Introduction
Apoptosis is a form of procedural cell death. It
plays a role in tissue growth and dynamic equilibrium, as well as
in numerous diseases, including cancer, autoimmune diseases, viral
infections and degenerative diseases (1,2).
Apoptosis is mediated by two integrated pathways, the extrinsic and
intrinsic apoptotic pathways. In the extrinsic pathway, the
combination of ligands and cell surface death receptors stimulates
formation of the death-inducing signaling complex (DISC), which
further activates upstream caspase-8. Activated caspase-8
stimulates downstream caspase molecules, inducing hydrolysis of a
large number of cellular proteins and eventually leads to cell
death. In the intrinsic pathway, a stimulus signal is delivered to
the mitochondria and endoplasmic reticulum (ER) through the
interaction of BH3-only and Bcl-2 family proteins, which in turn
triggers the release of apoptogen cytochrome c from
mitochondria to the cytoplasm. The cytochrome c in the
cytoplasm interplays with apoptosis-related factor 1 (Apaf-1) in
the presence of dATP and stimulates formation of apoptosomes and
activation of upstream caspase-9, which subsequently activates
downstream caspases, finally inducing apoptosis. Therefore, the
activation of the apoptotic pathway eventually leads to
mitochondrial dysfunction, caspase activation and cell death.
Although these pathways are mediated by death signal
stimulation and activation of various pro-apoptotic factors, each
stage of the apoptotic pathway is regulated at additional levels by
endogenous proteins that play a role in reverse adjustment. These
proteins include FLIP, which opposes the extrinsic pathway; Bcl-2
and Bcl-xL, which block the release of apoptogens from the
mitochondria; and IAPs, which inhibit the activation of downstream
caspases.
ARC (apoptosis repressor with caspase recruitment
domain) is an apoptotic inhibitor with a caspase enrichment of
functional domains. It is an endogenous CARD containing protein
that inhibits apoptosis by selectively binding to and inhibiting
caspase-8 and caspase-2, but not caspase-1, caspase-9 and caspase-3
(3,4). It can also directly combine with FADD
and Fas, and block the interaction of Fas and FADD, thereby
inhibiting the assembly of DISC (4). In addition, interactions between ARC
and pro-apoptotic factor Bax preclude activation of Bax and its
translocation to the mitochondria (5). In addition, the proline-glutamic
acid-riched region of ARC can be directly bound to the
tetramerization domain of p53, thereby blocking p53 tetramerization
and suppressing p53 transfer from the cytoplasm to the nucleus
(6). Actually, ARC can inhibit
apoptosis induced by death receptors, hypoxia, oxidative stress,
serum deprivation and ischemia-reperfusion (3,4,7–9).
As inhibition of apoptosis has been implicated in
carcinogenesis, ARC potently inhibits a wide array of death
signals, which suggests that it may play a functionally important
role in the process of carcinogenesis. It remains unknown whether
ARC plays a role in the development of nasopharyngeal carcinoma
(NPC). In the present study, we assessed the abundance of ARC in
NPC cell lines and NPC tissues and the relationship between its
expression and clinicopathological tumor grade. Moreover, we sought
to assess the effects of ARC expression, through downregulation and
overexpression of ARC, on the response of NPC cell lines with
diverse intrinsic radiation- and chemo-sensitivities and different
levels of ARC expression.
Materials and methods
Materials
Human NPC cell lines CNE-1, CNE-2, 5–8F, 6–10B and
HNE-2 have been previously described (10,11).
Formalin-fixed, paraffin-embedded tissues and clinicopathological
parameters were obtained from 82 patients with a pathological
diagnosis of poorly differentiated squamous cell carcinoma, from 14
patients with severe atypical hyperplasia and from 20 patients with
benign nasopharyngeal diseases at the Department of Otolaryngology
and Pathology of the Xiangya Hospital. The present study was
approved by the Ethics Committee of the Xiangya School of Medicine,
Central South University, China.
Cell culture
NPC cells were cultured in RMPI-1640 medium (Gibco,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco) at 37°C in an incubator with a humidified atmosphere
with 5% CO2 in air.
Immunohistochemical staining and counting
methods
Immunohistochemical staining and counting methods
were carried out as previously described (10,11).
Rabbit anti-human antibody against ARC (1:1,000 dilution; Abcam)
was used and its expression was detected with biotinylated goat
anti-rabbit IgG (1:1,000 dilution; Changxing Zhongshan Chemical
Industry Co., Ltd., Changxing, China). 3′,3′-Diaminobenzidine
(Fuzhou Maixin, Fuzhou, China) was used for chromogenesis. Sections
were blindly evaluated by two pathologists using light microscopy.
Both the intensity and the percentage of positive cells were
evaluated.
Western blotting
Western blotting was used as previously described
(10,11). A rabbit anti-human antibody against
ARC (1:2,000 dilution) and mouse anti-human antibodies against
caspase-8, caspase-2 and β-actin (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) were used and their expression was detected with
goat anti-rabbit or goat anti-mouse horseradish peroxidase (HRP) as
secondary antibody (Beyotime Biotechnology, Beijing, China),
respectively. Chemiluminescence reagent (Fermentas Biology) was
used for enhanced electrochemiluminescence detection. The signal
intensity was analyzed using GeneTools software (Syngene,
Frederick, MD, USA). β-actin was used as a loading control.
