Introduction
Gastric cancer (GC) is one of the most common and
lethal malignancies in Japanese and East Asian populations, and the
second most common cause of cancer-related death in the world
(1). Although the incidence and
mortality rate of GC located outside the cardia have decreased over
the last few decades, a considerable percentage of patients still
have advanced disease at diagnosis. Helicobacter pylori
(H. pylori) infection is now accepted as a crucial event in
the development of atrophic gastritis and is implicated in the
development of gastric carcinoma, particularly those not located in
the cardia (2–4). However, there is marked variation in
the extent of gastric inflammation among H. pylori-infected
patients, and only a small percentage of them actually develop GC.
That is, the occurrence and development of GC is a process
involving genetic and environmental factors, for example H.
pylori infection and other environmental factors. This suggests
that genetic factors play an important role in the long-term
outcome of H. pylori infection (5–9).
Lipopolysaccharide (LPS), which is a component of
the outer membrane of Gram-negative bacteria including H.
pylori, is a signaling molecule for the innate immune system
and is one of the main sources of inflammation (10). LPS binding to TLR4 activates signal
transduction through MyD88, IRAK and TRAF6 to activate the nuclear
factor (NF)-κB (11). Activation of
NF-κB by H. pylori induces nuclear translocation, which
causes an increase in IL-8 messenger RNA and protein levels
(12). In addition, the NF-κB
pathway is responsible for the generation of several cell adhesion
molecules including ICAM-1 (13).
Thus, H. pylori is a potent activator of NF-κB in gastric
epithelial cells, and NF-κB is a major molecule in H.
pylori-induced inflammation (14). On the other hand, NF-κB activation
is known to regulate cellular growth responses, including
apoptosis, and is required for the induction of inflammatory and
tissue-repair genes (15). These
facts suggest that NF-κB plays an important role in
inflammation-associated carcinogenesis. NFKB1 which is a
gene encoding 2 subunits (p50 and p105) of NF-κB is located on 4q24
(16). Recently, many studies have
reported the association between polymorphism rs28362491 (−94
ins/del ATTG of NFKB1) and cancer risk in various organs
(17). However, these results do
not always lead to the same conclusions, and there has been no
report concerning the risk of this polymorphism in the development
of GC in Japan. Furthermore, certain genetic variation in
rs72696119 (−449 C>G in 5′-UTR of NFKB1) has been
identified. We previously reported a closely association between
NFKB1 polymorphisms (rs28362491 and rs72696119) and aberrant
gene methylation in gastric mucosa, which is considered to be a
pre-malignant condition (18).
In the present study, we attempted to clarify the
association between −94 ins/del ATTG polymorphism (rs28362491) of
NFKB1 and GC development in Japanese subjects. In addition,
the −449 C>G polymorphism (rs72696119) in 5′-UTR of NFKB1
was also investigated.
Materials and methods
Clinical samples
Our gastric cancer group included 479 patients (GC
cases) enrolled at the Endoscopy Center of Fujita Health University
Hospital or Kanazawa Medical University Hospital between July 2006
and August 2012. As a control group, 880 subjects without a
malignant neoplasm, confirmed endoscopically and histologically,
were selected at random from our DNA biobank, collected over the
same period as that defined above (controls). Our final study
cohort comprised 1,359 subjects for whom polymorphisms could be
clearly analyzed.
All subjects underwent upper gastrointestinal
endoscopy, and patients with severe systemic diseases, malignancies
in other organs, and who had previously received non-steroidal
anti-inflammatory drugs, antibiotics, and H. pylori
eradication treatment were excluded. H. pylori infection
status was assessed by serology, histological examination, or the
urea breath test. Patients were diagnosed as having infection when
at least one of the diagnostic tests was positive.
The Ethics Committees of Fujita Health University
and Kanazawa Medical University approved the protocol, and written
informed consent was obtained from all of the participating
subjects.
Histological evaluation
In 778 of the 1,359 subjects (592 controls and 186
GC cases), the severity of chronic gastritis in non-cancerous
mucosa was classified according to the updated Sydney system
(19) by a pathologist who had no
access to any clinical information.
Genotyping of polymorphisms
DNA was isolated from biopsy specimens or peripheral
blood and genotyped using the PCR-SSCP method as reported
previously (18,20). We had previously confirmed that each
genotype was clearly determined by this method. To detect
NFKB1 rs28362491 (−94 ins/del ATTG) using the primer pairs
(94F, 5′-gctatggaccgcatgactctatcag-3′ and 94R,
5′-ggggctctggcttcctagcag-3′), PCR was carried out in a volume of 20
μl containing 0.1 μg of genomic DNA. The DNA was denatured at 95°C
for 3 min, followed by 35 cycles at 96°C for 15 sec, 58°C for 40
sec, and 72°C for 30 sec, with final extension at 72°C for 5 min.
