Introduction
Panax ginseng was discovered over 5,000 years
ago in China and has since been regarded as a highly venerated
medicinal herb. In recent years, natural products from plants,
including ginsenosides, have attracted increased attention due to
their favorable safety and efficacy profiles, suggesting a
potential therapeutic application in the treatment of cancers.
Recently, it has been reported that ginsenosides exhibit cytotoxic
activity in a variety of human tumor cell lines (1). Previous studies revealed that
ginsenoside Rg3 contributes to a reduction in proliferation
(2,3), metastasis (3,4) and
induction of cell death (5) in
several types of cancer cells in vivo and in vitro.
But few studies have focused on its effects on human osteosarcoma
cell lines. Moreover, ginsenoside Rh2 has been demonstrated to have
anticancer effects (5,6) and to be capable of inhibiting cell
growth and inducing cell cycle arrest and apoptosis in mammalian
tumor cells (7). Based on these
previous studies, ginseng compounds have been considered as
potential agents for cancer chemotherapy.
Structurally, most of the ginsenosides belong to the
protopanaxadiol (PPD), and protopanaxatriol (PPT) groups (8). Previous reports have identified that
ginsenoside Rg1 and Rh1 have the most potent effect on the
inhibition of cell proliferation in cancer cells (9,10). In
fact, steam treatment with increasing concentrations of ginsenoside
Rg1 and Rh1 augments the antiproliferative activities of those
ginsenosides. Ginsenoside Rf is a type of extract from the active
constituents of ginseng, and has a structure characteristic of the
presence of a hydroxyl group/sugar residue at C-6 in PPTs and is
unique to Asian ginseng (1). It has
been proposed that ginsenoside Rf may play an important role in
regulating cancer cell proliferation since this molecule has a
similar chemical structure with ginsenoside Rg1 and Rh1. However,
the effect of ginsenoside Rf on cancer cells remains to be
elucidated.
Osteosarcoma is the eighth most common form of
childhood cancer, comprising 2.4% of all malignancies in pediatric
patients and ~20% of all primary bone cancers. The 5-year survival
rate of osteosarcoma patients is only 50–70% due to the lack of
effective treatment options (11).
Concerning the clinical treatment of cancer, cell cycle control, a
major regulatory mechanism of cell proliferation, is one of the
most effective methods for cancer treatment through inhibition of
cancer cell growth (12). Thus,
there are many cytotoxic agents and/or DNA damaging agents that
arrest the cell cycle at the G0/G1, S or G2/M phase consequently
inducing apoptosis of cancer cells (13). The cyclin/Cdk families have been
found to play an important role in G2 to M phase progression,
particularly at the end of the G2 phase when a threshold level of
the active cyclin B1/CDK1 complex, also known as maturation
promoting factor (MPF), has been reached, followed by induction of
cell apoptosis (14). Apoptosis is
the result of a highly complicated cascade of cellular events that
result in cell rounding and shrinkage, chromatin condensation, DNA
fragmentation, shedding of smaller fragments from cells, and
detachment from the plate. In particular, the mitochondria play a
central role in the occurrence of apoptosis resulting from many
chemotherapeutic agents (15). It
is also well known that caspases, such as caspase-3, -8 and -9,
which are highly conserved cysteine-dependent aspartate-specific
proteases and the key components of the apoptotic machinery, play
the central role in apoptotic progression (16).
The present study was designed to clarify the
antitumor activity of ginsenoside Rf in a panel of established
cancer cell lines by MTT and colony forming assays. Furthermore, we
investigated cell cycle arrest and mitochondrial-associated
apoptotic events in the osteosarcoma MG-63 cell line, which is the
most sensitive to ginsenoside Rf, in order to elucidate the
mechanism of ginsenoside Rf-induced apoptosis in human
osteosarcoma.
Materials and methods
Drugs and materials
Ginsenoside Rf was purchased from the National
Institutes for Food and Drug Control (NIFDC) (CAS no. 111719) at a
purity of 99.69%. Dulbecco’s modified Eagle’s medium (DMEM), fetal
calf serum (FCS), trypan blue, penicillin G and streptomycin were
obtained from Gibco-BRL (Gaithersburg, MD, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO), ribonuclease (RNase), propidium iodide
(PI) and 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue
tetrazolium (BCIP/NBT) were purchased from Sigma Chemical (St.
Louis, MO, USA). The Apoptosis Detection Kit I was a product of BD
Pharmingen. Hoechst 33258, JC-1 and caspase assay kits were
purchased from Beyotime (Jiangsu, China). Human reactive monoclonal
antibodies against cyclin B1, Cdk1, Bcl-2, Bax, cytochrome c
and β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Cell lines and cell culture
Human osteosarcoma cell lines, MG-63 (wild-type),
OS732 (wild-type), U-2OS (wild-type), HOS (wild-type) and SAOS-2
(wild-type), were purchased from the Institute of Biochemistry and
Cell Biology, The Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in DMEM supplemented with 10% heat-inactivated
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. They were all
placed in a humidified atmosphere containing 5% CO2 at
37°C.
Analysis of cell proliferation
The cytotoxicity assay was analyzed with the MTT
assay and the trypan blue dye assay. Briefly, MG-63, OS732, U-2OS,
HOS and SAOS-2 cells were seeded in 96-well plates at a density of
1×104 cells/well with 100 μl of the cell culture medium.
After 12 h of incubation, the cells were treated with ginsenoside
Rf from 0 to 30 μM in medium for 24 h. MTT solution (5 mg/ml) was
then added to each well and incubated at 37°C for 4 h. The
supernatants were then removed and replaced by 100 μl DMSO. The
optical density (OD) was measured at a wavelength of 570 nm with an
enzyme immunoassay analyzer (Bio-Rad, USA). The cytotoxicity of
ginsenoside Rf was expressed as IC50 (concentration of
50% cytotoxicity, which was extrapolated from linear regression
analysis of the experimental data).
Colony forming assay
MG-63, OS732, U-2OS, HOS and SAOS-2 cells were
seeded at 3×102/well into 24-well culture plates,
respectively, and incubated overnight prior to treatment. Culture
medium containing increasing drug concentrations was added to the
cells, and incubation was carried out for 7 days in the presence of
the drug. After the wells were washed with phosphate-buffered
saline (PBS), the colonies were fixed with methanol, stained with
crystal violet and counted (>50 cells). The antiproliferative
activity was expressed as EC50 (50% inhibiting
concentration).
Analysis of cell cycle distribution by
flow cytometry
Cells (2×105) were seeded in a 6-well
culture plate. After a 12-h incubation, cells were treated with 0,
2.75, 5.5 and 11 μM of ginsenoside Rf for 0, 12, 24, 36 and 48 h,
respectively. Both floating and adherent cells were harvested,
combined and processed. Cells were collected by centrifugation,
fixed with ice-cold 70% ethanol, washed with PBS, and resuspended
in 0.5 ml of PBS containing PI (500 μg/ml) and RNase A (1 mg/ml).
