Introduction
In recent years, the discovery of new therapeutic
reagents for cancer treatment has been studied in several
countries. One of the most aggressive and malignant types of human
cancer, glioblastoma multiforme (GBM), is a common brain tumor in
humans (1,2). The prognosis of GBM after diagnosis
remains dismal, even after aggressive treatment such as
radiotherapy, chemotherapy and surgery (3–5). Thus,
it is necessary to identify new treatment modalities for GBM to
achieve more favorable results.
Korean red ginseng (KRG; Panax ginseng C.A.
Meyer), which is also generally known as Korean ginseng, is a
native herbal remedy commonly used in Korea and China (6,7). Red
ginseng has been recognized as a life prolonging herb in Asia for
thousands of years (8–10). The major active components in red
ginseng are the ginsenosides Rg3, Rg5 and Rk1, each of which has
unique pharmacological activities (11,12).
Ginsenosides have been reported to exert
cancer-preventive effects against various types of cancer, such as
lung cancer (13), nasopharyngeal
carcinoma (14) and prostate cancer
(15). Various components of
ginsenosides are expected to exert a similar preventive effect
against various types of cancer, but their effects against GBM
remain unknown. In the present study, we investigated the effects
of individual ginsenosides, particularly those of Rg3, on the human
glioblastoma cell line, U87MG, and their molecular signaling
mechanism.
Materials and methods
Reagents
Rg3 was purchased from NPC BioTech (Korea). DAPI
stain, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) and modified Hanks' balanced salt solution (HBSS) were
obtained from Sigma-Aldrich (USA). α-minimum essential medium (MEM)
and fetal bovine serum (FBS) were acquired from Invitrogen
(Canada). U0126, PD98059, SP600125, SB203580 and Z-VAD-fmk were
obtained from Calbiochem (Denmark). An enhanced chemiluminescence
(ECL) kit was purchased from Amersham Biosciences (UK). Bcl-2, Bax
and pro-caspase3 were acquired from Epitomics (USA). p-ERK, p-p38
and p-JNK were obtained from Cell Signaling Technology, Inc.
(USA).
Cell culture conditions
U87MG cells (human glioblastoma cell line) were
purchased from the Korean Cell Line Bank and cultured in α-MEM
supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin and
100 μg/ml streptomycin. The cells were plated in cell culture
dishes and cultivated at 37°C in a humidified 5% CO2
incubator. These cells were then sub-cultured until confluence for
3–5 days using 0.05% trypsin. Cells were cultivated under serum
starved conditions for 1–2 days before various reagents were
added.
Measurement of cell growth
MTT assay
U87MG cells were seeded in 96-well plates at
5×102 cells/well with various concentrations of Rg3 for
the indicated time periods. Following cultivation under various
conditions, 0.5 mg MTT/ml in α-MEM was added. The cells were then
cultivated for 2 h, after which they were dissolved in DMSO and the
absorbance of each well was measured at 570 nm using a 680
microplate ELISA reader (UK).
Measurement of cell death by a trypan
blue dye exclusion assay
Rg3-treated cells were harvested using 0.05% trypsin
solution and washed with HBSS buffer, after which they were
suspended in 0.4% trypan blue solution. Cells that excluded the dye
were considered viable. The cells were counted using a
hemocytometer under light microscopy.
Flow cytometry
Cells were plated in 6-well plates at
5×104 cells/well, after which they were treated with the
indicated reagents for 24 h at 37°C. The cells were then harvested
using 0.05% trypsin solution and centrifuged at 10,000 × g for 15
min, after which the pellets were washed in HBSS buffer twice and
fixing solution was added. The samples were then incubated
overnight at 4°C, stained with 50 μg propidium iodide/ml containing
100 μg RNase/ml for 20 min and analyzed using a FACSort
Becton-Dickinson Flow Cytometer (USA).
Staining of the apoptotic cells
DNA fragmentation was evaluated by terminal
deoxynucleotidyl transferase (TdT)-mediated deoxyuridine
triphosphate (dUTP) nick end labeling (TUNEL) assay using an in
situ Cell Death Detection kit (fluorescein) purchased from
Roche Applied Science (USA). Briefly, cells were plated at a
density of 5×104 cells/cover slip (25 mm size), and then
treated with Rg3. The cells were subsequently washed, after which
freshly prepared 4% paraformaldehyde was added and they were
incubated for 60 min at 37°C. Next, the samples were permeabilized
in permeabilization solution (0.1% Triton X-100 in 0.1% sodium
citrate) for 5 min on ice, then subjected to the TUNEL reaction at
37°C in a humidified atmosphere in the dark for 60 min. Finally,
the fluorescent signal was detected using a Zeiss fluorescence
microscope (Germany).
Immunocytochemistry
Cells were plated at 5×104 cells/well
cover slip (25 mm size), fixed in freshly prepared 4%
paraformaldehyde for 5 min and then washed. The cells were then
blocked in 1% BSA blocking reagent for 30 min at room temperature,
after which they were stained with primary antibodies such as Bax
(1:500) and Bcl-2 (1:500) overnight at 4°C and washed. Next,
secondary antibody was added and the cells were incubated for 2 h,
at which time they were subjected to DAPI staining. Fluorescent
signals were detected using a Zeiss fluorescence microscope.
Western blot analysis
The cells were plated in 6-well plates at
5×104 cells/cm2 and then lysed on ice using
lysis buffer (pH 7.4; 1 mM EGTA; 1 mM EDTA; 0.1 mM
phenylmethylsulfonyl fluoride; 10 mM NaCl; 20 mM Tris-HCl; 1%
Triton X-100). The lysates were then centrifuged at 10,000 × g for
20 min at 4°C and loaded onto a 15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, then
transferred to nitrocellulose membranes. Membranes were
immunoblotted with primary antibodies such as Bax (1:500), Bcl-2
(1:500) and β-tubulin (1:1,000). The membrane signals were
visualized using an ECL kit.
