Introduction
Multiple myeloma (MM) is a clonal plasma cell
malignant disease characterized by anemia, bone disease,
M-component and impaired renal function (1). The pathogenesis has yet to be fully
elucidated. Although new therapeutic modalities such as thalidomide
(2), bortezomib (3,4), and
lenalidomide (5,6) have been developed in recent years, MM
remains incurable.
The Bcl-2 family includes both death antagonists,
such as Bcl-2 and Bcl-xl, and death agonists such as Bax, Bak, Bid
(7–10). It is now recognized that Bcl-2 is
overexpressed in most MMs and it plays a major role in drug
resistance in myeloma cell lines and in freshly isolated myeloma
cells (11). Thus, inhibition of
the antiapoptotic function of Bcl-2 family member protein targeting
represents an attractive new strategy for the treatment of MM.
Antisense Bcl-2 and Bcl-xl studies have provided important
proof-of-the-concept that inhibition of Bcl-2 and Bcl-xl may be
effective for the treatment of MM (11).
Gossypol acetate is a naturally occurring
polyphenolic compound extracted from cotton plants. Since the
1960s, gossypol had been shown to possess antineoplastic activity
against a variety of malignant cell types. Some preclinical studies
have demonstrated that gossypol acetate has antitumoral activity
and induces apoptosis in several types of cancer, including lung
cancer (12), melanoma (13), colon carcinoma (14), breast cancer (15), and central nervous system tumor
(16). Gossypol has also been
demonstrated to be well-tolerated and has achieved few effects in
some clinical trials, but the mechanisms are not well understood
(17). Previous studies revealed
that gossypol was a nonpeptidic small-molecule inhibitor of
Bcl-2/Bcl-xl. It can bind to the BH3 (Bcl-2 homology domain 3)
binding site in Bcl-2/Bcl-xl, and then block the heterodimerization
of Bcl-2/Bcl-xl with proapoptotic members in the Bcl-2 protein
family such as Bad, Bak, Bid (18,19).
Nevertheless, whether gossypol acetate has antitumor
activity on MM cells remains unclear. In this study, we
investigated the effect of gossypol acetate on MM cells in
vitro and in vivo, and discussed the possible molecular
mechanisms of action.
Materials and methods
Reagents
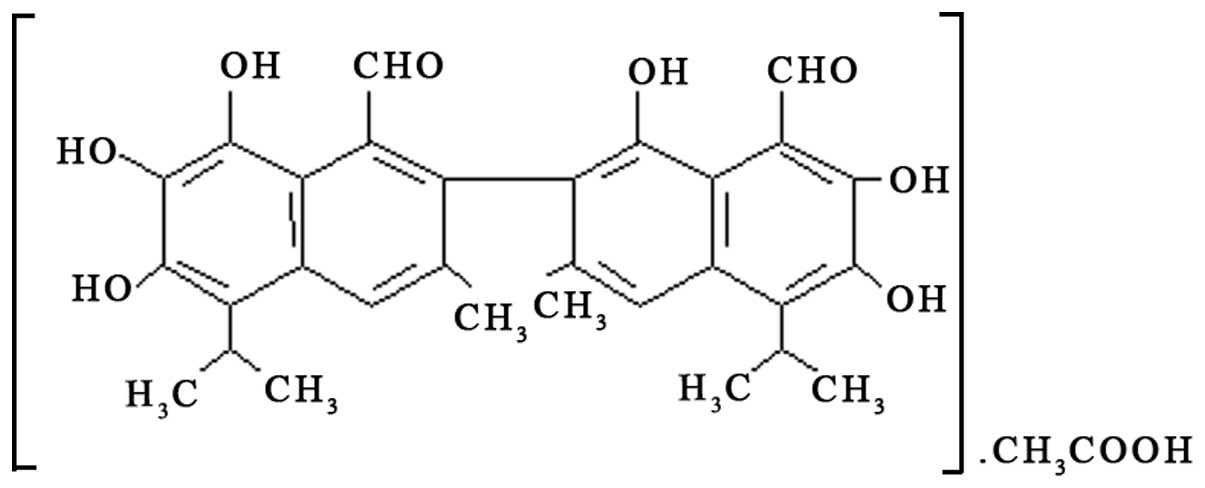
Gossypol acetate (provided by the University of
Michigan Comprehensive Cancer Center) was dissolved in dimethyl
sulfoxide (DMSO) to make a 50 mM stock solution and was preserved
at −20°C (Fig. 1). Treatment
solutions were prepared by the dilution of stock solution in
RPMI-1640.
Cell culture
Human MM cell lines U266 and Wus1 were obtained from
the hematology laboratory of Peking Union Medical College Hospital
(PUMCH). Both cell lines were cultured in RPMI-1640 medium
containing 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mmol/l
L-glutamine and 10% fetal bovine serum in a humidified incubator
(37°C, 5% CO2 and 95% air). Culture medium was changed
every 48–72 h.
Proliferation assay
MM cells were plated in 24-cell culture clusters at
a density of 1×105 viable cells/l per well. Triplicate
wells were treated with 1, 5, 10, 25 and 50 μmol/l gossypol
acetate, and the negative control group was supplemented with 0.1%
DMSO. Then, cell numbers at different treatment time points (0, 24,
48 and 72 h) were determined by using a hemocytometer and the
trypan blue dye-exclusion method. The trypan blue dye-exclusion
method was used to evaluate the cell viability. The cells were
examined in a counting chamber under a light microscope. Only
viable cells were recorded.
Apoptosis assay
Morphological observation
Cells were exposed to 25 μmol/l gossypol acetate for
24 h. Three methods were employed to confirm the morphological
changes of myeloma cells following exposure to gossypol acetate: i)
light microscopic examination: 106 cells were collected
and stained with Wright-Giemsa. Slides were analyzed under light
microscopy (Nikon); ii) fluorescence microscopic examination:
106 cells were collected and fixed in 4% formaldehyde at
4°C for 10 min. Then, Hoechst 33258 (100 μl) was added and stained
for 10 min. Cells were observed under fluorescence microscopy
(Olympus BX51) using 340 nm excitation wavelength; iii)
transmission electron microscopic examination: 106 cells
were collected and fixed in 2.5% glutaraldehyde and preserved at
−4°C. The work was completed by the Electron Microscopy Center,
Peking Union Medical College.
DNA fragmentation analysis
Cells (5×106) were collected after
exposure to gossypol acetate at a concentration of 25 μmol/l for 24
h. The cell pellet was washed twice with PBS and resuspended in 200
μl of 6 mol/l NaI solution, 400 μl chloroform and isoamyl alcohol
(24:1). Following centrifugation at 10,000 × g for 10 min,
supernatant was transferred to another centrifuge tube. Isopropanol
(400 μl) (100%) was added and the mixture was mixed vigorously and
incubated at room temperature for 10 min. DNA was pelleted by
centrifugation at 10,000 × g for 10 min and washed twice with 1.0
ml of 70% isopropanol. After centrifugation, DNA was air dried,
redissolved in 50 μl of TE, and preserved at −20°C. The control
group was supplemented with 0.1% DMSO.
DNA (10 μl) was mixed with 2 μl 6X loading buffer,
and was separated on a 1.5% agarose gel (including 0.5 μg/ml EB) by
electrophoresis at 80 V for 90 min in electrophoresis buffer (0.5X
TBE). DNA bands were photographed on a gel imaging system (UVI
GAS7001B).
DNA contents analysis by flow
cytometry
Cell apoptotic rate was quantitatively determined by
flow cytometry. The percentage of cells with a sub-G1
DNA content was taken as the fraction of apoptotic cell population.