Thereafter, 2 μl of the PCR product was denatured with 10 μl of
formamide (Sigma-Aldrich Co., St. Louis, MO, USA) at 90°C for 5
min. SSCP was carried out at 6°C using a GenePhor DNA separation
system with GeneGel Excel 12.5/24 (Amersham Biosciences Corp.,
USA), after which the denatured single-strand DNA bands were
detected using a DNA Silver Staining kit (Amersham Biosciences
Corp.).
To detect NFKB1 −449 C>G, using the primer
pairs (449F, 5′-cgtgtgtccgtctgtctgtatgctc-3′ and 449R,
5′-cgctggtgcacttctctctctttct-3′), PCR was carried out in a volume
of 20 μl containing 0.1 μg of genomic DNA. The DNA was denatured at
95°C for 3 min, followed by 35 cycles at 95°C for 30 sec, 57°C for
40 sec, and 72°C for 45 sec, with a final extension at 72°C for 5
min. Thereafter, SSCP was carried out in the same manner as
described above.
Statistical analysis
Data are expressed as means ± SD. Mean ages between
GC cases and controls were compared using the Student’s t-test. The
ratios of male/female and H. pylori infection were compared
between 2 groups using a 2×2 table and the Fisher’s exact test.
Allele and genotype frequencies were calculated by direct counting.
The allele counts and genotype distribution were also compared by
the Fisher’s exact test. Furthermore, the strength of association
between genotype frequencies and the disease was assessed by
calculating the odds ratio (OR) at 95% confidence intervals (CI).
Adjusted ORs were calculated with the use of logistical
multivariate regression analysis. The association of genotypes with
the progression of gastric cancer was assessed by ANCOVA using the
number of alleles as a covariate. Each updated Sydney system was
compared using Mann-Whitney U test. For all analyses, the level of
significance was set at p<0.05.
Results
Subject characteristics and genotype
frequencies
In the controls, rs72696119 was in Hardy-Weinberg
equilibrium (p=0.091), whereas rs28362491 was not (p=0.0495). The
mean age, male/female ratio and frequency of H. pylori
positivity of the controls were significantly lower than these
value in the GC cases (Table I).
The minor allele frequency and genotype distribution were not
significantly different between the controls and GC cases.
 | Table ISubject characteristics and genotype
frequencies. |
Table I
Subject characteristics and genotype
frequencies.
|
Characteristics | Controls | GC cases | p-value |
|---|
| No. of
subjects | 880 | 479 | |
| Mean age ± SD | 61.4±13.5 | 65.2±11.6 | <0.0001a |
| Male:female | 506:373 | 336:143 | <0.0001b |
| HP-positive
rate | 61.8% | 86.0% | <0.0001b |
| NFKB1
rs28362491 |
| ins/ins | 342 | 172 | |
| ins/del | 435 | 239 | |
| del/del | 103 | 68 | |
| del. allele
frequency | 36.4% | 39.1% | NS |
| NFKB1
rs72696119 |
| CC | 352 | 189 | |
| CG | 428 | 226 | |
| GG | 100 | 64 | |
| G allele
frequency | 35.7% | 37.0% | NS |
Association between gene polymorphisms
and gastric carcinogenesis
The rs28362491 del/del homozygote was significantly
but weakly associated with susceptibility to gastric carcinogenesis
(OR, 1.43; 95% CI, 1.01–2.02; p=0.045). This homozygote was
strongly associated with diffuse type of GC (OR, 1.85; 95% CI,
1.21–2.84; p=0.0049), whereas it was not significantly associated
with intestinal type of GC (Table
II). The rs72696119 GG homozygote appeared to be associated
with gastric carcinogenesis (p=0.089). This homozygote was also
strongly associated with diffuse type of GC (OR, 1.81; 95% CI,
1.17–2.78; p=0.0073).
 | Table IIAssociation between genetic
polymorphisms and gastric cancer. |
Table II
Association between genetic
polymorphisms and gastric cancer.
