Introduction
Esophageal carcinoma is the sixth most common cause
of cancer-related death among adult malignant tumors, and it is
considered to possess a relatively high malignant potential
(1–5). Squamous cell carcinoma is the
predominant cellular type of esophageal cancer worldwide, although
the incidence of esophageal adenocarcinoma has been increasing in
Western countries (6). More recent
advances in surgical techniques, chemotherapy and radiotherapy have
resulted in an improvement in the prognosis of esophageal squamous
cell carcinoma (ESCC) (7); however,
the long-term outcomes remain unacceptable. It is important to
detect more useful predictors of the prognosis and efficacy of
chemotherapy for the further improvement of the prognosis of ESCC
patients.
Many genetic and epigenetic alterations have been
reported in ESCC. In the present study, we focused on the
expression of glutathione S-transferase (GST) P1 protein, which is
the main GST expressed in the human esophagus. The GST family
consists of a group of important phase II drug-metabolizing enzymes
that is instrumental in providing cell protection or detoxification
of toxic substances and anticancer drugs through conjugation with
glutathione (GSH) (8). GSTP1 is
widely expressed in normal tissues, while the enzyme activity has
been shown to be highly overexpressed in several types of cancers,
for example, blood, head and neck, lung, colorectal, esophagus and
breast cancers (9–13). The high expression level of GSTP1
has been reported to be regulated by inflammation, various chemical
carcinogens, the expression of Nrf2 and other factors. Thus,
overexpression of GSTP1 may influence the clinical outcome or the
resistance to anticancer drugs. However, the available information
on the association between the degree of GSTP1 expression and the
prognosis of ESCC is conflicting (14).
In the present study, we simply evaluated the
immunohistochemical expression of GSTP1 in resected ESCC specimens
and investigated whether GSTP1 expression is associated with the
clinical outcome and the response to adjuvant chemotherapy.
Patients and methods
Study population
We selected 75 patients who underwent
macroscopically curative right-transthoracic esophagectomy with
extensive lymph node dissection for ESCC between January 2000 and
December 2009 at the Division of Digestive Surgery, Department of
Surgery, Kyoto Prefectural University of Medicine, Kyoto, Japan. Of
the 75 patients, 14 (19%) patients underwent three-field lymph node
dissection (cervical, mediastinal and abdominal nodes), while 61
(81%) patients underwent two-field lymph node dissection
(mediastinal and abdominal nodes) under pre- or intra-operative
diagnosis (15). Thirty-one of the
75 patients (41%) received post-operative adjuvant therapy,
high-dose FP or low-dose FP plus oral fluoropyrimidine [5-FU
(150–200 mg/body/day) or UFT (300–400 mg/body/day)] (16) and 4 patients were treated with
pre-operative chemotherapy, high-dose FP [5-FU (800
mg/m2/day, days 1–5) plus cisplatin (80
mg/m2/day, day 1)], followed by planned esophagectomy.
We excluded the patients who underwent neoadjuvant chemo-radiation
therapy. Staging was based principally on the International Union
Against Cancer (UICC)/TNM Classification of Malignant Tumors, 7th
edition (17). The macroscopic type
was classified as follows: type 0, macroscopic early cancer; type
1, polypoid tumors; type 2, ulcerated carcinomas with sharply
demarcated and raised margins; type 3, ulcerated carcinomas without
definite limits, infiltration into the surrounding wall; type 4,
diffusely infiltrating carcinomas in which ulceration is usually
not a marked feature.
Postsurgical management
Following discharge from the hospital, the patients
were regularly monitored in the outpatient clinic at intervals of 3
months for the first 2 years and at intervals of 6 months
thereafter. Multi-slice computed tomography of the neck, chest and
upper abdomen was performed every 6 months. Since 2007, positron
emission tomography with computed tomography has been used in
screening for recurrence (18,19).
All recurrent tumors were evaluated using imaging
studies. Of the 32 patients with recurrent tumors, 18 received
intensive treatment: 10 received chemoradiotherapy, 1 received
lymphadenectomy, 8 received chemotherapy; and 14 received the best
supportive care.
Cell lines and western blotting
Each cell line was purchased from the Cell Resource
Center of Tohoku University, Riken Bio Resource Center, or the
American Type Culture Collection. Whole-cell lysates were prepared
in SDS sample buffer, resolved by SDS-PAGE and transferred to PVDF
membranes. After blocking with TBS containing 0.05% Tween-20 and 5%
non-fat dry milk for 1 h, each membrane was incubated with an
antibody overnight. The primary antibodies and dilutions were
anti-GSTP1 (1/500, HPA019779; Sigma-Aldrich) and anti-GAPDH
(1/4,000, sc-25778; Santa Cruz Biotechnology, Inc.) antibodies. The
membrane was washed and exposed to horseradish
peroxidase-conjugated secondary antibody (at 1/5,000, #7074S; Cell
Signaling Technology). The bound antibodies were visualized with an
HRP staining solution or with an ECL Western Detection kit on an
Image Quant LAS 500 (GE Healthcare) according to the manufacturer’s
instructions (Cell Signaling Technology).