After a final incubation at 37°C for 30 min, cells were analyzed
using a FACScan flow cytometer (Becton-Dickinson). The percentages
of cells in G0/G1 phase, S phase, G2/M and sub-G1 phase were
analyzed using standard ModiFit and CellQuest software
programs.
Detection of apoptotic incidence by flow
cytometry
Apoptotic incidence was measured with the Annexin
V-FITC Apoptosis Detection Kit I according to the manufacturer’s
instructions. Briefly, cells were treated with 0, 2.75, 5.5 and 11
μM of ginsenoside Rf for 24 h, respectively. The cells were then
washed twice with cold PBS, and resuspended in 500 μl of binding
buffer at a concentration of 1×106 cells/ml. Annexin
V-FITC solution (5 μl) and 5 μl of PI (1 mg/ml) were added to the
cells at 37°C for 30 min. The cells were analyzed by flow cytometry
within 1 h. Apoptotic cells were counted, and data are expressed as
a percentage of the total cell count.
Assay of mitochondrial membrane potential
changes in situ
JC-1 probe was employed to measure mitochondrial
depolarization in MG-63 cells. Following treatment with 0, 2.75,
5.5 and 11 μM of ginsenoside Rf for 24 h, the MG-63 cells were
incubated at 37°C for 20 min with 5 mg/l JC-1, then washed twice
with PBS and placed in 2 ml culture medium. Green fluorescence
(JC-1 as a monomer at low membrane potentials) and red fluorescence
(JC-1 as ‘J-aggregates’ at higher membrane potentials) were
monitored under a fluorescence microscope. Mitochondrial
depolarization was indicated by a decrease in the red/green
fluorescence intensity ratio.
Hoechst 33258 staining of MG-63
cells
Cells were incubated with 0, 2.75, 5.5 and 11 μM of
ginsenoside Rf for 24 h, harvested, fixed with 4% paraformaldehyde
for 30 min at 25°C, washed 3 times with ice-cold PBS, and exposed
to 10 mg/l Hoechst 33258 in the dark at room temperature for 10
min. Finally, the stained nuclei were observed under a fluorescence
microscope (Olympus ×50) with excitation at 350 nm and emission at
460 nm.
Assays of caspase activity
Caspase activity was determined using caspase assay
kits. According to the manufacturer’s protocol, cells were placed
in 10-cm dishes at 1×106/dish. After a 12-h incubation,
cells were treated with 0, 2.75, 5.5 and 11 μM of ginsenoside Rf
for 24 h. After the different treatments, both floating and
adherent cells were collected and washed 3 times by PBS and were
lysed with lysis buffer (100 μl/2×106 cells) for 15 min
on ice. Cell lysates were centrifuged at 16,000 × g for 10 min at
4°C. Then, clear lysates containing 50 μg of protein were incubated
with 100 μM of enzyme-specific colorigenic substrates at 37°C for 1
h. The activities of caspase-3, -8 and -9 were described as the
cleavage of the colorimetric substrate by measuring the absorbance
at 405 nm with a microplate spectrophotometer (BioTek),
respectively.
Western blotting
Cells were placed in 10-cm dishes at
1×106/dish. After a 12-h incubation, cells were treated
with 0, 2.75, 5.5 and 11 μM of ginsenoside Rf for 24 h. Total
protein was extracted using a Western & IP cell lysis kit after
treatment, or cytoplasmic protein was extracted using a
Mitochondrial Isolation kit (Applygen). For total cell protein
extracts, the treated cells were washed in PBS, suspended in lysis
buffer and placed in ice for 30 min. After centrifugation for 15
min at 4°C, the supernatant was collected. Total proteins were
prepared by standard procedures and quantified by the BCA method
(Pierce Biotechnology, Inc., Rockford, IL, USA).
The western blot assay was performed as described
previously. Briefly, whole cell extracts were separated by 8%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electrotransferred to polyvinylidene difluoride
(PVDF) membranes (Bio-Rad, Hercules, CA, USA). After the PVDF
membranes were incubated with 10 mM TBS with 1.0% Tween-20 and 10%
dehydrated skim milk to block nonspecific protein binding, the
membranes were incubated with the appropriate primary antibodies in
blocking buffer overnight at 4°C. The membranes were then washed
with TBST and incubated with alkaline phosphatase-linked secondary
antibodies (Pierce Biotechnology, Inc.). After being washed with
TBST, immunoreactive bands were visualized using NBT/BCIP as
substrate. β-actin was used as a loading control.
RT-PCR analysis of cyclin B1 and Cdk1
mRNA
Total RNA of the ginsenoside Rf-treated cells
extracted using 1 ml TRIzol reagent (Invitrogen Life Technologies)
was dissolved in 0.1% diethylpyrocarbonate (DEPC) water and
quantified by spectrophotometry at 260 nm (absorbance). cDNA was
synthesized from 1 μg total RNA through reverse transcription using
a Takara RNA PCR kit ver. 2.1 (Takara Bio, Inc.) according to the
manufacturer’s protocol. Primers were obtained from Shanghai Sangon
Biotechnology Co. Ltd. (Shanghai, China), and the sequences for the
primers are as follows: 5′-CTTATACTAAGCACCAAATC-3′ (sense) and
5′-CTTGGCTAAATCTTGAACT-3′ (antisense) for cyclin B1;
5′-CTTATGCAGGATTCCAGGTT-3′ (sense) and 5′-GGTGCCTATACTCCAAATGTC-3′
(antisense) for Cdk1; and 5′-GGTCGGAGTCAACGGATTTG-3′ (sense) and
5′-ATGAGCCCCAGCCTTCTCCAT-3′ (antisense) for GAPDH. Quantitative
real-time PCR was performed using 1 μg of cDNA and SYBR-Green
(Bio-Bad) in 36-well plates in a LightCycler Rapid Thermal Cycler
system (Roche Diagnostics) according to the manufacturer’s
instructions. PCR products were subjected to melting curve
analysis, and the data were analyzed by the 2−δδCt
calculation method and standardized to GAPDH. PCR analysis was
performed in triplicate for each sample.
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Statistical analysis was performed using the SPSS
17.0 statistical software program to evaluate the significance of
differences between groups considered as p<0.05; p<0.01;
p<0.001. All data points represent the mean of triplicate
values.
Results
Effect of ginsenoside Rf on cell
proliferation in human osteosarcoma cell lines
To clearly identify the antiproliferative effect of
ginsenoside Rf on human osteosarcoma cell lines, we initially
treated 5 human osteosarcoma cell lines (MG-63, OS732, U-2OS, HOS
and SAOS-2) with ginsenoside Rf at different concentrations for 12,
24, 36 and 48 h, respectively. Cell proliferation and cell
viability were estimated by the MTT assay and trypan blue staining.