Cells (1×106) were collected after exposure to gossypol
acetate at different concentrations for different times. The
control group was supplemented with 0.1% DMSO. Cells were fixed
with 2 ml of ice-cold 70% ethanol and incubated overnight at −20°C.
Prior to analysis, cells were washed with ice-cold PBS and
resuspended in PBS, then incubated with 25 μg/ml RNase for 30 min
and 50 μg/ml propidium iodide (PI) for 30 min in the dark. The PI
fluorescence associated with DNA was measured on a flow cytometer
(Coulter EPICS XL). At least 10,000 cells were detected in each
sample.
Caspase fluorometric activity
assay
Caspase-3, -9 activity was measured with caspase-3
colorimetric assay kit and caspase-9 colorimetric assay kit
(R&D). Briefly, after incubating with 25 μmol/l gossypol
acetate for 0, 6, 12, 18 and 24 h, 2×106 cells were
collected and washed twice with PBS and then lysed at 4°C with 50
μl cell lysis buffer for 10 min. Following centrifugation at 2,000
× g at 4°C, 50 μl of supernatant was transferred to a 96-well
plate, 50 μl 2X reaction buffer containing 10 mmol/l DTT was added
to each sample. Then, 5 μl caspase-3 substrates (DEVD-pNA) or
caspase-9 (LEHD-pNA) was added to each sample and incubated for 120
min at 37°C. pNA fluorescence, released by caspase activity, was
measured on a fluorescence plate reader (Bio-Rad 450) set at 405-nm
excitation filter. The results are expressed as fold increase in
caspase activity of apoptotic cells over that of non-induced
cells.
Analysis of Bcl-2 and Bcl-xl protein
expression by flow cytometry
Cells (2×106) were collected after
exposure to 25 μmol/l gossypol acetate for 24 h. Cells was fixed
with 4% paraformaldehyde and incubated at room temperature for 40
min. Following centrifugation, 0.2% triton-X100 and PBS (including
5% FBS) 1 ml was added and cells were incubated on ice for 10–20
min. FITC-mouse anti-human Bcl-2 monoclonal antibody (40 μl) was
added and incubated for 40 min on ice. Cells were washed twice with
cold PBS and analyzed on a flow cytometer.
When analyzing Bcl-xl expression, primary antibody
rabbit anti human Bcl-xl was added first and, following incubation,
second antibody FITC-goat anti rabbit IgG was added. The other
steps were same as those of Bcl-2 analysis.
Human myeloma cell line Wus1
xenografts
Female Balb/c nude mice (4–6 weeks old) were
obtained from Charles River Laboratories Inc. Wus1 cells
(1×106) (in serum-free RPMI-1640) were injected
subcutaneously into the flanks of the mice. On the 4th day after
Wus1 cells were injected into mice, each mouse received gossypol
acetate i.p. Mice were randomized to 3 groups (4 mice per group)
including the blank group (normal mice not administered gossypol),
the control group and the gossypol acetate 40 mg/kg group (20
mg/kg, day 4 and 6).
Tumors were measured at their greatest length and
width, and the weight was calculated as tumor weight (mg) =
(AxB2)/2, where A and B are the tumor length and width
(millimeters), respectively. Tumor growth inhibition (T/C) was
calculated by using the median tumor weight in the treated group
(T) when the median tumor weight in the control group (C) reached
~1,000 mg. At day 8, liver and spleen were isolated to determine
the size and weight.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). SPSS12.0 was used for the statistical analysis. Statistical
differences between means were evaluated using the t-test and LSD
test followed by normality test and homogeneity test of variances.
A P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Gossypol acetate inhibits myeloma cell
proliferation in vitro
Cell viability was determined by trypan blue
exclusion assay. Gossypol acetate resulted in a dose- and
time-dependent inhibition of cell proliferation (Table I). Gossypol acetate inhibited the
growth of myeloma cell lines at concentrations >10 μmol/l.
Gossypol acetate (1 μmol/l) had no effect on the myeloma cell
lines. The IC50 of gossypol acetate on the myeloma cell
lines is listed in Table II.
 | Table IEffect of gossypol acetate on MM cell
growth. |
Table I
Effect of gossypol acetate on MM cell
growth.
| Cells | Gossypol
concentration (μM) | 24 h | 48 h | 72 h |
|---|
| U266 | 0 | 33.3±9.0 | 141.1±16.0 | 201.1±46.0 |
| 1 | 31.2±9.1 | 107.4±44.1 | 110.4±56 |
| 5 | 26.7±8.7 | 37.8±10.5a | 42.7±9.3a |
| 10 | 15.5±3.4a | 19.5±5.4a | 14.1±6.4a |
| 25 | 9.5±3.9a | 2.9±0.4a | 0.6±1.0a |
| 50 | 0.3±0.3a | 0.1±0.2a | 0.0a |
| Wus1 | 0 | 42.7±9.5 | 172.3±43.7 | 178.9±13.9 |
| 1 | 42.2±3.3 | 139.7±11.8 | 143.8±2.9 |
| 5 | 27.7±2.5a | 28.9±3.3a | 30.4±5.3a |
| 10 | 14.7±0.6a | 15.0±5.0a | 11.0±3.6a |
| 25 | 2±0.4a | 0.2±0.3a | 0.0a |
| 50 | 0.6±0.2a | 0.0a | 0.0a |
 | Table IIIC50 of gossypol acetate
on myeloma cells (unit, μmol/l). |
Table II
IC50 of gossypol acetate
on myeloma cells (unit, μmol/l).
| U266 | Wus1 |
|---|
|
|
|
|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
|
IC50 | 9.0 | 2.4 | 0.9 | 6.5 | 2.2 | 2.2 |
Induction of apoptosis in myeloma cell
line by gossypol acetate
Next we investigated whether gossypol acetate was
able to induce apoptosis in myeloma cell lines. Apoptosis is
distinguished from necrosis by characteristic morphological and
biochemical changes, including compaction and fragmentation of the
chromatin, the activation of certain proteases and nucleases and
the appearance of cells at sub-G0/G1 (the
subdiploid peak). In our study, evidence of apoptosis was assessed
by morphology, DNA ladder and analysis of cell DNA content by flow
cytometry.
Gossypol acetate causes morphological
changes in myeloma cells
After exposure to 25 μmol/l gossypol acetate for 24
h, typical apoptotic changes of U266 and Wus1 cells were found
under light microscopy, fluorescence microscopy and transmission
electron microscopy (Figs.
2–4).