RNA interference and plasmid
transfection
microRNA (miRNA) against ARC and negative control
miRNA (designed and synthesized by Invitrogen Biotechnology) were
used for gene knockdown. The double-stranded oligo (top strand:
5′-TGCTGTTCTGCTTCAGCCTCGGGTTCGTTTTGGCC
ACTGACTGACGAACCCGACTGAAGCAGAA-3′; bottom strand:
5′-CCTGTTCTGCTTCAGTCGGGTTCGTCAGTCA
GTGGCCAAAACGAACCCGAGGCTGAAGCAGAAC-3′) encoding the pre-miRNA for
ARC was inserted into the miRNA expression vector pcDNA™
6.2-GW/EmGFP-miR (Invitrogen, Carlsbad, CA, USA) for construction
of the expression plasmid. Expression of the plasmid was confirmed
by DNA sequencing. Cells at 60–80% confluence were transfected with
the miRNA plasmid at a final concentration of 100 nM of the
respective miRNA (ARC or control miRNA) using Lipofectamine™ 2000
transfection reagent (Invitrogen) according to the manufacturer’s
instructions. After 14 days of selection in RPMI-1640 containing
10% FBS and 10 μg/ml puromycin (Invitrogen), individual
puromycin-resistant colonies were isolated and expanded. The ARC
plasmid and control plasmids (EX-Z9301-M03, purchased from
GeneCopoeia™) were used for gene expression. Cells at 60–80%
confluence were transfected with 1.6 μg/ml of the plasmid using
Lipofectamine 2000 according to the manufacturer’s instructions.
After no less than 14 days of selection in RPMI-1640 containing 10%
FBS and neomycin (300 μg/ml; Santa Cruz Biotechnology), individual
neomycin-resistant colonies were isolated and expanded. The
expression of ARC was determined by western blot analysis.
Cell viability assay
Cell viability was detected using
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo
phenyl)-2H-tetrazolium, monosodium salt (CCK-8 Kit; Beyotime).
CCK-8 solution (10 μl) was added to each well of 96-well plates
with the same amount of culture fluid and drugs. CCK-8 solution
without cell as well as blank plus. Optical densities were
determined on a microtiter plate reader at 450 nm.
Cell apoptosis
Annexin V-FITC apoptosis detection kit (Beyotime)
was used to evaluate cell apoptosis. As previously described
(10), cells were stained using
Annexin-V-FITC for 10 min at room temperature and were then stained
using propidium iodide (PI) for 10 min in the dark. The cells were
analyzed immediately on a FACScan flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Radiation and drug treatment
Cells were seeded in triplicate in 96-well plates at
a density of 3,000 cells for CNE-2 and 2,000 cells for 6–10B per
well in 100 μl of the corresponding medium. Cells were exposed to
the indicated X-ray irradiation doses (2, 4, 6, 8 and 10 Gy). After
96 h, viable cells were detected using the CCK-8 assay kit. For
drug treatment, cells were seeded in triplicate in 96-well plates
at a density of 7,000 cells for CNE-2 and 3,000 cells for 6–10B per
well in 100 μl of the corresponding medium. On the following day,
the media were discarded and replaced with fresh media containing
the indicated concentrations of the following drug: cisplatin (0,
5, 10, 20, 30, 40 and 50 μmol/l for CNE-2; 0, 5, 10, 30, 70, 100
and 200 μmol/l for 6–10B; Santa Cruz Biotechnology). Cells were
incubated under standard culture conditions for 48 h, and viable
cells were assessed using the CCK-8 assay kit. The half-maximal
inhibitory concentration (IC50) values were determined
with SPSS 17.0 software.
Statistical analyses
All statistical analyses were carried out using SPSS
for Windows version 17.0 (SPSS). Log-rank test and one-way analysis
of variance (ANOVA) were used to analyze the significance of
differences. All cell culture experiments were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant result.
Results
ARC protein is abundant in several NPC
cell lines
Since ARC plays an important role in apoptosis
escape and the development of cancers, we hypothesized that ARC is
induced in NPC cell lines. To test this hypothesis, we assessed the
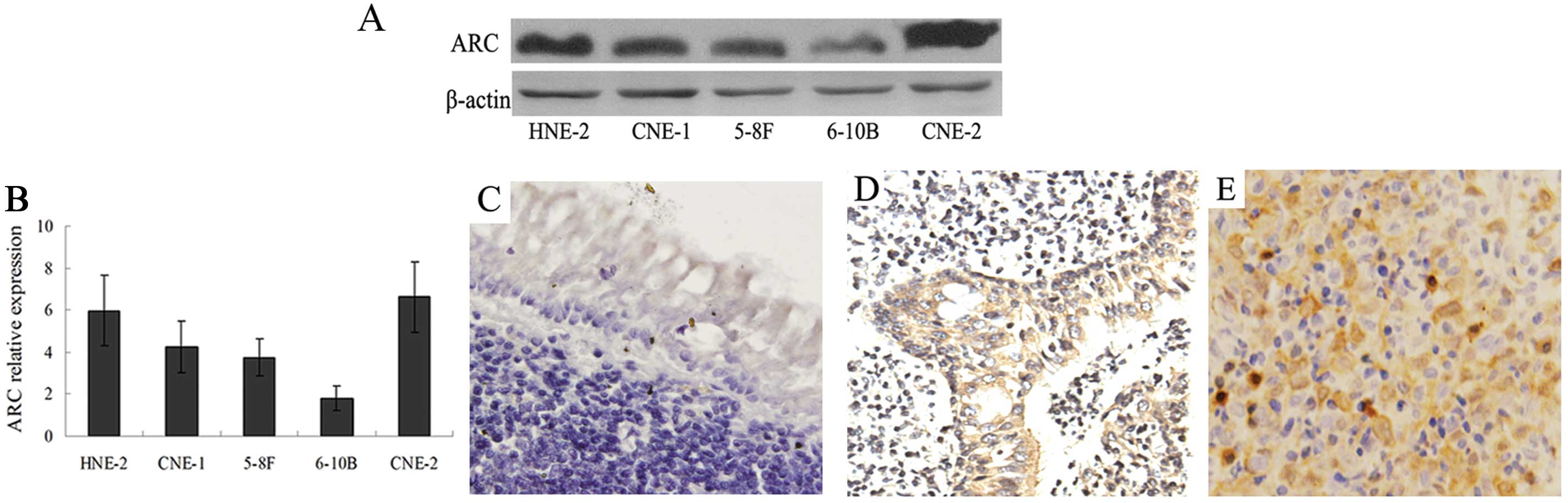
abundance of ARC in 5 cell lines using western blotting (Fig. 1A). ARC was detectable in most cell
lines but present to a variable extent. Quantitative analysis
revealed that levels were highest in CNE-2 and lowest in 6–10B
cells (Fig. 1B). ARC was also
present in the other NPC cell lines including CNE-1, 5–8F and
HNE-2.
