Introduction
E-cadherin is a calcium-regulated homophilic
cell-cell adhesion molecule and is expressed in most normal
epithelial tissues but is downregulated in most types of cancer
cells (1). The absence of
E-cadherin causes the dedifferentiation and invasiveness of human
cancers (2), indicating that
E-cadherin is a tumor suppressor (3). Either gene mutation or loss of the
wild-type allele leads to inactivation of E-cadherin (3,4).
Selective loss of E-cadherin is one of the hallmarks of invasive
breast cancer phenotypes (5).
Decreased levels of E-cadherin have been related to the distant
metastasis and poor prognosis of breast cancer (6,7).
Therefore, increasing the expression of functional E-cadherin is a
novel cancer therapeutic strategy. However, the potential
application of traditional Chinese medicine in inducing the
expression of E-cadherin in breast cancers is largely
unexplored.
Elemene
(1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane) is an active
anticancer component of the traditional Chinese medicine Curcuma
wenyujin(8). The extract of
elemene is a mixture of α-, β- and δ-elemene, with β-elemene (ELE)
as the main component, which accounts for 60–72% of the three
isoforms (9). ELE has shown
anticancer activities in the clinical treatment of leukemia and
carcinomas of the brain, breast and liver (8,10–12).
One formulation of ELE has been approved by the State Food and Drug
Administration of China for the treatment of primary and secondary
brain tumors.
ELE inhibits cell proliferation by inducing cell
cycle arrest and apoptosis, thereby reducing the metastasis or
tissue invasion of cancer cells (8,13,14).
We previously found that ELE upregulated estrogen receptor-α (ERα)
mRNA by downregulating the Ras/MAPK signaling pathway in the
tamoxifen (TAM)-resistant cell line MCF-7/TAM (15). As ERα suppresses the expression of
the nuclear transcription factor Snail, a negative transcription
factor of E-cadherin gene expression (16,17),
it is intriguing to propose that ELE may increase the expression of
E-cadherin via activating the re-expression of ERα and hence
inhibiting the gene transcription of Snail. In the present study,
we analyzed the levels of E-cadherin expression and cell motility
capacity of MCF-7 cells following ELE treatment.
Materials and methods
Chemicals and antibodies
ELE (98% purity) was purchased from Dalian Yuanda
Pharmaceuticals (Liaoning, China). The primary antibodies against
E-cadherin (ab1416), ERα (ab2746), metastasis-associated protein 3
(MTA3) (ab87275), Snail (ab53519) and β-actin were from Abcam
(Cambridge, UK). The secondary horseradish peroxidase
(HRP)-conjugated goat anti-mouse-IgG and anti-rabbit-IgG antibodies
were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell line and drug treatment
The MCF-7 human breast cancer cell line was obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and propagated in Dulbecco’s modified Eagle’s medium
(DMEM)/high glucose supplemented with 20% fetal bovine serum (FBS)
and 1% penicillin/streptomycin (Gibco, Carlsbad, CA, USA). MCF-7
cells were cultured at 37°C in a humidified incubator (Heraeus,
Germany) with 5% CO2 and seeded at 2.5×105
cells/ml in 6-well plates (Corning, Inc., Corning, NY, USA). The
cells were divided into three treatment groups (0, 50 and 100 μg/ml
ELE), observed and examined after 24 h.
Immunofluorescence assay
For E-cadherin staining, cells were plated on glass
coverslips in 6-well plates and treated with ELE for 24 h. The
cells were washed in phosphate-buffered saline (PBS), fixed in 4%
paraformaldehyde solution containing 0.1% Triton X-100 for 20 min
at room temperature (RT), incubated in 5% bovine serum albumin for
30 min at RT, and then treated with the anti-E-cadherin monoclonal
antibody for 16 h at 4°C. After being washed three times in PBS,
cells were further incubated with CY3 conjugated goat anti-mouse
IgG (Beyotime Biotechnology, Haimeng, China) for 1 h at RT. After
three washes with PBS, the cells were analyzed using a fluorescence
inverted microscope (IX71; Olympus, Japan). The cells were
counterstained with Hoechst 33258 (Beyotime Biotechnology) to label
the cell nuclei.
