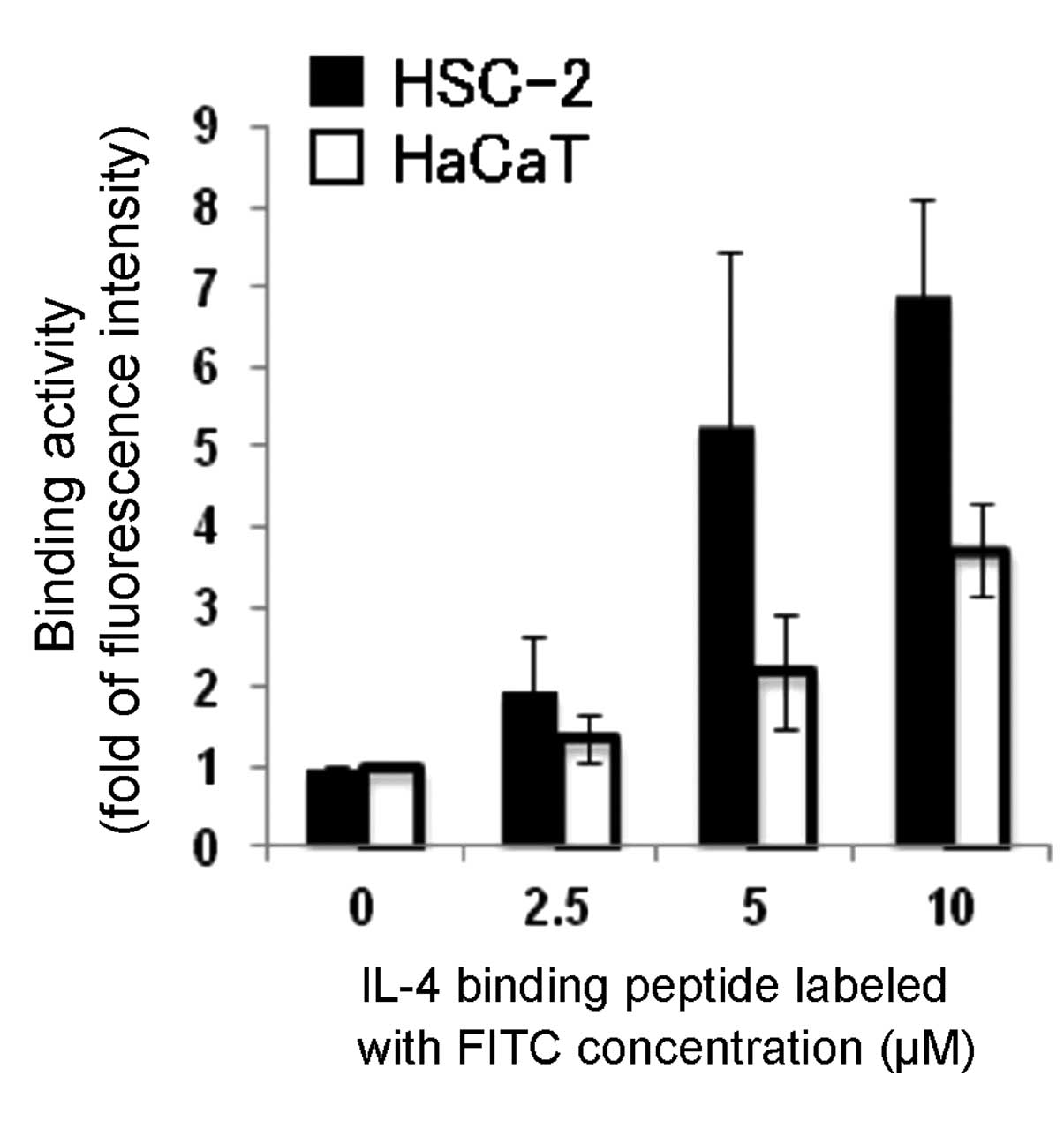
lytic peptide in the cancer cell lines (Table II). We examined the binding
activity of the IL-4 binding peptide labeled with FITC to both
HSC-2 and HaCaT cells by flow cytometry, and then found that
exposure of HSC-2 to this peptide resulted in the increased binding
activity of this peptide in a concentration-dependent manner
(Fig. 4). These results suggest
that the IL-4Rα-lytic hybrid peptide selectively kills cancer cells
expressing IL-4Rα.