ARC protein is increased in NPC
tissues
Given the high ARC levels in several NPC cell lines,
we assessed whether the ARC levels were increased in NPC tissues.
We next carried out an immunohistochemical analysis of an
independent group of 82 NPC, 14 severe atypical hyperplasia and 20
benign nasopharyngeal tissues. On a scale of 0–6, a score 0–2 was
considered to be negative; a score 3–6 was considered to be
positive. Quantitative analysis of data (Table I) revealed ARC staining in the
cytoplasm of 87.8% of NPC, 64.3% of severe atypical hyperplasia and
only 5.0% of the benign nasopharyngeal tissues (P<0.001;
Table I). The median staining
intensity of cytoplasmic ARC was 4 in NPC, 3 in atypical
hyperplasia and only 1 in benign nasopharyngeal tissues
(P<0.001; Table I). ARC was
present in the nucleus in 17.1% of NPC and in 7.2% of severe
atypical hyperplasia tissues and ARC was not detected in benign
nasopharyngeal tissue (P<0.001; Table I). The median staining intensity of
nuclear ARC was 2 in NPC and 1.5 in atypical hyperplasia tissues
(P<0.001, Table I). These data
demonstrate that the abundance of ARC was increased in the
cytoplasm of nearly all NPC tissues and a large part of the severe
atypical hyperplasia tissues, but was present only rarely and at
low levels in the benign nasopharyngeal tissues. Representative
examples of the differential presence of ARC in different
histopathological tissues are shown in Fig. 1C–E. In addition, we found that only
a small portion of the NPC tissues contained nuclear ARC, although
nuclear ARC was more prevalent in NPC than in the severe atypical
hyperplasia and benign nasopharyngeal tissues.
 | Table IARC in nasopharyngeal carcinoma
(NPC). |
Table I
ARC in nasopharyngeal carcinoma
(NPC).
| Classification | Cytoplasmic ARC
immunostaining positive (%); P50
[P25;P75] | P-value | Nuclear ARC
immunostaining positive (%); P50 [P25;
P75] | P-value |
|---|
| Benign nasopharyngeal
tissue | 1/20 (5.0); 1 [0;
1] | | 0/20 (0.0); 0 [0;
1] | |
| Severe atypical
hyperplasia | 9/14 (64.3); 3 [1.75;
4.25] |
χ2=42.446a | 1/14 (7.2); 1.5 [1;
2] |
χ2=34.025a |
| NPC | 72/82 (87.8); 4 [3;
5] | <0.001 | 14/82 (17.1); 2 [1;
2] | <0.001 |
| Gender (NPC) |
| Male | 41/47 (87.2); 4 [3;
5] | Z=−0.724b | 8/47 (17.0); 2 [1;
2] | Z=−0.650b |
| Female | 31/35 (88.6); 4 [3;
5] | 0.469 | 6/35 (17.1); 2 [1;
2] | 0.516 |
| Primary tumor (T)
stage (NPC) |
|
T1–2 | 33/43 (76.7); 3 [3;
5] | Z=−5.145b | 0/43 (0.0);2 [1;
2] | Z=−5.829b |
|
T3–4 | 39/39 (100.0); 5
[4; 6] | <0.001 | 14/39 (35.9);2 [2;
4] | <0.001 |
| Lymph node
metastasis (NPC) |
| Negative | 21/26 (80.8); 4 [3;
5] | Z=−1.289b | 6/26 (23.1); 2 [2;
2] | Z=−0.327b |
| Positive | 51/56 (91.1); 5 [3;
6] | 0.198 | 8/56 (14.3);2 [1;
2] | 0.743 |
We next explored the relationship between the
clinicopathological parameters of NPC and the subcellular
localization of ARC. The median intensity of cytoplasmic ARC
staining was 4 in the male patients compared to 4 in the female
patients (P>0.05; Table I), 5 in
stage T3–4 tumors compared to 3 in stage T1–2
tumors (P<0.001; Table I), 4 in
the patients with lymph node metastasis compared to 4 in patients
with no lymph node metastasis (P>0.05; Table I). The median intensity of nuclear
ARC staining was 2 in male compared to 2 in female patients
(P>0.05; Table I), 2 in stage
T3–4 tumors compared to 2 in stage T1–2
tumors (P<0.001; Table I), 2 in
patients with lymph node metastasis compared to 2 in patients with
no lymph node metastasis (P>0.05; Table I). These data demonstrate that both
cytoplasmic and nuclear ARC is present at a higher level in
advanced NPC than in early-stage NPC. However, neither cytoplasmic
nor nuclear ARC was associated with gender or lymph node
metastasis.