| NFKB1
rs28362491 | ins/ins | ins/del | del/del | del/del vs. others;
OR (95% CI) | p-value |
|---|
| Controls
(880)a | 342 | 435 | 103 | Reference | |
| Overall GC cases
(479)b | 172 | 239 | 68 | 1.43
(1.01–2.02) | 0.045 |
| Intestinal
(283) | 101 | 150 | 32 | 1.14
(0.724–1.78) | 0.58 |
| Diffuse (191) | 69 | 86 | 36 | 1.85
(1.21–2.84) | 0.0049 |
|
| NFKB1
rs72696119 | CC | CG | GG | GG vs. others; OR
(95% CI) | p-value |
|
| Controls (880) | 352 | 428 | 100 | Reference | |
| Overall GC cases
(479)b | 189 | 226 | 64 | 1.36
(0.955–1.94) | 0.089 |
| Intestinal
(283) | 116 | 138 | 29 | 1.05
(0.656–1.67) | 0.85 |
| Diffuse (191) | 71 | 85 | 35 | 1.81
(1.17–2.78) | 0.0073 |
In subjects younger than or 60 years of age, both
rs28362491 del/del and rs72696119 GG homozygotes conferred an
increased risk for the development of gastric cancer (OR, 2.24; 95%
CI, 1.33–3.75; p=0.0023 and OR, 1.95; 95% CI, 1.16–3.28; p=0.012,
respectively) (Table III). In
subjects older than 60 years of age, however, no significant
association was found between polymorphisms and gastric
carcinogenesis.
 | Table IIIRisk of gastric carcinogenesis with
genotype in young and old subjects. |
Table III
Risk of gastric carcinogenesis with
genotype in young and old subjects.
| NFKB1
rs28362491 | ins/ins | ins/del | del/del | del/del vs. others;
OR (95% CI) | p-value |
|---|
| ≤60 years of
age |
| Controls
(359)a | 144 | 171 | 44 | Reference | |
| GC cases
(167) | 57 | 73 | 37 | 2.24
(1.33–3.75) | 0.0023 |
|
| NFKB1
rs72696119 | CC | CG | GG | GG vs. others; OR
(95% CI) | p-value |
|
| ≤60 years of
age |
| Controls
(359) | 142 | 172 | 45 | Reference | |
| GC cases
(167) | 60 | 72 | 35 | 1.95
(1.16–3.28) | 0.012 |
|
| NFKB1
rs28362491 | ins/ins | ins/del | del/del | del/del vs. others;
OR (95% CI) | p-value |
|
| >60 years of
age |
| Controls