Measurement of reactive oxygen species
(ROS)
The intracellular generation of ROS was detected
using 2′,7′-dichlorofluorescin diacetate (DCFH-DA). Briefly, cells
were plated in 6-well plates at 5×104
cells/cm2, pre-treated with antioxidant enzymes and then
treated with Rg3. Following treatment, the cells were washed using
PBS and incubated with 30 μM DCFH-DA for 1 h at 37°C, after which
they were quickly washed, and the fluorescence signal intensity was
monitored using a Zeiss fluorescence microscope.
Statistical analysis
All experiments were performed at least three times
and statistical significance was determined using a Student’s
t-test (two-tailed). A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
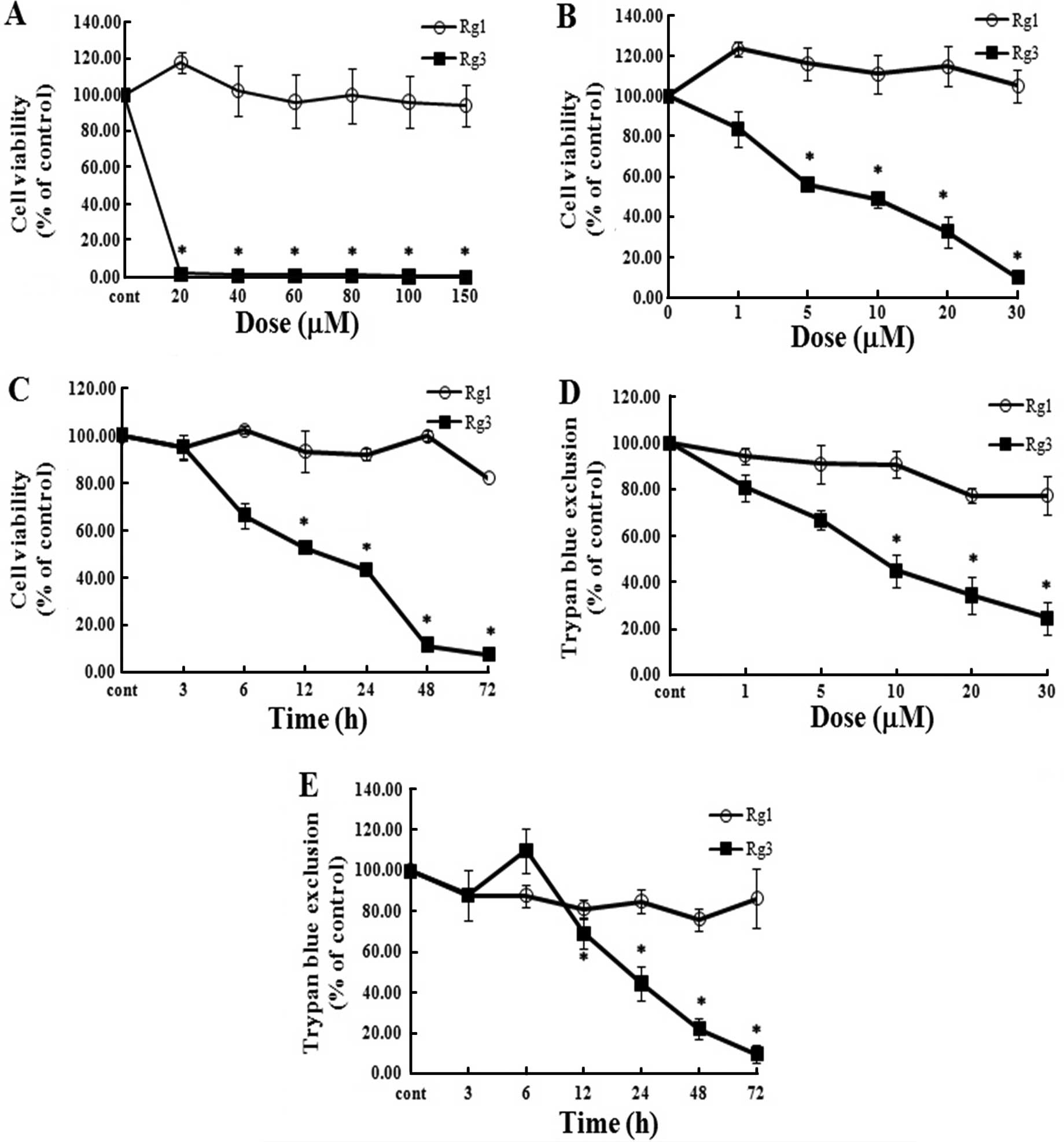
Rg3 exerts an inhibitory effect against
U87MG cells
To test whether ginseng components exert inhibitory
effects against U87MG cells, we conducted an MTT and a trypan blue
assay. Under high-concentration treatment conditions, Rg1 and Rg3
showed markedly different patterns (Fig. 1A). We also treated U87MG cells with
low concentrations of each of the two reagents. As shown in
Fig. 1B–E, Rg3 clearly decreased
the viability of cells in a dose- and time-dependent manner when
compared to Rg1 treatment. These results provide evidence that Rg3
exerts an inhibitory effect against U87MG cells.
