Introduction
Gastric cancer is the most common malignancy in Asia
and is the third leading cause of cancer-related deaths in Japan
(1–3). Endoscopic treatments, such as
endoscopic mucosal resection and endoscopic submucosal dissection
(ESD), have been recently performed in selected patients with early
gastric cancer. At present, the expanded ESD indication for
patients with early gastric cancer is under discussion (4). In regards to surgical procedures,
laparoscopic gastrectomy has been frequently carried out as
minimally invasive surgery in patients with early gastric cancer.
Furthermore, the 5-year survival rates of patients with mucosal and
submucosal gastric cancer are 95–100 and 85–95%, respectively
(5–7). Thus, these findings imply that there
is a spread of therapeutic options for the clinical management of
early gastric cancer patients with good outcome. On the other hand,
new anticancer agents for patients with unresectable advanced or
recurrent gastric cancer have been developed, and trastuzumab has
been focused on as a novel molecular-targeted drug for patients
with human epidermal growth factor receptor 2 (HER2)-positive
advanced gastric cancer (8).
Nevertheless, the prognosis of such patients is still poor even
when receiving novel chemotherapy. Therefore, it is important to
diagnose patients at an early stage of gastric cancer and to
identify post-operative patients at high risk for disease
recurrence in clinical management. Although tumor markers such as
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9) are currently used in the blood examination of patients
with gastric cancer, the identification of further molecular blood
markers is a prerequisite for the clinical management of patients
with gastric cancer.
Stanniocalcins (STCs) are glycoprotein hormones that
were originally identified in bony fish (9,10).
Moreover, several investigators have reported that STCs play an
important role in calcium and phosphate homeostasis (11–14).
STC2 is one member of the STC family, and recent findings have
demonstrated that it is a hypoxia-inducible factor 1 (HIF-1) target
gene that promotes cell proliferation, invasion and
epithelial-mesenchymal transition (EMT) in hypoxia (15,16).
STC2 is expressed at high levels in tumor cells of malignancies
such as neuroblastoma, esophageal, gastric, colorectal, ovarian,
prostate, breast and renal cell cancers (16–23).
The status of STC2 expression in these tumor cells of the primary
site is closely correlated with tumor progression, including
prognosis. However, the clinical significance of STC2 expression in
the blood of patients with gastric cancer has not yet been
determined.
The aim of the present study was to assess STC2
expression in blood specimens from patients with gastric cancer and
to investigate the relationship between STC2 expression and
clinicopathological factors including prognosis in patients with
gastric cancer.
Materials and methods
Gastric cancer cell lines
Four gastric cancer cell lines (MKN-7, MKN-45,
MKN-74 and KATO-III) were cultured in RPMI-1640 (Nissui
Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% fetal
calf serum (Mitsubishi Kasei, Tokyo, Japan), as well as 100
units/ml each of penicillin and streptomycin at 37°C in a
humidified atmosphere containing 5% CO2, as described
previously (24,25). These cell lines were used for
reverse transcription-polymerase chain reaction (RT-PCR) assay.
Patients
Blood specimens were obtained preoperatively from 93
patients (64 men and 29 women; age range, 35–87 years; average age,
68 years) with gastric cancer who underwent curative gastrectomy
with lymph node dissection at Kagoshima University Hospital
(Kagoshima, Japan) between 2003 and 2005. None of the patients had
received endoscopic mucosal resection, palliative resection,
preoperative chemotherapy, and/or radiotherapy in the present
study. Furthermore, patients who had synchronous or metachronous
cancer in other organs were excluded. Patients were classified and
staged on the basis of criteria of the tumor-node-metastasis (TNM)
classification of gastric carcinoma established by the
International Union Against Cancer (UICC) (26). Normal peripheral blood lymphocytes
(PBLs) isolated from 22 healthy volunteers were used as a control
group. After discharge, all patients were followed up every 3–6
months by blood tumor marker studies (CEA and CA19-9), radiography,
ultrasonography and computed tomography at Kagoshima University
Hospital. The median follow-up period after surgery was 25 months
(range, 1–74 months).
To investigate STC2 protein expression in gastric
cancer, 30 paraffin-embedded archival tissue (PEAT) specimens of
resected primary gastric tumors from patients enrolled in this
study were used for immunohistochemical analysis.
All specimens were collected from patients after
informed consent was obtained in accordance with the institutional
guidelines of our hospital.
Blood processing and RNA extraction for
RT-PCR analysis
Blood specimens (5 ml) from each patient were
preoperatively collected in tubes containing sodium citrate, and
then blood cells were separated in lymphocyte separation buffer
(Gentra Systems, Inc., Minneapolis, MN, USA). Total RNA was
extracted from the cell lines and blood specimens using Isogen
(Nippon Gene, Toyama, Japan). Total RNA was isolated and purified
using phenol-chloroform extraction as previously described
(24,25). The concentration and purity of the
total RNA were determined using a GeneQuant Pro UV/Vis
spectrophotometer (Amersham Pharmacia Biotech, Cambridge, UK).