(521) | 198 | 264 | 59 | Reference | |
| GC cases
(312) | 115 | 166 | 31 | 0.938
(0.583–1.51) | 0.79 |
|
| NFKB1
rs72696119 | CC | CG | GG | GG vs. others; OR
(95% CI) | p-value |
|
| >60 years of
age |
| Controls
(521) | 210 | 256 | 55 | Reference | |
| GC cases
(312) | 129 | 154 | 29 | 0.913
(0.559–1.49) | 0.71 |
Association between genetic polymorphisms
and the progression of gastric cancer
We further investigated the influence of genetic
polymorphisms on the progression of GC. According to the UICC (Unio
Internationalis Contra Cancrum) classification v. 7, gastric cancer
cases at stage 0–I are classified as early GC cases and those at
stage II–IV are classified as advanced GC cases. Thus, in this
study, early GC and advanced GC cases consisted of 210 and 262
cases, respectively (unknown, 7 cases). The minor allele rs28362491
was significantly correlated to the progression of GC by ANCOVA
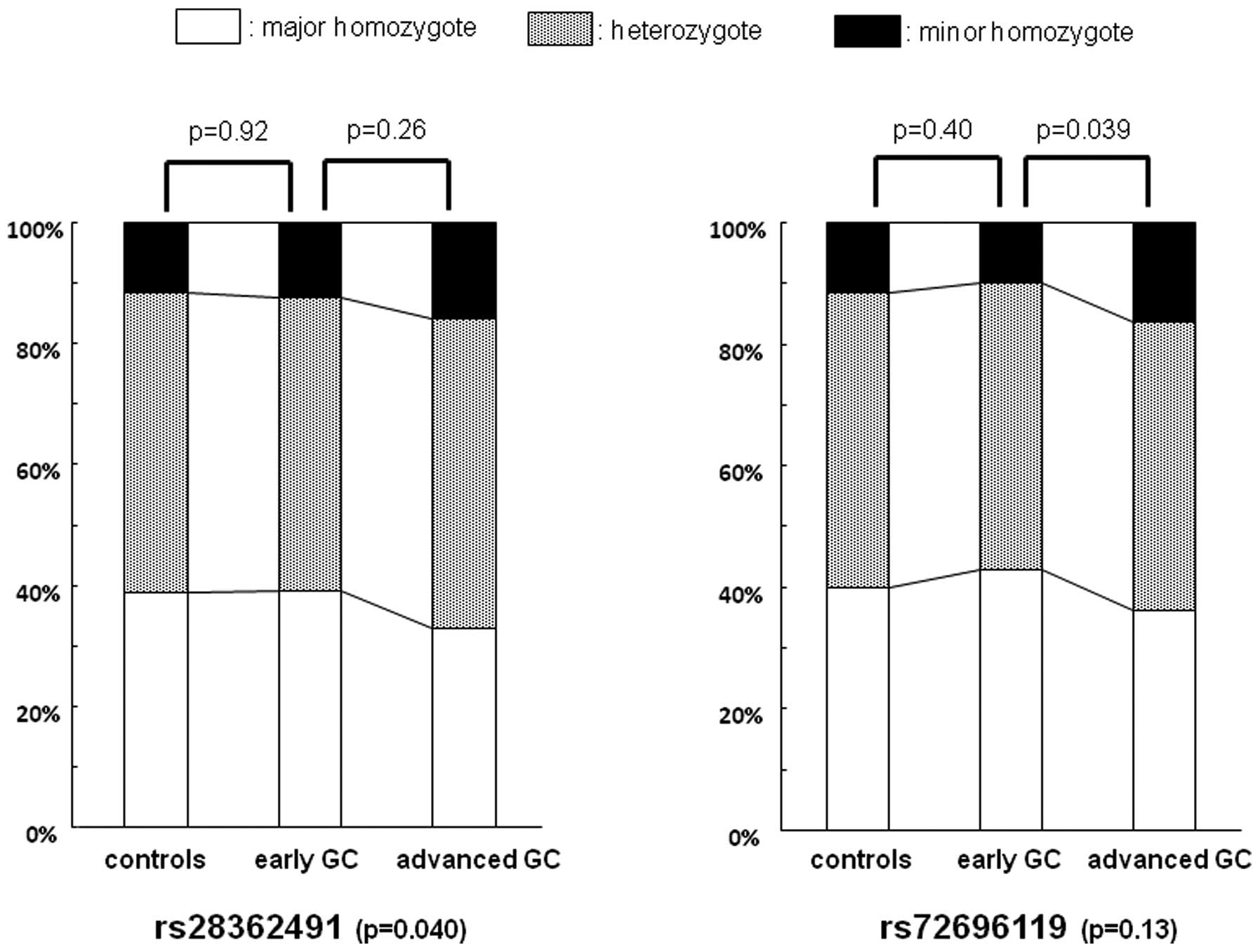
(p=0.040) (Fig. 1), whereas that of
rs72696119 was not. However, the distribution of the rs73696119
genotype was significantly different between advanced GC cases and
early GC cases (p=0.039). The risk of the rs73696119 GG homozygote
for advanced GC compared with early GC was OR, 1.78; 95% CI,
1.01–3.13 (p=0.046) (Table IV). In
addition, the rs28362491 del/del homozygote conferred an increased
risk for both tumor invasion over the muscle layer and lymph node
metastasis (OR, 1.75; 95% CI, 1.02–3.00; p=0.041 and OR, 1.71; 95%
CI, 1.01–2.89; p=0.047, respectively). The rs78366119 GG homozygote
was also associated with an increased risk for both factors (OR,
2.33; 95% CI,1.32–4.11; p=0.0036 and OR, 1.83; 95% CI, 1.07–3.15;
p=0.028, respectively).
 | Table IVAssociation between cancer
progression and genotype. |
Table IV
Association between cancer
progression and genotype.