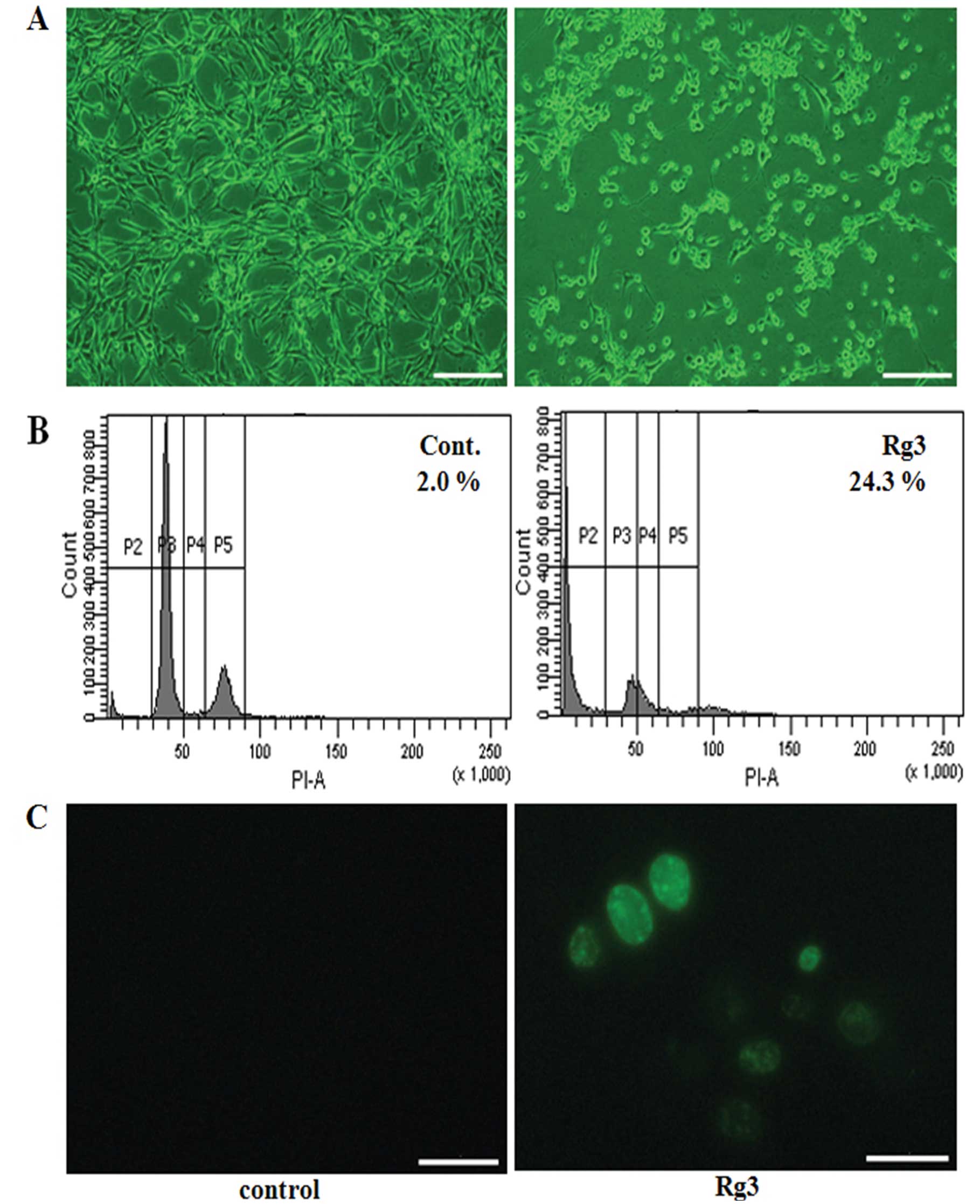
Rg3 induces apoptosis in U87MG cells
We used a variety of methods to clarify the findings
of the observed Rg3 effects on cell viability and morphology. Cells
that were treated with Rg3 exhibited cellular adhesion loss and
morphological change undergoing rounding and shrinkage (Fig. 2A). In addition, flow cytometry was
performed to reaffirm the inhibitory effects of Rg3 on the cells,
and Rg3-treated cells showed apoptosis, with a peak from 2.9 to
54.6 (Fig. 2B). To identify the
type of cell death, U87MG cells were treated with Rg3 and then
stained by TUNEL assay (Fig. 2C).
The numbers of positively stained cells (green) were visibly
increased after treatment with Rg3 when compared to the untreated
condition. These findings suggest that RG3 induced apoptotic
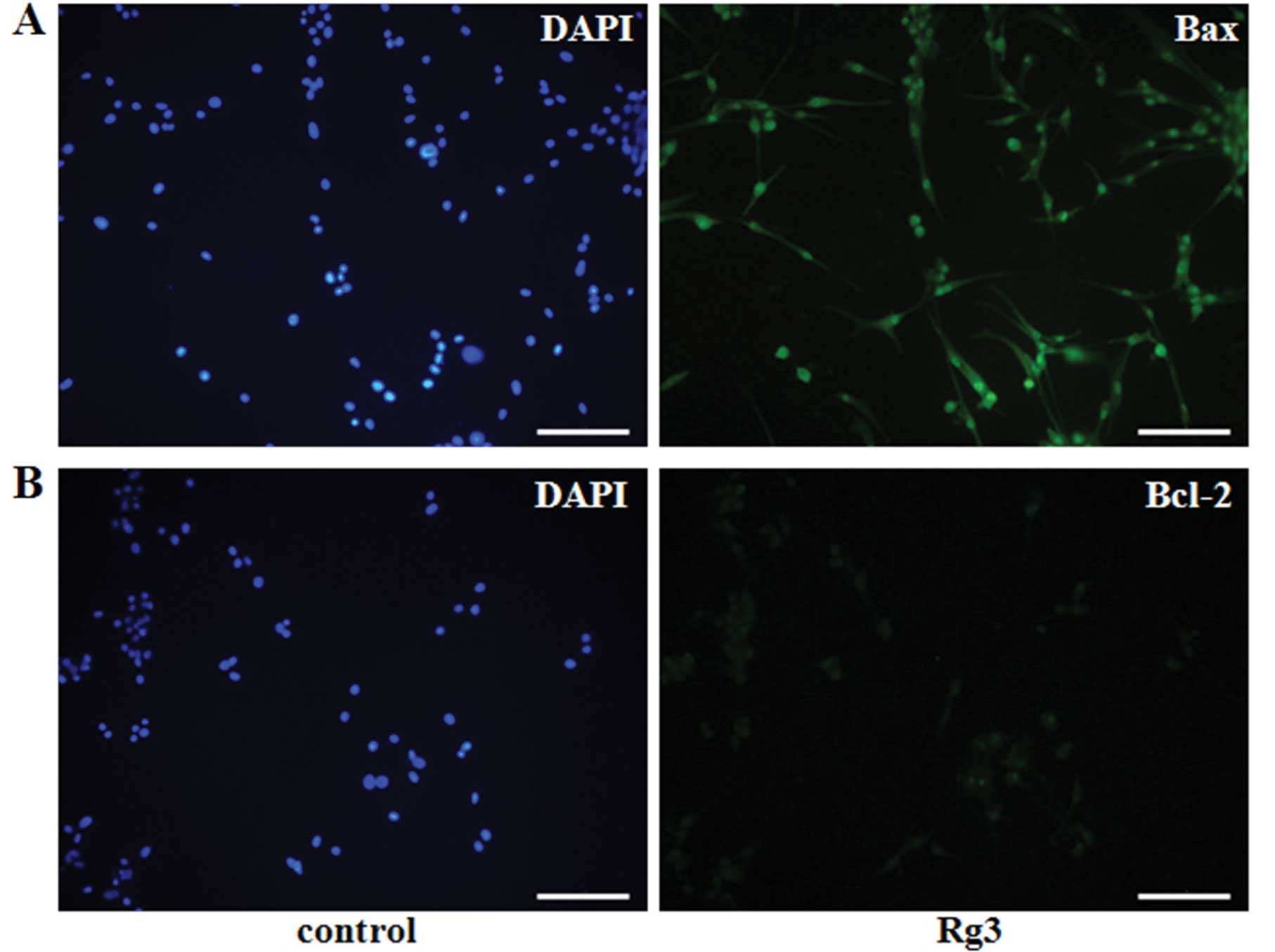
changes in the cells. Moreover, the cells treated with Rg3 were
stained by immunocytochemistry using a pro-apoptotic member, Bax,
and an anti-apoptotic member, Bcl-2 (16) (Fig. 3A
and B). The level of Bax expression was high, whereas Bcl-2
expression was very low. Similarly, western blot analysis indicated
that the levels of pro-caspase3 and Bcl-2 expression were
universally decreased in cells that were treated with different
concentrations of Rg3 for various lengths of time, but the level of
Bax expression increased in a dose- (Fig. 3C) and time- (Fig. 3D) dependent manner. These results
indicate that Rg3 induced apoptosis in U87MG cells.