Primers and probes
Primer and probe sequences of STC2 and
glyceraldehyde-3-phosphatase dehydrogenase (GAPDH) were designed
for RT-PCR assays of each marker. The forward primers, fluorescence
resonance energy transfer probe sequence, and reverse primers for
STC2 and GAPDH were as follows: STC2 forward,
5′-GACTTGCTGCTGCACGAAC-3′; probe,
5′-FAM-ACGTGGACCTCGTGAACTTGCTG-TAM RA-1-3′; reverse,
5′-TGCTCACACTGAACCTGCAC-3′ and GAPDH forward,
5′-GGGTGTGAACCATGAGAAGT-3′; probe,
5′-FAM-CAGCAATGCCTCCTGCACCACCAA-TA MRA-1-3′; reverse,
5′-GACTGTGGTCATGAGTCCT-3′. The RT-PCR products for STC2 and GAPDH
were resolved as 107- and 136- base pair fragments, respectively.
The integrity of the RNA was confirmed by RT-PCR assay using
GAPDH.
Quantitative RT-PCR assay
All total RNA samples were reverse-transcribed using
the Advantage RT-for-PCR kit (Clontech Laboratories, Inc., Palo
Alto, CA, USA) as previously described (24,25).
Quantitative RT-PCR (qRT-PCR) proceeded using the LightCycler
System (Roche Diagnostics, Mannheim, Germany). The reaction
mixtures contained cDNA transcribed from 250 ng of RNA using each
primer, probe, MgCl2, and LightCycler FastStart DNA
Master hybridization probes (Roche Diagnostics). The amplification
profile comprised precycling at 95°C for 10 min followed by 40
cycles of denaturation at 95°C for 10 sec, annealing for 20 sec
(60°C for STC2, and 55°C for GAPDH), and extension at 72°C for 10
sec. Plasmids for each marker were synthesized using pT7Blue-2
T-Vector (Novagen, Madison, WI, USA) according to the
manufacturer's instructions. Standard curves for each assay were
generated using the threshold cycles of 6 serial dilutions of
plasmid templates (106–101 copies). The mRNA
copy number was determined using LightCycler software (Roche
Diagnostics). Each assay was repeated in duplicate with positive
(cell line), negative (H2O), and reagent (without cDNA)
controls to evaluate the quality of the qRT-PCR assay. Absolute
copy numbers in qRT-PCR assays were computed on the basis of
standard curves of plasmid templates. Copy numbers of STC2 mRNA
were normalized by those of GAPDH mRNA (relative STC2 mRNA copies;
absolute STC2 mRNA copies/absolute GAPDH mRNA copies).
Cell spiking study for determining the
sensitivity of the RT-PCR assay
Serial dilutions (104, 103,
102, 101 and 0) of MKN-74 tumor cells mixed
with 1×107 PBLs, which were isolated from a healthy
volunteer without STC2 mRNA expression, were used for determining
the sensitivity of the qRT-PCR analysis. This in vitro assay
was repeated 3 times to verify its reproducibility.
Immunohistochemical staining
The PEAT sections (3 μm) of surgical primary gastric
tumors were incubated on slides at 50°C overnight, deparaffinized
with xylene and then rehydrated with a graded series of ethanol.
The sections were autoclaved in citrate buffer (0.01 mol/l, pH 6.0)
at 120°C for 10 min to activate the antigen. After cooling at room
temperature, endogenous peroxidase was blocked using a peroxidase
blocking reagent (DakoCytomation, Carpinteria, CA, USA) for 10 min.
Non-specific binding was blocked at room temperature for 30 min
with Protein Block Serum-Free (DakoCytomation). The sections were
incubated at room temperature for 60 min with an anti-human STC2
polyclonal antibody (Proteintech Group, Inc., Chicago, IL, USA)
diluted 1:200 in Dako antibody diluent with background reducing
components (DakoCytomation). After three 5-min washes in
phosphate-buffered saline (PBS), the reaction for STC2 was
developed by the ABC method (Vectastain ABC kit; Vector
Laboratories) and visualized using diaminobenzidine
tetrahydrochloride (DakoCytomation). Negative controls were treated
with PBS without the primary antibody under the same conditions. On
the basis of immunostainable intensity, STC2 immunoreactivity was
classified into 3 groups: negative, weak, and strong
immunoreactions.
Statistical analysis
Differences in STC2 mRNA expression between gastric
cancer cell lines and PBLs from healthy volunteers, and between
PBLs from patients with gastric cancer and healthy volunteers, were
evaluated by the Wilcoxon rank-sum test. The relationship between
the status of STC2 expression and categorical clinicopathological
factors was assessed by the Chi-square and Fisher's exact tests.
Survival curves were generated using the Kaplan-Meier method, and
differences in survival were examined using the log-rank test.
Prognostic factors were assessed by univariate and multivariate
analyses (Cox proportional hazard regression model). All
statistical calculations were performed using SAS statistical
software (SAS Institute Inc., Cary, NC, USA). A P-value of <0.05
was considered to indicate a statistically significant result.