Immunohistochemistry
Tumor samples were fixed with 10% formaldehyde in
PBS, embedded in paraffin, and sectioned into 5-μm slices. After
deparaffinization by xylene and rehydration with ethanol, antigen
retrieval was performed by boiling in Dako REAL Red Target
Retrieval Solution (pH 6.0) at 95°C for 30 min and the sections
were treated with 0.3% hydrogen peroxide in methanol at room
temperature for 20 min to inactivate the endogenous peroxidase.
After treatment with Block Ace (Vectastain Elite ABC universal kit;
Vector Laboratories, Inc., Burlingame, CA, USA) for 30 min at room
temperature, the sections were incubated at 4°C overnight with a
primary antibody against GSTP1 (1:500 dilution). The
avidin-biotin-peroxidase complex system (Vectastain Elite ABC
universal kit) was used for secondary antibody detection and color
development with diaminobenzidine tetrahydrochloride at room
temperature for 30 min. The slides were lightly counterstained with
hematoxylin.
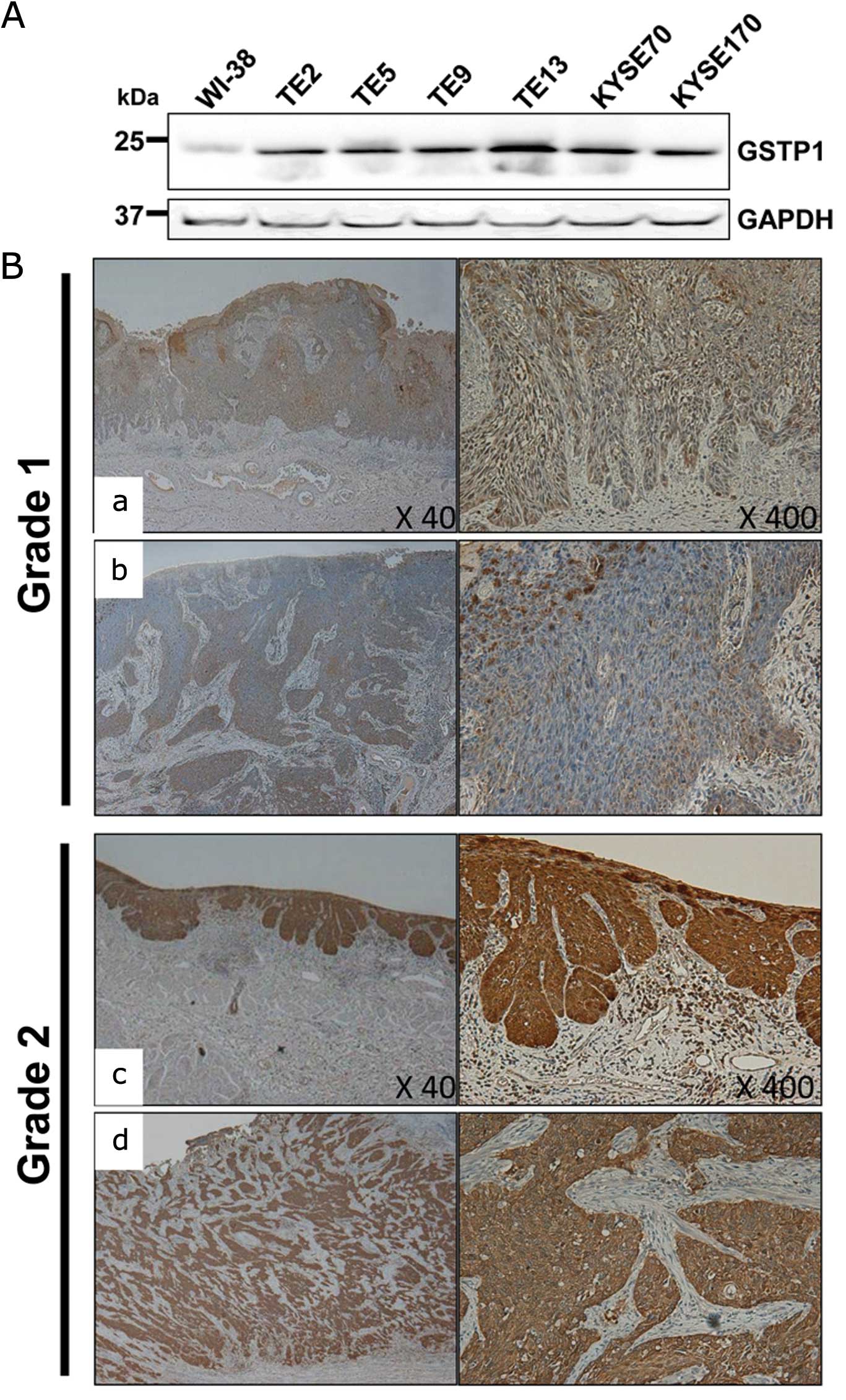
The patients were divided into two groups, grade 1
and 2, according to the degree of GSTP1 expression. Tissues of
grade 2 were defined as those with even staining of at least 90% of
the cancer area (Fig. 1B–a and –b)
and tissues of grade 1 were defined as areas with spotty staining
corresponding to <90% of the cancer area (Fig. 1B–c and –d).