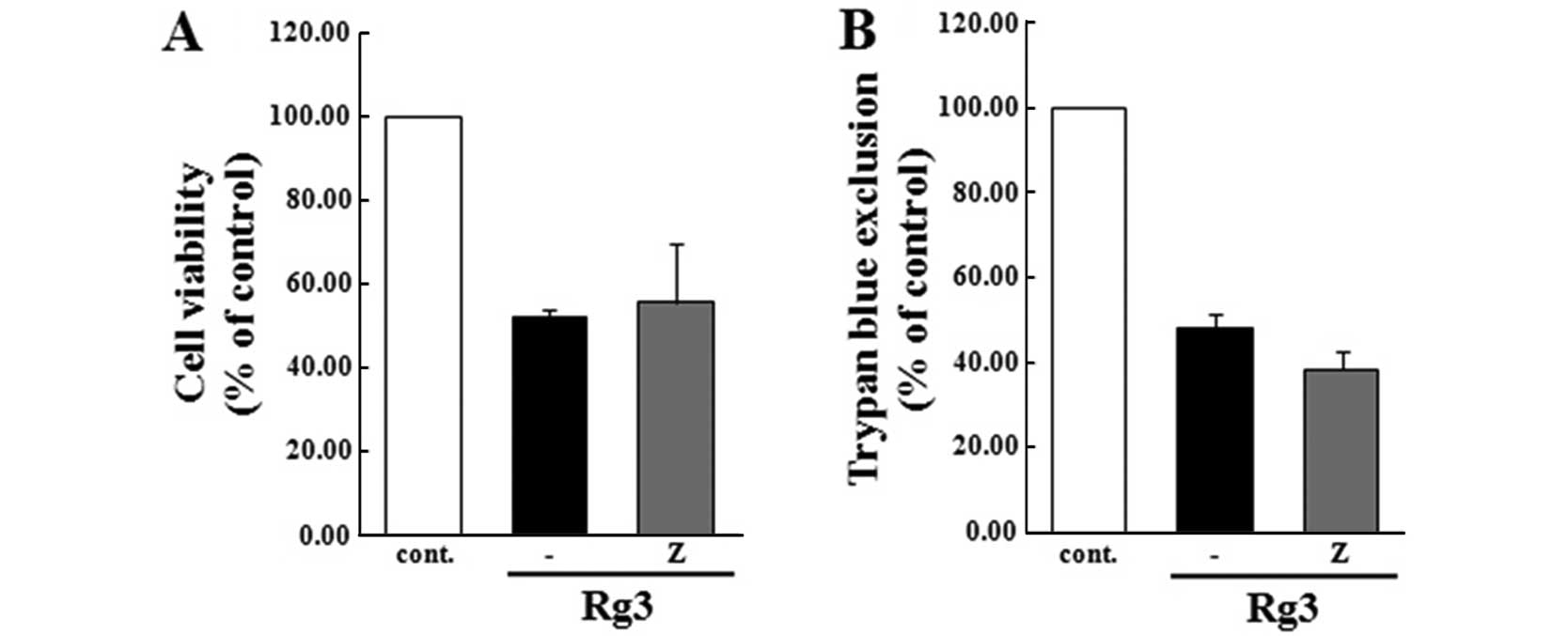
Effect of Z-VAD-fmk on U87MG cells during
Rg3-induced apoptosis
To verify the involvement of the caspase cascade in
Rg3-induced apoptosis, the cells were pre-exposed to the general
caspase inhibitor, Z-VAD-fmk. Caspases, the interleukin-1
β-converting enzyme family proteases, are one of the major
executors of the apoptotic process, and they convey the apoptotic
signal via induction of death receptors (17,18).
Caspases have been reported as a class of cysteine proteases
including several representatives involved in apoptosis (19). However, the results of our present
study indicate that the cell viability of the group that was
pre-treated with the caspase cascade inhibitor was sustained in the
Rg3-treated group (Fig. 4),
suggesting that the caspase cascade does not regulate Rg3-induced
apoptosis in U87MG cells.
Effect of mitogen-activated protein
kinases (MAPKs) on U87MG cells during Rg3-induced apoptosis
MAPK signaling cascades are composed of a large
group of serine/threonine kinases. The MAPKs mediate signal
transduction from the cell surface to the nucleus and are actively
involved in converting a wide variety of extracellular stimuli
commonly expressed in various cell types (20,21).
Several studies have shown that the MAPK signaling pathway plays an
important role in the regulation of cellular growth, survival,
apoptosis and differentiation (22–24).
