Introduction
Renal cell carcinoma (RCC) is one of the most
commonly diagnosed urological malignancies in China. Although
surgery is the main therapy for RCC, some patients are already
considered to have metastatic RCC (mRCC) at time of diagnosis
(1). For those particular patients,
the treatment relies mainly on systemic therapy including
chemotherapy, radiation therapy and immune therapy, but their
effects are limited (1,2). RCC expresses multidrug resistance
transporters and is refractory to chemotherapy once it becomes
metastatic (3). With this concern,
novel agents are being developed to target mRCC; one approach to
control RCC is growth inhibition wherein the disease is prevented,
slowed by the administration of one or more non-toxic naturally
occurring or synthetic agents (4).
Kaempferol, a flavonoid, is a yellow compound with a
low molecular weight (MW:286.2 g/mol) (5). Kaempferol has been identified in many
botanical families, and several epidemiological studies have
evaluated the possible association between the consumption of foods
containing kaempferol and a reduced risk of developing several
disorders (6–8). For anticancer activity, kaempferol
induced apoptosis in ovarian cancer (9), oral cavity cancer (10), osteosarcoma (11) and colon cancer (12). Kaempferol was also able to inhibit
cell growth (13) and angiogenesis
(14,15), which is necessary for solid tumor
formation.
Epidermal growth factor receptor (EGFR) is one of
the members of the ErbB receptor tyrosine kinase family and plays a
critical role in a wide variety of cellular functions, including
proliferation, differentiation and apoptosis (16). Several studies have shown that EGFR
and other members of their family together with the growth factors
that activate them are overexpressed in RCC tissue and cell lines
(17,18). Mitogen-activated protein kinases
(MAPKs) are serine/threonine-specific protein kinases. p38 is one
of the MAPKs, and could regulate cell proliferation. EGFR could
mediate p38 activation (19). In
this study, we found kaempferol could functions mainly through the
EGFR/p38 pathway to inhibit RCC cell growth.
Materials and methods
Cell culture
Human RCC cell lines (786-O and 769-P) were
purchased from ATCC and were maintained in RPMI-1640 containing 10%
fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/ml penicillin,
50 μg/ml streptomycin. All cells were cultured at 37°C, in a
humidified atmosphere containing 5% CO2.
Reagents
Kaempferol was a gift from Dr Defeng Xu of Changzhou
University; it was dissolved in DMSO at 50 mM and stored at −20°C.
Antibodies against human Chk1, CDK2, p35, c-jun, cyclin B1 and
GAPDH were purchased from Santa Cruz Biotechnology, Inc. Antibodies
against p-EGFR, EGF, p-MEK, MEK and p-p38 were purchased from Cell
Signaling Technology, Inc. AG1478 was from Calbiochem and SB203580
was purchased from Cell Signaling Technology, Inc. Annexin V-FITC
Apoptosis Detection kit was from Nanjing Jiancheng Bioengineering
Institute.
Cell viability
Cell viability was assessed using a
tetrazolium-based assay (MTT assay). One thousand cells in 50 μl of
media per well were plated in 96-well plates. Cells treated with or
without different doses of kaempferol were incubated for various
times, and then incubated with 0.5 mg/ml of MTT at 37°C for 1 h.
Subsequently, the supernatant was dropped and dissolved with DMSO.
Colorimetric analysis using a 96-well microplate reader was
performed at the wavelength of 490 nm. The experiments were
performed in triplicate.
Colony formation assay
RCC cells (786-O and 769-P) were respectively seeded
in 24-well plates (100 cells/well) and cultured with different
doses of kaempferol (50, 100 and 150 μM) for 14 days before
staining. The colonies were stained with crystal violet and
counted.
Quantitative detection of apoptosis
Cells (786-O and 769-P) were exposed to different
doses of kaempferol (50, 100 and 150 μM) for 48 h. The cells were
collected and subjected to Annexin V and propidium iodide (PI)
staining using an Annexin V-FITC Apoptosis Detection kit, following
the protocol provided by the manufacturer. Apoptotic cells were
then analyzed by flow cytometry.