Inter-observer reproducibility for
identifying the immunohistological characteristics
The reproducibility of the grading classification
was tested by asking another independent observer (K.H.) to blindly
review all of the examples. This observer was not provided with any
clinical information regarding the outcomes of the patients. The
reproducibility was tested by obtaining the κ-scores according to
the widely used statistical chart that grades the strength of
agreement to six categories [poor (κ-score, <0.00), slight
(0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial
(0.61–0.80) and almost perfect (0.81–1.00)] (20).
Statistical analysis
We performed univariate analyses of the 15 clinical
and pathologic factors that were potentially associated with
overall survival. Survival was calculated using the Kaplan Meier
method and compared between groups using the log-rank test. A
multivariate analysis using the Cox hazards model was performed to
identify independent predictors of survival.
The relationship of GSTP1 expression and 10
pathological factors was compared using the Chi-squared test with
Yates correction. All significant factors determined by the
univariate analysis were entered into a multivariate regression
analysis to identify independent factors. These tests were
one-tailed and a p<0.05 was considered to be statistically
significant. All statistical analyses were performed using the SPSS
for Windows 11.5 software program (SPSS, Chicago, IL, USA).
Results
GSTP1 protein expression in the ESCC cell
lines
We performed a western blot analysis with an
antibody against GSTP1 in 6 ESCC cell lines (TE2, TE5, TE9, TE13,
KYSE70 and KYSE170) and a normal fibroblast cell line, WI-38. The
GSTP1 protein expression level was upregulated in all of the ESCC
cell lines compared with the level in the WI-38 cells (Fig. 1A).
Overall survival and clinicopathological
features according to GSTP1 grade
Thirty-six patients had tumors that showed a low
GSTP1 protein expression level defined as grade 1 (Fig. 1B-a and -b), whereas 39 had tumors
that showed a high GSTP1 protein expression level defined as grade
2 (Fig. 1B-c and -d). The κ-value
between the classifications of the two reviewers was 0.893 (almost
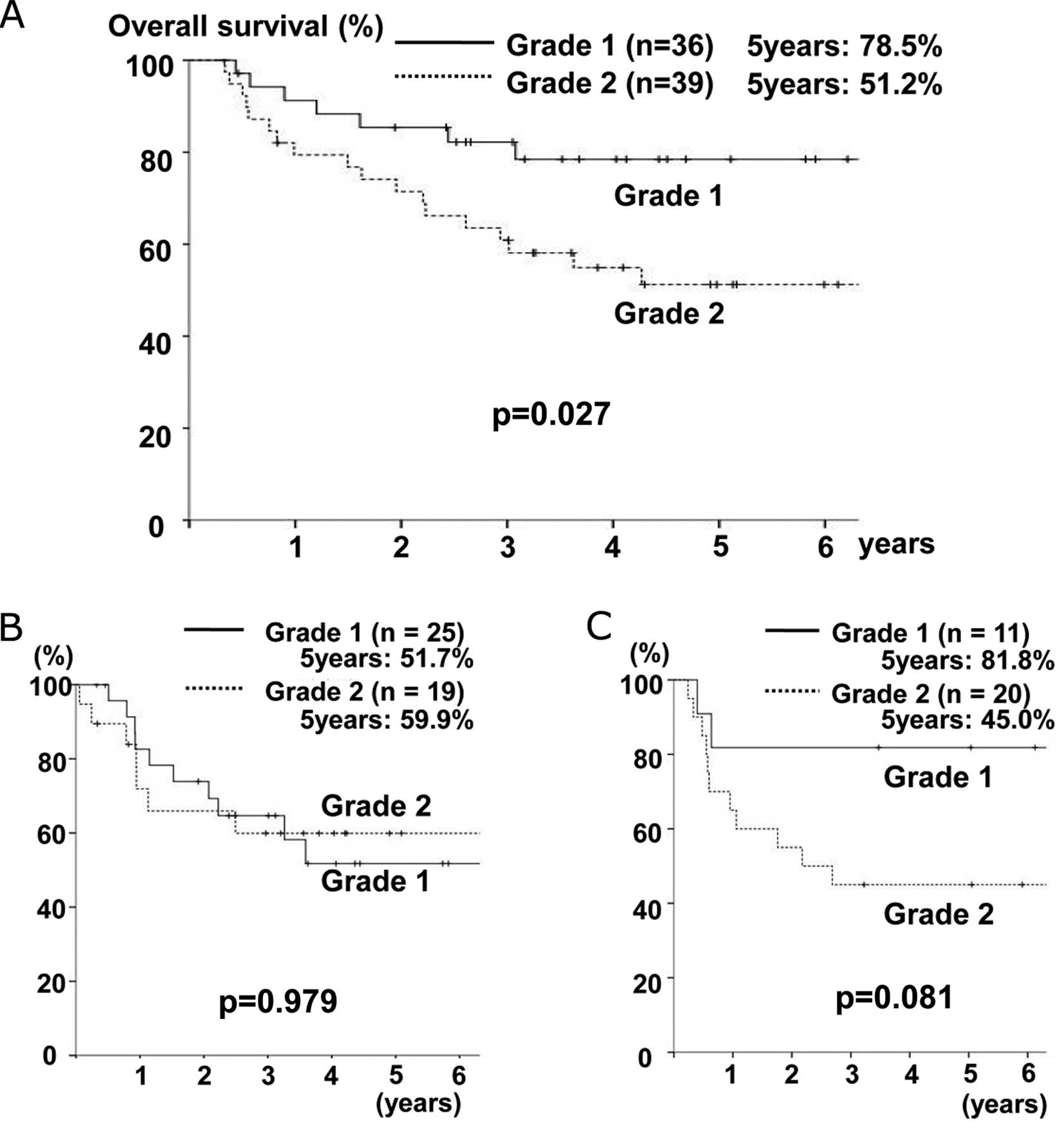
perfect). The overall 5-year survival rate of all patients was
63.5% and Fig. 2A showed the
results of a survival analysis according to the GSTP1 grade. The
5-year survival rate was 78.5% for grade 1 patients and 51.2% for
grade 2 patients. There were significant differences in the
survival rates between both groups (p=0.027).
Table I summarizes