Results
STC2 mRNA expression determined by RT-PCR
in cell lines and clinical blood specimens
STC2 mRNA expression in 4 gastric cancer cell lines,
blood specimens from 93 patients with gastric cancer, and from 22
healthy volunteers without cancers was assessed by qRT-PCR.
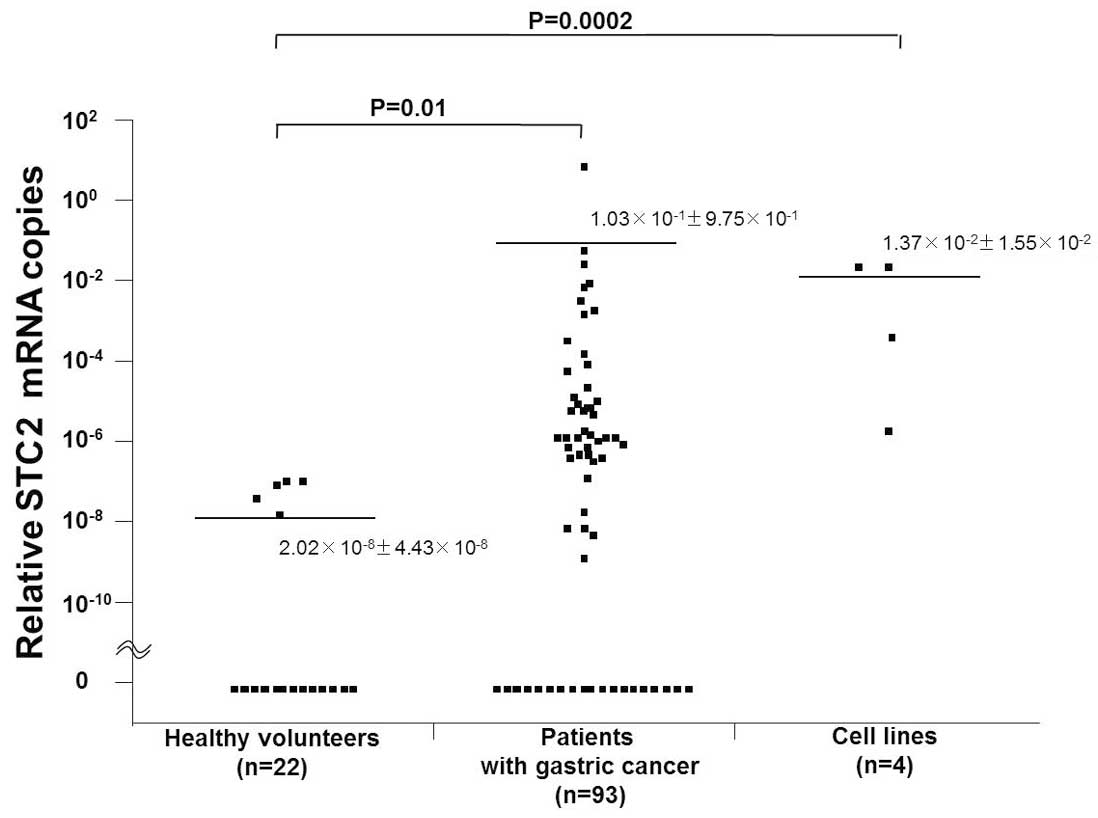
The range of relative STC2 mRNA copies was
2.49×10−6 to 2.75×10−2 in gastric cancer cell
lines, 0 to 9.4 in blood from patients, and 0 to
1.35×10−7 in normal PBLs from healthy volunteers. The
mean relative numbers of STC2 mRNA copies (± SD) were
1.37×10−2±1.55×10−2 in gastric cancer cell
lines, 1.03×10−1±9.75×10−1 in blood from
patients, and 2.02×10−8±4.43×10−8 in normal
PBLs (Fig. 1). Accordingly, the
relative numbers of STC2 mRNA copies were significantly higher in
the gastric cancer cell lines and in blood from patients than in
normal PBLs (P=0.0002 and P=0.01, respectively). STC2 mRNA
expression was identified in 43 (46.2%) of the 93 patients with
gastric cancer.