Overexpression of ARC increases the
resistance to X-radiation
Since ARC is known to inhibit apoptosis through
extrinsic and intrinsic apoptotic pathways and presents at high
levels in most NPC tissues, we hypothesized that this protein may
contribute to radiation-resistance in NPC. To confirm this
hypothesis, ARC-overexpressing 6–10B cells, ARC-silenced CNE-2
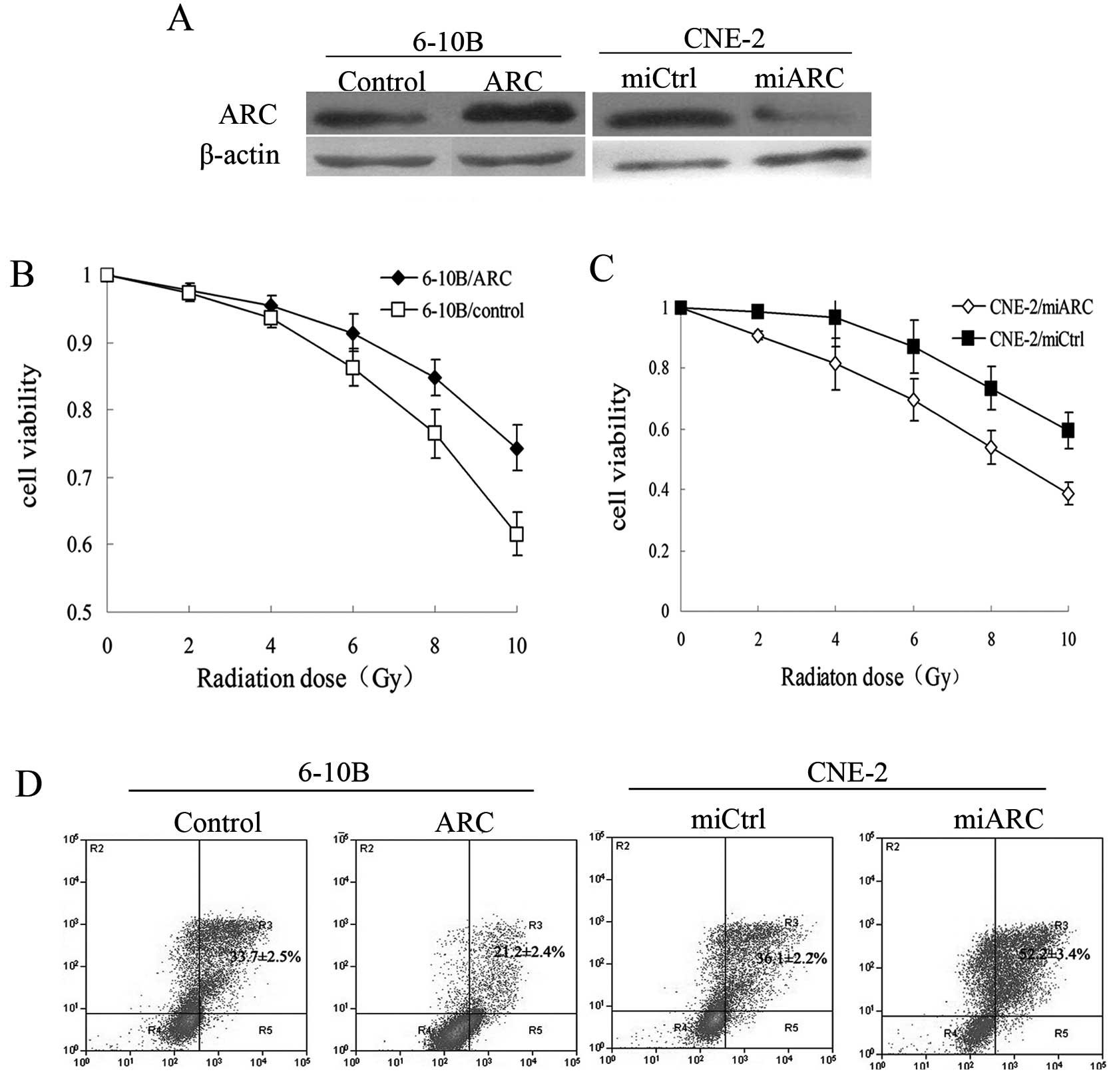
cells and their respective control cells (Fig. 2A) were exposed individually to X-ray
radiation (2–10 Gy). Cell viability was then examined after 96 h.
We found that cell viability in the ARC-overexpressing 6–10B cells
was significantly increased at each indicated dose compared with
the control cells (P<0.001; Fig.
2B). The ARC-transfected 6–10B cells were less sensitive to
radiation than the control cells. Furthermore, the ARC-silenced
CNE-2 cells exhibited decreased cell viability and increased
sensitivity to radiation when compared to the control cells
(P<0.001; Fig. 2C).