| NFKB1
rs28362491 | ins/ins | ins/del | del/del | del/del vs. others;
OR (95% CI) | p-value |
|---|
| Early GC cases
(210)a | 82 | 102 | 26 | Reference | - |
| Advanced GC cases
(262) | 86 | 134 | 42 | 1.34
(0.783–2.29) | 0.28 |
| ≤ T1 (234) | 92 | 116 | 26 | Reference | - |
| ≥ T2 (238) | 76 | 120 | 42 | 1.75
(1.02–3.00) | 0.041 |
| N(−) (266) | 91 | 144 | 31 | Reference | - |
| N(+) (206) | 77 | 92 | 37 | 1.71
(1.01–2.89) | 0.047 |
|
| NFKB1
rs72696119 | CC | CG | GG | GG vs. others; OR
(95% CI) | p-value |
|
| Early GC cases
(210) | 90 | 99 | 21 | Reference | - |
| Advanced GC cases
(262) | 95 | 124 | 43 | 1.78
(1.01–3.13) | 0.046 |
| ≤ T1 (234) | 100 | 113 | 21 | Reference | - |
| ≥ T2 (238) | 85 | 110 | 43 | 2.33
(1.32–4.11) | 0.0036 |
| N(−) (266) | 103 | 135 | 28 | Reference | - |
| N(+) (206) | 82 | 88 | 36 | 1.83
(1.07–3.15) | 0.028 |
Risk of the subjects with the rs28362491
del/del or rs72696119 GG genotype for gastric inflammation and
carcinogenesis
When assessing the risk of the rs28362491 del/del or
rs72696119 GG genotype (del/G group) for gastric carcinogenesis, a
stronger association with GC was found (OR, 1.44; 95% CI,
1.02–2.03; p=0.037), particularly with the diffuse type of GC (OR,
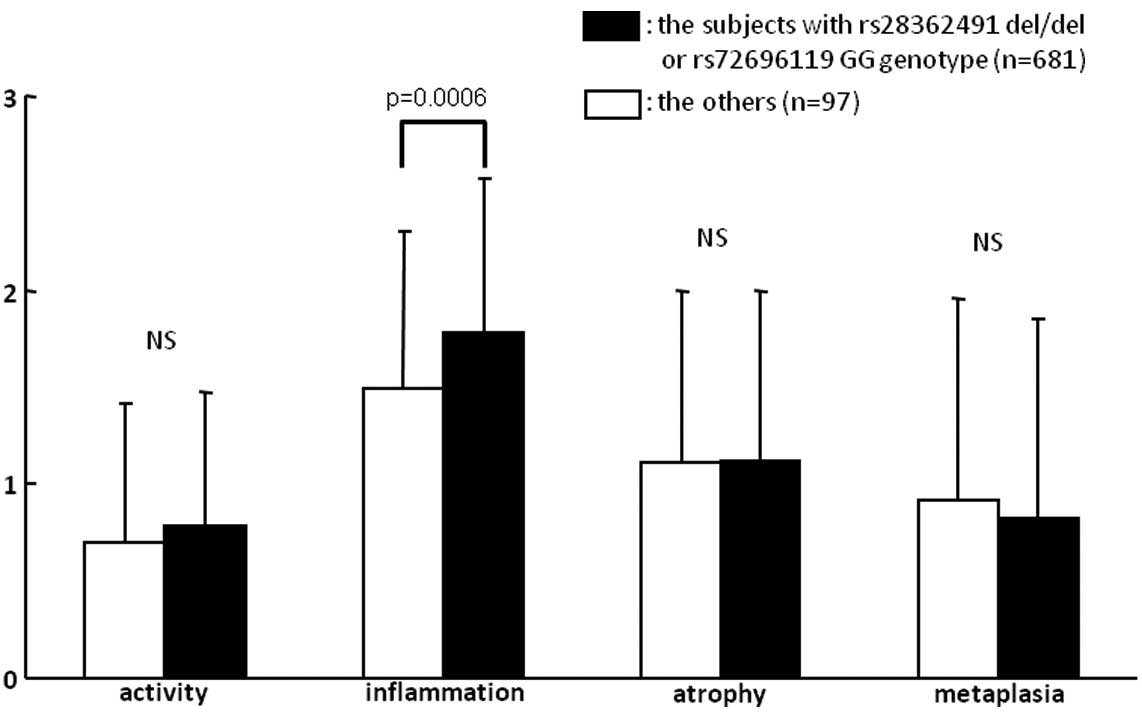
1.88; 95% CI, 1.23–2.85; p=0.0033) (Table V). In addition, the inflammation
score of the del/G group was significantly higher than that of the
non-del/G group (p=0.0006), whereas the other updated Sydney system
scores did not differ between the two groups (Fig. 2).
 | Table VAssociation of del/del or GG genotype
with gastric cancer. |
Table V
Association of del/del or GG genotype
with gastric cancer.
| del/del or GG | Others | del/del or GG vs.
others; OR (95% CI) | p-value |
|---|
| Controls
(880)a | 108 | 772 | Reference | |
| Overall GC cases
(479)b | 71 | 408 | 1.44
(1.02–2.03) | 0.037 |
| Intestinal
(283) | 33 | 250 | 1.13
(0.726–1.77) | 0.58 |
| Diffuse (191) | 38 | 153 | 1.88
(1.23–2.85) | 0.0033 |
Discussion
In the present study, we demonstrated the
association between NFKB1 polymorphisms and gastric cancer
risk. We revealed that the rs28362941 del/del genotype was
significantly associated with an increased risk for the development
of GC, and that both rs28362941 del/del and rs72696119 GG genotypes
were closely associated with development of the diffuse type of GC.
In addition, in the subjects with the rs28362941 del/del or
rs73696119 GG genotype, the frequency of inflammatory cell
infiltration into non-cancerous gastric mucosa was higher than this
frequency in the other genotypes. Based on the fact that rs28362941
and rs73696119 are in linkage disequilibrium (18), these results suggest that more
severe gastric inflammation may be induced in the homozygote of
NFKB1 minor allele, subsequently developing diffuse type of
GC.