STC2 mRNA expression in the cell spiking
study
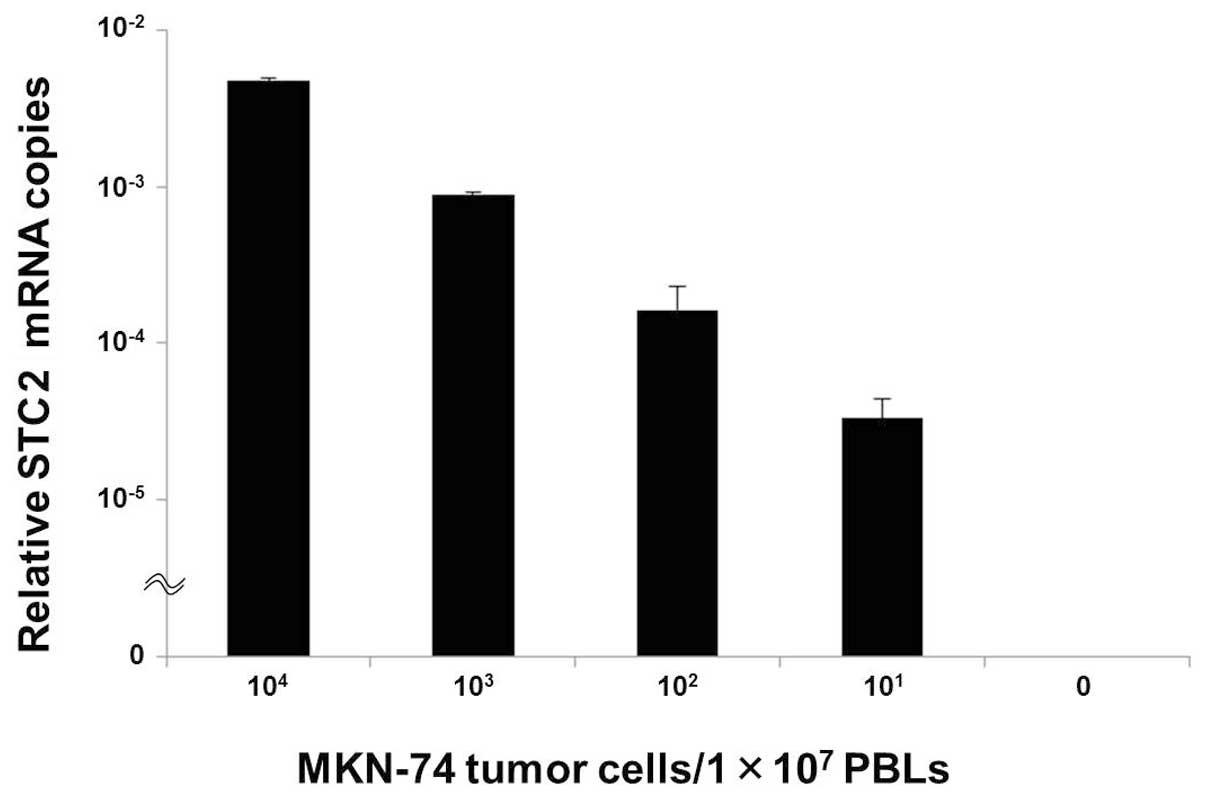
This spiking study was planned to investigate the
relationship between STC2 mRNA expression and the number of tumor
cells in an in vitro assay.
STC2 expression was identified in MKN-74 cells at a
density of 101 tumor cells/1×107 PBLs, and
the relative numbers of STC2 mRNA copies gradually decreased as the
numbers of tumor cells within normal PBLs decreased (Fig. 2).
Relationship between STC2 expression and
the clinicopathological features of the gastric cancer
patients
All patients were classified into 2 groups based on
the status of STC2 expression (positive, n=43; negative, n=50) to
evaluate the relationship between STC2 expression and
clinicopathological features.
STC2 expression was significantly correlated with
age, depth of tumor invasion, lymph node metastasis, stage and
venous invasion (P=0.023, P=0.045, P=0.035, P=0.007 and P=0.027,
respectively; Table I).
 | Table IRelationship between STC2 expression
and clinicopathological features in patients with gastric
cancer. |
Table I
Relationship between STC2 expression
and clinicopathological features in patients with gastric
cancer.
| STC2 expression, n
(%) | |
|---|
|
| |
|---|
| Features | Negative (n=50) | Positive (n=43) | P-value |
|---|
| Gender |
| Male | 35 (70.0) | 29 (67.4) | 0.825 |
| Female | 15 (30.0) | 14 (32.6) | |
| Age (years) |
| ≤70 | 31 (62.0) | 16 (37.2) | 0.023 |
| >70 | 19 (38.0) | 27 (62.8) | |
| Tumor location |
| Upper | 15 (30.0) | 16 (37.2) | 0.540 |
| Middle | 18 (36.0) | 11 (25.6) | |
| Lower | 17 (34.0) | 16 (37.2) | |
| Histological
type |
|
Differentiated | 27 (54.0) | 19 (44.2) | 0.408 |