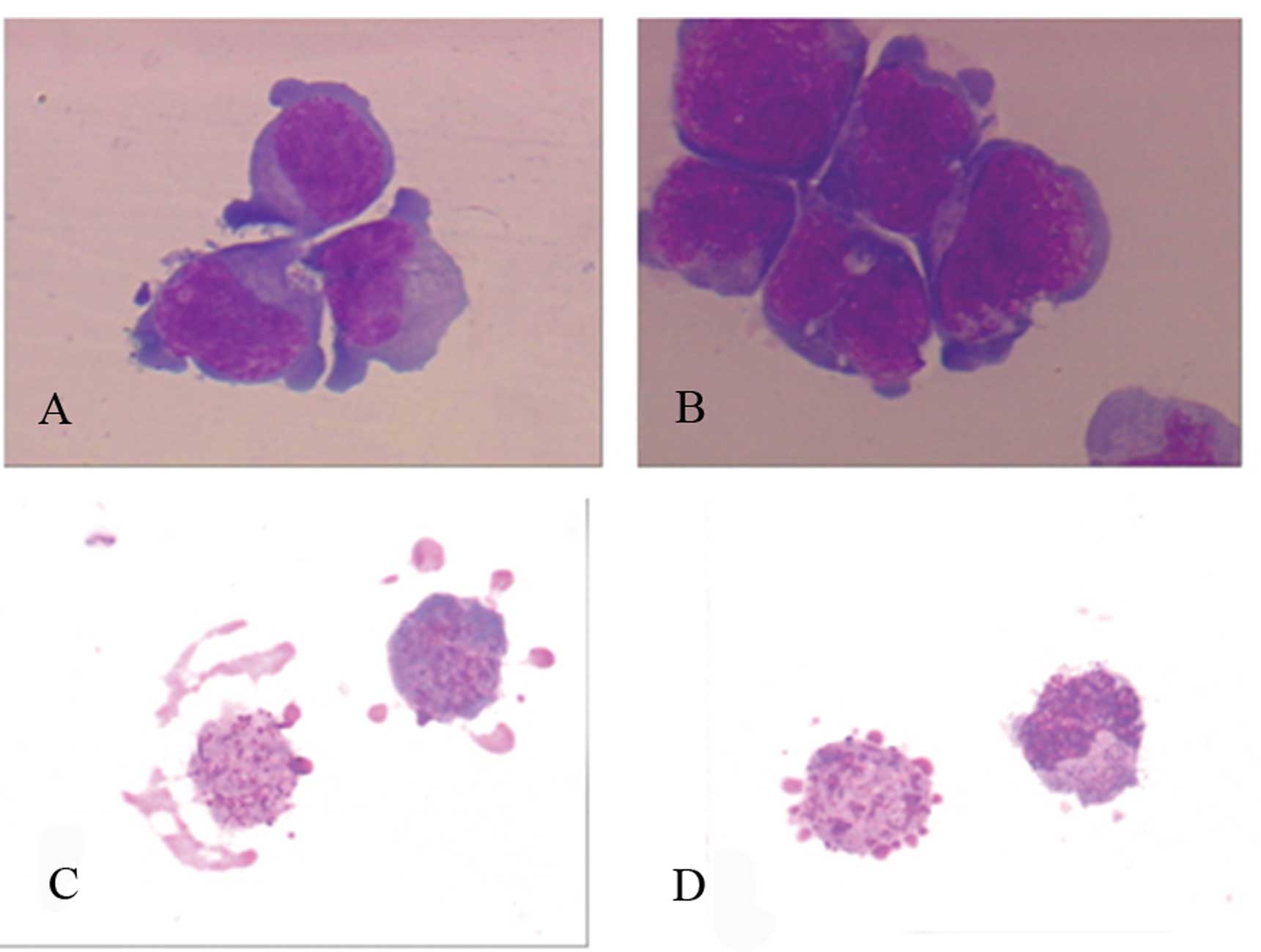 | Figure 2Morphological characteristics of
apoptotic cells under light microscopy in MM cells after treatment
with gossypol acetate 25 μmol/l for 24 h. Untreated MM cells showed
large, round and irregular cell body, pseudopodium was seen in
partial cells, neucleo is large, mostly round, oval or kidney
shape, nucleoli are observed in some cells. Characteristics of
apoptotic MM included diminished size, integrated cytomembrane,
enhanced acidophilia, condensed chromatin, formation of apoptotic
body. (A) Untreated U266 cells; (B) untreated Wus1 cells; (C)
apoptotic U266 cells; (D) apoptotic Wus1 cells (magnification,
×1,000). |
Gossypol acetate induces DNA
fragmentation
The results indicated that DNA from the control
group showed no degradation (Fig.
5, lane 4 and 5). By contrast, DNA ladder can be observed after
incubation with gossypol acetate for 24 h (Fig. 5, lane 2 and 3). This typical pattern
of DNA degradation with oligonucleosome-sized fragments of 180–200
bp and multiples thereof is the biochemical hallmark of apoptotic