the results of the univariate and multivariate analyses of the
prognostic factors associated with overall survival. Macroscopic
type 3 or 4 disease (p=0.001), lymph node metastasis (p=0.010) and
grade 2 GSTP1 expression (p=0.029) were independently associated
with a poor prognosis.
 | Table IResults of the univariate and
multivariate analyses of the prognostic factors associated with
overall survival. |
Table I
Results of the univariate and
multivariate analyses of the prognostic factors associated with
overall survival.
| | | Univariate
analysis | Multivariate
analysis |
|---|
| | |
|
|
|---|
| n | 5-year OS (%) | p-value | HR (95% CI) | p-value |
|---|
| Gender | | | 0.715 | | |
| Male | 64 | 62.7 | | | |
| Female | 11 | 68.6 | | | |
| Age, years | | | 0.094 | | |
| <65 | 50 | 56.8 | | | |
| ≥65 | 25 | 77.4 | | | |
| Curability | | | 0.013 | | |
| Curative
resection | 54 | 70.0 | | | |
| Non-curative
resection | 21 | 45.4 | | | |
| Main tumor
location | | | 0.048 | | |
| Lower thoracic
esophagus | 26 | 48.9 | | | |
| Middle or upper
thoracic esophagus | 49 | 71.9 | | | |
| Neoadjuvant
chemotherapy | | | 0.241 | | |
| Absent | 71 | 65.4 | | | |
| Present | 4 | 40.0 | | | |
| Macroscopic
type | | | 0.001 | | 0.001 |
| Type 0–2 | 64 | 70.2 | | 1 | |
| Type 3 and 4 | 11 | 27.3 | | 4.200
(1.751–10.071) | |
| Predominant
differentiation | | | 0.358 | | |
| Well or moderately
differentiated | 52 | 69.3 | | | |
| Poorly
differentiated or others | 23 | 51.7 | | | |
| Depth of tumor
invasion (pT) | | | 0.094 | | |
| pT1 or pT2 | 44 | 68.4 | | | |
| pT3 or pT4 | 31 | 55.7 | | | |
| Tumor size
(mm) | | | 0.022 | | |
| <40 | 39 | 74.5 | | | |
| ≥40 | 36 | 51.4 | | | |
| Lymphatic
invasion | | | 0.007 | | |
| Absent | 34 | 77.9 | | | |
| Present | 41 | 51.8 | | | |
| Venous
invasion | | | 0.019 | | |
| Absent | 42 | 76.3 | | | |
| Present | 33 | 47.5 | | | |
| Infiltrative growth
pattern | | | 0.181 | | |
| Expansive
pattern | 16 | 78.6 | | | |
| Intermediate or
infiltrative pattern | 58 | 59.2 | | | |
| Intramural
metastasis | | | 0.336 | | |
| Absent | 71 | 64.2 | | | |
| Present | 4 | 50.0 | | | |
| Lymph node
metastasis | | | 0.006 | | 0.010 |
| Absent | 34 | 79.6 | | 1 | |
| Present | 41 | 50.0 | | 3.396
(1.335–8.637) | |
| GSTP1 | | | 0.029 | | 0.029 |
| Grade 1 | 36 | 78.5 | | 1 | |
| Grade 2 | 39 | 51.2 | | 2.704
(1.107–6.604) | |
The 10 clinicopathological factors related to the
GSTP1 grade in tumor specimens were analyzed (Table II, the Chi-squared test and the
logistic regression model). A tumor size >40 mm (p=0.007) and
venous invasion (p=0.011) were identified as independent factors
associated with grade 2 GSTP1 expression.