|
Undifferentiated | 23 (46.0) | 24 (55.8) | |
| Depth of tumor
invasion |
| pT1–T2 | 21 (42.0) | 9 (20.9) | 0.045 |
| pT3–T4 | 29 (58.0) | 34 (79.1) | |
| Lymph node
metastasis |
| Negative | 25 (50.0) | 12 (27.9) | 0.035 |
| Positive | 25 (50.0) | 31 (72.1) | |
| Distant
metastasis |
| Negative | 43 (86.0) | 36 (83.7) | 0.779 |
| Positive | 7 (14.0) | 7 (16.3) | |
| Stage |
| I–II | 32 (64.0) | 15 (34.9) | 0.007 |
| III–IV | 18 (36.0) | 28 (65.1) | |
| Lymphatic
invasion |
| Negative | 20 (40.0) | 10 (23.3) | 0.119 |
| Positive | 30 (60.0) | 33 (76.7) | |
| Venous
invasion |
| Negative | 22 (44.0) | 9 (20.9) | 0.027 |
| Positive | 28 (56.0) | 34 (79.1) | |
Relationship between STC2 expression and
prognosis
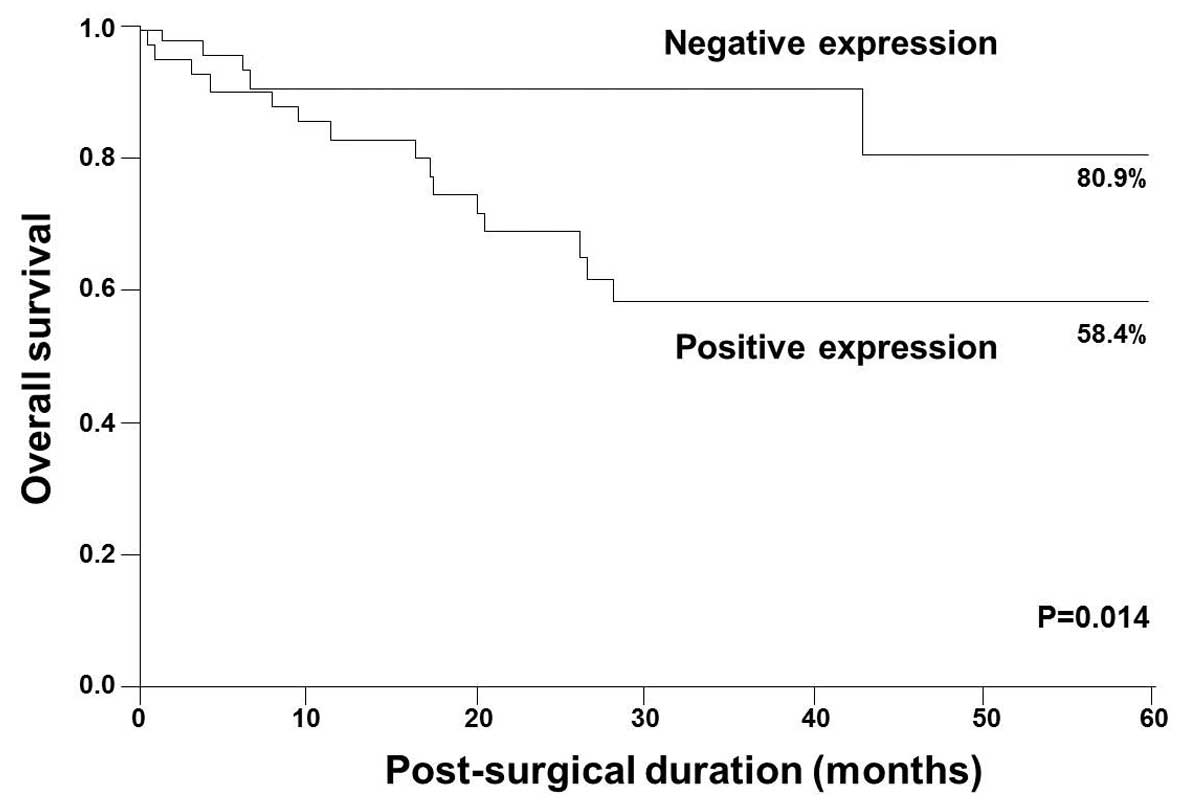
The 5-year survival rates were 58.4 and 80.9% in
patients with STC2-positive and -negative expression, respectively
(Fig. 3). Five-year survival rates
were significantly lower in patients with STC2-positive expression
than in those with STC2-negative expression (P=0.014; Fig. 3).
Univariate analysis demonstrated that age, depth of
tumor invasion, lymph node metastasis, distant metastasis,
lymphatic invasion, venous invasion and STC2 expression were
significantly correlated with postoperative survival (P=0.020,
P=0.003, P=0.002, P=0.046, P=0.030, P=0.041 and P=0.012,
respectively; Table II).
Multivariate analysis demonstrated that age, lymph node metastasis
and distant metastasis were independent prognostic factors
(P=0.002, P=0.024 and P=0.047, respectively; Table II). Consequently, STC2 expression
was not an independent prognostic factor in the multivariate
analysis (P=0.433).