Cell cycle detection assay
After cells reached 60–80% confluence, they were
treated with different doses of kaempferol (50, 100 and 150 μM).
After 48 h, cells were washed twice with PBS and fixed with 70%
ethanol for 1 h at 4°C, and then washed with PBS and resuspended
with PI solution (0.05 mg/ml) containing RNase, and incubated at
room temperature in the dark for 30 min. DNA content was then
analyzed using the flow cytometer.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris-HCl/pH
7.4, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM
Na3VO4, 1 mM NaF, 1 mM okadaic acid, and 1
mg/ml aprotinin, leupeptin, and pepstatin) with Protease Inhibitor
Cocktail (Roche Inc.). Individual samples (25 μg protein) were
prepared for electrophoresis run on 12–15% SDS-PAGE gel and then
transferred onto PVDF membranes (Millipore). After blocking the
membranes with 5% BSA in PBS for 1 h at room temperature, the
membranes were incubated with appropriate dilutions of specific
primary antibodies overnight at 4°C. After washing, the blots were
incubated with anti-rabbit, anti-mouse or anti-goat IgG HRPs for 1
h. The blots were developed in ECL mixture (Thermo Fisher
Scientific Inc.).
Statistical analyses
All statistical analyses were performed using SPSS
16.0. Quantitative data are presented as mean ± SE and the
differences among various treatment groups were compared by one-way
ANOVA, followed by Dunnett’s t-test for separate comparisons. When
the comparison involved only 2 groups, Student’s t-test was used.
P<0.05 was considered to indicate statistically significant
differences.
Results
Kaempferol inhibits cell growth and
induces cell death in RCC cells
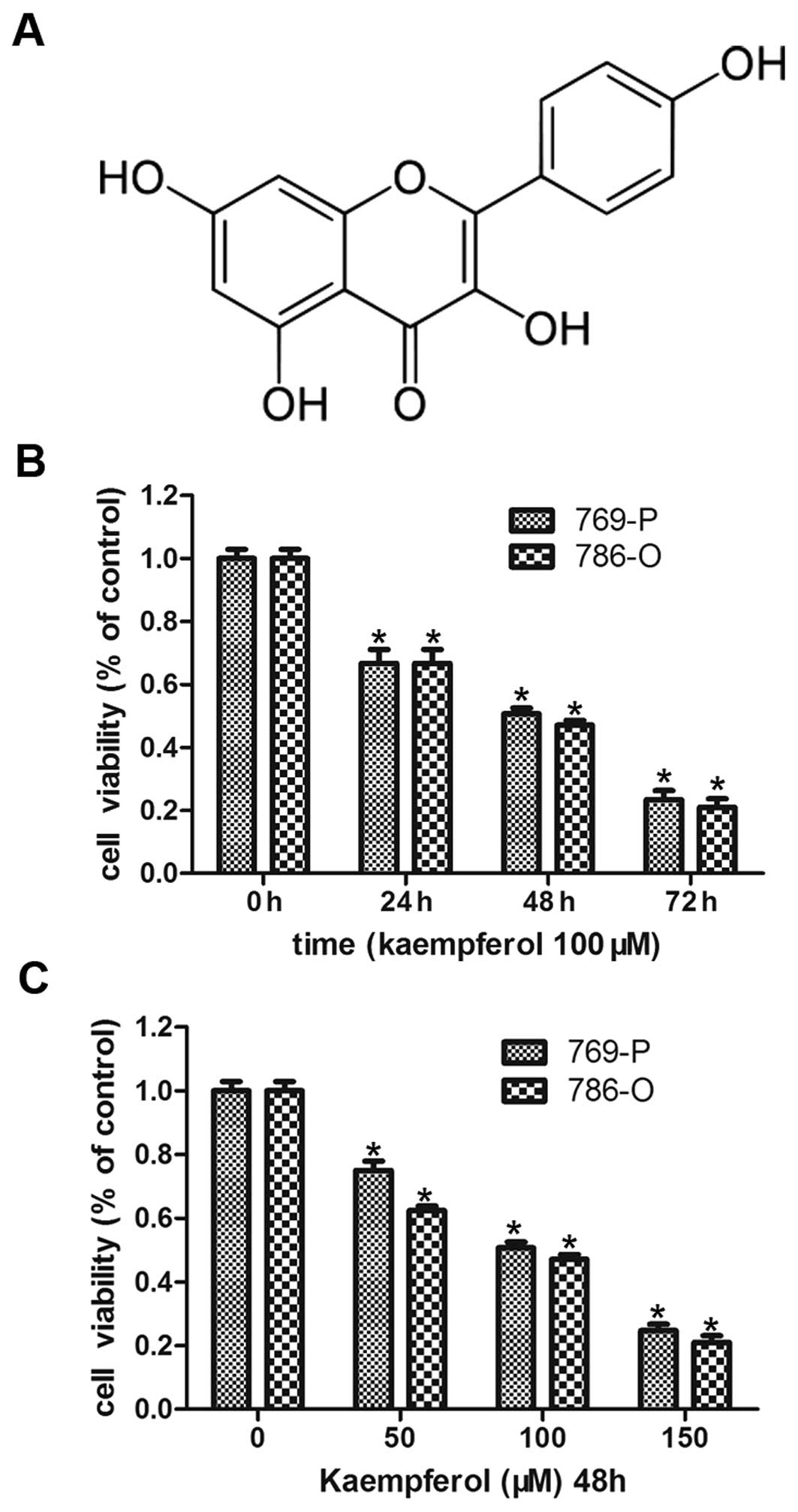