Western blot analysis
Cells were washed twice with ice-cold PBS and lysed
in 1% Triton buffer [1% Triton X-100, 50 mM Tris-Cl (pH 7.4), 150
mM NaCl, 10 mM EDTA, 100 mM NaF, 1 mM Na3VO4
(Sigma, St. Louis, MO, USA), 1 mM phenylmethanesulfonyl fluoride
(PMSF) and 2 μg/ml aprotinin] on ice. Total proteins were
quantified using the Lowry method. Proteins (50 μg) were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
electrophoretically transferred to polyvinylidene fluoride (PVDF)
membranes (Immobilon-P; Millipore, USA). The membranes were blocked
with 5% bovine serum albumin (Sigma) at RT for 1 h and incubated
overnight at 4°C with the following primary antibodies: E-cadherin
(1:1,000), ERα (1:1,000), MTA3 (1:2,000) and β-actin (1:500). After
washing with Tris-buffered saline with Tween-20 (TBST) buffer, the
membranes were probed with HRP-conjugated secondary antibodies and
developed with enhanced chemiluminescence reagent (Beyotime
Biotechnology). To detect Snail, nuclear proteins were extracted
with a nuclear extraction kit (Beyotime Biotechnology) and probed
with the anti-Snail antibody (1:2,000). The images were analyzed by
NIH ImageJ software.
Real-time RT-PCR
Total RNA was extracted by homogenization in 1 ml of
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), followed by
chloroform re-extraction and isopropanol precipitation. RT-PCR was
performed in a final volume of 20 μl containing 1.6 ml of cDNA
template, 1 ml of primer (10 mM) and 10 ml of SYBR-Green Master Mix
(Takara, Dalian, China). Primers used were 5′-TCCCATCAGCT
GCCCAGAAA-3′ (sense) and 5′-TGACTCCTGTGTTCCTG TTA-3′ (antisense)
for E-cadherin; 5′-GCACCGTCAAGG CTGAGAAC-3′ (sense) and
5′-TGGTGAAGACGCCAGT GGA-3′ (antisense) for human GAPDH. GAPDH was
used as an endogenous housekeeping gene.
Transwell migration and invasion
assay
Cell invasion was measured using a Transwell
chamber. In brief, 2×105 cells were added per Transwell
invasion chamber coated with 1–2 mg/ml Matrigel (reconstituted
basement membrane; BD Biosciences, Mississauga, ON, Canada). MCF-7
cells were treated with ELE. Twenty-four hours later, the cells in
the upper chamber were removed with a cotton swab. The remaining
cells on the membrane were fixed for 10 min in methanol, stained
with 1% crystal violet solution and washed with PBS. The number of
invaded cells was counted for 5 fields/field of view at ×200
magnification.
Cell viability assay
Cell viability was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells were seeded at 5×104 cells/well in
96-well plates, incubated overnight and then exposed to the
indicated concentrations of ELE for the indicated times. Next, 20
μl of MTT (Sigma, St. Louis, MO, USA) solution (5 mg/ml) was added
to each well, and the cells were incubated for another 4 h at 37°C.
After removal of the culture medium, the cells were lysed in 200 μl
of dimethyl sulfoxide (DMSO), and the optical density (OD) was
measured at 570 nm with a microplate reader (Model 550; Bio-Rad
Laboratories, USA). The following formula was used: Cell viability
= (OD of the experimental sample/OD of the control group) ×
100%.
Statistical analysis
The experiments were repeated at least three times.
Data are expressed as the means ± standard deviation. Differences
in the results between two groups were evaluated by the Student’s
t-test. P<0.05 was considered to indicate a statistically
significant result.
Results
Effect of ELE on the cell proliferation
of MCF-7 cells
To examine the effect of ELE on the cell viability
of MCF-7 cells, we treated the cells with 50 or 100 μg/ml ELE for
0, 12, 24, 36, 48, 60 and 72 h and determined the rate of cell
survival with an MTT assay. MCF-7 cell proliferation was not
inhibited at 50 or 100 μg/ml ELE for 24 h (P>0.05), whereas
treatment of cells with both doses of ELE for 36 h significantly
inhibited cell survival (Fig. 1)
(P<0.05). Therefore, for our subsequent experiments, we treated
cells with 50 and 100 μg/ml ELE for 24 h, respectively.
ELE transforms the phenotype of MCF-7
cells
We used a microscope to approximately evaluate the
cell motility capacity with the change in cell morphology of MCF-7
cells treated with 50 and 100 μg/ml ELE for 24 h, respectively.
ELE-treated cells demonstrated a loosely aggregated cell phenotype
that switched gradually to a tight adherence, indicating increased
cell-cell interactions and reduced capacity for motility (Fig. 2).