 | Table IIUnivariate and multivariate analyses
of survival in patients with gastric cancer. |
Table II
Univariate and multivariate analyses
of survival in patients with gastric cancer.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Independent
factor | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (years) |
| ≤70/>70 | 1.719 | 1.088–2.890 | 0.020 | 2.272 | 1.342–4.110 | 0.002 |
| Depth of tumor
invasion |
| pT1–T2/pT3–T4 | 2.417 | 1.300–6.066 | 0.003 | 2.281 | 0.920–7.145 | 0.080 |
| Lymph node
metastasis |
|
Negative/positive | 2.257 | 1.306–4.674 | 0.002 | 2.812 | 1.126–9.038 | 0.024 |
| Distant
metastasis |
|
Negative/positive | 1.700 | 1.010–2.690 | 0.046 | 1.813 | 1.008–3.186 | 0.047 |
| Lymphatic
invasion |
|
Negative/positive | 1.821 | 1.055–3.771 | 0.030 | 0.851 | 0.429–2.035 | 0.687 |
| Venous
invasion |
|
Negative/positive | 1.763 | 1.021–3.650 | 0.041 | 0.491 | 0.182–1.486 | 0.200 |
| STC2
expression |
|
Negative/positive | 1.820 | 1.132–3.190 | 0.012 | 1.234 | 0.740–2.225 | 0.433 |
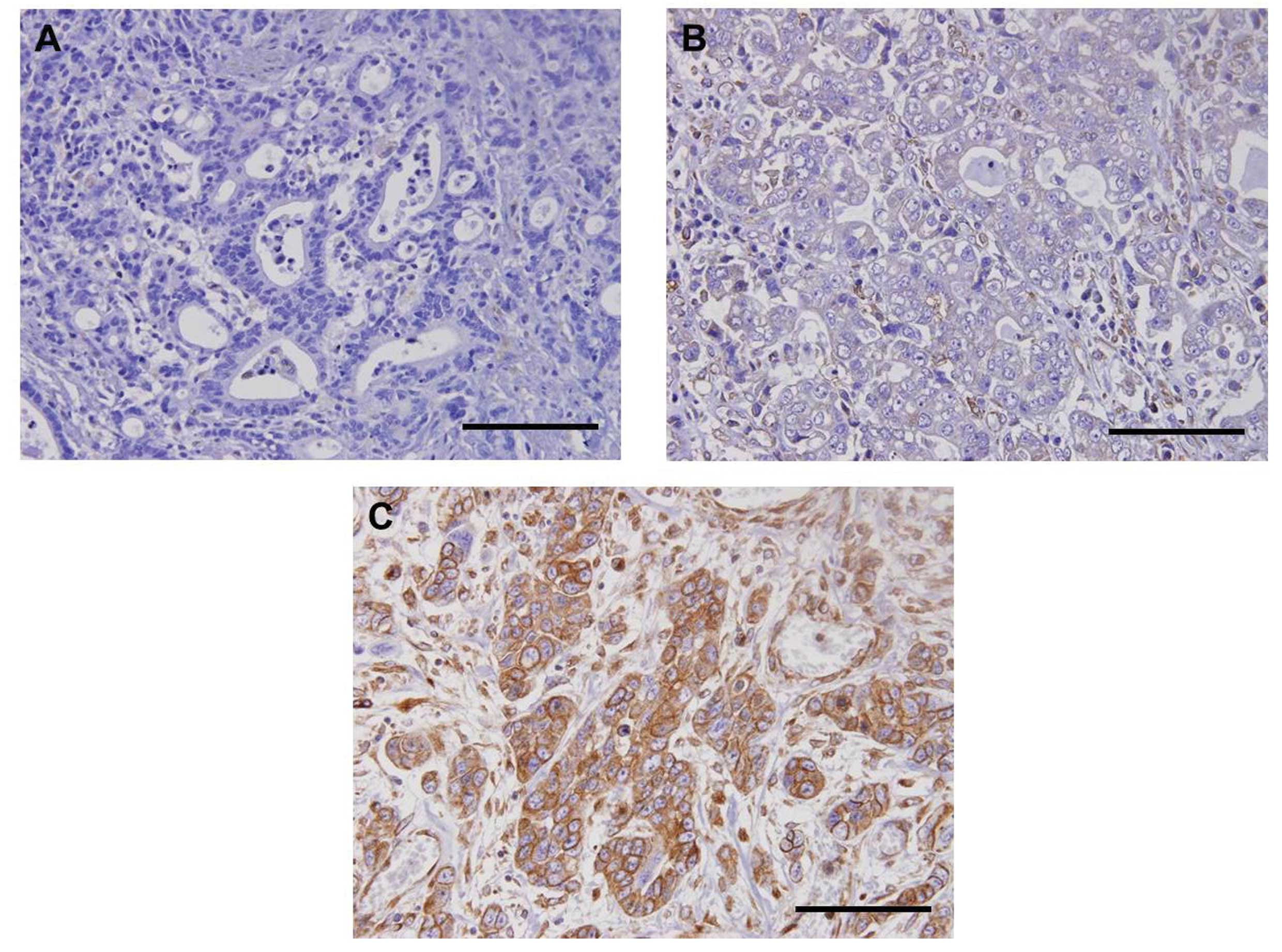
STC2 protein expression as determined by
immunohistochemistry in primary tumor specimens
To confirm STC2 expression in primary tumor sites,
immunohistochemical evaluation was carried out on 30 surgical PEAT
specimens of primary gastric tumors. Immunohistochemical analysis
showed that STC2 protein expression was identified in the membrane
and/or cytoplasm of gastric tumor cells (Fig. 4). Furthermore, primary gastric
tumors had various immunoreactions for STC2 (Fig. 4A–C). Finally, strong immunoreaction
was identified in 46.7% (7/15) and 26.7% (4/15) of patients with
STC2-positive and -negative mRNA expression, respectively.