cell death, and it suggested that gossypol acetate induced myeloma
cell apoptosis.
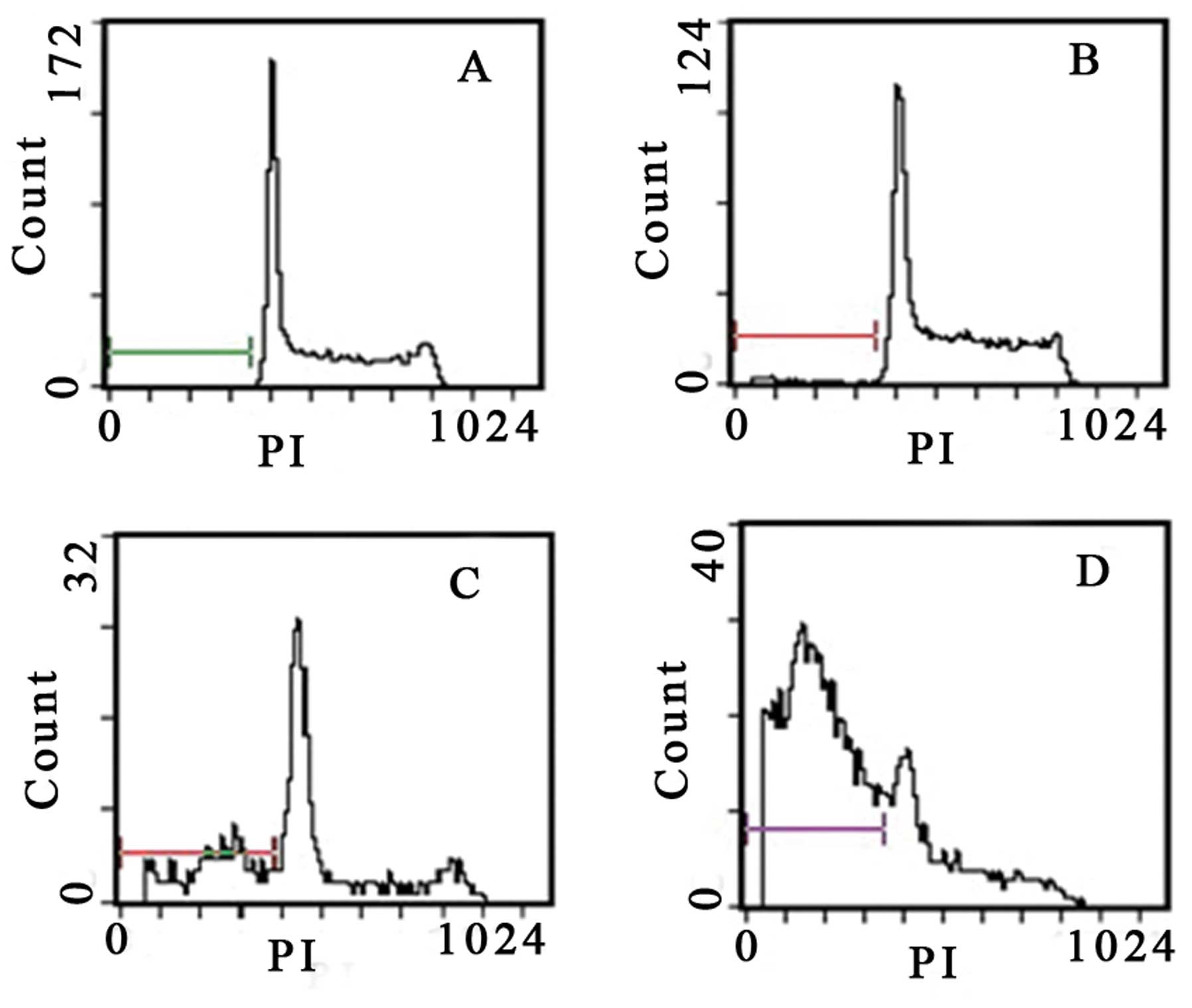
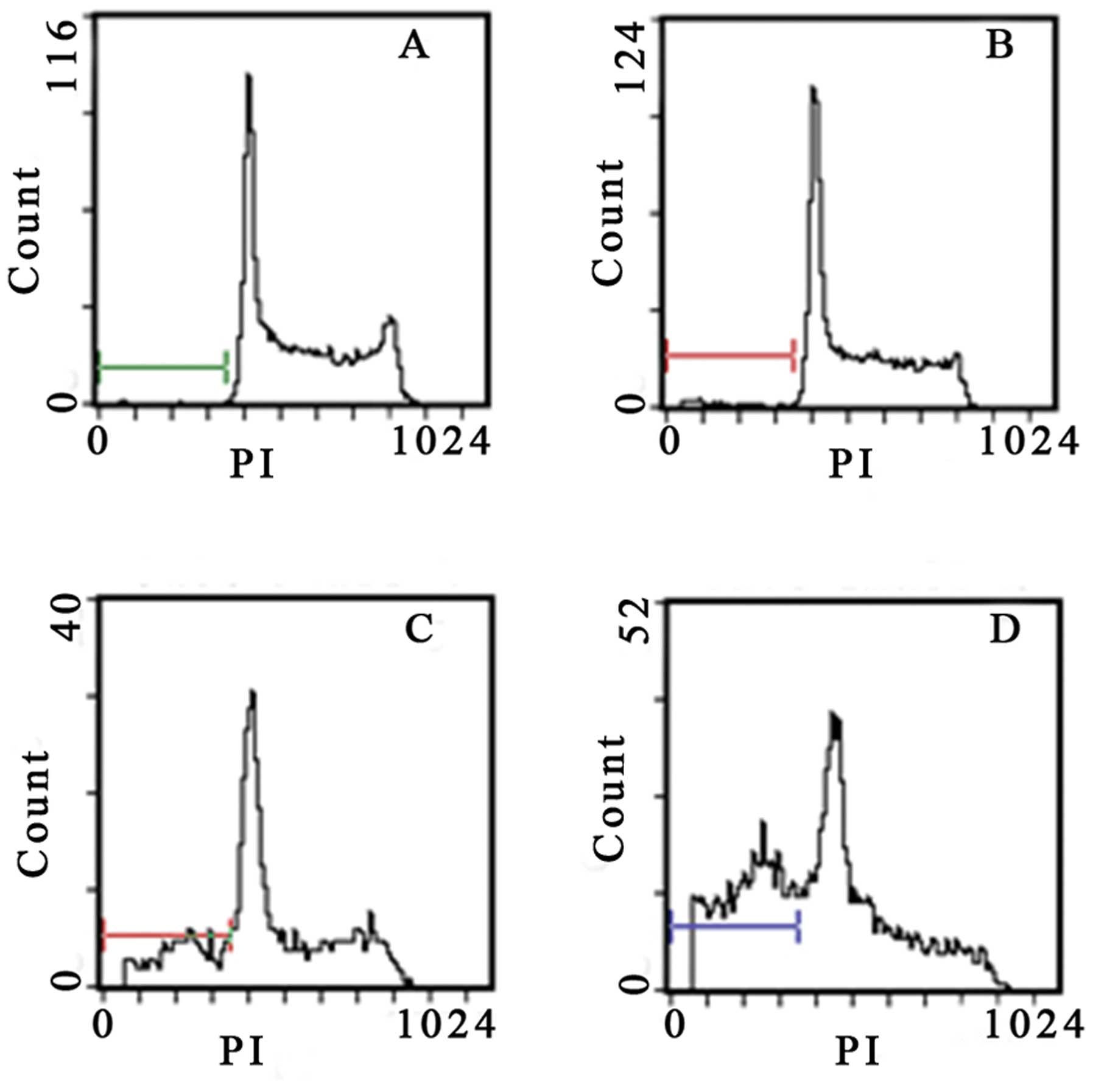
Gossypol acetate induces appearance of
subdiploid DNA of myeloma cells
Flow cytometry analysis revealed the hallmark
features of apoptosis, with the appearance of subdiploid DNA.
Quantitative analysis revealed that gossypol acetate induced the
apoptosis of myeloma cells in a time- and dose-dependent manner
(Table III). The subdiploid peak
began to appear in the DNA content distribution after treatment
with gossypol acetate at the concentration of >25 μmol/l for 24
h or 5 μmol/l for 48 h (Figs. 6 and
7).
 | Table IIIApoptotic rate of myeloma cells after
induction with gossypol acetate at different concentrations for 24
and 48 h. |
Table III
Apoptotic rate of myeloma cells after
induction with gossypol acetate at different concentrations for 24
and 48 h.
| Time | Gossypol acetate
concentration (μM) | U266 (%) | Wus1 (%) |
|---|