Moreover, it has been established that MAPK consists of three
parallel kinase modules, ERK, JNK and p-38-MAPK. Overall, MEK, a
key kinase, is responsible for the upstream signals from Ras and
Raf via activation of ERK (25).
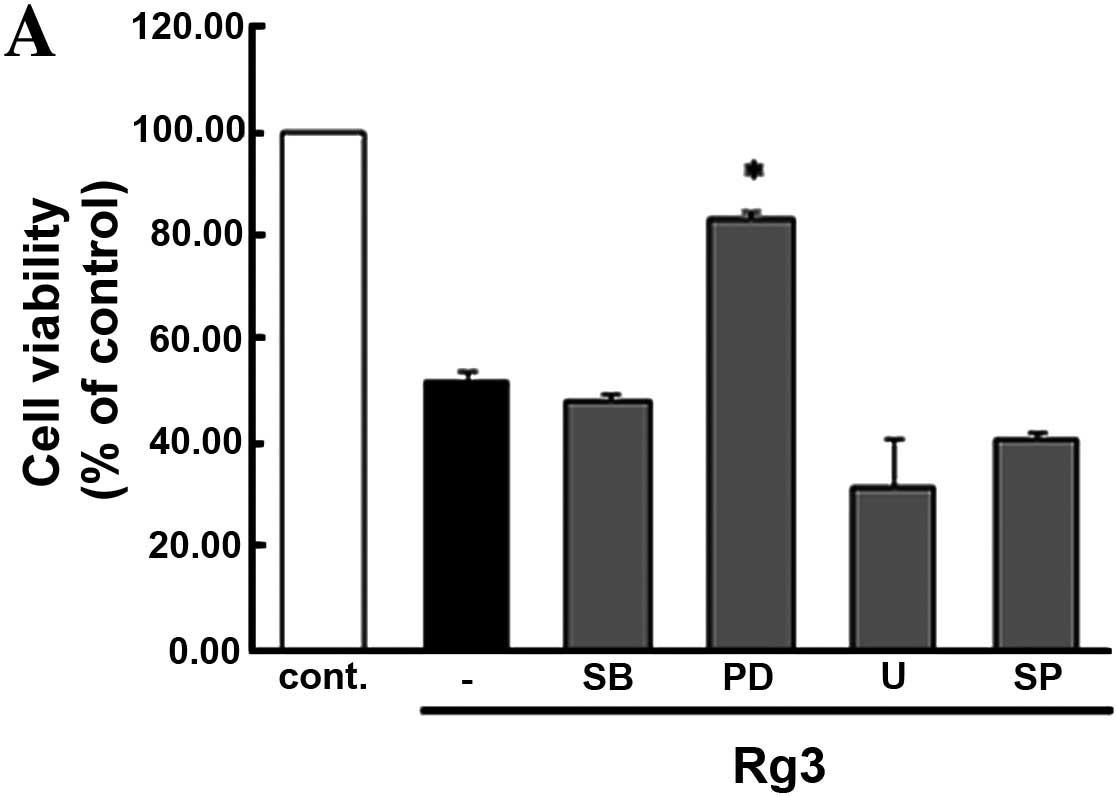
Based on these findings, we examined the effects of Rg3 on the
viability of U87MG cells by conducting a variety of methods. Cells
pre-treated with inhibitors of MAPK family members were measured by
MTT assay (Fig. 5A) and trypan
exclusion assay (Fig. 5B). To
confirm these results, we conducted flow cytometry (Fig. 5C) to compare the inhibitor treatment
group to the untreated control group. Our data clearly show that
Rg3-induced apoptosis in U87MG cells through PD98059 (specific
inhibitor of MEK1/2).
Effect of antioxidant enzyme system on
U87MG cells during Rg3-induced apoptosis
Antioxidant enzymes are endogenous proteins that are
well known for their involvement in protecting cells from ROS
damage (26). ROS are extremely
toxic to organisms (27,28) and oxidative stress can lead to
damage to cellular structures and is related to a number of
diseases, including cancer (29).
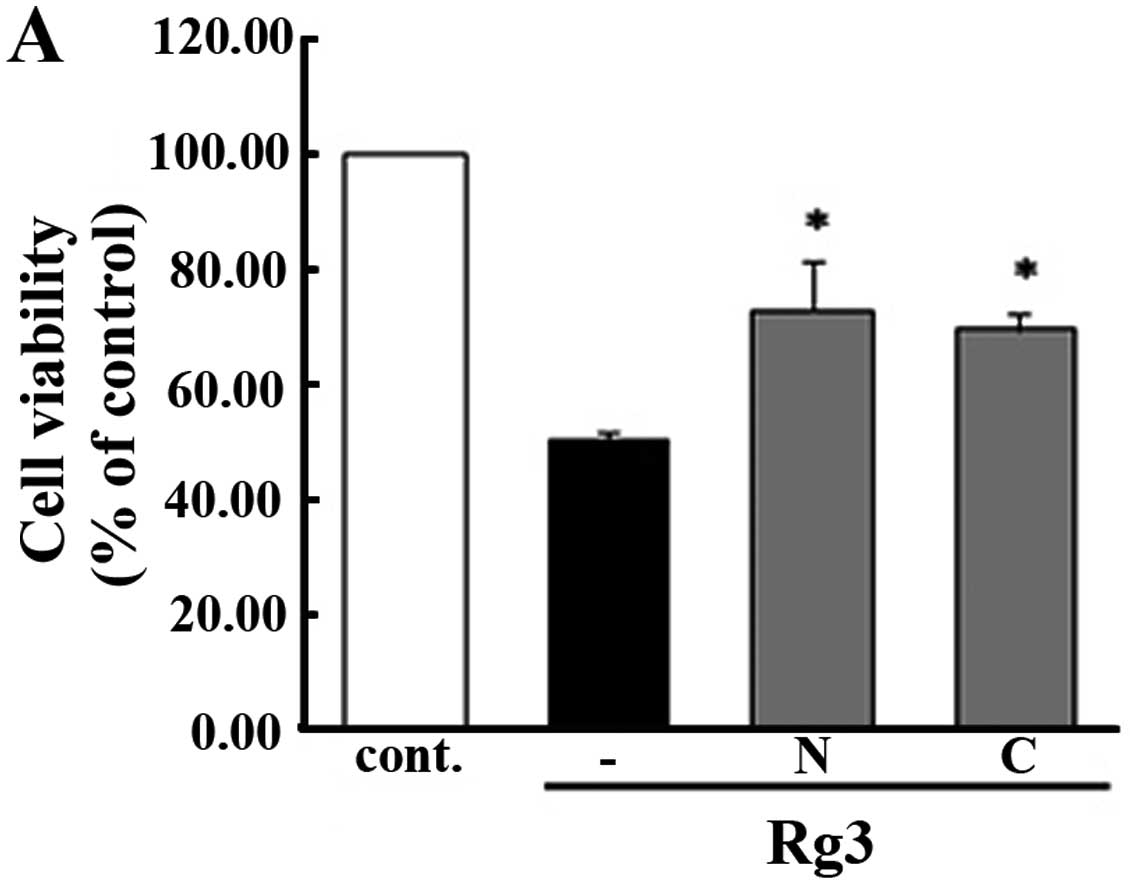
The present study was conducted to verify whether ROS is associated
with the regulation of Rg3-induced cellular apoptosis. To
accomplish this, we used antioxidant enzyme inhibitor for the
treatment of cells. As shown in Fig. 6A
and B, in the group pre-treated with antioxidant enzymes such
as N-acetylcysteine (NAC) and catalase (CAT), all cells were
sustained as in the control group, except for those that received
the Rg3 treatment, which showed decreased cell viability. To
confirm these results, we conducted flow cytometry (Fig. 6C) to compare the group that received
the antioxidant pre-treatment to the untreated control group.