ARC decreases X-radiation-induced
apoptosis
We next investigated the effect of ARC on
radiation-induced cell apoptosis. Cells were exposed to 8-Gy
radiation and the proportion of apoptotic cells was assessed using
the Annexin V-FITC apoptosis detection kit. Compared to the control
cells, overexpression of ARC in 6–10B cells resulted in a lower
proportion of apoptotic cells. The percentage of apoptotic cells in
the ARC-overexpressing 6–10B cells was significantly decreased when
compared to the controls (21.2±2.4 vs. 33.7±2.5%, P<0.001).
Similarly, when ARC was knocked down in CNE-2 cells, the number of
apoptotic cells after radiation treatment was significantly
increased when compared to the control cells (52.2±3.4 vs.
36.1±2.2%, P<0.001) (Fig. 2D).
These results indicate that ARC attenuates radiation-induced
apoptosis in NPC cells.
Overexpression of ARC increases the
resistance to cisplatin
As an apoptotic inhibitor, ARC may also imply a
change of cell survival capacity against chemotherapeutic
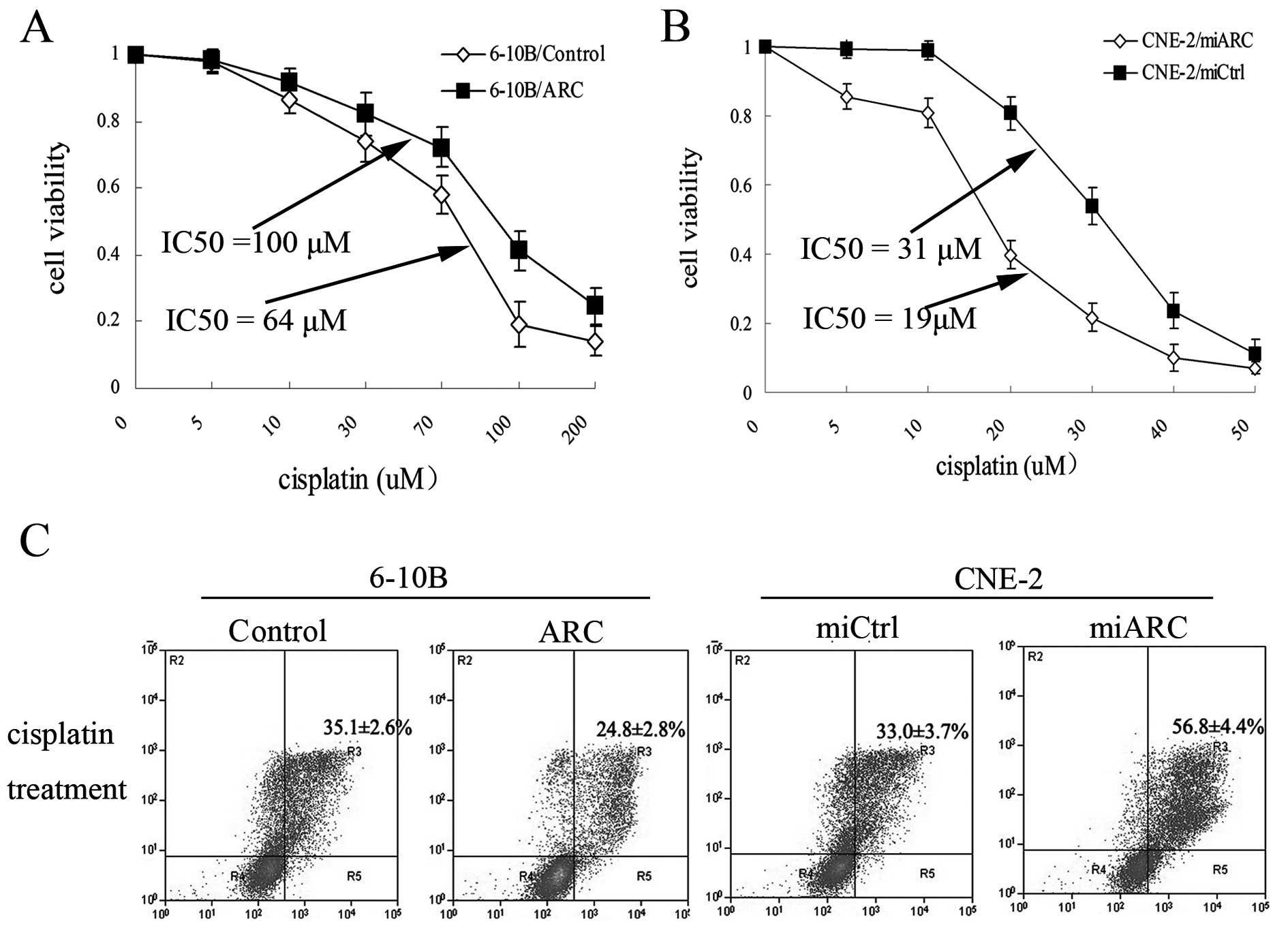
treatment. To test this hypothesis, the ARC-overexpressing 6–10B
cells, ARC-silenced CNE-2 cells and their respective control cells
were treated individually with cisplatin for 48 h and the cell
viability was evaluated. When the cells were exposed to cisplatin,
the viability of ARC-overexpressing 6–10B cells was significantly
increased at each indicated dose compared with the control cells
(P<0.001; Fig. 3A). The
ARC-transfected 6–10B cells were less sensitive to cisplatin
(IC50, 100 μM) than the control cells (IC50,
64 μM). Furthermore, when ARC was silenced in CNE-2 cells, the
CNE-2 cells showed an increased sensitivity to cisplatin
(IC50, 19 μM) compared to the control cells
(IC50, 31 μM) (P<0.001; Fig. 3B).