First, we demonstrated the effect of kaempferol
(structure shown in Fig. 1A) on the
growth of RCC cells (786-O and 769-P), as shown in Fig. 1. Kaempferol treatment inhibited the
growth of 786-O and 769-P cells in both a dose- and a
time-dependent manner. We fixed its concentration at 100 μM and
treated RCC cells for 24, 48 and 72 h, resulting in ~78, 50 and 30%
cell survival respectively (Fig.
1B), and then kaempferol treatment at 50, 100 and 150 μM doses
resulted in the same tendency decreasing cell survival.
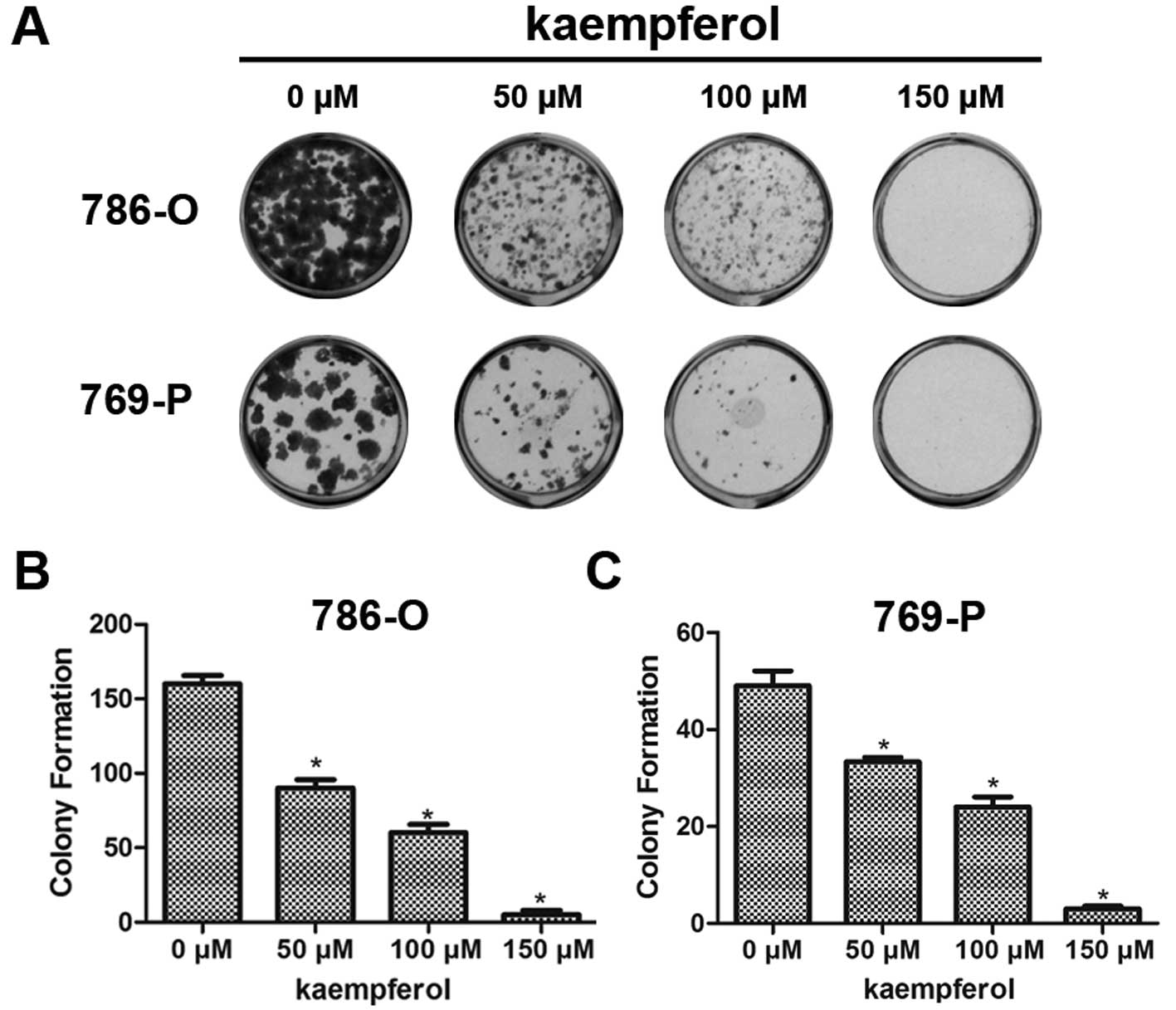
Kaempferol inhibits colony formation of
RCC cells
We also tested the effect of kaempferol on colony
formation, which is another type of proliferation assay. One
hundred of each RCC cell type (786-O and 769-P) were seeded into
24-well plates, and cultured with kaempferol at 50, 100 and 150 μM