Moreover, we measured the fluorescence activation of ROS using
DCFH-DA staining (Fig. 6D) as
DCFH-DA is generally used for detection of ROS formation (30). DCFH-DA expression was not detected
in cells that were pre-treated with CAT and NAC, while the
Rg3-treated group showed increased DCFH-DA expression when compared
to the control. Taken together, these findings indicate that ROS is
involved in Rg3-induced apoptosis, particularly through the work of
antioxidant enzymes system.
Discussion
Studies of the chemo-preventive effect of anticancer
reagents have recently begun to focus on naturally-occurring
chemical compounds in plants and animals. KRG is well-recognized in
traditional Korean medicine as having a pharmacological effect
(6–8). Among various components of KRG,
ginsenosides are the most widely known. These compounds have
diverse, beneficial biochemical activities, including
chemo-preventive effects against diseases. Rg3 has negative effects
on cancer growth, such as inhibition of metastasis and angiogenesis
(31), confirming its usefulness as
a novel anticancer agent (32). Rg3
is also an effective chemical reagent of a saponin, a unique
component of KRG that can be extracted from ginseng (12,33).
It has been reported that Rg3 has potential cancer-preventive
effects owing to its suppression of invasion, metastasis and growth
of various forms of cancer and neovascularization (32,34).
Moreover, Rg3 has been reported to induce the reduction of
metastasis and the amount of tumor development in colon and ovarian
cancer (35), as well as to inhibit
angiogenesis in prostate (15) and
lung cancer (36).
Effective and less-invasive alternatives for cancer
treatment are actively being developed. To date, surgical
techniques and chemotherapies such as reagents targeting specific
molecules have been applied to improve the prognosis of cancer
treatment.
Despite several novel trials for its treatment, GBM
is one of the most aggressive and invasive malignant tumor forms of
human cancer (1,2). As standard treatment methods for
cancer such as chemotherapy, microsurgical techniques and
radiotherapy have been shown to be ineffective at ameliorating GBM,
the prognosis of GBM remains poor (5,37,38).
Consequently, recent studies have focused on the development of new
treatment modalities for cancer such as biological therapy and
chemotherapy using novel substances that can be extracted from a
variety of foods.
This study was conducted to investigate the effects
of Rg3 on U87MG cells. Furthermore, we attempted to identify the
molecular mechanisms of cell death induced by Rg3 using various
inhibitory agents.
U87MG cells were used to explore the effects and
regulatory mechanisms of Rg3 on the human glioblastoma cell line.
First, we treated cells with Rg3 (panaxadiol group) and Rg1
(panaxatriol group). The results of these experiments revealed
different effects on the cells, particularly under high
concentration conditions (9).
Specifically, the inhibition of U87MG cell growth was much greater
when cells were treated with Rg3 than with Rg1, and these
differences occurred in a dose-dependent manner (Fig. 1A). We also found that Rg1 had no
inhibitory effect on cell proliferation or viability, while Rg3 did
(Fig. 1B–E).
Some studies have reported that Rg1 promotes
angiogenesis in vivo and in vitro by inducing
vascular endothelial growth factor (VEGF), a mediator of
angiogenesis (9). These results
suggest that Rg1 may be the main candidate for angiotherapy due to
its potential to induce wound healing and tissue regeneration.
We verified whether the inhibitory effect of Rg3 was
related to cell apoptosis. Observation of morphological changes in
the cells, flow cytometry, TUNEL assay and expression of Bax or
Bcl-2 indicated that Rg3 led to apoptosis (Figs. 2 and 3). Apoptosis or programmed cell death is a
common type of cell death, and is one of the principal mechanisms
involved in tissue homeostasis (39–41).
This physiological ‘cell suicide’ program is essential for diverse
cellular processes, particularly for the elimination of damaged,
infected and redundant cells. Apoptosis is induced by a disparate
variety of pathways that can be further divided into intrinsic and
extrinsic apoptotic pathways. The general methods for effectively
detecting apoptotic cells are the TUNEL assay and flow cytometry
(42,43).
We conducted a TUNEL assay using an in situ
Cell Death Detection kit. As expected, positive-stained cells
(green) were highly detected by DNA fragmentation labeling of the
terminal end of nucleic acids. Cells treated with Rg3 showed
morphological changes ranging from rounding shape to shrinkage.
Furthermore, the level of Bax expression was largely detected in
the cells, but that of Bcl-2 was not. Collectively, these results
indicate that Rg3 induced the death of U87MG cells through
apoptosis.
The relationship between GBM and Rg3 was uncertain,
therefore we investigated whether Rg3 conducted molecular
mechanisms during apoptosis of the cells.