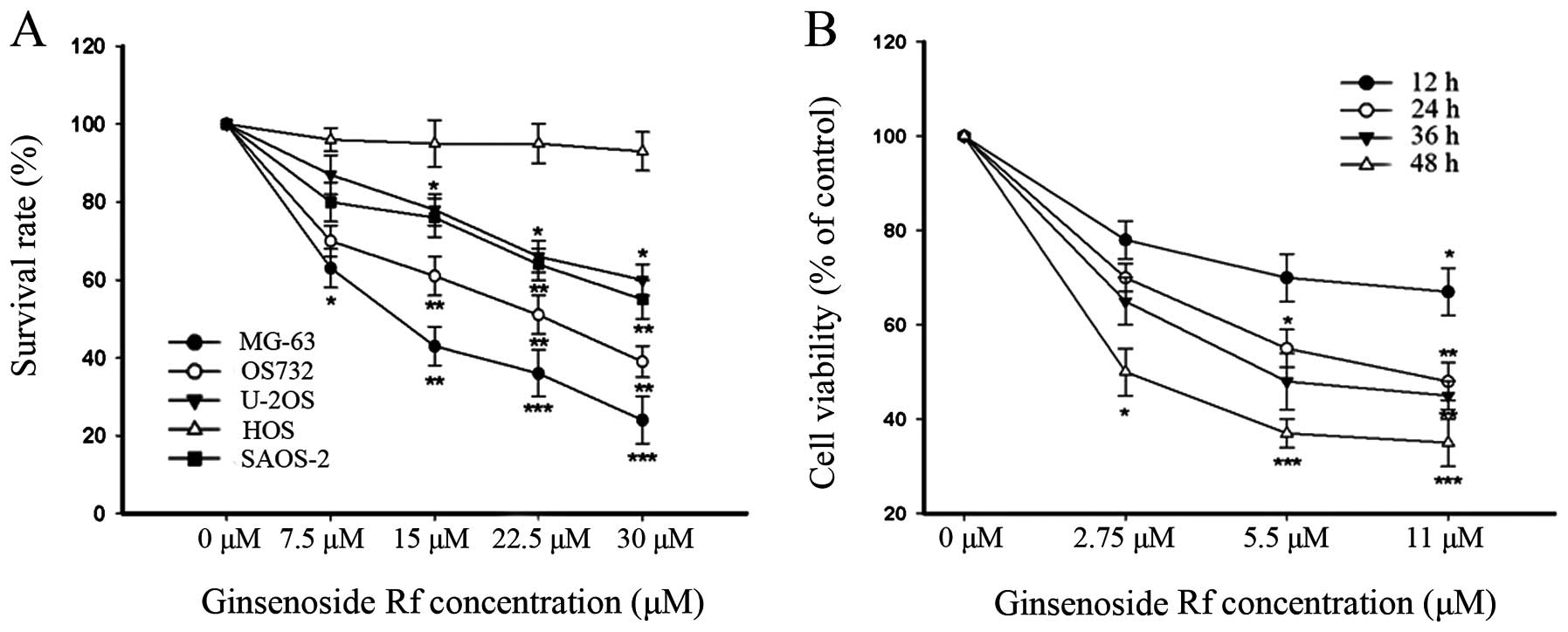
As shown in Fig. 1, the
cytotoxicity of ginsenoside Rf to the human osteosarcoma cell lines
was dose-dependent, and each cell line exhibited a different
sensitivity to ginsenoside Rf. Notably, the MG-63 cells were the
most sensitive to ginsenoside Rf. The IC50 values for
the cytotoxic effect are showed in Table I. The IC50 value for the
MG-63 cells treated with ginsenoside Rf was 11 μM at 24 h. This
dose- and time-point were referred to in the following experiments.
Doses of 0, 2.75 and 5.5 μM were used in the following assays. Our
findings demonstrated that ginsenoside Rf inhibited cellular
proliferation in a time- and dose-dependent manner.
 | Table IGrowth inhibitory effect of
ginsenoside Rf on 5 osteosarcoma cell lines. |
Table I
Growth inhibitory effect of
ginsenoside Rf on 5 osteosarcoma cell lines.
| OS732 | MG-63 | U-2OS | HOS | SAOS-2 |
|---|
| IC50
(μM) | 20.43 | 11.36 | 25.02 | >30 | >30 |
| EC50
(μM) | 6.84 | 0.93 | 8.58 | / | / |
| TI | 2.98 | 12.21 | 2.91 | / | / |
Effect of ginsenoside Rf on the colony
forming ability of human osteosarcoma cell lines
The EC50 values for colony formation are
showed in Table I. The therapeutic
indices (the ratio of IC50 vs. EC50) for the
5 human osteosarcoma cell lines were in the following order: MG-63,
OS732, U-2OS, HOS and SAOS-2 successively. This suggested that
MG-63 cells, which were used in the following experiments, were
most sensitive to ginsenoside Rf with a therapeutic index
(IC50 vs. EC50) equal to 12.21.