doses for 14 days. As shown in Fig.
2, it is evident that kaempferol significantly inhibited colony
formation of both 786-O (Fig. 2B)
and 769-P (Fig. 2C) cells.
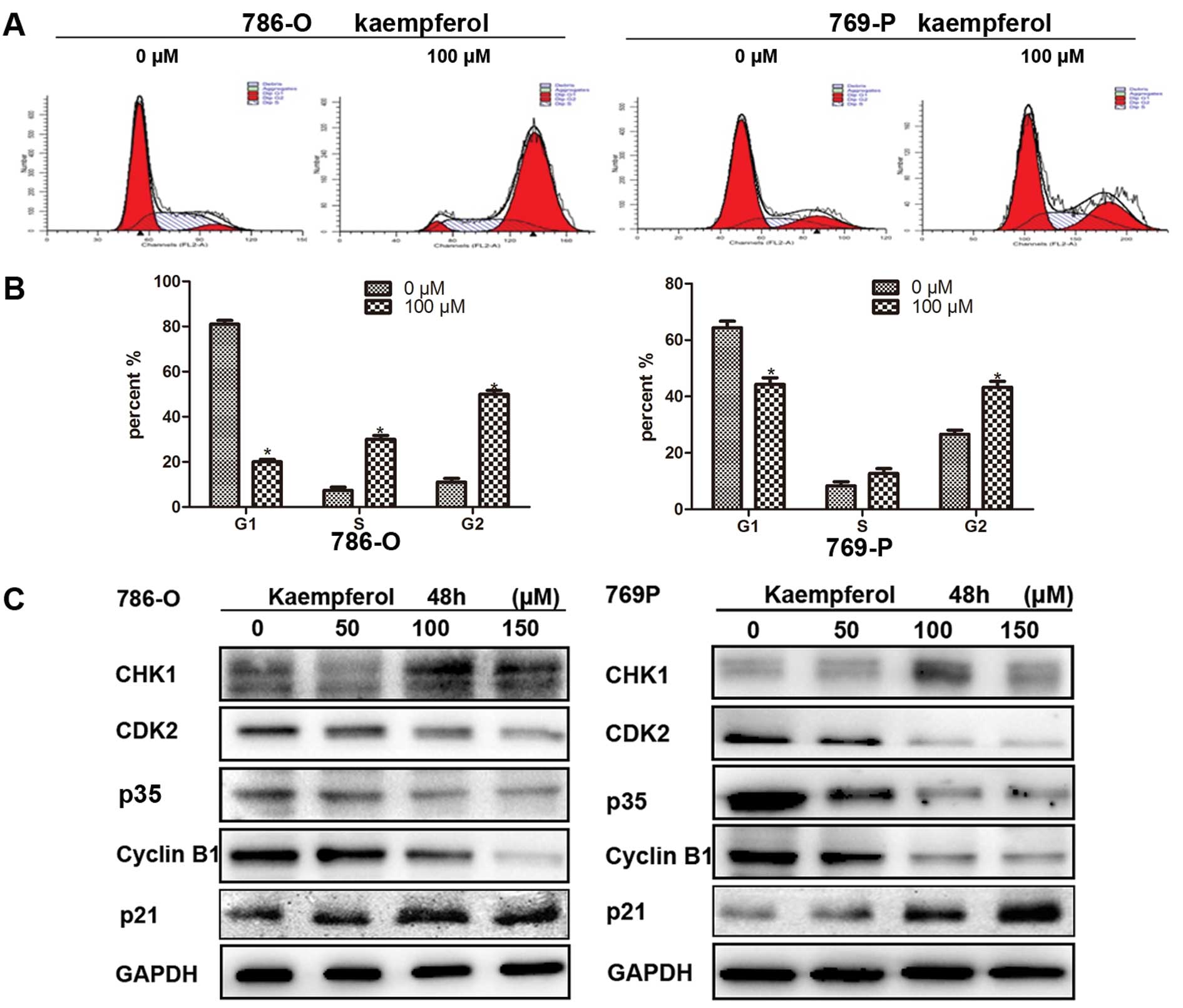
Kaempferol induces cell cycle arrest in
RCC cells
We further detected the effect of kaempferol on cell
cycle arrest by flow cytometry. After kaempferol treatment for 24 h
at 100 μM, most cells arrested mainly at phase G2-M stage 52.36% in
786-O cell and 43.45% in 769-P cells (Fig. 3A and B). Consistently, we observed
that several cell cycle related gene expressions were altered, for
example, Chk1 and p21waf1/Cip1 increased, while CDK2, p35 and
cyclin B1 decreased in 786-O and 769-P cells after treatment for 48
h with different doses of kaempferol (Fig. 3C). The results of Fig. 3A–C show that kaempferol induced cell
cycle arrest.