ARC decreases cisplatin-induced
apoptosis
We also investigated the effect of ARC on
cisplatin-induced cell apoptosis. Cells were exposed to cisplatin
(IC30) for 48 h and the proportion of apoptotic cells
was assessed using the Annexin V-FITC apoptosis detection kit.
Compared to the control cells, overexpression of ARC in 6–10B cells
resulted in a lower proportion of apoptotic cells. The percentage
of apoptotic cells in the ARC-overexpressing 6–10B cells was
significantly decreased when compared to the controls (24.8±2.8 vs.
35.1±2.6%, P<0.001). Consistent with the reduced viability,
knockdown of ARC promoted cell apoptotic activity. The prercentage
apoptotic cells after repression of ARC was increased compared to
that observed in the cells without silencing of ARC (56.8±4.4 vs.
33.0±3.7%, P<0.001) (Fig. 3C).
These results indicate that ARC protects cells from
cisplatin-induced apoptosis.
Exogenous ARC inhibits radiation and
cisplatin-induced caspase-2 and caspase-8 activation
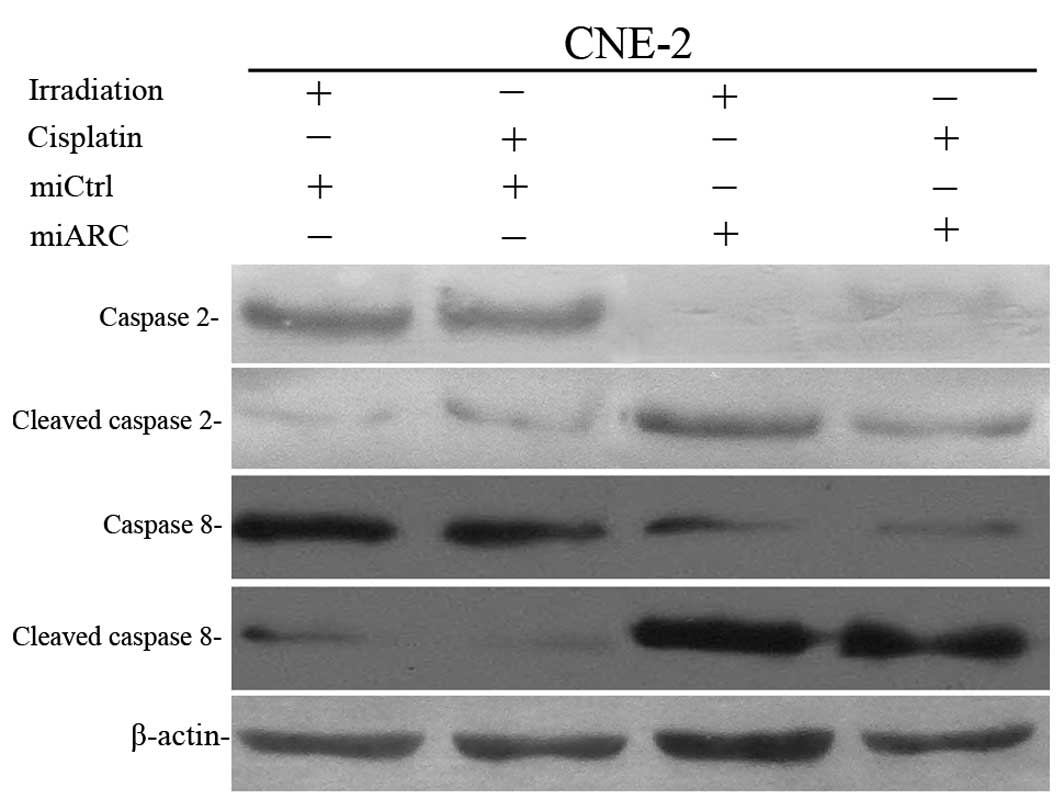
To understand the mechanism(s) by which exogenous
ARC protects NPC cells from radiation- or cisplatin-induced
apoptosis, we detected caspase-2 and caspase-8 activation in the
ARC-silenced CNE-2 cells after exposure to X-radiation or
cisplatin. As shown in Fig. 4,
miRNA knockdown of ARC enhances X-radiation- or cisplatin-induced
caspase-2 and caspase-8 activation.
Discussion
ARC is primarily expressed in terminally
differentiated cells, such as heart, skeletal muscle and nerve
cells (4,12). Recent research suggests that ARC is
highly expressed in a variety of non-terminally differentiated
tissues and in human cancer cell lines, including those derived
from breast, lung, pancreas, colon, cervix, prostate, kidney and
lymph (13). Expression of ARC in
primary epithelial-derived breast cancer and colon cancer was also
higher than that in the respective benign epithelial tissues
(14,15), but the applicability of this finding
to NPC remains unknown.
The present study assessed the expression of ARC in
NPC cell lines and NPC tissues. Western blot analysis results
demonstrated that ARC was present to a variable extent in several
NPC cell lines. ARC level was high in CNE-2, HNE-2 and CNE-1 cells;
in contrast, its level was low in 5–8F and 6–10B cells. Because of
the inherent limitations of cell lines, we assessed ARC levels in
primary NPC, severe atypical hyperplasia and benign nasopharyngeal
tissues by immunohistochemistry. We found that the amount of
cytoplasmic ARC was increased in NPC and severe atypical
hyperplasia but not in benign nasopharyngeal tissues. Moreover,
cytoplasmic and nuclear ARC was increased in T3–4 stage
NPC, which indicates that high expression of ARC provides a direct
correlation with advanced local invasion. In addition, we found
that only a small amount of nuclear ARC was expressed in NPC and
severe atypical hyperplasia tissues in contrast to cytoplasmic ARC,
which is similar to expression in human breast cancer (14). These results demonstrate that ARC
may serve as a novel marker of NPC and suggest that it may be
important in the pathogenesis of NPC.