Ginsenoside Rf induces G2/M phase arrest
and apoptosis in MG-63 cells
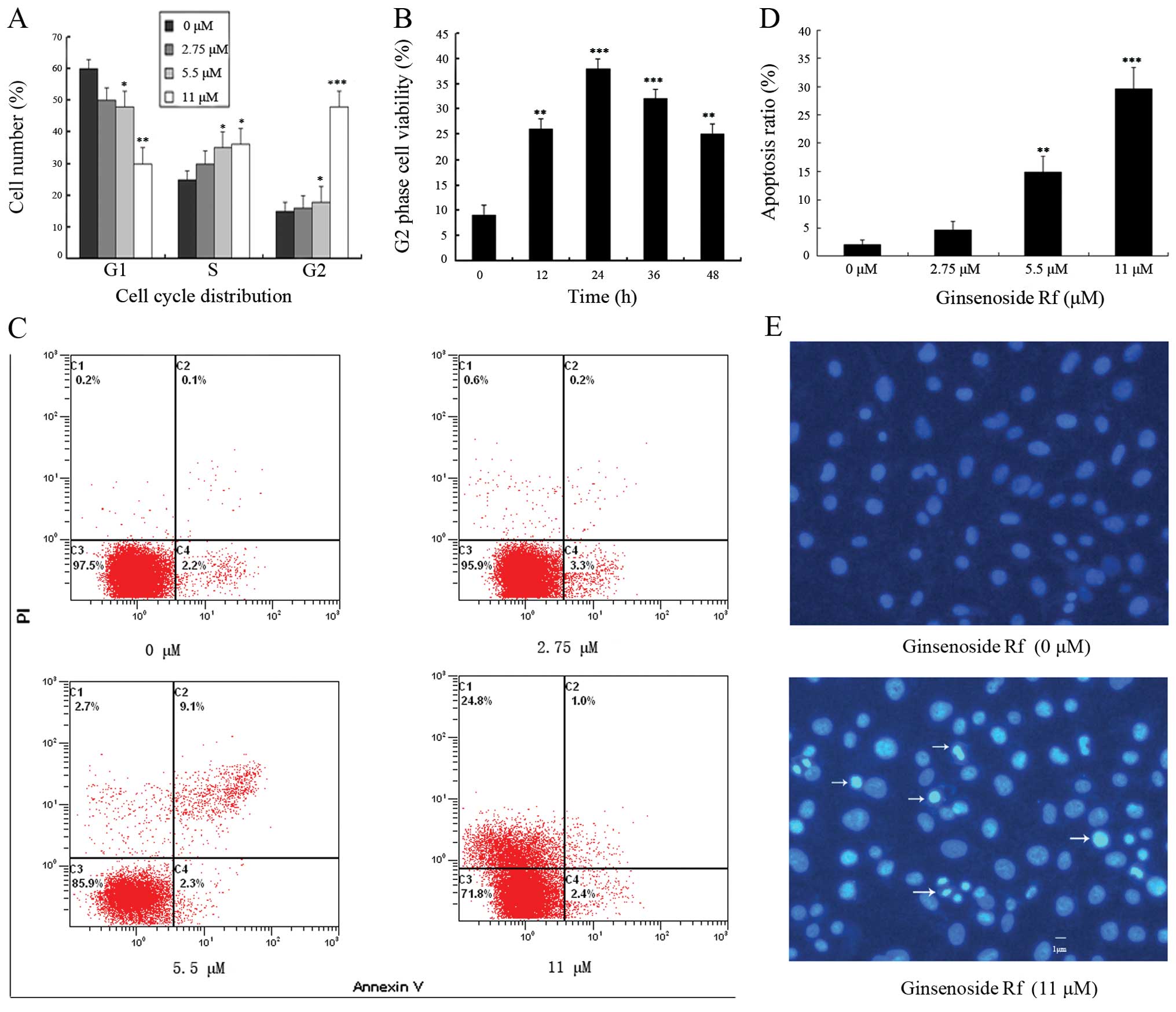
To determine whether changes in cell cycle
distribution were involved in the decrease in cell viability, we
investigated the effect of ginsenoside Rf on cell cycle
distribution by flow cytometric analysis. The exposure of cells to
0, 2.75, 5.5 and 11 μM ginsenoside Rf for 24 h resulted in the
accumulation of the proportion of cells in the G2/M phase from
13.26±2.6% to 15.63±2.9, 19.22±4.2 and 49.55±4.7%, respectively
(Fig. 2A). Ginsenoside Rf resulted
in a time-dependent G2/M phase cell cycle arrest, and G2/M phase
accumulation peaked following treatment with 11 μM ginsenoside Rf
at 24 h (Fig. 2B). These results
suggest that ginsenoside Rf inhibits the cellular proliferation via
G2/M phase cell cycle arrest in a time- and dose-dependent
manner.
The rate of cell apoptosis was detected by flow
cytometry by double labeling with Annexin V and PI. Representative
graphs obtained by flow cytometric analysis of cells treated with
ginsenoside Rf at different concentrations for 24 h after double
staining with Annexin V-FITC and PI are shown in Fig. 2C. The apoptotic rate in the control
cells was 2.1±0.7%. There was a dose-dependent increase in the
apoptotic rate of MG-63 cells exposed to ginsenoside Rf. The
apoptotic rates in the MG-63 cells were increased to 4.6±1.6,
14.9±2.9 and 29.6±3.8% following treatment with ginsenoside Rf at
2.75, 5.5 and 11 μM for 24 h, respectively (Fig. 1D). Using Hoechst 33258 staining, we
further confirmed that ginsenoside Rf induced MG-63 cell death
through the apoptosis pathway (Fig.
2E). MG-63 cells treated with 11 μM ginsenoside Rf for 24 h
exhibited an increase in chromatin condensation and nuclear
fragmentation. The results indicate that cell death occurred
through apoptosis.
Effect of ginsenoside Rf on mitochondrial
membrane potential in MG-63 cells
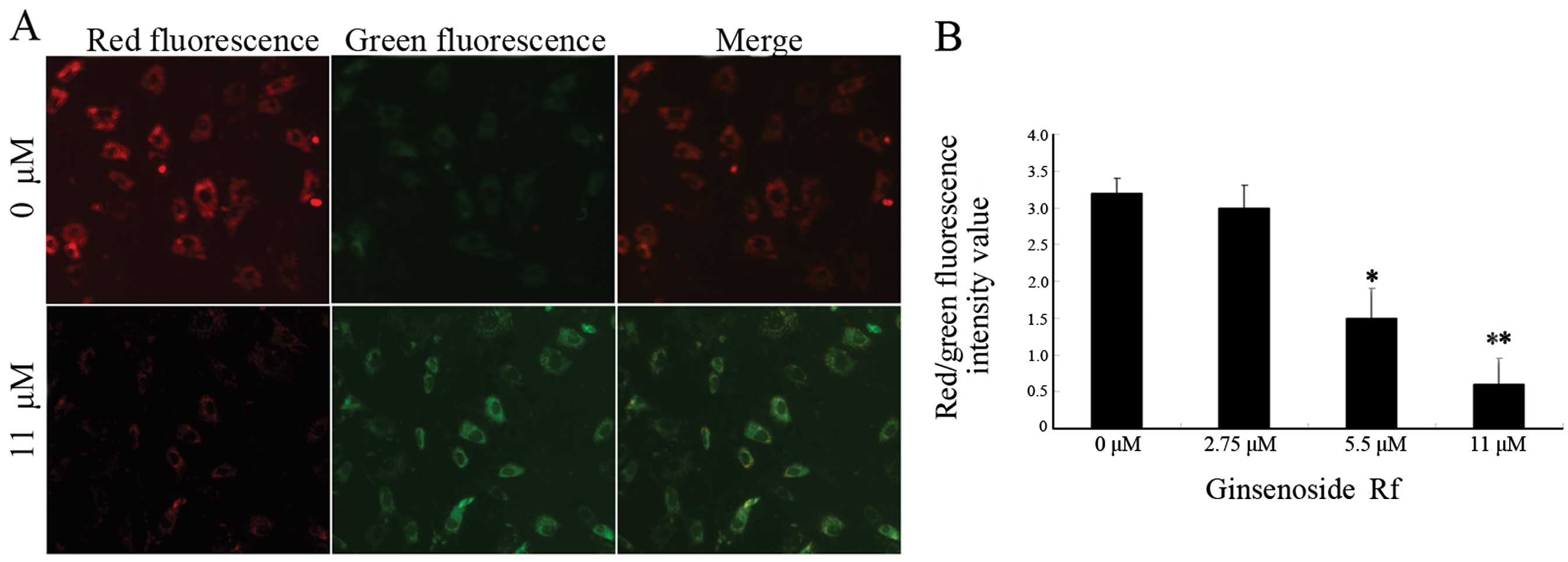
In order to investigate whether the mitochondrial
pathway is involved in the apoptosis induced by ginsenoside Rf, we
analyzed MG-63 cells treated with ginsenoside Rf at different
concentrations for 24 h using the membrane potential sensing dye
JC-1. The control cells stained with JC-1 emitted mitochondrial red
fluorescence with slight green fluorescence, indicative of a normal
polarized state. The JC-1 aggregates were dispersed to the
monomeric form (green fluorescence) in the cells exposed to 11 μM
ginsenoside Rf for 24 h (Fig. 3A).