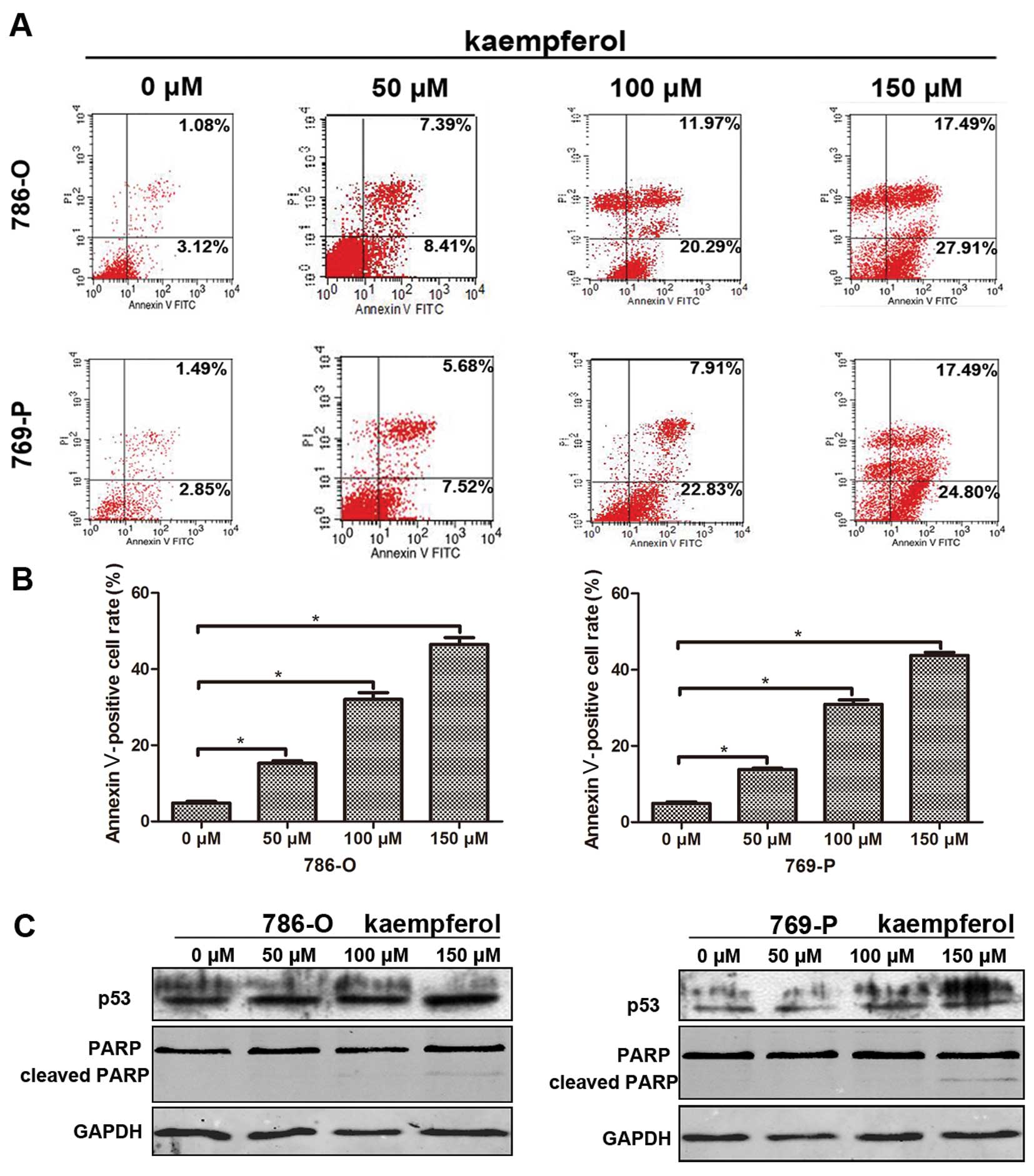
Kaempferol induces RCC cell
apoptosis
We also investigated the effects of kaempferol on
apoptosis in 786-O and 769-P cells after they were treated with
kaempferol for 48 h at 50, 100 and 150 μM. The flow cytometry data
(Fig. 4A) showed Annexin V positive
cells increased after treatment with kaempferol and there are
significant differences compared with the control group, with
Annexin V positive cells increased to 15.8, 32.26 and 45.4% in
786-O cells, and 13.2, 30.74 and 42.29% in 769-P cells (Fig. 4B). Next, we used western blotting to
detect apoptosis related gene expression, and we found p53 and
cleaved PARP increased after treatment with kaempferol (Fig. 4C).