| 24 h | 0 | 1.0±0.5 | 1.2±1.4 |
| 5 | 4.2±2.2 | 2.4±1.1 |
| 10 | 4.7±2.1 | 3.0±0.3 |
| 25 | 16.6±9.6a | 39.4±8.8a |
| 48 h | 0 | 1.0±0.8 | 1.7±2.7 |
| 5 | 8.3±1.9a | 12.5±3.4a |
| 10 | 28.9±3.9a | 44.3±6.9a |
| 25 | 80.1±5.3a | 82.4±8.4a |
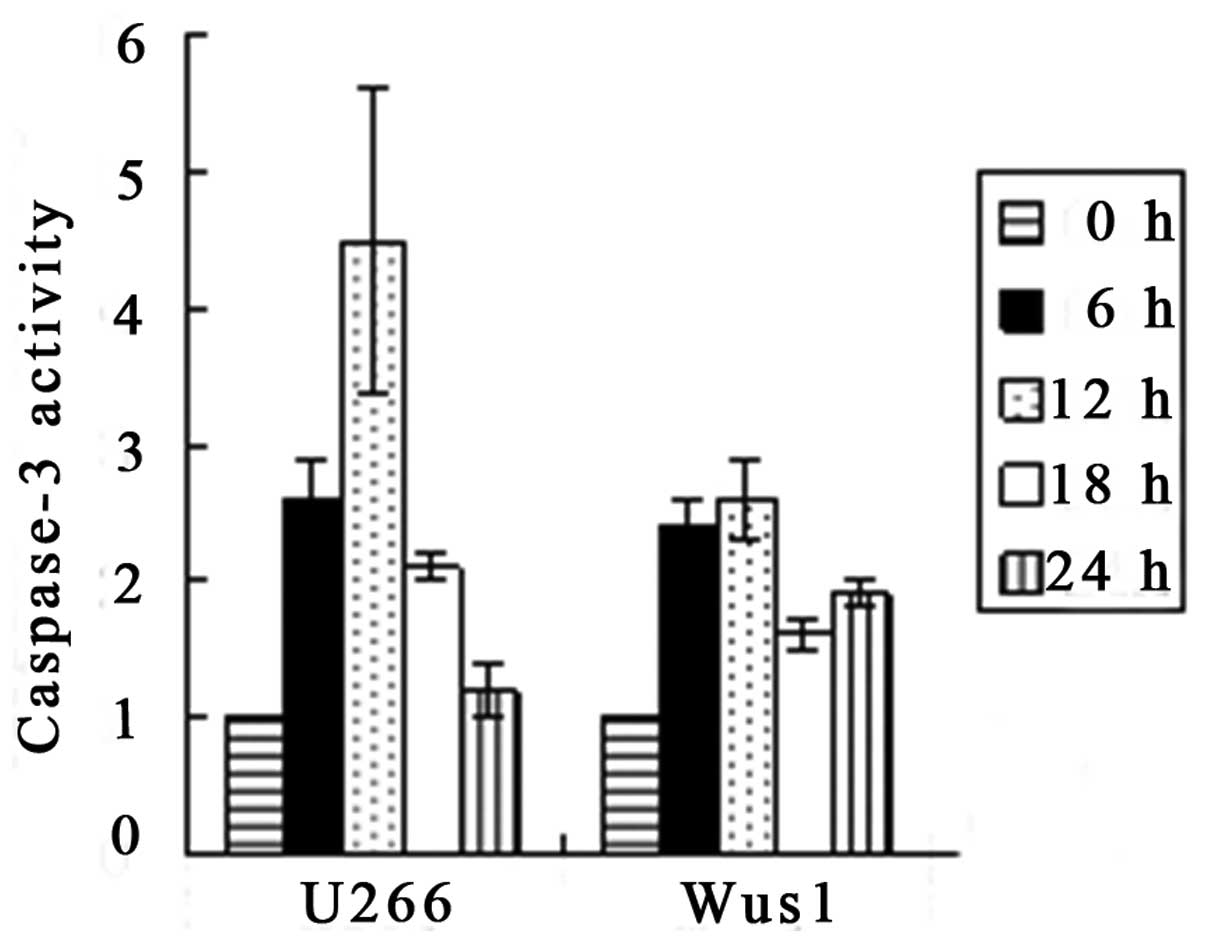
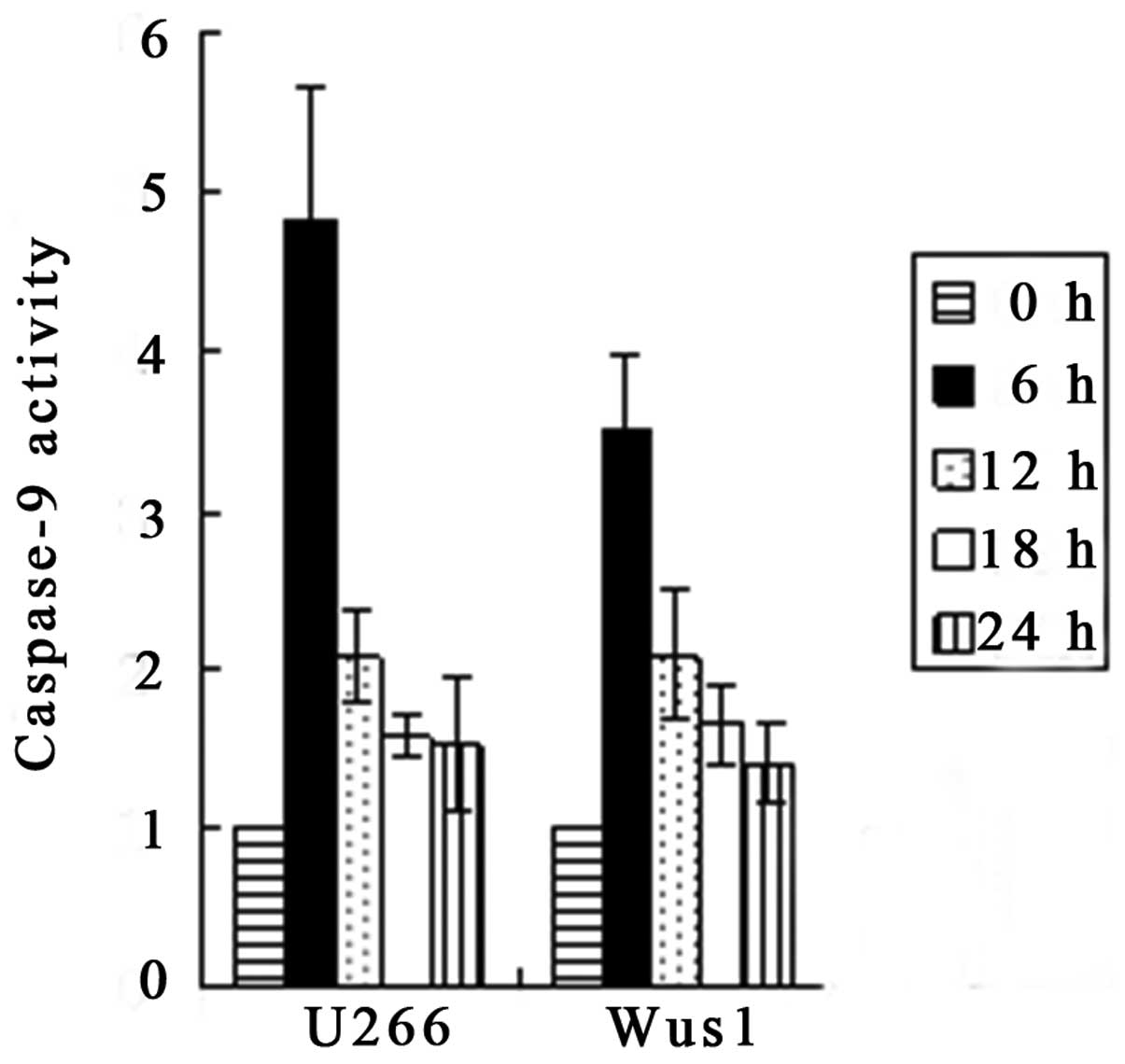
Activation of caspase-3 and -9 by
gossypol acetate
Apoptosis is associated with the activation of
specific cysteine proteases referred to as caspases. We assessed
whether gossypol acetate activated specific caspases during
apoptosis of myeloma cells. Treatment of U266 and Wus1 with 25
μmol/l gossypol acetate for 0,6,12,18,24 h resulted in the
activation of caspase-9 and -3 as early as 6 h (Fig. 8). The maximum increase in caspase-9
activity was observed at 6 h, whereas the maximum caspase-3
activity was seen at 12 h with treatment of 25 μmol/l gossypol
acetate (Fig. 9). The time to
maximum caspase-3 activity is longer than that of caspase-9. The
results indicated that gossypol acetate activated caspase-3 and -9
of myeloma cell lines.