The results showed that ginsenoside Rf reduced the ratio of red
(JC-1 aggregates) fluorescence to green (JC-1 monomers)
fluorescence, indicating that ginsenoside Rf caused the dissipation
of mitochondrial membrane potential (Fig. 3B).
Effect of ginsenoside Rf on Cdk1 and
cyclin B1 expression
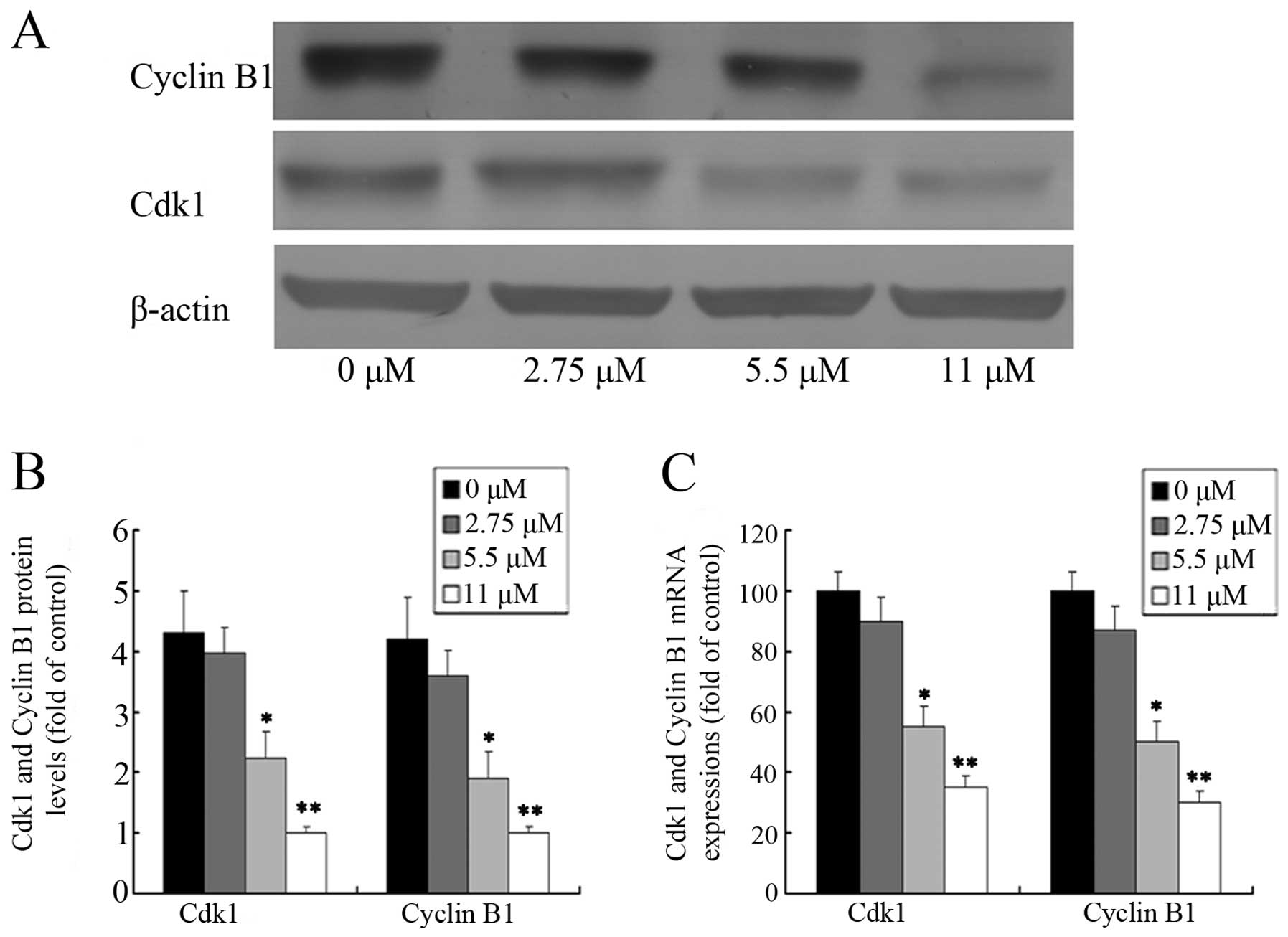
In an attempt to investigate the molecular events
involved in the activity of ginsenoside Rf activity on the cell
cycle, we assessed the effect of ginsenoside Rf on the expression
of Cdk1 and cyclin B1 in MG-63 cells. The protein levels of Cdk1
and cyclin B1 were detected by western blotting after MG-63 cells
were treated with 0, 2.75, 5.5 and 11 μM ginsenoside Rf for 24 h.
As shown in Fig. 4A and B, the
level of cyclin B1 protein in the cells treated with 11 μM
ginsenoside Rf increased almost 1/5-fold over the control group at
24 h. As expected, we also found that the expression of Cdk1
protein was attenuated in the cells treated with 11 μM ginsenoside
Rf, which was only ~1/4 times more than the NC group. The changes
in Cdk1 and cyclin B1 mRNA expression, as measured by real-time
PCR, were almost parallel with that of the corresponding protein
(Fig. 4C). Our results suggest that
downregulation of the expression of G2 phase-regulating proteins
may contribute to the ginsenoside Rf-mediated cell cycle arrest in
MG-63 cells.