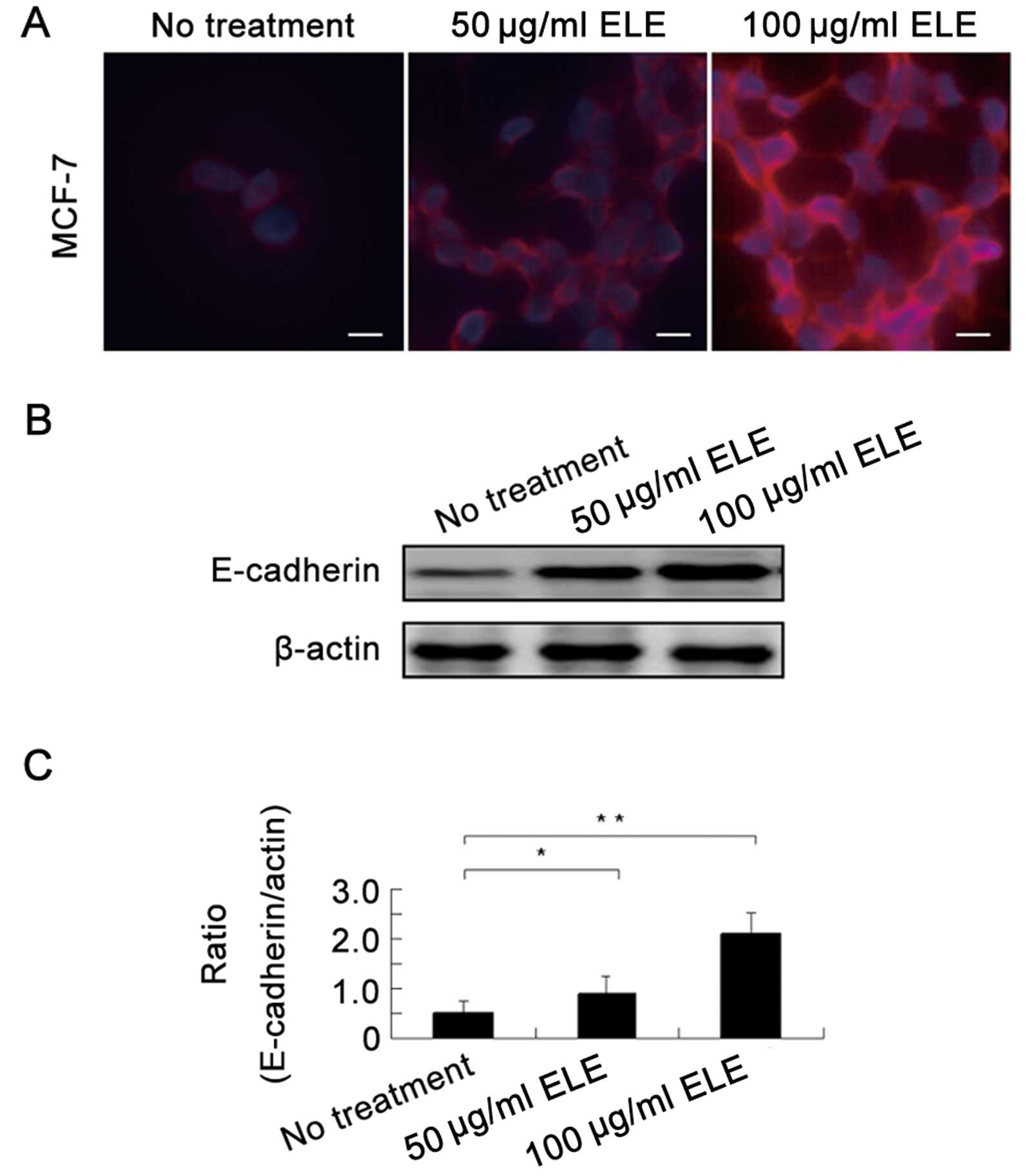
ELE increases the E-cadherin protein
level in MCF-7 cells
To support the above-mentioned alteration in cell
morphology, we next determined the E-cadherin protein levels in the
MCF-7 cells by an immunofluorescence assay and found that
E-cadherin protein was upregulated by ELE (Fig. 3A). Further western blot analysis
showed that 50 and 100 μg/ml ELE increased E-cadherin in a
concentration-dependent manner (Fig. 3B
and C) (P<0.05 and <0.01, respectively). Thus, ELE
upregulates E-cadherin protein levels.