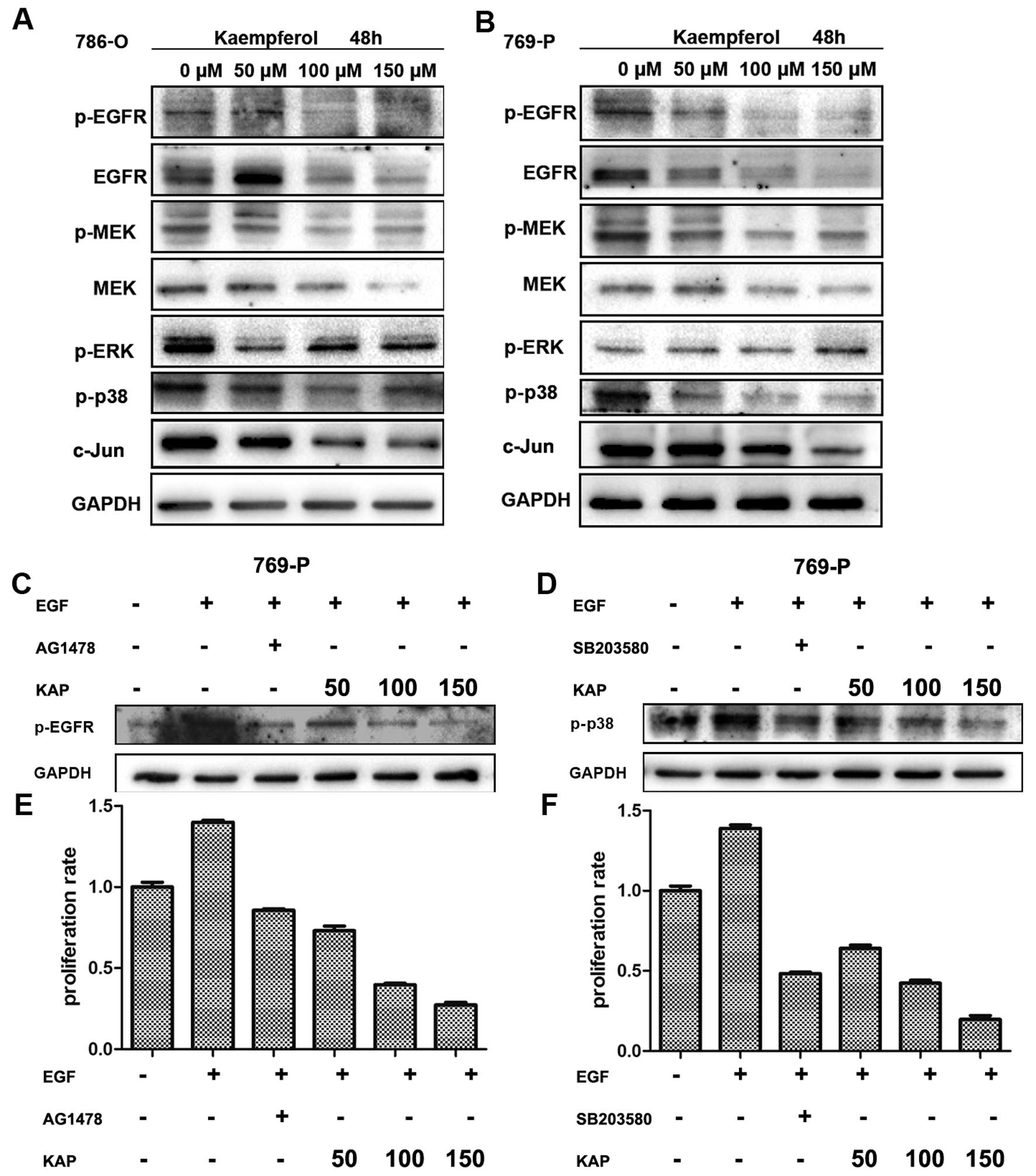
Kaempferol inhibits cell growth through
the EGFR/p38 MAPK signaling pathway
Since we showed that kaempferol inhibits cell
proliferation, the underlying mechanisms were then further
investigated. We screened the expression of some growth related
genes, and binding kaempferol significantly inhibited the
activation of the EGFR pathway, as shown in Fig. 5A and B, EGFR and phospho-EGFR, MEK
and phospho-MEK, phospho-p38 and its downstream c-jun, decreased
after treatment with kaempferol. However, we also found that the
expression of ERK increased, which may be related to stress, since
we know kaempferol induces (endoplasmic reticulum) stress.