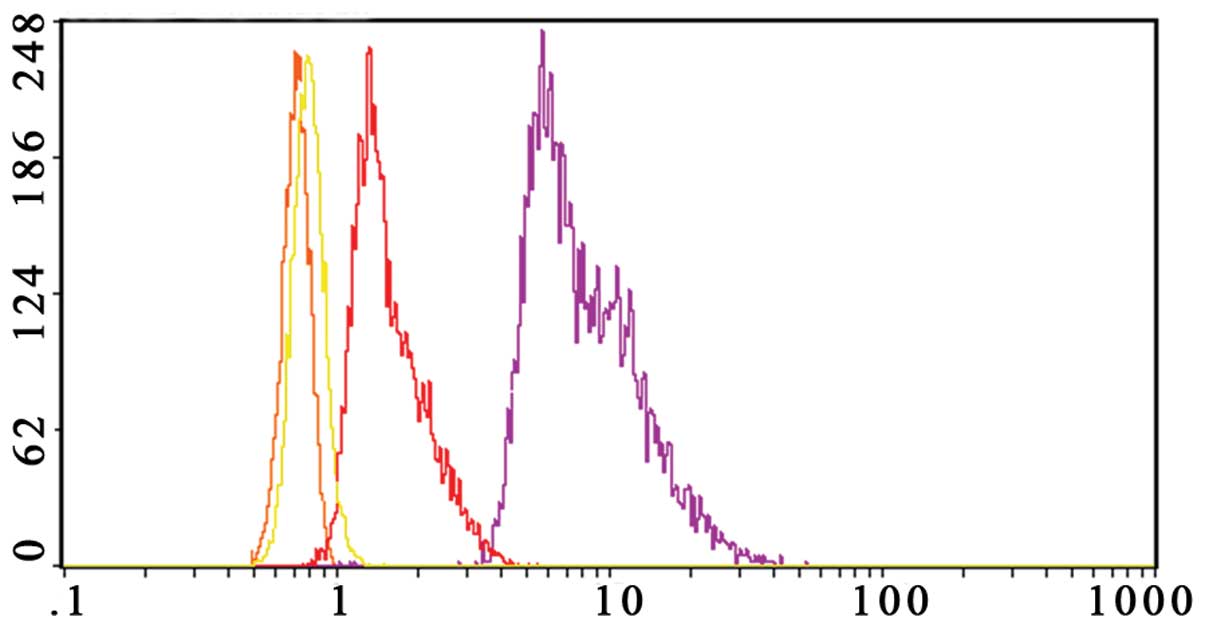
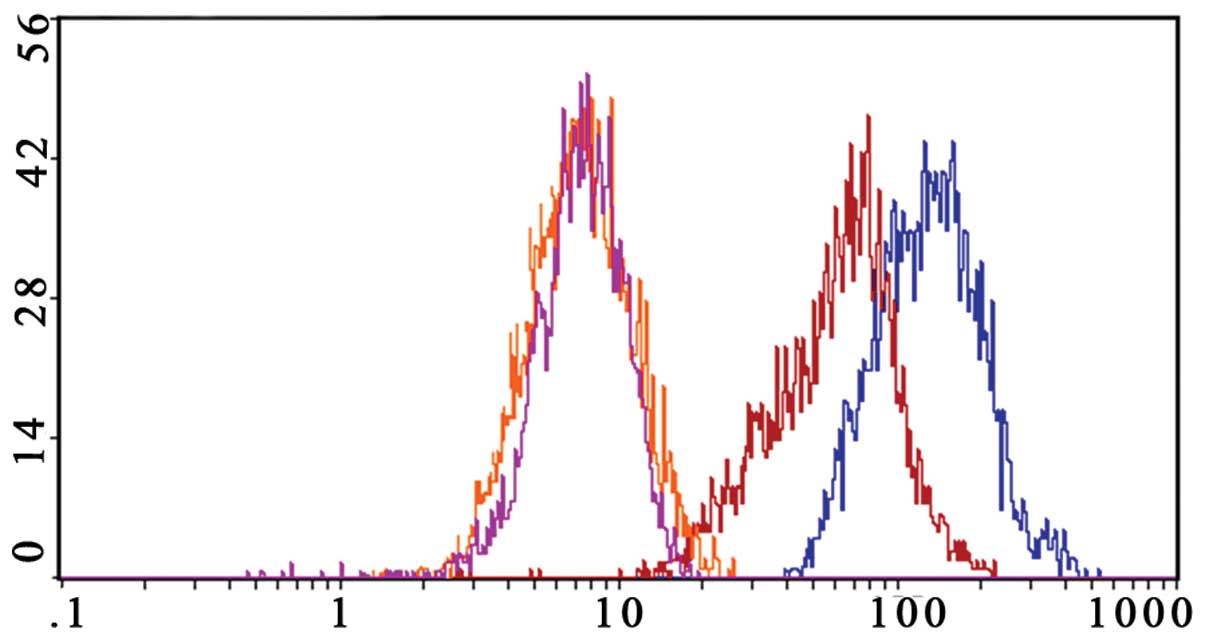
Gossypol acetate downregulates Bcl-xl and
Bcl-2 expression of myeloma cells
In our study, we found that Bcl-2 was not expressed
in normal peripheral blood mononuclear cells and the U266 cell
line, but was highly expressed in the Wus1 cell line. Bcl-xl was
not expressed in normal peripheral blood mononuclear cells, but was
highly expressed in the U266 and the Wus1 cell line. Thus, we only
analyzed Bcl-2 and Bcl-xl changes in Wus1 cells after treating with
gossypol acetate.
The Bcl-2 level of Wus1 was decreased by 86.5±1.2%
after treating with 25 μmol/l gossypol acetate for 24 h (Fig. 10). Gossypol acetate at a
concentration of 25 μmol/l on Wus1 for 24 h downregulated
expression of the Bcl-xl level by 35.9±3.6% (Fig. 11).
The results suggested that gossypol acetate
downregulates Bcl-2 and Bcl-xl expression of MM cells, and the
effect of Bcl-2 downregulation was more evident than that of
Bcl-xl.
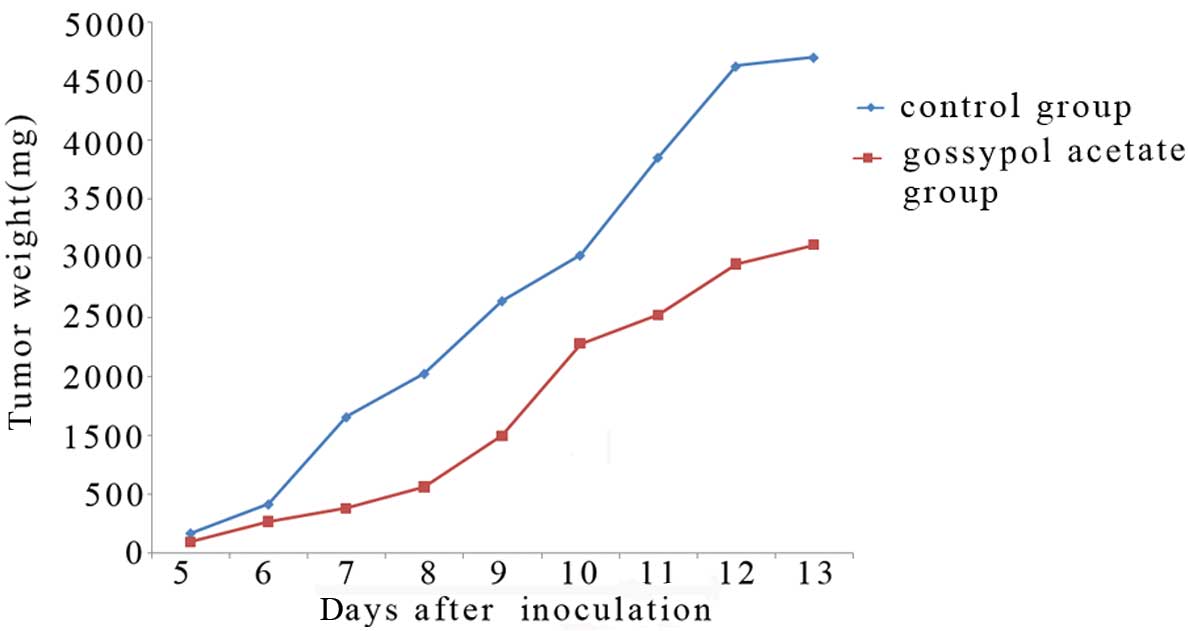
Gossypol acetate has antitumor effects in
Balb/c mice bearing Wus1 cells
We next tested the toxicity and antitumor activity
of gossypol acetate in Wus1 Balb/c mice model. Antitumor activity
of gossypol acetate against Wus1-bearing mice was observed
(Fig. 12). T/C was 30.9% (gossypol
acetate 40 mg/kg). Enlarged liver and spleen contributed to myeloma
can be diminished after gossypol acetate treatment (Table IV). In addition, there was no body
weight loss for the treated group in comparison with the vehicle
treated mice. Of note, mouse survival was not prolonged.