Carcinogenesis often involves defects in apoptosis
that is mediated by an increase in apoptotic inhibitors. It was
previously found that multiform endogenous apoptosis inhibitors are
significantly present at high levels in numerous types of cancers.
For example, survivin, cIAP2 and XIAP which belong to the IAP
family, were significantly more highly expressed in colon cancer,
prostate cancer and leukemia, and high expression of these
molecules indicated a poor prognosis (16–18).
Similarly, Bcl-2 and Bcl-xl were highly expressed in leukemia,
breast, colon, esophageal, pancreatic and uterine cancer (19–24).
Thus, the abnormal expression of ARC may provide NPC cells with a
survival mechanism that normally serves to protect cancer cell
populations.
Given such strong evidence implicating the role of
ARC in enhancing the malignant potential of NPC, in the present
study, we investigated whether ARC also plays a direct role in
mediating radiation- and chemo-resistance in NPC. Regarding
radiotherapy, we showed that attenuation of ARC expression by miRNA
treatment resulted in a decreased rate of cell viability as well as
increased X-radiation-induced apoptosis in CNE-2 cells. In
contrast, overexpression of ARC in 6–10B cells resulted in an
increased rate of cell viability and decreased X-radiation-induced
apoptosis. Similarly, regarding chemotherapy, silencing of ARC
resulted in a decreased rate of cell viability and increased
apoptosis following cisplatin exposure in CNE-2 cells. In contrast,
overexpression of ARC increased the rate of cell viability and
decreased apoptosis following cisplatin exposure in 6–10B cells.
Moreover, the mechanism(s) of ARC in the protection of NPC cells
from X-radiation- or cisplatin-induced apoptosis was shown by miRNA
knockdown of ARC in CNE-2 cells, which revealed that ARC suppressed
the activation of caspase-2 and caspase-8. Thus, ARC expression
seems to be a marker of radiation- and chemo-resistance in
vitro.
Radiation therapy has been thought to induce cell
death mainly by apoptosis (25,26).
Similarly, cisplatin exerts its cytotoxic effect by inducing DNA
damage and activating apoptosis (27). ARC, as an apoptosis inhibitor
through mediating both the extrinsic and intrinsic pathways, is
likely to suppress radiation- and cisplatin-induced cell apoptosis.
ARC was found to protect against doxorubicin and γ-radiation in
breast cancer cells, and inhibition of ARC promoted apoptosis and
sensitized cytosine arabinoside-induced cell death in OCI-AML3
cells (14,28).
Caspase-8 and caspase-2 are initiator caspases that
are activated following various forms of genotoxic stress,
including that induced by radiation and cisplatin (29–32).
Caspase-8 plays an important role in transducing the apoptosis
signal to downstream death effectors, including CD95, TRAIL-DR4 or
TRAIL-DR5, which results in formation of the DISC (33). Caspase-2 possesses a caspase
recruitment domain (CARD) that enables it to interact with other
CARD carrying proteins such as TRAF-2 and PIDD (p53 induced protein
with a death domain), which eventually leads to activation of Bid
and mitochondrial injury (34,35).
ARC can interact with caspase-2 and caspase-8 and inhibit their
activation to protect against cell death.
In summary, the present study demonstrated that ARC
is expressed at high levels in NPC cell lines. Moreover, abundant
ARC was present in most NPC and severe atypical hyperplasia
tissues, but not in benign nasopharyngeal tissues. Furthermore, we
demonstrated the function of ARC in exerting X-radiation resistance
and cisplatin resistance by inhibiting apoptosis through blocking
the activation of caspase-2 and caspase-8.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (30672296).
References
|
1
|
Vaux DL and Strasser A: The molecular
biology of apoptosis. Proc Natl Acad Sci USA. 93:2239–2244. 1996.
View Article : Google Scholar
|
|
2
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nam YJ, Mani K, Ashton AW, et al:
Inhibition of both the extrinsic and intrinsic death pathways
through nonhomotypic death-fold interactions. Mol Cell. 15:901–912.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koseki T, Inohara N, Chen S, et al: ARC,
an inhibitor of apoptosis expressed in skeletal muscle and heart
that interacts selectively with caspases. Proc Natl Acad Sci USA.
95:5156–5160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gustafsson AB, Tsai JG, Logue SE, et al:
Apoptosis repressor with caspase recruitment domain protects
against cell death by interfering with Bax activation. J Biol Chem.
279:21233–21238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foo RS, Nam YJ, Ostreicher MJ, et al:
Regulation of p53 tetramerization and nuclear export by ARC. Proc
Natl Acad Sci USA. 104:20826–20831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ekhterae D, Lin Z, Lundberg MS, et al: ARC
inhibits cytochrome c release from mitochondria and protects
against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ
Res. 85:e70–e77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neuss M, Monticone R, Lundberg MS, et al:
The apoptotic regulatory protein ARC (apoptosis repressor with
caspase recruitment domain) prevents oxidant stress-mediated cell
death by preserving mitochondrial function. J Biol Chem.