 | Table IICytotoxic activity of each peptide in
HNSCC and HaCaT cell lines. |
Table II
Cytotoxic activity of each peptide in
HNSCC and HaCaT cell lines.
| IC50
(μM) | IC50
ratio |
|---|
|
|
|
|---|
| Cell lines | IL-4Rα-lytic hybrid
peptide | Lytic peptide | Lytic/IL-4Rα-lytic
hybrid peptide |
|---|
| HNSCC cells |
| HSC-2 | 1.9±0.1 | 8.2±0.2 | 4.4 |
| HSC-3 | 2.3±0.6 | 17.4±1.2 | 7.4 |
| HSC-4 | 2.8±0.8 | 33.2±6.1 | 11.9 |
| Ca9-22 | 2.2±0.7 | 29.6±5.8 | 13.2 |
| OSC-19 | 8.2±5.4 | 33.3±13.1 | 4.0 |
| Normal cells |
| HaCaT | 37.6±15.3 | 43.4±13.6 | 1.2 |
In vivo antitumor activity of the
IL-4Rα-lytic hybrid peptide in a human HNSCC xenograft mouse
model
Following the observation that the IL-4Rα-lytic
hybrid peptide exhibits a marked cytotoxic effect on HNSCC cells
in vitro (Fig. 3), the
antitumor activity of the hybrid peptide was assessed in a
xenograft model of human HNSCC. HSC-2 cells were inoculated
subcutaneously into athymic nude mice, and the animals were
subsequently treated with the IL-4Rα-lytic hybrid peptide by
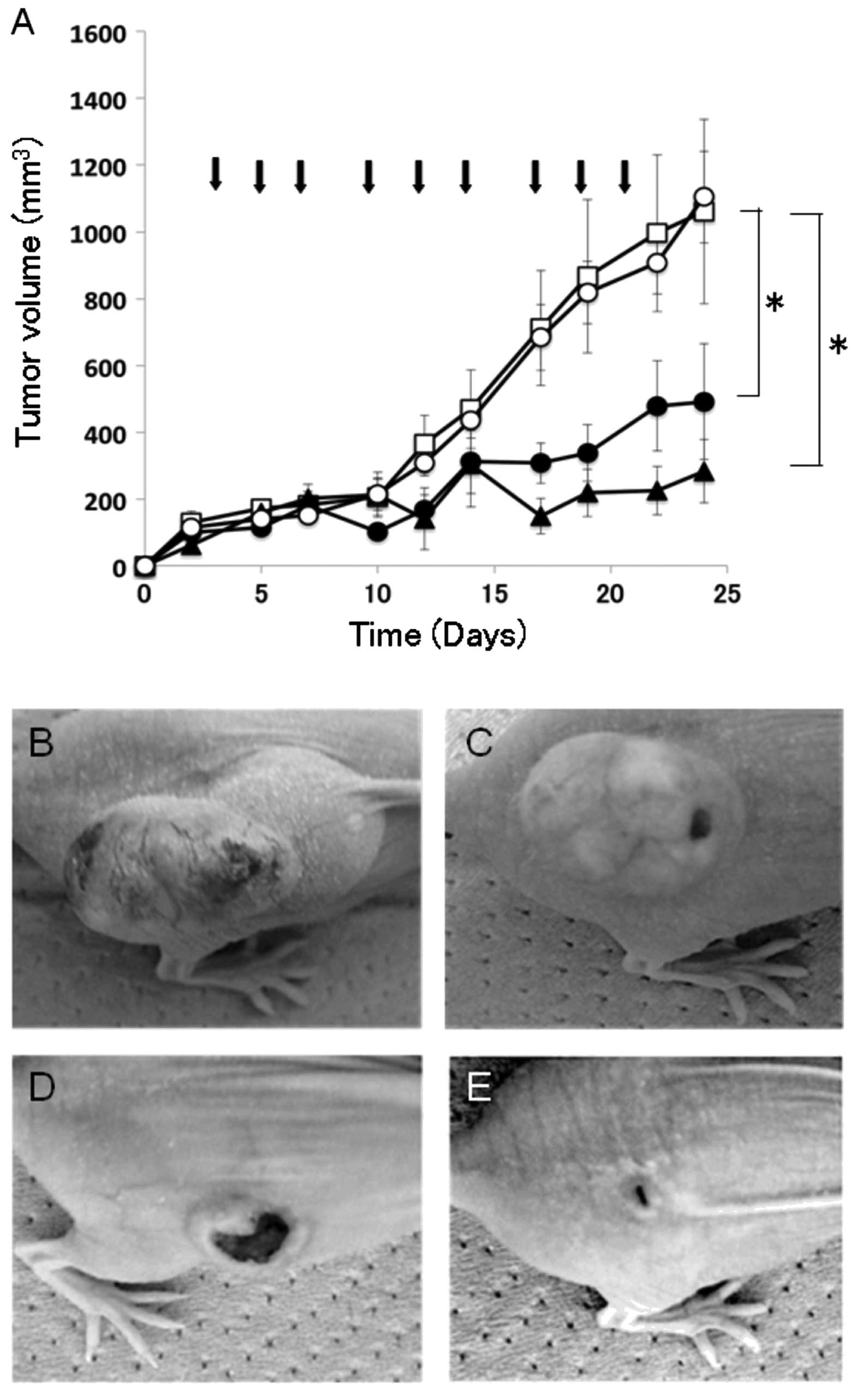
intratumoral injection. As shown in Fig. 5, tumors grew aggressively in the
control mice injected with saline alone, reaching a volume of
>1000 mm3 by day 24. In contrast, mice treated with
the IL-4Rα-lytic hybrid peptide showed significant tumor regression
at both dosages: mean tumor volumes were 491 mm3 (5
mg/kg) and 283 mm3 (10 mg/kg) on day 24. Moreover,
tumors in mice injected with the lytic peptide grew rapidly similar
to tumor growth in the control mice with saline alone, reaching a
volume of 1104 mm3 (Fig.
5). No other abnormalities, such as loss of appetite and body
weight, were observed in mice injected with the IL-4Rα-lytic hybrid
peptide (data not shown). Histological analysis also showed no side
effects in tissues from the major organs, including the liver and
kidney, which were obtained from mice treated with intratumoral
administration of the IL-4Rα-lytic hybrid peptide (data not shown).