According to the Lauren classification (21), there are two histologically distinct
types of GC, which is still widely accepted. The intestinal type
consists of gland-like structures that mimic the intestinal glands,
and a series of precancerous lesions are recognized. The diffuse
type of gastric cancer lacks any glandular structures and arises
closer to the advancing edge of gastric mucosal inflammation
without any identifiable histological precursor lesion (22). The former develops in stomachs
affected by chronic inflammation by passing through the
intermediate steps of atrophic gastritis or intestinal metaplasia
(23). On the other hand, the
severity of mucosal inflammation and various host features may
directly induce mutagenetic events that ultimately lead to the
onset of the latter. Moreover, the diffuse type of GC develops in
comparatively younger subjects (24). Therefore, we suspect that
NFKB1 polymorphisms were significantly associated with
susceptibility to GC in young subjects, not elder subjects, in the
present study.
It has been reported that the NFKB1 −94 ATTG
deletion mutant in the promoter region destroys a transcription
factor binding site, resulting in lower expression of NF-κB
(25). One study reported that the
NFKB1 −94 deletion mutant had a reduced risk for auto-immune
disorders in China (26). In the
stomach, Lo et al(27)
showed that the −94 deletion mutant had a significantly reduced
risk for gastric carcinogenesis in China. Contrary to these
results, several studies in Caucasians have shown that the −94
deletion mutant is associated with an increased risk for the
development of inflammatory or auto-immune diseases (25,28).
In colorectal carcinogenesis, Andersen et al(29) demonstrated that carriers of the
NFKB1 −94 deletion were at a 1.45-fold higher risk than
homozygous carriers of the insertion allele. On the other hand,
other studies found no association of NFKB1 −94 ins/del
polymorphism with inflammatory or auto-immune diseases (30–32).
These contrasting observations may be explained by differences in
the genotypic composition of populations in different countries
with different racial groups. In fact, the frequency of the −94
deletion allele appears to be rather higher in Chinese healthy
subjects (45–55%). However, in our Japanese subjects, it was
approximately 35–36%, similar to the value in Caucasians. Our
Japanese study indicates, as well as the Caucasian study, that the
−94 deletion allele may be an inflammation promoting allele.
NF-κB regulates a number of different transcription
factors that are homodimers or heterodimers of p65, p50, p105,
C-rel and relB (33). NF-κB is
involved in both the inflammatory and the anti-inflammatory process
(34). The role of NF-κB in
inflammation is determined by subunit type. NFKB1 encodes
both subunits p105 and p50 of the transcription factor NF-κB by
alternative splicing (35). As part
of the p65/p50 NF-κB transcription factor complex, it is
pro-inflammatory, controlling transcription of pro-inflammatory
cytokines (36). Conversely, since
p50 lacks this COOH-terminal transactivation domain which is
necessary for the positive regulation of gene expression, p50 has
anti-inflammatory properties in the p50 homodimer by repressing
transcription (37). The relative
abundance of p65/p50 heterodimers and p50 homodimers may determine
the magnitude of inflammation by balancing the pro-inflammatory and
anti-inflammatory response (33).
In fact, p50-deficient mice have an increased sensitivity to LPS
and have increased LPS-induced inflammation (38,39).
In subjects with the del/del genotype, decreased p50 synthesis may
lead to decreased repressive homodimers and increased active
heterodimers of the NF-κB complex. This balance may promote gastric
inflammation, resulting in cancer development.
In the present study, NFKB1 polymorphisms
appeared to be associated with GC progression. Sasaki et
al(40) reported that NF-κB
activation was correlated with gastric cancer invasion and
lymphatic invasion. It has been shown that the NF-κB pathway has an
important role in GC cell growth and metastatic function in
vitro(41,42). In a study in a Korean population by
Kim et al(43), however, no
correlation was observed between the genotype or allelic frequency
of rs28362941 and the T, N or M stage of gastric cancer. The
distribution of genotype in their study was 107 ins/ins, 80 ins/del
and 274 del/del, which was entirely different from that in the
present study. The minor allele frequency in our controls was
almost equal to that in JSA426 (426 anonymous unrelated Japanese
individuals, from NCBI dbSNP). It is unclear why there is such a
discrepancy between the two Asian studies. Further large scale
study is needed, since the association observed in this study was
significant but weak.
The present study was a hospital-based case-control
study. Therefore, sample selection may have affected the outcome as
our controls included patients who came to the hospital in order to
seek treatment for various complaints and were not completely
healthy subjects. Another limitation of this study was that the
effect of type II error cannot be excluded in relatively small
sample sizes.