 | Table IIResults of the univariate and
multivariate analyses of the 10 clinicopathological factors as
related to the GSTP1 expression in the tumor specimens. |
Table II
Results of the univariate and
multivariate analyses of the 10 clinicopathological factors as
related to the GSTP1 expression in the tumor specimens.
| n | Grade 1 (n=36) | Grade 2 (n=39) | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| p-value | RR (95% CI) | p-value |
|---|
| Gender | | | | 0.855 | | |
| Male | 64 | 31 | 33 | | | |
| Female | 11 | 5 | 6 | | | |
| Age, years | | | | 0.327 | | |
| <65 | 50 | 22 | 28 | | | |
| ≥65 | 25 | 14 | 11 | | | |
| Main tumor
location | | | | 0.091 | | |
| Lower thoracic
esophagus | 26 | 9 | 17 | | | |
| Middle or upper
thoracic esophagus | 49 | 27 | 22 | | | |
| Macroscopic
type | | | | 0.403 | | |
| Type 0–2 | 64 | 32 | 32 | | | |
| Type 3 and 4 | 11 | 4 | 7 | | | |
| Tumor size
(mm) | | | | 0.015 | | 0.007 |
| <40 | 39 | 24 | 15 | | 1 | |
| ≥40 | 36 | 12 | 24 | | 4.193
(1.478–11.897) | |
| Lymphatic
invasion | | | | 0.030 | | |
| Absent | 34 | 21 | 13 | | | |
| Present | 41 | 15 | 26 | | | |
| Venous
invasion | | | | 0.024 | | 0.011 |
| Absent | 42 | 25 | 17 | | 1 | |
| Present | 33 | 11 | 22 | | 3.918
(1.367–11.230) | |
| Infiltrative growth
pattern | | | | 0.211 | | |
| Expansive
pattern | 16 | 10 | 6 | | | |
| Intermediate or
infiltrative pattern | 58 | 26 | 32 | | | |
| Intramural
metastasis | | | | 0.344 | | |
| Absent | 71 | 35 | 36 | | | |
| Present | 4 | 1 | 3 | | | |
| Lymph node
metastasis | | | | 0.088 | | |
| Absent | 34 | 20 | 14 | | | |
| Present | 41 | 16 | 25 | | | |
Relationship between the GSTP1 expression
level and the efficacy of adjuvant chemotherapy
With regard to the subgroup analysis among the 44
patients who did not undergo adjuvant chemotherapy, there were no
significant differences in survival between the grade 1 and grade 2
groups (5-year rate, 51.7 vs. 59.9%, p=0.979) (Fig. 2B). On the other hand, among the 31
patients undergoing adjuvant chemotherapy, the grade 1 group had a
better prognosis than the grade 2 group (5-year rate, 81.8 vs.
45.0%). There were, however, no significant differences between the
groups (p=0.081) (Fig. 2C).
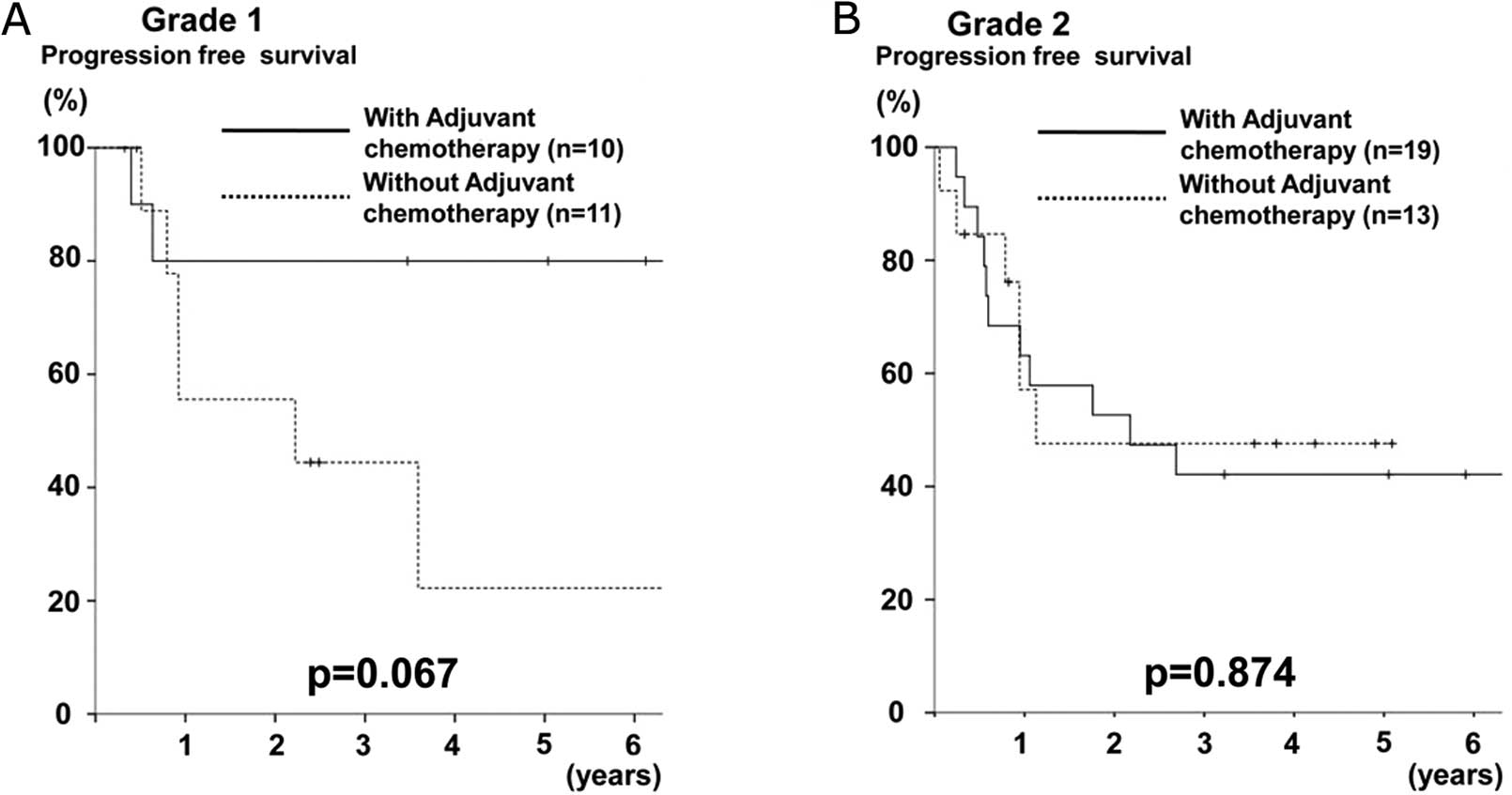
Furthermore, a subgroup analysis of progression-free
survival among the pStage II, III, or IV patients according to the
GSTP1 grade was performed. In the grade 1 group, the patients who
underwent adjuvant chemotherapy were shown to have a better
prognosis than those who did not undergo adjuvant chemotherapy
(p=0.067) (Fig. 3A), whereas no
difference was observed in the grade 2 group (p=0.874) (Fig. 3B).
Discussion
GST expression has been widely detected in various
organs and tumors, such as prostate cancer, colorectal cancer,
breast cancer, gastric cancer and esophageal cancer (21,22).