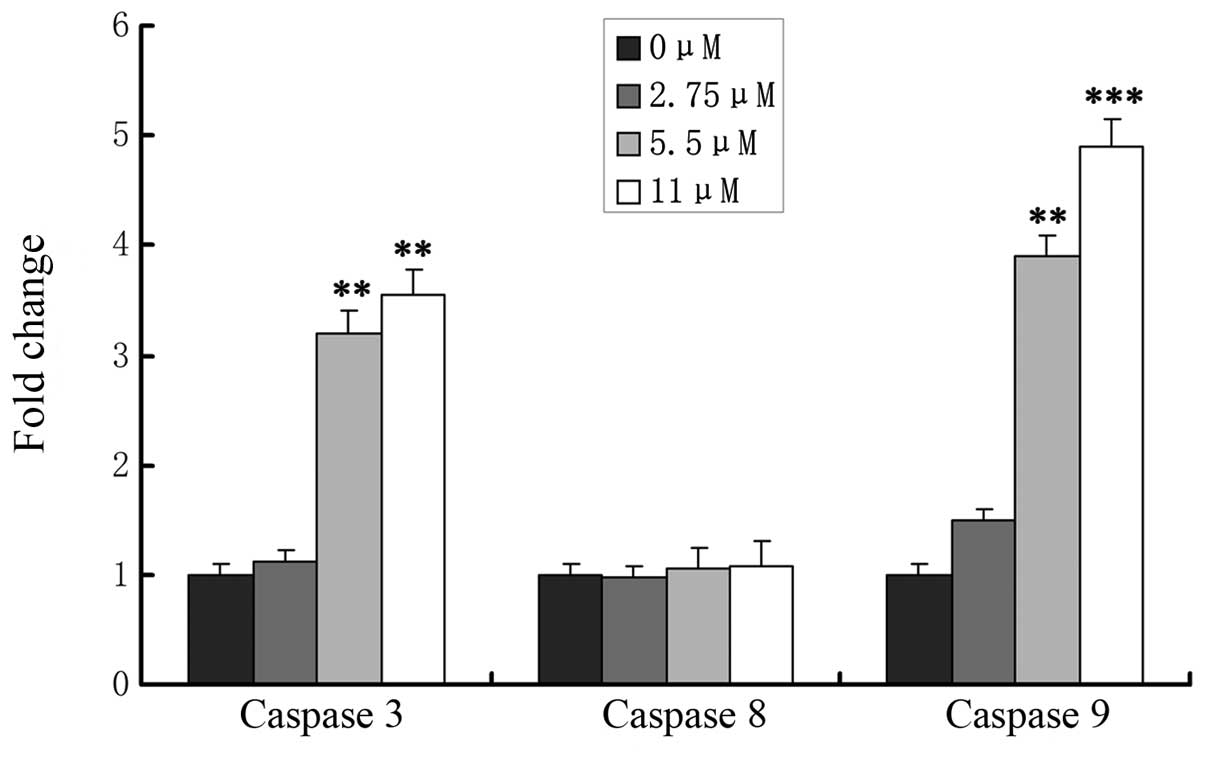
Effects of ginsenoside Rf on caspase
activation
The caspase cascade reaction is one of the most
important events in the process of apoptosis through the
mitochondrial pathway. To determine whether release of caspase-3,
-8 and -9 is involved in ginsenoside Rf-induced apoptosis, MG-63
cells were treated with 0, 2.75, 5.5 and 11 μM ginsenoside Rf, and
caspase-8, -9 and caspase-3 activation was determined at 24 h
(Fig. 5). We assessed the caspase-3
and -9 levels in cell lysates and found that caspase-3 and -9
levels increased significantly in the MG-63 cells in a
dose-dependent manner, which indicated that mitochondrial pathways
were involved in ginsenoside Rf-induced apoptosis. Simultaneously,
we found that activity of caspase-8 was not changed in the cells
treated with ginsenoside Rf.
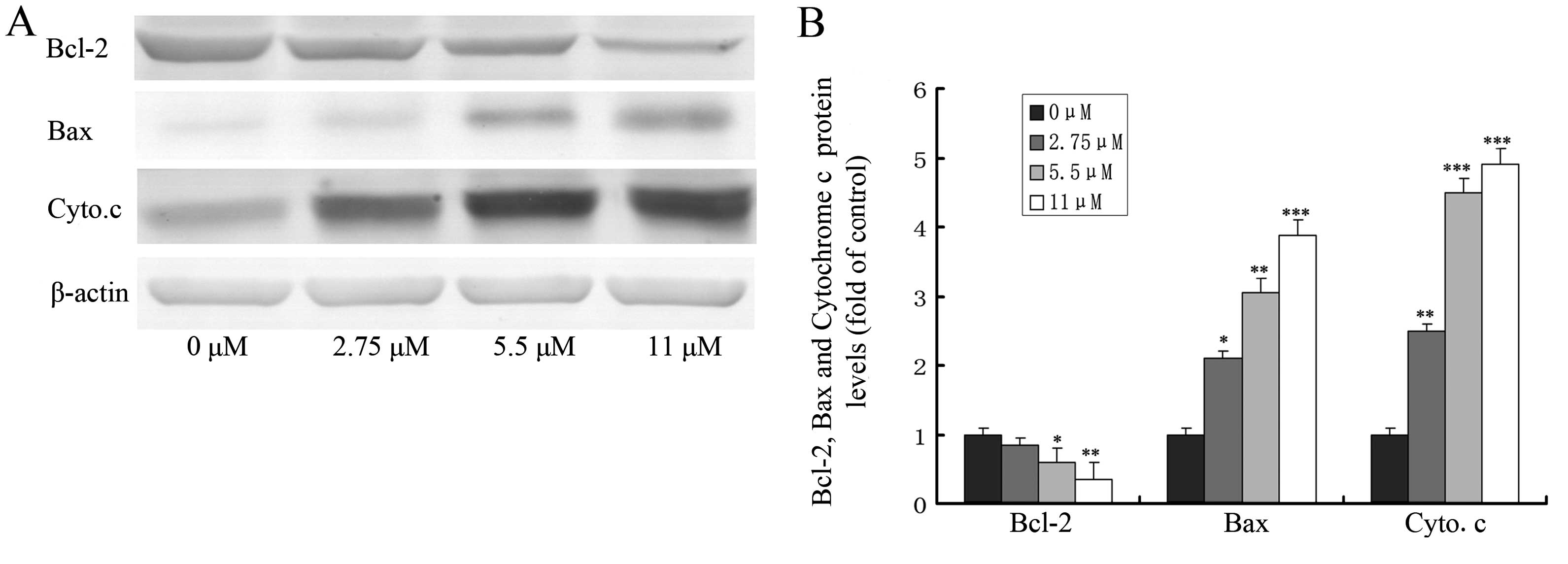
Effect of ginsenoside Rf on the
expression of apoptosis-related proteins
The loss of mitochondrial transmembrane potential
and the release of cytochrome c from mitochondria to the
cytosol are pivotal events in the mitochondrial apoptosis pathway,
which is regulated by Bcl-2 family members (such as Bcl-2 and Bax)
during this process (17,18). To explore the possible role of Bcl-2
family members and cytochrome c in ginsenoside Rf-induced
apoptosis, we investigated the effect of ginsenoside Rf at 0, 2.75,
5.5 and 11 μM for 24 h on the expression of proteins including
Bcl-2, Bax and the release of cytochrome c by western
blotting. As shown in Fig. 6,
treatment of MG-63 cells with ginsenoside Rf caused a marked
increase in Bax proteins and the release of cytochrome c,
and a decrease in Bcl-2 protein, when compared to these levels in
the control.