In conclusion, the functional promoter polymorphism
of NFKB1 is associated with an increased risk of gastric
cancer, in particular, with the development of diffuse type of
gastric cancer via severe gastric inflammation. In addition, this
polymorphism appears to be associated with gastric cancer
progression.
References
|
1
|
Parkin DM, Pisani P and Ferlay J:
Estimates of the worldwide incidence of 25 major cancers in 1990.
Int J Cancer. 80:827–841. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parsonnet J, Friedman GD, Vandersteen DP,
et al: Helicobacter pylori infection and the risk of gastric
carcinoma. N Engl J Med. 325:1127–1131. 1991. View Article : Google Scholar
|
|
3
|
No authors listed. NIH Consensus
Conference: Helicobacter pylori in peptic ulcer disease. NIH
Consensus Development Panel on Helicobacter pylori in Peptic
Ulcer Disease. JAMA. 272:65–69. 1994.
|
|
4
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar
|
|
5
|
El-Omar EM, Carrington M, Chow WH, et al:
Interleukin-1 polymorphisms associated with increased risk of
gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT and
Lin JT: Interleukin-10 genotypes associate with the risk of gastric
carcinoma in Taiwanese Chinese. Int J Cancer. 104:617–623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohyauchi M, Imatani A, Yonechi M, et al:
The polymorphism interleukin 8 −251 A/T influences the
susceptibility of Helicobacter pylori related gastric
diseases in the Japanese population. Gut. 54:330–335. 2005.
|
|
8
|
Arisawa T, Tahara T, Shibata T, et al:
Functional promoter polymorphisms of the macrophage migration
inhibitory factor gene in gastric carcinogenesis. Oncol Rep.
19:223–228. 2008.PubMed/NCBI
|
|
9
|
Arisawa T, Tahara T, Shiroeda H, et al:
Genetic polymorphisms of IL17A and pri-microRNA-938,
targeting IL17A 3′-UTR, influence susceptibility to gastric
cancer. Hum Immunol. 73:747–752. 2012.
|
|
10
|
Kiechl S, Lorenz E, Reindl M, et al:
Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med.
347:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoshino K, Takeuchi O, Kawai T, et al:
Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are
hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps
gene product. J Immunol. 162:3749–3752. 1999.PubMed/NCBI
|
|
12
|
Keates S, Hitti YS, Upton M and Kelly CP:
Helicobacter pylori infection activates NF-kappaB in gastric
epithelial cells. Gastroenterology. 113:1099–1109. 1997. View Article : Google Scholar
|
|
13
|
Hatz RA, Rieder G, Stolte M, et al:
Pattern of adhesion molecule expression on vascular endothelium in
Helicobacter pylori-associated antral gastritis.
Gastroenterology. 112:1908–1919. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maeda S, Yoshida H, Ogura K, et al: H
pylori activates NF-kappaB through a signaling pathway
involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and
TRAF6 in gastric cancer cells. Gastroenterology. 119:97–108. 2000.
View Article : Google Scholar
|
|
15
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006.
|
|
16
|
Le Beau MM, Ito C, Cogswell P, Espinosa R
III, Fernald AA and Baldwin AS Jr: Chromosomal localization of the
genes encoding the p50/p105 subunits of NF-kappaB (NFKB2)
and the I kappaB/MAD-3 (NFKBI) inhibitor of NF-kappaB to
4q24 and 14q13, respectively. Genomics. 14:529–531. 1992.PubMed/NCBI
|
|
17
|
Zou YF, Yuan FL, Feng XL, et al:
Association between NFKB1 −94ins/del ATTG promoter
polymorphism and cancer risk: a meta-analysis. Cancer Invest.
29:78–85. 2011.
|
|
18
|
Arisawa T, Tahara T, Shiroeda H, et al:
NFKB1 polymorphism is associated with age-related gene
methylation in Helicobacter pylori-infected subjects. Int J
Mol Med. 30:255–262. 2012.
|
|
19
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated Sydney
System International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996.PubMed/NCBI
|
|
20
|
Hayashi R, Tahara T, Yamaaki T, et al:
−449 C>G polymorphism of NFKB1 gene, coding nuclear
factor-kappaB, is associated with the susceptibility to ulcerative
colitis. World J Gastroenterol. 47:6981–6986. 2012.
|
|
21
|
Lauren P: The two histologic main types of
gastric carcinoma: diffuse and so-called intestinal type carcinoma.