GST enzymes catalyze the conjugation of toxic and carcinogenic
electrophilic molecules to GSH and their activities constitute an
important cellular protection mechanism for many types of damage,
such as those that involve the activation of phase II
detoxification enzymes (11,23).
In mammals, 7 classes of GSTs have been identified on the basis of
amino acid sequence similarities, substrate specificity and
immunological cross reactivity, such as GSTA, GSTM and GSTP
(23). In the normal esophageal
epithelium, several GST variants are expressed and GSTP1 is the
main one (23).
For many types of cancers, including ESCC, the role
of the GST protein has been discussed in the context of the
variants of GST and the polymorphisms of each variant. In ESCC,
many authors have reported several genotypes of GSTP1, such as lle
105 Val and Ala 114 Val (13,22–24).
However, these differences among the several variants or genotypes
are conflicting between reports. In the present study, we simply
assessed the relationship between GSTP1 protein expression and
various clinicopathological parameters in ESCC (Fig. 1; Table
II) and we found that high levels of GSTP1 expression, which
were significantly associated with severe venous invasion and
larger tumor size, led to poor prognosis (Fig. 2A; Table
I). Moreover, the reproducibility of our classification of
GSTP1 expression was found to be ‘almost perfect’ (κ-value, 0.893)
when all slides were assessed by another independent observer.
These findings indicate that our classification of GSTP1 expression
is a simple and reproducible predictor of the prognosis of
ESCC.
There are two important reasons that we focused on
GSTP1 expression in ESCC. The first is that GSTP1 has two
tumor-protective roles involving the deactivation of anticancer
reagents and the inhibition of signaling pathways that lead to
apoptosis through the inhibition of the c-Jun N-terminal kinase
(JNK). The deactivation of anticancer reagents by GST expression
has been reported in many types of cancers, including ESCC, and it
has been considered one of the main reasons for the acquisition of
resistance to chemotherapeutic agents (25,26).
Furthermore, several GSTs have been shown to be associated with
members of the mitogen-activated protein kinase (MAPK) pathway,
such as JNK and p38, which is involved in cell survival and cell
death signaling (23,27,28).
Based on these two major roles, we aimed to ascertain whether GSTP1
also affects cancer prognosis in ESCC.
The second reason is that GSTP1 expression is
partially regulated by the Nrf2 gene, which is a transcriptional
factor that is frequently upregulated in lung cancer, esophageal
cancer and several other squamous cell carcinomas (29,30).
In ESCC cell lines and primary samples, mutations in Nrf2 are
frequently reported (31).