 | Table IVChanges of liver and spleen weight in
Wus1 Balb/c mice at day 8 after gossypol acetate treatment. |
Table IV
Changes of liver and spleen weight in
Wus1 Balb/c mice at day 8 after gossypol acetate treatment.
| Normal mice | DMSO group | Gossypol acetate 40
mg/kg group |
|---|
| Liver weight
(g) | 0.53±0.03 | 0.70±0.16 | 0.55±0.08a |
| Spleen weight
(g) | 0.03±0.01 | 0.07±0.03 | 0.03±0.01a |
Discussion
MM is a malignant plasma cell disease. The clinical
manifestation includes hyperglobulinemia, renal dysfunction, bone
damage and cytopenia. Advances in the therapy have been made in
recent years, but MM remains incurable and the overall survival has
not improved.
Bcl-2, frequently expressed in follicular lymphomas
bearing the t(14;18) chromosomal translocation, is also widely
expressed in several other B- and T-cell lymphomas such as
lymphoma, chronic lymphocytic leukemia, MM (20). The study from Pettersson et
al showed that Bcl-2 was expressed in most MM cell lines such
as U266–1970, U266–1984, U1958, U1996, L363, Karpas 707, OPM1, OPM2
(21). Our results revealed that
the human MM Wus1 cell line which was established in our laboratory
expressed a high level of Bcl-2. It was controversial whether U266
expressed Bcl-2 highly. Some studies found that during the
continuous cultivations, U266 had undergone cytogenetic changes and
Bcl-2 expression changed (21–23).
Overexpression of Bcl-xl has been observed in 30% MM (24). Our studies found that human MM cell
lines U266 and Wus1 expressed a high level of Bcl-xl.
There is increasing evidence that high expression of
Bcl-2, Bcl-xl, or both, may play a critical role in MM progression
and resistance to chemotherapeutic agents (25). Therefore, therapy targeting Bcl-2 or
Bcl-xl has become a promising strategy. There are several methods
that could inhibit Bcl-2 such as antisense nucleotide or monoclonal
antibody of Bcl-2. Antisense Bcl-2 and Bcl-xl studies showed that
inhibition of Bcl-2 and Bcl-xl may be an effective treatment of MM
(11). Previous studies revealed
that gossypol was a nonpeptidic small-molecule inhibitor of
Bcl-2/Bcl-xl. It can bind to the BH3 binding site in Bcl-2/Bcl-xl,
and then block the heterodimerization of Bcl-2/Bcl-xl with
proapoptotic members in the Bcl-2 protein family such as Bad, Bak,
Bid and Bas, and can initiate downstream apoptosis pathways
(18).
Gossypol has antiproliferative and antimetastatic
effects on several tumors. Its mechanism and molecular targets may
be variable, for example inhibition of protein kinase C, regulation
of cell cyclin-related protein Rb and cyclin D1, antiangiogenesis,
impacting signal transduction pathway (26,27).
Gossypol has now been found to have inhibitory effects on
proliferation or to induce apoptosis in ovarian cancer, endometrial
cancer, adrenal cortical tumor, thyroid cancer, lung cancer, colon
carcinoma, leukemia, pancreatic cancer, melanoma and lymphoma. In
addition, gossypol can increase the sensitivity of drug-resistant
tumor cells to chemotherapy and radiotherapy (12,13,15,26,28–36).
Some clinical trials showed gossypol was well-tolerated, and
partial responses were observed in some patients (17,37–39).
We found gossypol acetate was able to induce dose- and
time-dependent apoptosis of MM cells, as evidenced by typical
morphological changes, DNA ladder and increase in the percentage of
cells in subdiploid peak.
The mitochondrial pathway is a key pathway in cell
apoptosis. DNA-damaging agents signal cell death by altering the
mitochondrial transmembrane protein, activating Bcl-2 family
members with subsequent cyto c release, and activating the
caspase family of proteins. Caspase-3 is a central component in
cell apoptosis caused by exogenous or endogenous apoptotic signal.
We revealed that caspase-3 and caspase-9 were activated, and
Bcl-2/Bcl-xl expression was downregulated in the process of MM cell
apoptosis induced by gossypol acetate. Therefore, induction of MM
cell apoptosis by gossypol acetate may be through the mitochondrial
pathway, inhibiting Bcl-2/Bcl-xl expression and activating
caspase-9, caspase-3. Our results are consistent with previous
findings (14,36,40,41).
As a new antitumor agent, the effect of gossypol
acetate should be confirmed in vivo. Our in vivo
experiments proved that gossypol acetate could inhibit tumor growth
in Wus1-bearing mice, but the survival of mice was not prolonged,
and tumor grew rapidly after short inhibition. Some studies
reported that the effect may improve if gossypol is combined with
the other chemotherapeutics. Thus, whether these effects may be
improved when gossypol acetate is combined with other antimyeloma
drugs, such as dexamethasone, thalidomide, requires further
investigation.
In the present study, we demonstrated that gossypol
acetate had an antiproliferative effect and induced apoptosis of MM
cells for the first time. The mechanisms might involve
downregulation of Bcl-2/Bcl-xl expression and affecting the cell
cycle. Gossypol acetate was also able to inhibit tumor growth in
Wus1-bearing mice. Our findings provide evidence that gossypol
acetate warrants further preclinical and clinical research in the
treatment of MM.
Acknowledgements
The authors thank Yumei Li at the Institute of Basic
Theory of Traditional Chinese Medicine, China Academy of
Traditional Chinese Medicine for flow cytometry analysis, Electron
Microscopy Center, Peking Union Medical College for transmission
electron microscopy examination. This study was supported by
Innovation Funds in PLA General Hospital (no. 12KMM34), Clinical
Research Supportive Funds in PLA General Hospital (no.
2012FC-TSYS-1020), and Innovation Funds in Nan Lou at PLA General
Hospital (no. 2012NLQN03).
References
|
1
|
Bird JM, Owen RG, D’Sa S, et al:
Guidelines for the diagnosis and management of multiple myeloma
2011. Br J Haematol. 154:32–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singhal S, Mehta J, Desikan R, et al:
Antitumor activity of thalidomide in refractory multiple myeloma. N
Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson PG, Barlogie B, Berenson J, et
al: A phase 2 study of bortezomib in relapsed, refractory myeloma.
N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajkumar SV, Hayman SR, Lacy MQ, et al:
Combination therapy with lenalidomide plus dexamethasone (Rev/Dex)
for newly diagnosed myeloma. Blood. 106:4050–4053. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richardson PG, Sonneveld P, Schuster MW,
et al: Bortezomib or high-dose dexamethasone for relapsed multiple
myeloma. N Engl J Med. 352:2487–2498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richardson PG, Blood E, Mitsiades CS, et
al: A randomized phase 2 study of lenalidomide therapy for patients
with relapsed or relapsed and refractory multiple myeloma. Blood.
108:3458–3464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelekar A and Thompson CB: Bcl-2-family
proteins: the role of the BH3 domain in apoptosis. Trends Cell
Biol. 8:324–330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pulley H and Mohammad R: Small-molecule
inhibitors of Bcl-2 protein. Drugs Future. 29:369–381. 2004.