An attempt at a histo-clinical classification. Acta Pathol
Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
22
|
Yoshimura T, Shimoyama T, Fukuda S, Tanaka
M, Axon AT and Munakata A: Most gastric cancer occurs on the distal
side of the endoscopic atrophic border. Scand J Gastroenterol.
34:1077–1081. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Go MF: Review article: Natural history and
epidemiology of Helicobacter pylori infection. Aliment
Pharmacol Ther. 16(Suppl 1): S3–S15. 2002. View Article : Google Scholar
|
|
24
|
Kong X, Wang JL, Chen HM and Fang JY:
Comparison of the clinicopathological characteristics of young and
elderly patients with gastric carcinoma: a meta analysis. J Surg
Oncol. 106:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karban AS, Okazaki T, Panhuysen CI, et al:
Functional annotation of a novel NFKB1 promoter polymorphism
that increases risk for ulcerative colitis. Hum Mol Genet.
13:35–45. 2004.PubMed/NCBI
|
|
26
|
Li H, Gao L, Shen Z, et al: Association
study of NFKB1 and SUMO4 polymorphisms in Chinese patients with
psoriasis vulgaris. Arch Dermatol Res. 300:425–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lo SS, Chen JH, Wu CW and Lui WY:
Functional polymorphism of NFKB1 promoter may correlate to
the susceptibility of gastric cancer in aged patients. Surgery.
145:280–285. 2009.
|
|
28
|
Kurylowicz A, Hiromatsu Y,
Jurecka-Lubieniecka B, et al: Association of NFKB1
−94ins/del ATTG promoter polymorphism with susceptibility to and
phenotype of Graves’ disease. Genes Immun. 8:532–538. 2007.
|
|
29
|
Andersen V, Christensen J, Overvad K,
Tjønneland A and Vogel U: Polymorphisms in NFκB, PXR, LXR and risk
of colorectal cancer in a prospective study of Danes. BMC Cancer.
10:4842010.
|
|
30
|
Mirza MM, Fisher SA, Onnie C, et al: No
association of the NFKB1 promoter polymorphism with
ulcerative colitis in a British case control cohort. Gut.
54:1205–1206. 2005.PubMed/NCBI
|
|
31
|
Glas J, Török HP, Tonenchi L, et al: Role
of the NFKB1 −94ins/delATTG promoter polymorphism in IBD and
potential interactions with polymorphisms in the CARD15/NOD2, IKBL,
and IL-1RN genes. Inflamm Bowel Dis. 12:606–611. 2006.
|
|
32
|
Bajwa EK, Cremer PC, Gong MN, et al: An
NFKB1 promoter insertion/deletion polymorphism influences
risk and outcome in acute respiratory distress syndrome among
Caucasians. PLoS One. 6:e194692011.PubMed/NCBI
|
|
33
|
Pereira SG and Oakley F: Nuclear
factor-kappaB1: regulation and function. Int J Biochem Cell Biol.
40:1425–1430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Winther MP, Kanters E, Kraal G and
Hofker MH: Nuclear factor kappaB signaling in atherogenesis.
Arterioscler Thromb Vasc Biol. 25:904–914. 2005.PubMed/NCBI
|
|
35
|
Lin L, DeMartino GN and Greene WC:
Cotranslational biogenesis of NF-kappaB p50 by the 26S proteasome.
Cell. 92:819–828. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gadjeva M, Tomczak MF, Zhang M, et al: A
role for NF-kappaB subunits p50 and p65 in the inhibition of
lipopolysaccharide-induced shock. J Immunol. 173:5786–5793. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han W, Joo M, Everhart MB, et al: Myeloid
cells control termination of lung inflammation through the
NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol.
296:L320–L327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sasaki N, Morisaki T, Hashizume K, et al:
Nuclear factor-kappaB p65 (RelA) transcription factor is
constitutively activated in human gastric carcinoma tissue. Clin
Cancer Res. 7:4136–4142. 2001.PubMed/NCBI
|
|
41
|
Kang MH, Oh SC, Lee HJ, et al: Metastatic
function of BMP-2 in gastric cancer cells: the role of PI3K/AKT,
MAPK, the NF-κB pathway, and MMP-9 expression. Exp Cell Res.
317:1746–1762. 2011.PubMed/NCBI
|
|
42
|
Qin Y, Li L, Chen J, et al: Fentanyl
inhibits progression of human gastric cancer MGC-803 cells by NF-κB
downregulation and PTEN upregulation in vitro. Oncol Res. 20:61–69.
2012.PubMed/NCBI
|
|
43
|
Kim JG, Sohn SK, Chae YS, et al: No
association of the NFKB1 insertion/deletion promoter
polymorphism with survival in patients with gastric cancer. Jpn J
Clin Oncol. 39:497–501. 2009.
|