Therefore, we considered whether the high expression of GSTP1 may
be partially affected by the activation of Nrf2.
In our study, the majority of patients treated by
adjuvant chemotherapy with a low GSTP1 protein expression level
showed better prognosis than the patients with a high GSTP1
expression level. This tendency was not shown in the patients with
high GSTP1 protein expression level (Fig. 3). These results may indicate the
role of GSTP1 for the detoxification of anticancer drugs in
ESCC.
Since the publication of the results of the JCOG
9907 study, neoadjuvant chemotherapy followed by radical
esophagectomy has been accepted as the standard therapeutic
approach for resectable cStage II/III esophageal cancer in Japan
(32). However, the clinical
response rate of preoperative chemotherapy is only 38%, which is
not sufficient. Therefore, it seems to be quite important to
develop predictive factors for the response to chemotherapy for
patients who are unlikely to derive benefits from such therapy.
Retrospective analysis in a relatively small case
series was a limitation of the present study, and a conflicting
result regarding the utility of GSTP1 expression has also been
reported (14). Therefore, our
results need to be confirmed through additional studies of a large
number of patients, and the clinical significance of GSTP1 in the
prediction of the response to chemotherapy must be established in
ESCC. If the relationship between the GSTP1 expression level and
efficacy of chemotherapy is clinically confirmed, then the
expression level may also have the potential to be used as a
preoperative marker for the effect of neoadjuvant chemotherapy.
In conclusion, we showed that high GSTP1 protein
expression in ESCC tissue is a sensitive marker of poor prognosis,
and the resistance to adjuvant chemotherapy may be influenced by
high GSTP1 expression. These findings indicate that classification
of GSTP1 expression may be used as a simple, accurate, and
reproducible predictor of prognosis and drug resistance for ESCC
patients.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Vita F, Di Martino N, Orditura M,
Cosenza A, Galizia G, Del Genio A and Catalano G: Preoperative
chemoradiotherapy for squamous cell carcinoma and adenocarcinoma of
the esophagus: a phase II study. Chest. 122:1302–1308.
2002.PubMed/NCBI
|
|
3
|
Hofstetter W, Swisher SG, Correa AM, Hess
K, Putnam JB Jr, Ajani JA, Dolormente M, Francisco R, Komaki RR,
Lara A, Martin F, Rice DC, Sarabia AJ, Smythe WR, Vaporciyan AA,
Walsh GL and Roth JA: Treatment outcomes of resected esophageal
cancer. Ann Surg. 236:376–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medical Research Council Oesophageal
Cancer Working Group. Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: a randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, Asbell SO, Graham MV and Leichman LL:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85–01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999.PubMed/NCBI
|
|
6
|
McKinney A, Sharp L, Macfarlane GJ and
Muir CS: Oesophageal and gastric cancer in Scotland 1960–90. Br J
Cancer. 71:411–415. 1995.
|
|
7
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surg. 232:225–232. 2000.PubMed/NCBI
|
|
8
|
Coles BF and Kadlubar FF: Detoxification
of electrophilic compounds by glutathione S-transferase catalysis:
determinants of individual response to chemical carcinogens and
chemotherapeutic drugs? Biofactors. 17:115–130. 2003. View Article : Google Scholar
|
|
9
|
Matthias C, Jahnke V, Fryer AA and Strange
RC: Influence of glutathione s-transferase and cytochrome p450
polymorphisms on prognosis of head and neck cancer.
Laryngorhinootologie. 81:406–412. 2002.(In German).
|
|
10
|
Anderer G, Schrappe M, Brechlin AM, Lauten
M, Muti P, Welte K and Stanulla M: Polymorphisms within glutathione
S-transferase genes and initial response to glucocorticoids in
childhood acute lymphoblastic leukaemia. Pharmacogenetics.
10:715–726. 2000. View Article : Google Scholar
|
|
11
|
Stoehlmacher J, Park DJ, Zhang W, Groshen
S, Tsao-Wei DD, Yu MC and Lenz HJ: Association between glutathione
S-transferase P1, T1, and M1 genetic polymorphism and survival of
patients with metastatic colorectal cancer. J Natl Cancer Inst.
94:936–942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sweeney C, Ambrosone CB, Joseph L, Stone
A, Hutchins LF, Kadlubar FF and Coles BF: Association between a
glutathione S-transferase A1 promoter polymorphism and survival
after breast cancer treatment. Int J Cancer. 103:810–814. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JM, Wu MT, Lee YC, Yang SY, Chen JS,
Hsu HH, Huang PM, Kuo SW, Lee CJ and Chen CJ: Association of GSTP1
polymorphism and survival for esophageal cancer. Clin Cancer Res.