These results demonstrated that the IL-4Rα-lytic hybrid peptide
exhibited effective antitumor activity in a mouse xenograft model
of HNSCC.
Discussion
The main treatment options for patients with HNSCC
currently involve surgery, radiotherapy and chemotherapy, alone or
in combination. Despite significant advances in HNSCC treatment,
survival rates and prognosis have improved only moderately over the
years (16). Systemic chemotherapy
remains the only effective treatment option, but it is associated
with significant rates of toxicity in HNSCC patients, who usually
have a high prevalence of co-morbidities and problematic lifestyle
habits (17). Ideally, future
therapies should act over the short term, to minimize damage to
healthy cells and target tumor compartments that have the highest
sensitivity.
The concept of a ‘magic bullet’ proposed by Paul
Ehlrich over 100 years ago has led to the search for agents that
can selectively target cancer cells (18). Immunotoxins are proteins used to
treat cancer and are composed of an antibody fragment linked to a
toxin (19). Several disadvantages
of these conventional immunotoxins for clinical use include
immunogenicity, undesirable toxicity, manufacturing difficulties,
short half-lives and neutralizing antibody production (20,21).
However, peptides can be produced affordably by chemical synthesis,
with a cost comparable to that of producing protein drugs.
Moreover, since peptides are easy to produce, a wide variety of
candidate peptides combining moieties for targeting and for
toxicity can be tested in preclinical settings. We previously
linked two functional peptide domains to produce a novel chimerical
peptide termed a ‘hybrid peptide’, which was designed as a
bifunctional peptide that binds to receptors or proteins
overexpressing in cancer cells and consequently disrupts the cancer
membrane (14,22,23).
In the present study, we focused on IL-4Rα as recent evidence
suggests that IL-4Rα is preferentially expressed on the surface of
a variety of solid tumors including HNSCC (24).