Discussion
The aim of the present study was to clarify whether
ginsenoside Rf has a cytotoxic effect on human osteosarcoma cells
and to explore the molecular mechanisms involved during this
process. The main findings of our study are as follows. First, we
demonstrated that the cytotoxicity of ginsenoside Rf to human
osteosarcoma cell lines was dose-dependent and that the MG-63 cells
were the most sensitive to ginsenoside Rf. Second, we showed that
ginsenoside Rf induced G2/M phase cell cycle arrest and cell death
through the apoptosis pathway. Furthermore, we found that the
mitochondrial pathway participated in the process of apoptosis
induced by ginsenoside Rf.
Conventional chemotherapeutic agents kill cancer
cells as well as normal cells, limiting their therapeutic use in
the clinic. Previous studies have shown that natural bioactive
reagents as an approach to effective cancer prevention can
selectively kill cancer cells, based on their favorable safety and
efficacy profiles (19). Ginseng, a
widely used traditional Chinese medicine, has been reported to
exhibit various biological effects including antitumor activity
(1). The antitumor efficacy of
ginseng is attributed mainly to the presence of saponins, known as
ginsenosides. Previous studies have demonstrated that several
ginsenosides such as the ginsenoside Rg3 and Rh2 lead to the
accumulation of cells in the G2/M phase and cell apoptosis by DNA
damaging agents (20,21). However, whether ginsenoside Rf,
which is unique to Asian ginseng, influences human osteosarcoma
cell viability and regulates cell apoptosis has not been reported.
Here, we first investigated the cytotoxicity of ginsenoside Rf to
MG-63, OS732, U-2OS, HOS and SAOS-2 cells. According to our
results, ginsenoside Rf inhibited cell proliferation in the human
osteosarcoma cell lines in a dose-dependent manner. MG-63 cells
were the most sensitive to ginsenoside Rf. The value of
IC50 in the MG-63 cells following treatment with
ginsenoside Rf was found to be 11 μM at 24 h. Therefore, we chose
this dose- and time-point as a reference for MG-63 cells in other
experiments.
The cell cycle is mediated by the activation of a
highly conserved protein kinase family, the cyclin-dependent
kinases (Cdks) (22). Cyclins can
activate Cdks by forming complexes with Cdks, and these cyclin/Cdk
complexes are cell cycle regulators. Among them, the cyclin B1/Cdk1
complex, in which B-type cyclins associate with Cdk1, is the
primary regulator of transition from the G2 to M phase. This
complex was originally discovered and defined as the
maturation-promoting factor or M phase-promoting factor (MPF). The
cell cycle arrests at the G2 phase and mitosis cannot occur without
activation of the cyclin B1/Cdk1 complex. In the present study,
flow cytometric analysis showed that ginsenoside Rf caused G2/M
phase cell cycle arrest, and G2/M phase accumulation peaked at 24 h
of treatment. Subsequently, the apoptotic sub-G1 phase increased
obviously after >24 h of treatment, suggesting the occurrence of
sequential events of cell cycle arrest followed by apoptosis; it is
known that cell cycle dysregulation is a hallmark of tumor cells.
Evidence indicates that G2/M phase arrest is mediated by a limited
supply of Cdk1 and cyclin B1 for Cdk1/cyclin B1 complex formation,
resulting in the shortage of the cyclin B1/Cdk1 complex during cell
cycle progression (23). In the
present study, we found that ginsenoside Rf arrested MG-63 cells in
the G2/M phase through a decrease in the cyclin B1/Cdk1 complex
resulting from inhibition of cyclin B1 and Cdk1 expression in a
dose-dependent manner at both the transcriptional and protein
levels. According to the results mentioned above, ginsenoside Rf
induced G2/M phase arrest in MG-63 cells by the downregulation of
Cdk1 and cyclin B1.
Apoptosis, first recognized by Kerr et
al(24), is originally
described as a series of morphological events that give rise to
controlled cell death and is executed by an active cellular
process. This fundamental process is essential for both development
and maintenance of tissue homeostasis. There are two principal
signaling transduction pathways involved in the process of
apoptosis: one is the cell surface death receptor pathway, and the
other is the mitochondrial pathway initiated by the upregulation of
wild-type p53, followed by suppression of Bcl-2. Moreover, these
two pathways can be connected via Bid, one of the Bcl-2 family
members (25). In the present
study, the inhibition of cell viability was observed in ginsenoside
Rf-treated MG-63 cells. After treatment, cells gradually exhibited
cell rounding and shrinkage, vacuolization and even detachment from
the bottom in a time-dependent manner, which are important
characteristics of cell apoptosis. This was further supported by
our findings of increased chromatin condensation and nuclear
fragmentation over time as observed by Hoechst 33258 staining. Our
findings demonstrated that ginsenoside Rf may serve as a protective
agent in cancer treatment as we observed that the apoptosis ratio
increased to 29.6±3.8% in MG-63 cells following treatment with
ginsenoside Rf at 11 μM for 24 h when compared to control cells
(2.1±0.7%), which is consistent with previous reports on other
ginsenosides (such as ginsenosides Rg1) (26).
The Bcl-2/Bax ratio appears to be a critical
determinant of a cell’s threshold for undergoing apoptosis through
the mitochondrial pathway (27).
Our results showed that upregulation of Bax and downregulation of
Bcl-2 occurred simultaneously in the human osteosarcoma cell line
MG-63 following treatment with ginsenoside Rf, which triggered
progression of apoptosis. Therefore, we inferred that the decrease
in the Bcl-2/Bax ratio modulated by ginsenoside Rf induced
apoptosis of MG-63 cells. This hypothesis was further supported by
nuclear morphological changes using Hoechst 33258 staining. The
mitochondrial-mediated apoptosis pathway is triggered by various
cellular stress stimuli and is dependent on mitochondrial outer
membrane permeabilization, resulting in the loss of mitochondrial
transmembrane potential and the release of cytochrome c from
the mitochondria to the cytosol (18). Thus, cells use cytosolic cytochrome
c as a cue to activate the apoptotic machinery (28). Our results demonstrate that MG-63
cells treated with ginsenoside Rf exhibited a reduction in
mitochondrial transmembrane potential. Using western blot analysis,
a dose-dependent increase in cytochrome c accumulation was
found in the cytosol of ginsenoside Rf-treated MG-63 cells. These
results suggest that ginsenoside Rf stimulates the reduction in
mitochondrial transmembrane potential and the release of
mitochondrial cytochrome c, finally activating the
mitochondrial-mediated apoptosis pathway.