Rg3 was previously reported to exert anticancer
activity through various molecular pathways including Wnt/β-catenin
signaling (44), the
caspase-dependent signaling cascade (45) and the mitochondrial pathway
(46) in different cell lines.
Therefore, we examined the involvement of the caspase cascade in
the effects of Rg3 treatment. The intracellular cysteine enzymes
mediating the caspase cascade are well defined as a family of
proteins that are major executors of apoptosis processes (17,18).
Although the caspase cascade has been shown to activate apoptosis,
the roles of the individual caspases remain uncertain. The results
of the present study showed that the caspase cascade was not
involved in the apoptosis signal induced by Rg3 (Fig. 4).
We demonstrated that MAPK signaling may be related
to Rg3-induced anticancer activity. MAPK cascades are the main
signaling pathways involved in various cellular responses including
proliferation, survival, inflammation and differentiation (21,47).
The key factors involved in these cascades are MAPK/ERK, SAPK/JNK,
and p38-MAPK. Moreover, the MAPK/ERK signaling cascade is well
known to occur in response to Raf and MEK in cancer progression and
cancer growth (23,25,48).
The MAPK ERK signaling cascade starts with the phosphorylation and
activation of MEK by Raf, which is followed by the phosphorylation
and activation of ERK by MEK (22,49).
MEK plays a specific dual role in phosphorylation of tyrosine and
activation of threonine residues on ERKs 1 and 2. However, the
relationship between JNK and p38 MAPK and their involvement in
cancer signaling pathways is less clearly established.
As shown in Fig. 5,
pretreatment of PD98059 on the U87MG cells showed maintained cell
viability markedly better than other inhibitor pretreatment groups
compared with control. MEK signaling has relevance to Rg3-induced
apoptosis, while other inhibitors of MAPKs had only a slight effect
on the viability of U87MG cells. We indicated if the MEK signaling
pathway could be controlled in living systems, cancer could be also
controlled in the same system. The results of the present study
suggest that efficient cancer treatment can inhibit the MEK
signaling pathway.
ROS occur naturally in organisms as side-products
from the homeostatic intracellular signaling and as a part of the
defense mechanism of the immune system. Moreover, ROS have been
found to directly injure cells and to play an important role in the
physiological and pathological processes of diseases including
ischemia, tissue damage, endocrine dysfunction and cancer (26,50,51).
To accelerate oxidative stress to exert cell damage, antioxidant
systems and capacity must fail and decline. ROS are neutralized by
antioxidant enzymes such as superoxide dismutase (SOD), glutation
peroxidase (GSHPx), glutation reductase (GR) and CAT (52–54).
Moreover, antioxidant enzyme imbalances induce the arrest of cell
proliferation and growth, leading to cell cycle arrest being
switched on by activated p53 proteins, resulting in apoptosis
(55,56).
We found that antioxidant enzyme systems have
significant control over molecular signaling pathways and the
mechanism of Rg3-induced apoptosis in U87MG cells. As shown in
Fig. 6A–C, cells that were treated
with Rg3 displayed decreased viability. However, the group
subjected to antioxidant enzyme pretreatment showed sustained cell
viability, similar to the results observed in the control group.
CAT is a tetrameric heme containing an enzyme that degrades
hydrogen peroxide into oxygen and water (53,57).
N-acetylcysteine and N-acetyl-L-cysteine (NAC) are thiols that act
as mucolytic agents and are derived from sulfhydryl groups in cells
and scavengers that interact with the ROS of free radicals
(58,59). These are the main parts of the
enzymatic and protective system against ROS, and are known to be
indispensable to ROS neutralization under oxidative stress
conditions (56).
To confirm that the antioxidant enzyme system exerts
an effect on the molecular mechanism during Rg3-induced apoptosis,
we stained the cells with DCFH-DA (Fig.
6D). The results revealed that Rg3-induced apoptosis signaling
was regulated by CAT, and that this association was triggered by
the antioxidant enzyme system.
There have been few studies on Rg3 for the treatment
of GBM, and there have been few investigations of its molecular
mechanism in such treatments. The results of our present study
indicated that Rg3 has a greater inhibitory effect on the U87MG
human glioblastoma cell line, and that its apoptotic mechanism was
regulated through MEK and ROS. We expect Rg3 to be the major
candidate for natural treatment of GBM, as well as other types of
cancer. Further studies using various cancer cells should be
conducted to verify the anticancer effects of Rg3 and its molecular
mechanism on these cells.
Acknowledgements
The present study was supported by the Medical
Research Institute and Pusan Cancer Center Grant (2011–29), Pusan
National University Hospital.
References
|
1
|
Chamberlain MC: Treatment options for
glioblastoma. Neurosurg Focus. 20:E192006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chamberlain M: Evolving strategies: future
treatment of glioblastoma. Expert Rev Neurother. 11:519–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schratter-Sehn AU and Marosi C: Treatment
of glioblastoma recurrences. Wien Med Wochenschr. 161:1–2. 2011.(In
German).
|
|
4
|
Kala M, Srámek V, Houdek M, Vaverka M and
Zmrzlík P: Treatment of glioblastoma multiforme. Cas Lek Cesk.
132:653–656. 1993.(In Czech).
|
|
5
|
Holland EC: Glioblastoma multiforme: the
terminator. Proc Natl Acad Sci USA. 97:6242–6244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun TK, Lee YS, Lee YH, Kim SI and Yun HY:
Anticarcinogenic effect of Panax ginseng C.A. Meyer and
identification of active compounds. J Korean Med Sci. 16(Suppl):
S6–S18. 2001.
|
|
7
|
De Souza LR, Jenkins AL, Sievenpiper JL,
Jovanovski E, Rahelić D and Vuksan V: Korean red ginseng (Panax
ginseng C.A. Meyer) root fractions: differential effects on
postprandial glycemia in healthy individuals. J Ethnopharmacol.
137:245–250. 2011.
|
|
8
|
Varjas T, Nowrasteh G, Budán F, et al:
Chemopreventive effect of Panax ginseng. Phytother Res.