Discussion
In the present study, we investigated STC2 mRNA
expression in blood from patients with gastric cancer using qRT-PCR
assay. Furthermore, we also compared the relationship between the
status of STC2 expression and clinicopathological findings to
assess the clinical impact of STC2 expression as a useful blood
marker in patients with gastric cancer. To our knowledge, this is
the first study regarding STC2 expression in circulating tumor
cells (CTCs) obtained from patients with gastric cancer.
To date, STC2 expression has been identified in
various malignant diseases (16–23).
In the present study, STC2 protein expression in gastric tumor
cells was verified by immunohistochemical analysis. Furthermore, we
confirmed STC2 mRNA expression in all gastric cancer cell lines and
demonstrated STC2 mRNA high expression in blood from patients with
gastric cancer. Although STC2 mRNA expression was detected in a few
blood specimens from healthy volunteers, the level of STC2 mRNA
expression in these specimens was extremely low in comparison with
the level in blood specimens from gastric cancer patients. These
results indicate the clinical utility of RT-PCR assay against the
STC2 molecule for discriminating gastric cancer patients from
healthy volunteers.
Previous studies have demonstrated the clinical
efficacy of CTC detection for predicting the potential for tumor
progression and the response to chemotherapy in patients with
various types of malignancies (27–30).
Furthermore, a CTC assay system using immunomagnetics has been
developed as a promising novel tool in recent years (31–33).
In gastric cancer, epithelial markers such as cytokeratin and CEA
are usually used for CTC detection in RT-PCR assay (28). Miyazono et al(28) reported that the CEA mRNA-positive
rate in patients with gastric cancer was 36.8%, and CEA mRNA
positivity was significantly correlated with the depth of tumor
invasion and recurrence. In the present study, we showed the
intensive relationship between the status of STC2 expression and
tumor progression, such as depth of tumor invasion, lymph node
metastasis, stage and venous invasion. Moreover, we verified the
positive correlation between STC2 mRNA copies and the number of
gastric tumor cells in a cell spiking study. At present, potential
blood markers for monitoring CTCs are limited in the clinical
management of patients with gastric cancer. Consequently, STC2
could be one of the surrogate markers for predicting tumor
progression in patients with gastric cancer.
Although the functional role of STC2 remains
unclear, several investigators have demonstrated the positive
effects of STC2 induced by hypoxia in an in vitro study
(15,16). Law and Wong (16) reported that STC2 overexpression
enhanced the process of EMT via an increase in N-cadherin and
vimentin and a reduction in E-cadherin in hypoxic human ovarian
cancer cells. Additionally, a potentially relevant association was
found between STC2 expression and matrix metalloproteases involved
in tumor invasion in hypoxia (16).
In the present study, STC2 overexpression was significantly
correlated with tumor aggressiveness and poorer prognosis. These
results suggest that STC2 is a promising marker for new targeted
therapies that suppress tumor progression in patients with gastric
cancer.
In conclusion, we demonstrated that STC2 is
overexpressed in blood from patients with gastric cancer and that
its expression is positively associated with malignant behavior.
Therefore, STC2 is available for predicting tumor progression by
monitoring CTCs in patients with gastric cancer. Further
understanding of its functional role would allow the development of
an attractive treatment strategy for patients with gastric
cancer.
Acknowledgements
We thank Ms. Y. Nishizono and Ms. M. Motomura for
their technical assistance. This study was supported in part by a
Grant-in-Aid (no. 25461955) for Scientific Research from the
Ministry of Education, Science, Sports and Culture, Japan.
References
|
1
|
Statistics and Information Department,
Ministry of Health, Labour, and Welfare. Vital Statistics of Japan
2004. Tokyo: Health and Welfare Statistics Association; 2006
|
|
2
|
Katanoda K and Yako-Suketomo H: Comparison
of time trends in stomach cancer incidence (1973–2002) in Asia,
from Cancer Incidence in Five Continents, Vols IV–IX. Jpn J Clin
Oncol. 39:71–72. 2009.
|
|
3
|
Li ZX and Kaminishi M: A comparison of
gastric cancer between Japan and China. Gastric Cancer. 12:52–53.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee H, Yun WK, Min BH, et al: A
feasibility study on the expanded indication for endoscopic
submucosal dissection of early gastric cancer. Surg Endosc.
25:1985–1993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maehara Y, Orita H, Okuyama T, Moriguchi
S, Tsujitani S, Korenaga D and Sugimachi K: Predictors of lymph
node metastasis in early gastric cancer. Br J Surg. 79:245–247.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai J, Ikeguchi M, Maeta M and Kaibara N:
Micrometastasis in lymph nodes and microinvasion of the muscularis
propria in primary lesions of submucosal gastric cancer. Surgery.
127:32–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DY, Joo JK, Ryu SY, Kim YJ and Kim SK:
Factors related to lymph node metastasis and surgical strategy used
to treat early gastric carcinoma. World J Gastroenterol.