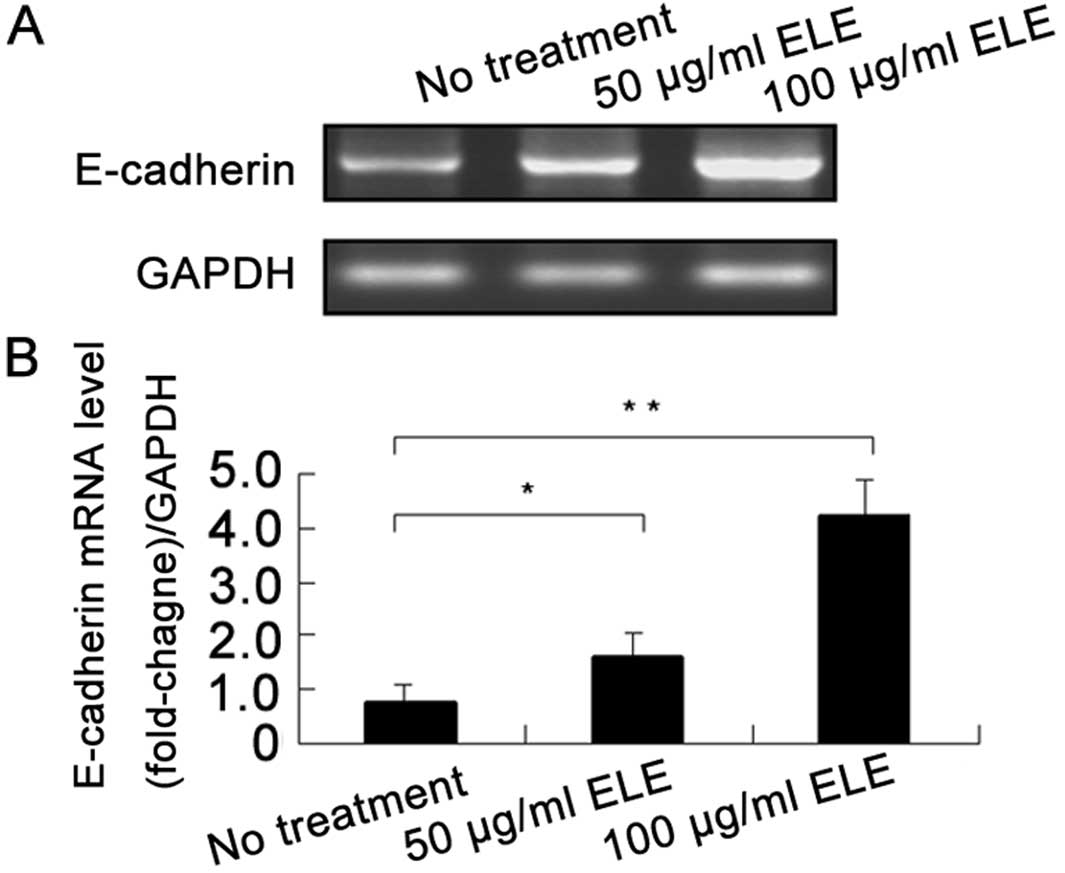
ELE increases the E-cadherin mRNA level
in MCF-7 cells
To investigate whether ELE enhances the gene
transcription of E-cadherin, we determined the E-cadherin mRNA
levels in MCF-7 cells using an RT-PCR assay. ELE at 50 and 100
μg/ml significantly increased the mRNA levels of E-cadherin in a
concentration-dependent manner (Fig.
4) (P<0.05 and 0.01, respectively), which was consistent
with the upregulation of E-cadherin protein (Fig. 3). These results showed that ELE
increased E-cadherin gene transcription.