11:4749–4753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, He W, Yang G, Wang J, Wang Z,
Nesland JM, Holm R and Suo Z: Decreased expression of GST pi is
correlated with a poor prognosis in human esophageal squamous
carcinoma. BMC Cancer. 10:3522010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueda Y, Shiozaki A, Itoi H, Okamoto K,
Fujiwara H, Ichikawa D, Kikuchi S, Fuji N, Itoh T, Ochiai T,
Komatsu S and Yamagishi H: Intraoperative pathological
investigation of recurrent nerve nodal metastasis can guide the
decision whether to perform cervical lymph node dissection in
thoracic esophageal cancer. Oncol Rep. 16:1061–1066. 2006.
|
|
16
|
Shiozaki A, Yamagishi H, Itoi H, Fujiwara
H, Kikuchi S, Okamoto K, Ichikawa D, Fuji N, Ochiai T, Sonoyama T
and Ueda Y: Long-term administration of low-dose cisplatin plus
5-fluorouracil prolongs the postoperative survival of patients with
esophageal cancer. Oncol Rep. 13:667–672. 2005.PubMed/NCBI
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer. TNM Classification of
Malignant Tumours. 7th edition. Hoboken, NJ: Wiley-Blackwell; pp.
73–77. 2010
|
|
18
|
Kato H, Miyazaki T, Nakajima M, Fukuchi M,
Manda R and Kuwano H: Value of positron emission tomography in the
diagnosis of recurrent oesophageal carcinoma. Br J Surg.
91:1004–1009. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roedl JB, Harisinghani MG, Colen RR,
Fischman AJ, Blake MA, Mathisen DJ and Mueller PR: Assessment of
treatment response and recurrence in esophageal carcinoma based on
tumor length and standardized uptake value on positron emission
tomography-computed tomography. Ann Thorac Surg. 86:1131–1138.
2008. View Article : Google Scholar
|
|
20
|
Fleiss JL: Measuring nominal scale
agreement among many rates. Psychol Bull. 76:378–382. 1971.
View Article : Google Scholar
|
|
21
|
Nakajima T, Wang RS, Nimura Y, Pin YM, He
M, Vainio H, Murayama N, Aoyama T and Iida F: Expression of
cytochrome P450s and glutathione S-transferases in human esophagus
with squamous-cell carcinomas. Carcinogenesis. 17:1477–1481. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laborde E: Glutathione transferases as
mediators of signaling pathways involved in cell proliferation and
cell death. Cell Death Differ. 17:1373–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossini A, Rapozo DC, Soares Lima SC,
Guimarães DP, Ferreira MA, Teixeira R, Kruel CD, Barros SG,
Andreollo NA, Acatauassú R, Matos HJ, Albano RM and Pinto LF:
Polymorphisms of GSTP1 and GSTT1, but not of CYP2A6, CYP2E1 or
GSTM1, modify the risk for esophageal cancer in a western
population. Carcinogenesis. 28:2537–2542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li D, Dandara C and Parker MI: The 341C/T
polymorphism in the GSTP1 gene is associated with increased risk of
oesophageal cancer. BMC Genet. 11:472010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peklak-Scott C, Smitherman PK, Townsend AJ
and Morrow CS: Role of glutathione S-transferase P1-1 in the
cellular detoxification of cisplatin. Mol Cancer Ther. 7:3247–3255.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyake T, Nakayama T, Naoi Y, Yamamoto N,
Otani Y, Kim SJ, Shimazu K, Shimomura A, Maruyama N, Tamaki Y and
Noguchi S: GSTP1 expression predicts poor pathological complete
response to neoadjuvant chemotherapy in ER-negative breast cancer.
Cancer Sci. 103:913–920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adler V, Yin Z, Fuchs SY, Benezra M,
Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR,
Davis RJ and Ronai Z: Regulation of JNK signaling by GSTp. EMBO J.
18:1321–1334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sau A, Filomeni G, Pezzola S, D’Aguanno S,
Tregno FP, Urbani A, Serra M, Pasello M, Picci P, Federici G and
Caccuri AM: Targeting GSTP1-1 induces JNK activation and leads to
apoptosis in cisplatin-sensitive and -resistant human osteosarcoma
cell lines. Mol Biosyst. 8:994–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Ju Y, Lin D, Wang Z, Huang Y, Zhang
S, Wu C and Jiao S: Mutation of the Nrf2 gene in non-small cell
lung cancer. Mol Biol Rep. 39:4743–4747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YR, Oh JE, Kim MS, Kang MR, Park SW,
Han JY, Eom HS, Yoo NJ and Lee SH: Oncogenic NRF2 mutations in
squamous cell carcinomas of oesophagus and skin. J Pathol.
220:446–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibata T, Kokubu A, Saito S,
Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y,
Kushima R, Kiyono T and Yamamoto M: NRF2 mutation confers malignant
potential and resistance to chemoradiation therapy in advanced
esophageal squamous cancer. Neoplasia. 13:864–873. 2011.PubMed/NCBI
|
|
32
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K,
Kanda T, Tsujinaka T, Nakamura K and Fukuda H: A randomized trial
comparing postoperative adjuvant chemotherapy with cisplatin and
5-fluorouracil versus preoperative chemotherapy for localized
advanced squamous cell carcinoma of the thoracic esophagus
(JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|