The high degree of antitumor activity of the
IL-4Rα-lytic hybrid peptide in HNSCC correlated with the expression
of IL-4Rα in vitro. All HNSCC tumor specimens showed
specific immunohistochemical staining for IL-4Rα, and western blot
analysis revealed expression of IL-4Rα. However, IL-4Rα expression
was not observed in normal tissue specimens from the same patients
(Fig. 1). These data are consistent
with previous reports that HNSCC cells express IL-4Rα on their cell
surface and confirm that IL-4Rα is expressed in
situ(25,26). These results also indicate that this
receptor may be an attractive target for the treatment of
HNSCC.
Previous results suggest that IL-4 receptor-targeted
cytotoxin may provide an effective therapeutic option for HNSCC
(24,26). In the present study, the in
vitro cytotoxicity of the IL-4Rα-lytic hybrid peptide was
examined in five HNSCC cell lines (Fig.
3). HSC-2 cells, which showed the highest level of IL-4Rα
expression in western blot analysis, also showed the highest
sensitivity to the IL-4Rα-lytic hybrid peptide (Figs. 2 and 3). Normal HaCaT cells with low IL-4Rα
expression were not sensitive to the IL-4Rα-lytic hybrid peptide
(Fig. 3). These results suggest
that the cytotoxic effect of the IL-4Rα-lytic hybrid peptide
correlates well with the level of IL-4Rα expression.
In the present study, although the growth rate of
HSC-2 was rapid, it was found that intratumoral administration of
the hybrid peptide at 10 mg/kg, dramatically inhibited the growth
of HSC-2 tumors in vivo (Fig.
5). Histological analysis also showed no abnormal changes in
the tissues of major organs obtained from the mice injected with
the hybrid peptide (data not shown). For clinical use, local
injection may be effective for tumors such as HNSCC, and
combination with prior chemoradiotherapy should be developed. These
observations indicate that abundant IL-4Rα expression in HNSCC
tumors would facilitate efficient targeting by the IL-4Rα-lytic
hybrid peptide.
In conclusion, IL-4Rα was overexpressed in both
tumor specimens from patients with HNSCC and in HNSCC cell lines
in vitro. The overexpressed IL-4Rα on HNSCC cells could be
successfully targeted with the IL-4Rα-lytic hybrid peptide in
vitro and in vivo. Future investigations using cancer
progenitor cells isolated from primary malignant tissues at
different stages during cancer progression and metastatic disease
may help to identify new biomarkers for the development of more
effective diagnostic and prognostic methods and targeted therapies
(27). Additional studies should be
performed to reveal the antitumor activity of the IL-4Rα-hybrid
peptide in animal models, and perhaps a phase I clinical trial
should be undertaken to study its antitumor activity.
Acknowledgements
We thank Megumi Kawamoto, Kumi Kodama, Nana Ohkubo,
Aya Torisawa (Department of Pharmacoepidemiology, Kyoto
University), and Airi Ueda (Department of Clinical Sciences, and
Molecular Cellular Physiology, Tsukuba University) for technical
assistance or advice with the tissue culturing and in vivo
experiments. This study was supported by Grants-in-Aid for
Scientific Research (B) (grant no. 24390449), Young Scientists (A)
(grant no. 2368009), and Challenging Exploratory Research (grant
no. 23659934) from the Japan Society for the Promotion of Science
(JSPS).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Seiwert TY and Cohen EE: State-of-the-art
management of locally advanced head and neck cancer. Br J Cancer.
92:1341–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forastiere AA, Goepfert H, Maor M, et al:
Concurrent chemotherapy and radiotherapy for organ preservation in
advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dietz A, Boehm A, Mozet C, Wichmann G and
Giannis A: Current aspects of targeted therapy in head and neck
tumors. Eur Arch Otorhinolaryngol. 265(Suppl 1): S3–S12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Price KA and Cohen EE: Current treatment
options for metastatic head and neck cancer. Curr Treat Options
Oncol. 13:35–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirby AM, A’Hern RP, D’Ambrosio C, et al:
Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or
metastatic head and neck cancer. Br J Cancer. 94:631–636.
2006.PubMed/NCBI
|
|
7
|
Herbst RS, Arquette M, Shin DM, et al:
Phase II multicenter study of the epidermal growth factor receptor
antibody cetuximab and cisplatin for recurrent and refractory
squamous cell carcinoma of the head and neck. J Clin Oncol.