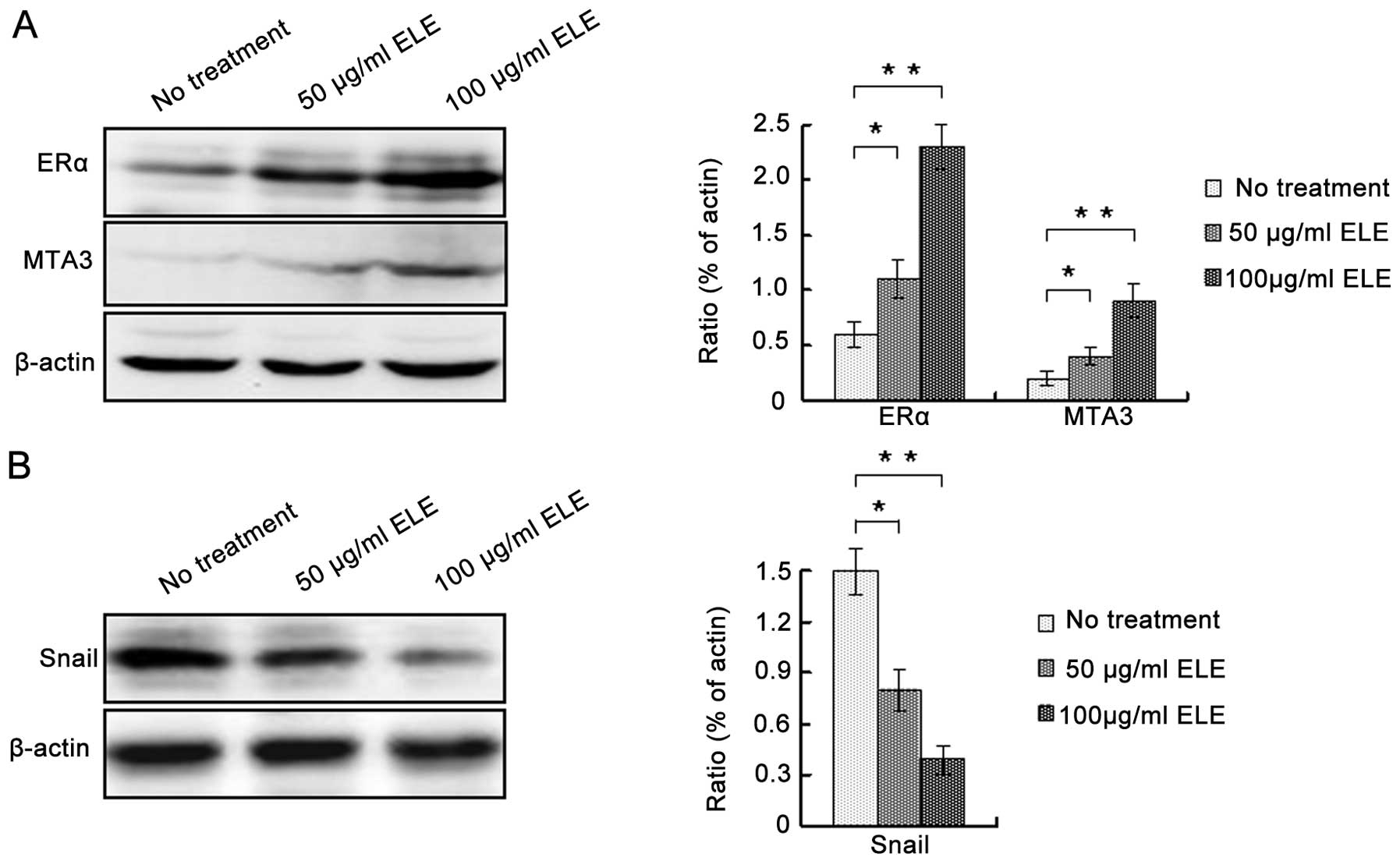
ELE regulates E-cadherin expression via
ERα/MTA3/Snail signaling
To determine the underlying mechanism by which ELE
increases the expression of E-cadherin, we determined the protein
levels of ERα, MTA3 and Snail. ELE at 50 and 100 μg/ml increased
both ERα and MTA3, whereas it decreased Snail (Fig. 5A and B) (P<0.05 and <0.01,
respectively). In addition, increasing the concentration of ELE
apparently enhanced these effects. Thus, ELE regulates E-cadherin
expression via the ERα/MTA3/Snail signaling pathway.