In order to clarify the effect of caspases in the
apoptotic process, we determined the activation of caspase-3, -8
and -9. Caspases, a family of cysteine proteases, play essential
roles in apoptosis, necrosis and inflammation. The activation of
caspase-8, which in turn activates downstream caspases, leads to
apoptosis through the death receptor pathway, whereas the
activation of caspase-9, which forms the apoptosome with released
cytochrome c and Apaf-1, activates the executioner caspase-3
and induces apoptosis through the mitochondrial pathway. In the
present study, consistent with the study of Rannou et
al(29), we also found that
ginsenoside Rf had no effect on caspase-8 activity, suggesting that
the death receptor-mediated apoptotic pathway was not involved in
the apoptosis induced by ginsenoside Rf in MG-63 cells. Notably, we
found that ginsenoside Rf enhanced caspase-3 and -9 activities in a
dose-dependent manner, indicating that the mitochondrial death
pathway was involved in the process of apoptosis induced by
ginsenoside Rf. These results are in concordance with those
reported in another study, showing a linkage between activation of
caspase-9 cleavage and activation of procaspase-3 consequently
leading to activation of the caspase cascade and apoptosis
(30).
In conclusion, our results demonstrated that
ginsenoside Rf inhibits cell proliferation and induces cell cycle
arrest at the G2/M phase and apoptosis in human osteosarcoma cells
in a dose- and time-dependent manner. The cell cycle arrest induced
by ginsenoside Rf was associated with a reduction in the protein
and mRNA levels of Cdk1 and cyclin B1. Furthermore, we found that
ginsenoside Rf decreased the Bcl-2/Bax ratio and the mitochondrial
transmembrane potential, resulting in the release of cytochrome
c and activation of caspase-9 and -3, suggesting that the
mitochondrial pathway may be involved in the process of apoptosis
induced by ginsenoside Rf in MG-63 cells. In view of the above
arguments and the new data presented herein, ginsenoside Rf can
induce G2/M phase arrest and apoptosis in human osteosarcoma MG-63
cells through the mitochondrial pathway. It may be a new effective
and promising therapeutic agent for human osteosarcoma, although
further research must be carried out to fully investigate these
possibilities.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81000779), the Excellent
College Teacher Foundation of Shanghai Education Commission, and
the Shanghai Jiaotong University SMC Foundation.
References
|
1
|
Nag SA, Qin JJ, Wang W, et al:
Ginsenosides as anticancer agents: in vitro and in vivo activities,
structure-activity relationships, and molecular mechanisms of
action. Front Pharmacol. 3:252012.PubMed/NCBI
|
|
2
|
Fishbein AB, Wang CZ, Li XL, et al: Asian
ginseng enhances the anti-proliferative effect of 5-fluorouracil on
human colorectal cancer: comparison between white and red ginseng.
Arch Pharm Res. 32:505–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HS, Lee EH, Ko SR, et al: Effects of
ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer
cells. Arch Pharm Res. 27:429–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iishi H, Tatsuta M, Baba M, et al:
Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal
metastasis of intestinal adenocarcinomas induced by azoxymethane in
Wistar rats. Clin Exp Metastasis. 15:603–611. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Peng H, Ou-Yang X and He X:
Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma
cells. Melanoma Res. 18:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu N, Wu GC, Hu R, Li M and Feng H:
Ginsenoside Rh2 inhibits glioma cell proliferation by targeting
microRNA-128. Acta Pharmacol Sin. 32:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Yu H and Hou J: Effects of 20 (S)
-ginsenoside Rh2 and 20 (R) -ginsenoside Rh2 on proliferation and
apoptosis of human lung adenocarcinoma A549 cells. Zhongguo Zhong
Yao Za Zhi. 36:1670–1674. 2011.(In Chinese).
|
|
8
|
Qi LW, Wang CZ and Yuan CS: American
ginseng: potential structure-function relationship in cancer
chemoprevention. Biochem Pharmacol. 80:947–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung JS, Kim DH and Kim HS: Ginsenoside
Rh1 suppresses inducible nitric oxide synthase gene expression in
IFN-γ-stimulated microglia via modulation of JAK/STAT and ERK
signaling pathways. Biochem Biophys Res Commun. 397:323–328.
2010.PubMed/NCBI
|
|
10
|
Wang CZ, Aung HH, Ni M, et al: Red
American ginseng: ginsenoside constituents and antiproliferative
activities of heat-processed Panax quinquefolius roots.
Planta Med. 73:669–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bielack SS, Kempf-Bielack B, Delling G, et
al: Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: an analysis of 1,702 patients treated on
Neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin
Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
12
|
Torres K and Horwitz SB: Mechanisms of
Taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
13
|
Gamet-Payrastre L, Li P, Lumeau S, et al:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
14
|
Hindley C and Philpott A: Co-ordination of
cell cycle and differentiation in the developing nervous system.
Biochem J. 444:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robertson JD and Orrenius S: Role of
mitochondria in toxic cell death. Toxicology. 181–182:491–496.
2002.PubMed/NCBI
|
|
16
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in intervertebral disc degeneration. Apoptosis.
11:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Autret A and Martin SJ: Bcl-2 family
proteins and mitochondrial fission/fusion dynamics. Cell Mol Life
Sci. 67:1599–1606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukherjee AK, Basu S, Sarkar N and Ghosh
AC: Advances in cancer therapy with plant based natural products.
Curr Med Chem. 8:1467–1486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibits hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis - the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome
c release from mitochondria in response to activation of
cell surface death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li QF, Shi SL, Liu QR, et al: Anticancer
effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in
osteosarcoma MG-63 cells: nuclear matrix downregulation and
cytoplasmic trafficking of nucleophosmin. Int J Biochem Cell Biol.
40:1918–1929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rello S, Stockert JC, Moreno V, et al:
Morphological criteria to distinguish cell death induced by
apoptotic and necrotic treatments. Apoptosis. 10:201–208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Autret A and Martin SJ: Emerging role for
members of the Bcl-2 family in mitochondrial morphogenesis. Mol
Cell. 36:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rannou F, Lee TS, Zhou RH, et al:
Intervertebral disc degeneration: the role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fribley A, Zhang K and Kaufman RJ:
Regulation of apoptosis by the unfolded protein response. Methods
Mol Biol. F559:191–204. 2009. View Article : Google Scholar : PubMed/NCBI
|