276:33915–33922. 2001. View Article : Google Scholar
|
|
9
|
Donath S, Li P, Willenbockel C, et al:
Apoptosis repressor with caspase recruitment domain is required for
cardioprotection in response to biomechanical and ischemic stress.
Circulation. 113:1203–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu P, Zhang H, Qi L, et al: Identification
of ERp29 as a biomarker for predicting nasopharyngeal carcinoma
response to radiotherapy. Oncol Rep. 27:987–994. 2012.PubMed/NCBI
|
|
11
|
Qi L, Wu P, Zhang X, et al: Inhibiting
ERp29 expression enhances radiosensitivity in human nasopharyngeal
carcinoma cell lines. Med Oncol. 29:721–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geertman R, McMahon A and Sabban EL:
Cloning and characterization of cDNAs for novel proteins with
glutamic acid-proline dipeptide tandem repeats. Biochim Biophys
Acta. 1306:147–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Qanungo S, Crow MT, et al:
Apoptosis repressor with caspase recruitment domain (ARC) is
expressed in cancer cells and localizes to nuclei. FEBS Lett.
579:2411–2415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mercier I, Vuolo M, Madan R, et al: ARC,
an apoptosis suppressor limited to terminally differentiated cells,
is induced in human breast cancer and confers chemo- and
radiation-resistance. Cell Death Differ. 12:682–686. 2005.
View Article : Google Scholar
|
|
15
|
Mercier I, Vuolo M, Jasmin JF, et al: ARC
(apoptosis repressor with caspase recruitment domain) is a novel
marker of human colon cancer. Cell Cycle. 7:1640–1647. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarela AI, Macadam RC, Farmery SM, et al:
Expression of the antiapoptosis gene, survivin, predicts death from
recurrent colorectal carcinoma. Gut. 46:645–650. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krajewska M, Krajewski S, Banares S, et
al: Elevated expression of inhibitor of apoptosis proteins in
prostate cancer. Clin Cancer Res. 9:4914–4925. 2003.PubMed/NCBI
|
|
18
|
Tamm I, Kornblau SM, Segall H, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
19
|
Hanada M, Delia D, Aiello A, et al: bcl-2
gene hypomethylation and high-level expression in B-cell chronic
lymphocytic leukemia. Blood. 82:1820–1828. 1993.PubMed/NCBI
|
|
20
|
Olopade OI, Adeyanju MO, Safa AR, et al:
Overexpression of BCL-x protein in primary breast cancer is
associated with high tumor grade and nodal metastases. Cancer J Sci
Am. 3:230–237. 1997.PubMed/NCBI
|
|
21
|
Friess H, Lu Z, Andren-Sandberg A, et al:
Moderate activation of the apoptosis inhibitor bcl-Xl worsens the
prognosis in pancreatic cancer. Ann Surg. 228:780–787. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marone M, Scambia G, Mozzetti S, et al:
bcl-2, bax, bcl-XL and bcl-XS expression in normal and neoplastic
ovarian tissues. Clin Cancer Res. 4:517–524. 1998.PubMed/NCBI
|
|
23
|
Biroccio A, Benassi B, D’Agnano I, et al:
c-Myb and Bcl-x overexpression predicts poor prognosis in
colorectal cancer: clinical and experimental findings. Am J Pathol.
158:1289–1299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takayama T, Nagao M, Sawada H, et al:
Bcl-X expression in esophageal squamous cell carcinoma: association
with tumor progression and prognosis. J Surg Oncol. 78:116–123.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verma YK, Raghav PK, Raj HG, et al:
Enhanced heterodimerization of Bax by Bcl-2 mutants improves
irradiated cell survival. Apoptosis. 18:212–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang S, Benavente S, Armstrong EA, et al:
p53 modulates acquired resistance to EGFR inhibitors and radiation.
Cancer Res. 71:7071–7079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong JG, Costanzo A, Yang HQ, et al: The
tyrosine kinase c-Abl regulates p73 in apoptotic response to
cisplatin-induced DNA damage. Nature. 399:806–809. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carter BZ, Qiu YH, Zhang N, et al:
Expression of ARC (apoptosis repressor with caspase recruitment
domain), an antiapoptotic protein, is strongly prognostic in AML.
Blood. 117:780–787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen T, Chen M and Chen J: Ionizing
radiation potentiates dihydroartemisinin-induced apoptosis of A549
cells via a caspase-8-dependent pathway. PLoS One. 8:e598272013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paul I, Chacko AD, Stasik I, et al:
Acquired differential regulation of caspase-8 in
cisplatin-resistant non-small-cell lung cancer. Cell Death Dis.
e4492012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao X, Bennett RL and May WS: c-Myc and
caspase-2 are involved in activating Bax during cytotoxic
drug-induced apoptosis. J Biol Chem. 283:14490–14496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanoux V, Pairault C, Bakalska M, et al:
Caspase-2 involvement during ionizing radiation-induced oocyte
death in the mouse ovary. Cell Death Differ. 14:671–681. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cuenin S, Tinel A, Janssens S, et al:
p53-induced protein with a death domain (PIDD) isoforms
differentially activate nuclear factor-kappaB and caspase-2 in
response to genotoxic stress. Oncogene. 27:387–396. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lassus P, Opitz-Araya X and Lazebnik Y:
Requirement for caspase-2 in stress-induced apoptosis before
mitochondrial permeabilization. Science. 297:1352–1354. 2002.
View Article : Google Scholar : PubMed/NCBI
|