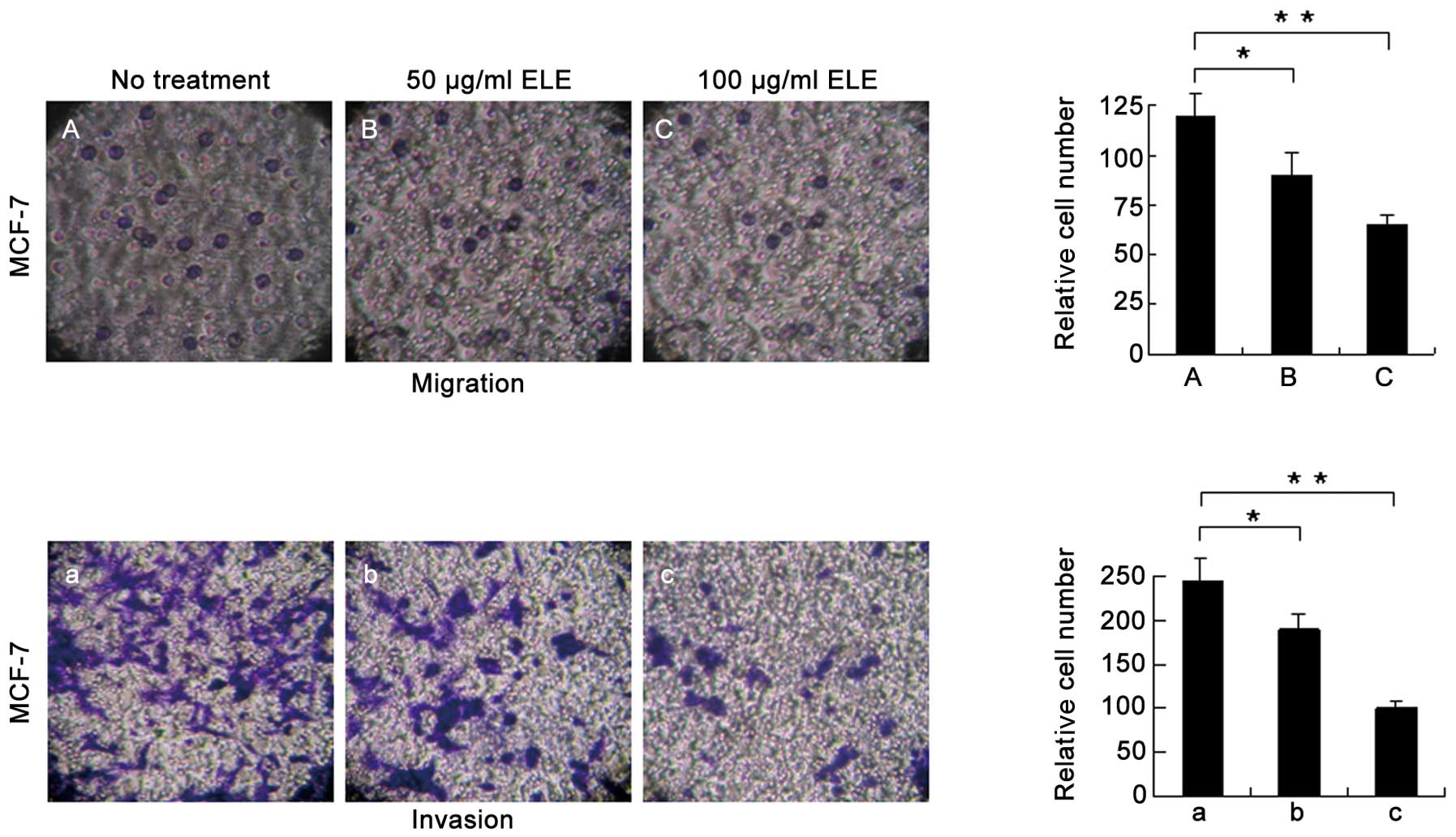
ELE affects the cell migration and
invasion of MCF-7 cells
An increase in E-cadherin may explain the increased
cell-cell interactions and decreased capacity for motility of the
MCF-7 cells following ELE treatment (Fig. 2). To further support this
conclusion, we measured the cell migration and invasion of MCF-7
cells treated with ELE with a Transwell assay and found that cells
successfully penetrated the basement membrane-coated chambers;
however, the number of cells that penetrated the membrane was
reduced significantly in the ELE-treated cells (Fig. 6) (P<0.05 and <0.01,
respectively, for the 50 and 100 μg/ml treatment groups). These
data demonstrated that ELE inhibits both cell migration and
invasion.
Discussion
E-cadherin is a cell adhesion molecule that is
expressed in normal breast tissue, and decreased E-cadherin
expression is correlated with the poor clinical prognosis of breast
cancer. The status of the E-cadherin gene, including promoter
methylation and mutation, is related to the capacity for cell
motility of breast cancers (18).
Loss of E-cadherin expression results in increased cellular
motility, invasiveness, and resistance to apoptosis, which leads to
epithelial-mesenchymal transition (EMT) (19,20).
Thus, reversing the functional protein levels of E-cadherin is an
alternative strategy for cancer therapy. Reversing CpG
hypermethylation in the promoter region of the E-cadherin gene
could re-activate E-cadherin gene expression (21,22).