23:1399–1403. 2009. View
Article : Google Scholar
|
|
9
|
Helms S: Cancer prevention and
therapeutics: Panax ginseng. Altern Med Rev. 9:259–274.
2004.
|
|
10
|
Shin HR, Kim JY, Yun TK, Morgan G and
Vainio H: The cancer-preventive potential of Panax ginseng:
a review of human and experimental evidence. Cancer Causes Control.
11:565–576. 2000. View Article : Google Scholar
|
|
11
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yun TK: Experimental and epidemiological
evidence on non-organ specific cancer preventive effect of Korean
ginseng and identification of active compounds. Mutat Res.
523–524:63–74. 2003.PubMed/NCBI
|
|
13
|
Lu P, Su W, Miao ZH, Niu HR, Liu J and Hua
QL: Effect and mechanism of ginsenoside Rg3 on postoperative life
span of patients with non-small cell lung cancer. Chin J Integr
Med. 14:33–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji C, Ren F and Xu M: Caspase-8 and
p38MAPK in DATS-induced apoptosis of human CNE2 cells. Braz J Med
Biol Res. 43:821–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HS, Lee EH, Ko SR, Choi KJ, Park JH
and Im DS: Effects of ginsenosides Rg3 and Rh2 on the proliferation
of prostate cancer cells. Arch Pharm Res. 27:429–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olsson M and Zhivotovsky B: Caspases and
cancer. Cell Death Differ. 18:1441–1449. 2011. View Article : Google Scholar
|
|
19
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
20
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
22
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: a family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
24
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kolch W: Meaningful relationships: the
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stadtman ER: Protein oxidation and aging.
Science. 257:1220–1224. 1992. View Article : Google Scholar
|
|
28
|
Stadtman ER: Role of oxidant species in
aging. Curr Med Chem. 11:1105–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar
|
|
30
|
Gomes A, Fernandes E and Lima JL:
Fluorescence probes used for detection of reactive oxygen species.
J Biochem Biophys Methods. 65:45–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue PY, Wong DY, Wu PK, et al: The
angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem
Pharmacol. 72:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keum YS, Han SS, Chun KS, et al:
Inhibitory effects of the ginsenoside Rg3on phorbol
ester-induced cyclooxygenase-2 expression, NF-κB activation and
tumor promotion. Mutat Res. 523–524:75–85. 2003.
|
|
33
|
Lee JI, Ha YW, Choi TW, et al: Cellular
uptake of ginsenosides in Korean white ginseng and red ginseng and
their apoptotic activities in human breast cancer cells. Planta
Med. 77:133–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Christensen LP: Ginsenosides chemistry,
biosynthesis, analysis, and potential health effects. Adv Food Nutr
Res. 55:1–99. 2009.PubMed/NCBI
|
|
35
|
Xu TM, Cui MH, Xin Y, et al: Inhibitory
effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J
(Engl). 121:1394–1397. 2008.PubMed/NCBI
|
|
36
|
Liu TG, Huang Y, Cui DD, et al: Inhibitory
effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis
and growth of lung cancer in mice. BMC Cancer. 9:2502009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Henson JW: Treatment of glioblastoma
multiforme: a new standard. Arch Neurol. 63:337–341. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wong ET and Yamaguchi NH: Treatment
advances for glioblastoma. Expert Rev Neurother. 11:1343–1345.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yue TL, Ohlstein EH and Ruffolo RR Jr:
Apoptosis: a potential target for discovering novel therapies for
cardiovascular diseases. Curr Opin Chem Biol. 3:474–480. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hickman JA: Apoptosis induced by
anticancer drugs. Cancer Metastasis Rev. 11:121–139. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nagata S: DNA degradation in development
and programmed cell death. Annu Rev Immunol. 23:853–875. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wieder R: TUNEL assay as a measure of
chemotherapy-induced apoptosis. Methods Mol Med. 111:43–54.
2005.PubMed/NCBI
|
|
44
|
He BC, Gao JL, Luo X, et al: Ginsenoside
Rg3 inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011.PubMed/NCBI
|
|
45
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.PubMed/NCBI
|
|
47
|
Dhanasekaran DN and Johnson GL: MAPKs:
function, regulation, role in cancer and therapeutic targeting.
Oncogene. 26:3097–3099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kyriakis JM, App H, Zhang XF, et al: Raf-1
activates MAP kinase-kinase. Nature. 358:417–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mavria G, Vercoulen Y, Yeo M, et al:
ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell
survival and sprouting during angiogenesis. Cancer Cell. 9:33–44.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Allen CL and Bayraktutan U: Oxidative
stress and its role in the pathogenesis of ischaemic stroke. Int J
Stroke. 4:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wiseman H and Halliwell B: Damage to DNA
by reactive oxygen and nitrogen species: role in inflammatory
disease and progression to cancer. Biochem J. 313:17–29.
1996.PubMed/NCBI
|
|
52
|
Bannister JV, Bannister WH and Rotilio G:
Aspects of the structure, function, and applications of superoxide
dismutase. CRC Crit Rev Biochem. 22:111–180. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chelikani P, Fita I and Loewen PC:
Diversity of structures and properties among catalases. Cell Mol
Life Sci. 61:192–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rhee SG, Chae HZ and Kim K:
Peroxiredoxins: a historical overview and speculative preview of
novel mechanisms and emerging concepts in cell signaling. Free
Radic Biol Med. 38:1543–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oberley TD and Oberley LW: Antioxidant
enzyme levels in cancer. Histol Histopathol. 12:525–535.
1997.PubMed/NCBI
|
|
56
|
Kong Q and Lillehei KO: Antioxidant
inhibitors for cancer therapy. Med Hypotheses. 51:405–409. 1998.
View Article : Google Scholar
|
|
57
|
Bechtel W and Bauer G: Catalase protects
tumor cells from apoptosis induction by intercellular ROS
signaling. Anticancer Res. 29:4541–4557. 2009.PubMed/NCBI
|
|
58
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar
|
|
59
|
Aruoma OI, Halliwell B, Hoey BM and Butler
J: The antioxidant action of N-acetylcysteine: its reaction with
hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous
acid. Free Radic Biol Med. 6:593–597. 1989. View Article : Google Scholar : PubMed/NCBI
|