23:5578–5587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimamura T, Royal RE, Kioi M, Nakajima A,
Husain SR and Puri RK: Interleukin-4 cytotoxin therapy synergizes
with gemcitabine in a mouse model of pancreatic ductal
adenocarcinoma. Cancer Res. 67:9903–9912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishige K, Shoda J, Kawamoto T, et al:
Potent in vitro and in vivo antitumor activity of
interleukin-4-conjugated Pseudomonas exotoxin against human
biliary tract carcinoma. Int J Cancer. 123:2915–2922. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastan I, Hassan R, Fitzgerald DJ and
Kreitman RJ: Immunotoxin therapy of cancer. Nat Rev Cancer.
6:559–565. 2006. View
Article : Google Scholar
|
|
11
|
Weber F, Asher A, Bucholz R, et al: Safty,
tolerability, and tumor response of IL4-Pseudomonas exotoxin
(NBI-3001) in patients with recurrent malignant glioma. J
Neurooncol. 64:125–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Attia P, Powell DJ Jr, Maker AV, Kreitman
RJ, Pastan I and Rosenberg SA: Selective elimination of human
regulatory T lymphocytes in vitro with the recombinant immunotoxin
LMB-2. J Immunother. 29:208–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choudhary S, Mathew M and Verma RS:
Therapeutic potential of anticancer immunotoxins. Drug Discov
Today. 16:495–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kohno M, Horibe T, Haramoto M, et al: A
novel hybrid peptide targeting EGFR-expressing cancers. Eur J
Cancer. 47:773–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garland L, Gitlitz B, Ebbinghaus S, et al:
Phase I trial of intravenous IL-4 Pseudomonas exotoxin
protein (NBI-3001) in patients with advanced solid tumors that
express the IL-4 receptor. J Immunother. 28:376–381.
2005.PubMed/NCBI
|
|
16
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
17
|
Goon PK, Stanley MA, Ebmeyer J, et al: HPV
& head and neck cancer: a descriptive update. Head Neck Oncol.
1:362009.
|
|
18
|
Bosch F and Rosich L: The contributions of
Paul Ehrlich to pharmacology: a tribute on the occasion of the
centenary of his Nobel Prize. Pharmacology. 82:171–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pastan I, Hassan R, FitzGerald DJ and
Kreitman RJ: Immunotoxin treatment of cancer. Annu Rev Med.
221–237. 2007. View Article : Google Scholar
|
|
20
|
Kreitman RJ: Immunotoxins for targeted
cancer therapy (review). AAPS J. 8:532–551. 2006. View Article : Google Scholar
|
|
21
|
Li Z, Yu T, Zhao P and Ma J: Immunotoxins
and cancer therapy. Cell Mol Immunol. 2:106–112. 2005.PubMed/NCBI
|
|
22
|
Horibe T, Kohno M, Haramoto M, Ohara K and
Kawakami K: Designed hybrid TPR peptide targeting Hsp90 as a novel
anticancer agent. J Transl Med. 9:82011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tada N, Horibe T, Haramoto M, Ohara K,
Kohno M and Kawakami K: A single replacement of histidine to
arginine in EGFR-lytic hybrid peptide demonstrates the improved
anticancer activity. Biochem Biophys Res Commun. 407:383–388. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strome SE, Kawakami K, Alejandro D, et al:
Interleukin 4 receptor-directed cytotoxin therapy for human head
and neck squamous cell carcinoma in animal models. Clin Cancer Res.
8:281–286. 2002.PubMed/NCBI
|
|
25
|
Mehrotra R, Varricchio F, Husain SR and
Puri RK: Head and neck cancers, but not benign lesions, express
interleukin-4 receptors in situ. Oncol Rep. 5:45–48.
1998.PubMed/NCBI
|
|
26
|
Kawakami K, Leland P and Puri RK:
Structure, function, and targeting of interleukin 4 receptors on
human head and neck cancer cells. Cancer Res. 60:2981–2987.
2000.PubMed/NCBI
|
|
27
|
Mimeault M, Hauke R, Mehta PP and Batra
SK: Recent advances in cancer stem/progenitor cell research:
therapeutic implications for overcoming resistance to the most
aggressive cancers. J Cell Mol Med. 11:981–1011. 2007. View Article : Google Scholar : PubMed/NCBI
|