It has been proposed that targeting signal transduction pathways
such as EGFR and ER may be a more promising method with which to
restore E-cadherin levels (23–26).
In the present study, we discovered that ELE
positively regulated the expression of E-cadherin in MCF-7 cells.
Consistently, ELE induced a switch in cell morphology and reduced
cell migration and invasion. Estrogen and its receptors regulate
the expression of E-cadherin and EMT in breast cancer cells
(27). The ERα maintains the
epithelial morphology of breast cancer cells by activating CDH-1,
the E-cadherin encoding gene (28).
It has been reported that ER signaling upregulates MTA3 levels to
negatively modulate Snail-mediated repression of E-cadherin
(29). Further studies have
demonstrated that activation of the ER-MTA3-Snail-E-cadherin
pathway in breast cancer is generally associated with a more
favorable clinical outcome (30–33).
We previously found that ELE upregulates ERα mRNA
and promotes the re-expression of ERα through downregulating the
Ras/MAPK/ERK signaling pathway in MCF-7/TAM cells (15). In the present study, we showed that
ELE increased ERα and MTA3, while reducing Snail. Thus, we
demonstrated that ELE enhances the E-cadherin system and decreases
the cell motility capacity by mediating the
ER/MTA3/Snail/E-cadherin pathway in the breast cancer cell line
MCF-7. These results suggest that the traditional Chinese medicine
β-elemene is a promising agent for the treatment of breast
cancers.
Acknowledgements
We thank Luping Zheng (The Research Institute of
Integrated Traditional and Western Medicine, Dalian Medical
University) for the technical assistance. This study was approved
by the Ethics Committee of the Second Affiliated Hospital of Dalian
Medical University.
References
|
1
|
Hazan RB and Norton L: The epidermal
growth factor receptor modulates the interaction of E-cadherin with
the actin cytoskeleton. J Biol Chem. 273:9078–9084. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng CW, Wu PE, Yu JC, et al: Mechanisms
of inactivation of E-cadherin in breast carcinoma: modification of
the two-hit hypothesis of tumor suppressor gene. Oncogene.
20:3814–3823. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berx G, Cleton-Jansen AM, Nollet F, et al:
E-cadherin is a tumour/invasion suppressor gene mutated in human
lobular breast cancers. EMBO J. 14:6107–6115. 1995.PubMed/NCBI
|
|
4
|
Vos CB, Cleton-Jansen AM, Berx G, et al:
E-cadherin inactivation in lobular carcinoma in situ of the breast:
an early event in tumorigenesis. Br J Cancer. 76:1131–1133. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida R, Kimura N, Harada Y and Ohuchi
N: The loss of E-cadherin, α- and β-catenin expression is
associated with metastasis and poor prognosis in invasive breast
cancer. Int J Oncol. 18:513–520. 2001.
|
|
6
|
Parker C, Rampaul RS, Pinder SE, et al:
E-cadherin as a prognostic indicator in primary breast cancer. Br J
Cancer. 85:1958–1963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kowalski PJ, Rubin MA and Kleer CG:
E-cadherin expression in primary carcinomas of the breast and its
distant metastases. Breast Cancer Res. 5:R217–R222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu JJ, Dang YY, Huang M, et al:
Anti-cancer properties of terpenoids isolated from Rhizoma
Curcumae - a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Li X, Huang F, et al: Antitumor
effect of β-elemene in non-small-cell lung cancer cells is mediated
via induction of cell cycle arrest and apoptotic cell death. Cell
Mol Life Sci. 62:881–893. 2005.
|
|
10
|
Yao YQ, Ding X, Jia YC, et al: Anti-tumor
effect of β-elemene in glioblastoma cells depends on p38 MAPK
activation. Cancer Lett. 264:127–134. 2008.
|
|
11
|
Liu J, Zhang Y, Qu J, et al:
β-Elemene-induced autophagy protects human gastric cancer cells
from undergoing apoptosis. BMC Cancer. 11:1832011.
|
|
12
|
Li X, Wang G, Zhao J, et al:
Antiproliferative effect of β-elemene in chemoresistant ovarian
carcinoma cells is mediated through arrest of the cell cycle at the
G2-M phase. Cell Mol Life Sci. 62:894–904. 2005.
|
|
13
|
Chen W, Lu Y, Wu J, et al: Beta-elemene
inhibits melanoma growth and metastasis via suppressing vascular
endothelial growth factor-mediated angiogenesis. Cancer Chemother
Pharmacol. 67:799–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Z, Wang R, Xu L, et al: β-Elemene
piperazine derivatives induce apoptosis in human leukemia cells
through downregulation of c-FLIP and generation of ROS. PLoS One.