10:737–740. 2004.PubMed/NCBI
|
|
8
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
9
|
Chang AC, Janosi J, Hulsbeek M, de Jong D,
Jeffrey KJ, Noble JR and Reddel RR: A novel human cDNA highly
homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol.
112:241–247. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang AC, Jeffrey KJ, Tokutake Y, et al:
Human stanniocalcin (STC): genomic structure, chromosomal
localization, and the presence of CAG trinucleotide repeats.
Genomics. 47:393–398. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wagner GF, Jaworski EM and Haddad M:
Stanniocalcin in the seawater salmon: structure, function, and
regulation. Am J Physiol. 274:R1177–R1185. 1998.PubMed/NCBI
|
|
12
|
Chang AC and Reddel RR: Identification of
a second stanniocalcin cDNA in mouse and human: stanniocalcin 2.
Mol Cell Endocrinol. 141:95–99. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishibashi K and Imai M: Prospect of a
stanniocalcin endocrine/paracrine system in mammals. Am J Physiol
Renal Physiol. 282:F367–F375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang AC, Jellinek DA and Reddel RR:
Mammalian stanniocalcins and cancer. Endocr Relat Cancer.
10:359–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Law AY and Wong CK: Stanniocalcin-2 is a
HIF-1 target gene that promotes cell proliferation in hypoxia. Exp
Cell Res. 316:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Law AY and Wong CK: Stanniocalcin-2
promotes epithelial-mesenchymal transition and invasiveness in
hypoxic human ovarian cancer cells. Exp Cell Res. 316:3425–3434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouras T, Southey MC, Chang AC, et al:
Stanniocalcin 2 is an estrogen-responsive gene coexpressed
with the estrogen receptor in human breast cancer. Cancer Res.
62:1289–1295. 2002.
|
|
18
|
Meyer HA, Tölle A, Jung M, et al:
Identification of stanniocalcin 2 as prognostic marker in renal
cell carcinoma. Eur Urol. 55:669–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamura K, Furihata M, Chung SY, et al:
Stanniocalcin 2 overexpression in castration-resistant prostate
cancer and aggressive prostate cancer. Cancer Sci. 100:914–919.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ieta K, Tanaka F, Yokobori T, et al:
Clinicopathological significance of stanniocalcin 2 gene
expression in colorectal cancer. Int J Cancer. 125:926–931.
2009.
|
|
21
|
Volland S, Kugler W, Schweigerer L,
Wilting J and Becker J: Stanniocalcin 2 promotes invasion and is
associated with metastatic stages in neuroblastoma. Int J Cancer.
125:2049–2057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokobori T, Mimori K, Ishii H, et al:
Clinical significance of stanniocalcin 2 as a prognostic
marker in gastric cancer. Ann Surg Oncol. 17:2601–2607. 2010.
|
|
23
|
Kita Y, Mimori K, Iwatsuki M, et al:
STC2: a predictive marker for lymph node metastasis in
esophageal squamous-cell carcinoma. Ann Surg Oncol. 18:261–272.
2011. View Article : Google Scholar
|
|
24
|
Arigami T, Natsugoe S, Uenosono Y, et al:
Lymphatic invasion using D2–40 monoclonal antibody and its
relationship to lymph node micrometastasis in pN0 gastric cancer.
Br J Cancer. 93:688–693. 2005.
|
|
25
|
Arigami T, Natsugoe S, Uenosono Y, et al:
Evaluation of sentinel node concept in gastric cancer based on
lymph node micrometastasis determined by reverse
transcription-polymerase chain reaction. Ann Surg. 243:341–347.
2006. View Article : Google Scholar
|
|
26
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on Cancer (AJCC).
Cancer Staging Manual. 7th edition. Springer; New York, NY: pp.
6492010
|
|
27
|
Hoon DS, Bostick P, Kuo C, Okamoto T, Wang
HJ, Elashoff R and Morton DL: Molecular markers in blood as
surrogate prognostic indicators of melanoma recurrence. Cancer Res.
60:2253–2257. 2000.PubMed/NCBI
|
|
28
|
Miyazono F, Natsugoe S, Takao S, et al:
Surgical maneuvers enhance molecular detection of circulating tumor
cells during gastric cancer surgery. Ann Surg. 233:189–194. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uen YH, Lin SR, Wu DC, et al: Prognostic
significance of multiple molecular markers for patients with stage
II colorectal cancer undergoing curative resection. Ann Surg.
246:1040–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ignatiadis M, Kallergi G, Ntoulia M, et
al: Prognostic value of the molecular detection of circulating
tumor cells using a multimarker reverse transcription-PCR assay for
cytokeratin 19, mammaglobin A, and HER2 in early breast cancer.
Clin Cancer Res. 14:2593–2600. 2008. View Article : Google Scholar
|
|
31
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen SJ, Punt CJ, Iannotti N, et al:
Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krebs MG, Sloane R, Priest L, et al:
Evaluation and prognostic significance of circulating tumor cells
in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|