View Article : Google Scholar
|
|
9
|
Reed JC: Bcl-2 family proteins. Oncogene.
17:3225–3236. 1998. View Article : Google Scholar
|
|
10
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Q and Gazitt Y: Potentiation of
dexamethasone-, paclitaxel-, and Ad-p53-induced apoptosis by Bcl-2
antisense oligodeoxynucleotides in drug-resistant multiple myeloma
cells. Blood. 101:4105–4114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JS, Hsu YL, Kuo PL, Chiang LC and
Lin CC: Upregulation of Fas/Fas ligand-mediated apoptosis by
gossypol in an immortalized human alveolar lung cancer cell line.
Clin Exp Pharmacol Physiol. 31:716–722. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuszynski GP and Cossu G: Differential
cytotoxic effect of gossypol on human melanoma, colon carcinoma,
and other tissue culture cell lines. Cancer Res. 44:768–771.
1984.PubMed/NCBI
|
|
14
|
Zhang M, Liu H, Guo R, et al: Molecular
mechanism of gossypol-induced cell growth inhibition and cell death
of HT-29 human colon carcinoma cells. Biochem Pharmacol. 66:93–103.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaroszewski JW, Kaplan O and Cohen JS:
Action of gossypol and rhodamine 123 on wild type and
multidrug-resistant MCF-7 human breast cancer cells: 31P nuclear
magnetic resonance and toxicity studies. Cancer Res. 50:6936–6943.
1990.PubMed/NCBI
|
|
16
|
Le Blanc M, Russo J, Kudelka AP and Smith
JA: An in vitro study of inhibitory activity of gossypol, a
cottonseed extract, in human carcinoma cell lines. Pharmacol Res.
46:551–555. 2002.PubMed/NCBI
|
|
17
|
Stein RC, Joseph AE, Matlin SA, Cunningham
DC, et al: A preliminary clinical study of gossypol in advanced
human cancer. Cancer Chemother Pharmacol. 30:480–482. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Enyedy IJ, Ling Y, Nacro K, et al:
Discovery of small-molecule inhibitors of Bcl-2 through
structure-based computer screening. J Med Chem. 44:4314–4324. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitada S, Leone M, Sareth S, et al:
Discovery, characterization and structure-activity relationships
studies of proapoptotic polyphenols targeting B-cell
lymphocyte/leukemia-2 proteins. J Med Chem. 46:4259–4264. 2003.
View Article : Google Scholar
|
|
20
|
Yunis JJ, Frizzera G, Oken MM, et al:
Multiple recurrent genomic defects in follicular lymphoma, a
possible model for cancer. N Engl J Med. 316:79–84. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pettersson M, Jernberg-Wiklund H, Larsson
LG, et al: Expression of the bcl-2 gene in human multiple myeloma
cell lines and normal plasma cells. Blood. 79:495–502.
1992.PubMed/NCBI
|
|
22
|
Graninger WB, Seto M, Boutain B, Goldman P
and Korsmeyer SJ: Expression of bcl-2 and bcl-2-Ig fusion
transcripts in normal and neoplastic cells. J Clin Invest.
80:1512–1515. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jernberg H, Zech L and Nilsson K:
Cytogenetic studies on human myeloma cell lines. Int J Cancer.
40:811–817. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peeters SD, Hovenga S, Rosati S and
Vellenga E: Bcl-xl expression in multiple myeloma. Med Oncol.
22:183–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Linden M, Kirchhof N, Carlson C and Van
Ness B: Targeted overexpression of Bcl-xl in B-lymphoid cells
results in lymphoproliferative disease and plasma cell
malignancies. Blood. 103:2779–2786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu L, Yang D, Wang S, et al: (−)-Gossypol
enhances response to radiation therapy and results in tumor
regression of human prostate cancer. Mol Cancer Ther. 4:197–205.
2005.
|
|
27
|
Jiang J, Sugimoto Y, Liu S, et al: The
inhibitory effects of gossypol on human prostate cancer cells-PC3
are associated with transforming growth factor beta1 (TGFbeta1)
signal transduction pathway. Anticancer Res. 24:91–100.
2004.PubMed/NCBI
|
|
28
|
Band V, Hoffer AP, Band H, et al:
Antiproliferative effect of gossypol and its optical isomers on
human reproductive cancer cell lines. Gynecol Oncol. 32:273–277.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coyle T, Levante S, Shetler M and Winfield
J: In vitro and in vivo cytotoxicity of gossypol against central
nervous system tumor cell lines. J Neurooncol. 19:25–35. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benz CC, Keniry MA, Ford JM, et al:
Biochemical correlates of the antitumor and antimitochondrial
properties of gossypol enantiomers. Mol Pharmacol. 37:840–847.
1990.PubMed/NCBI
|
|
31
|
Shelley MD, Hartley L, Groundwater PW and
Fish RG: Structure-activity studies on gossypol in tumor cell
lines. Anticancer Drugs. 11:209–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jarvis WD, Turner AJ, Povirk LF, Traylor
RS and Grant S: Induction of apoptotic DNA fragmentation and cell
death in HL-60 human promyelocytic leukaemia cells by
pharmacological inhibitors of protein kinase C. Cancer Res.
54:1707–1714. 1994.PubMed/NCBI
|
|
33
|
Blackstaffe L, Shelley MD and Fish RG:
Cytotoxicity of gossypol enantiomers and its quinone metabolite
gossypolone in melanoma cell lines. Melanoma Res. 7:364–372. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohammad RM, Wang S, Aboukameel A, et al:
Preclinical studies of a nonpeptidic small-molecule inhibitor of
Bcl-2 and Bcl-X(L) [(−)-gossypol] against diffuse large cell
lymphoma. Mol Cancer Ther. 4:13–21. 2005.
|
|
35
|
Ergun MA, Konac E, Erbas D and Ekmekci A:
Apoptosis and nitric oxide release induced by thalidomide, gossypol
and dexamethasone in cultured human chronic myelogenous leukemic
K-562 cells. Cell Biol Int. 28:237–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hou DX, Uto T, Tong X, et al: Involvement
of reactive oxygen species-independent mitochondrial pathway in
gossypol-induced apoptosis. Arch Biochem Biophys. 428:179–187.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bushunow P, Reidenberg MM, Wasenko J, et
al: Gossypol treatment of recurrent adult malignant gliomas. J
Neurooncol. 43:79–86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Flack MR, Pyle RG, Mullen NM, Lorenzo B,
et al: Oral gossypol in the treatment of metastatic adrenal cancer.
J Clin Endocrinol Metab. 76:1019–1024. 1993.PubMed/NCBI
|
|
39
|
Van Poznak C, Seidman AD, Reidenberg MM,
et al: Oral gossypol in the treatment of patients with refractory
metastatic breast cancer: a phase I/II clinical trial. Breast
Cancer Res Treat. 66:239–248. 2001.PubMed/NCBI
|
|
40
|
Oliver CL, Miranda MB, Shangary S, et al:
(−)-Gossypol acts directly on the mitochondria to overcome Bcl-2-
and Bcl-X(L)-mediated apoptosis resistance. Mol Cancer Ther.
4:23–31. 2005.
|
|
41
|
Oliver CL, Bauer JA, Wolter KG, et al: In
vitro effects of the BH3 mimetic, (−)-gossypol, on head and neck
squamous cell carcinoma cells. Clin Cancer Res. 10:7757–7763.
2004.
|