6:e158432011.
|
|
15
|
Zhang B, Zhang X, Tang B, et al:
Investigation of elemene-induced reversal of tamoxifen resistance
in MCF-7 cells through oestrogen receptor α (ERα) re-expression.
Breast Cancer Res Treat. 136:399–406. 2012.PubMed/NCBI
|
|
16
|
Oesterreich S, Deng W, Jiang S, et al:
Estrogen-mediated down-regulation of E-cadherin in breast cancer
cells. Cancer Res. 63:5203–5208. 2003.PubMed/NCBI
|
|
17
|
Scherbakov AM, Andreeva OE, Shatskaya VA
and Krasil’nikov MA: The relationships between snail1 and estrogen
receptor signaling in breast cancer cells. J Cell Biochem.
113:2147–2155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Horssen R, Hollestelle A, Rens JA, et
al: E-cadherin promotor methylation and mutation are inversely
related to motility capacity of breast cancer cells. Breast Cancer
Res Treat. 136:365–377. 2012.PubMed/NCBI
|
|
19
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nam JS, Ino Y, Kanai Y, et al:
5-aza-2′-deoxycytidine restores the E-cadherin system in
E-cadherin-silenced cancer cells and reduces cancer metastasis.
Clin Exp Metastasis. 21:49–56. 2004.
|
|
22
|
Peng G, Wargovich MJ and Dixon DA:
Anti-proliferative effects of green tea polyphenol EGCG on
Ha-Ras-induced transformation of intestinal epithelial cells.
Cancer Lett. 238:260–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu Z, Ghosh S, Wang Z and Hunter T:
Downregulation of caveolin-1 function by EGF leads to the loss of
E-cadherin, increased transcriptional activity of beta-catenin, and
enhanced tumor cell invasion. Cancer Cell. 4:499–515. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mauro L, Pellegrino M, Lappano R, et al:
E-cadherin mediates the aggregation of breast cancer cells induced
by tamoxifen and epidermal growth factor. Breast Cancer Res Treat.
121:79–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Belguise K, Guo S and Sonenshein GE:
Activation of FOXO3a by the green tea polyphenol
epigallocatechin-3-gallate induces estrogen receptor α expression
reversing invasive phenotype of breast cancer cells. Cancer Res.
67:5763–5770. 2007.PubMed/NCBI
|
|
26
|
Helguero LA, Lindberg K, Gardmo C, et al:
Different roles of estrogen receptors α and β in the regulation of
E-cadherin protein levels in a mouse mammary epithelial cell line.
Cancer Res. 68:8695–8704. 2008.
|
|
27
|
Planas-Silva MD and Waltz PK: Estrogen
promotes reversible epithelial-to-mesenchymal-like transition and
collective motility in MCF-7 breast cancer cells. J Steroid Biochem
Mol Biol. 104:11–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardamone MD, Bardella C, Gutierrez A, et
al: ERα as ligand-independent activator of CDH-1 regulates
determination and maintenance of epithelial morphology in breast
cancer cells. Proc Natl Acad Sci USA. 106:7420–7425. 2009.
|
|
29
|
Kumar R: Another tie that binds the MTA
family to breast cancer. Cell. 113:142–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita N, Kajita M, Taysavang P and Wade
PA: Hormonal regulation of metastasis-associated protein 3
transcription in breast cancer cells. Mol Endocrinol. 18:2937–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mishra SK, Talukder AH, Gururaj AE, et al:
Upstream determinants of estrogen receptor-α regulation of
metastatic tumor antigen 3 pathway. J Biol Chem. 279:32709–32715.
2004.
|
|
32
|
Yu JC, Hsu HM, Chen ST, et al: Breast
cancer risk associated with genotypic polymorphism of the genes
involved in the estrogen-receptor-signaling pathway: a multigenic
study on cancer susceptibility. J Biomed Sci. 13:419–432. 2006.
View Article : Google Scholar
|
|
33
|
Toh Y and Nicolson GL: The role of the MTA
family and their encoded proteins in human cancers: molecular
functions and clinical implications. Clin Exp Metastasis.
26:215–227. 2009. View Article : Google Scholar : PubMed/NCBI
|