As is well known, EGFR goes through the MAPK pathway
to influence cell proliferation (20). To delineate the growth inhibition of
RCC cells upon kaempferol treatment was mainly through the
EGFR/MAPK signaling pathway, we used EGFR inhibitor AG1478
(21) and p38 inhibitor SB203580
(22) to study their effects on
cell growth. First, as shown in Fig. 5C
and D, kaempferol blocked EGF induced p-EGFR and activation of
p38, similar to their inhibitors. This indicated that kaempferol
blocked the EGFR/p38 signaling pathway.
We used the MTT assay to detect cell viability of
786-O and 769-P after treatment with EGR as well as EGFR inhibitor
AG1478, or p38 inhibitor SB203580 and kaempferol for 48 h, as shown
in Fig. 5E and F. Kaempferol
significantly inhibited cell growth simulated EGFR inhibitor or p38
inhibitor. Collectively, we suggest kaempferol inhibited cell
growth via the EGFR/p38 signaling pathway.
Discussion
Kaempferol has been reported to inhibit the cell
growth of several types of cancer and to induce apoptosis. However,
in RCC, the effect of kaempferol remains unclear. This study is the
first to demonstrate that kaempferol inhibits RCC cell line growth
in vitro.
As our results show, kaempferol inhibited the cell
growth of two different RCC cell lines, 786-O and 769-P. In
previous studies, kaempferol was shown to inhibit prostate cancer
cell (13), hepatocyte (23) and lung cancer cell (24) growth. This suggests kaempferol has a
wide anti-proliferation capacity. In addition, our data showed 50
μM kaempferol has an effect on cell growth, which is μM grade and a
relatively low concentration, suggesting it has a low toxicity and
is relatively safe; however, in vivo studies to test and
verify this are required.
Cell cycle progression is tightly controlled by a
subfamily of cyclin-dependent kinases (CDKs), the activity of which
is regulated by several activators (cyclins) and cyclin-dependent
kinase inhibitors (CDKIs) (25). In
several other types of cancer, kaempferol was demonstrated to cause
G2/M phase arrest (26–29), but in our results we also observed
G1/S phase arrest in RCC cell lines, which is associated with
p21waf1/cip1 upregulation in RCC cells. Kaempferol could induce DNA
damage, increase ATM, and lead to CHK1/2 activation, which has
several effector substrates, including cyclin B1.
An aberrant activation of several growth signaling
pathways and evasion of apoptosis have been recognized as hallmarks
of cancer cell survival and growth including RCC (30,31)
cells and the inhibition of growth mediated by blocking the
EGFR/p38 pathway. We screened some growth related genes and found
phospho-p38 and its downstream c-jun was downregulated, but,
notably, phospho-ERK increased after treatment with kaempferol in
our study, which may mediate cell apoptosis (24) or may be related to cell stress
related with ER stress (32). Huang
et al have already found that kaempferol could induce ER
stress in osteosarcoma cells (11),
and ERK acts as a stress consequence. Next, we detected some
candidates upstream of p38, such as c-Met (20), and EGFR (33), which could lead to DNA synthesis and
cell proliferation. Therefore, we suggest that kaempferol may
function through the EGFR/p38 signaling pathway to inhibit cell
growth. We utilized EGFR inhibitor AG1478 and p38 inhibitor
SB203580 to confirm whether kaempferol has the same effects with
inhibitors. Our results support the hypothesis that the EGFR/p38
signaling pathway is involved in growth inhibition by kaempferol in
RCC.
In conclusion, the present study showed that
kaempferol causes a strong inhibition of the activation of EGFR/p38
signaling pathways, upregulation of p21 expression, and
downregulation of cyclin B1 expression in human RCC cells together
with activation of PARP cleavages, induction of apoptotic death,
and inhibition of cell growth. Further studies are required to
establish the efficacy of kaempferol in pre-clinical RCC models,
which may be useful in supporting a rationale for a clinical trial
in RCC patients.
References
|
1
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yagoda A, Abi-Rached B and Petrylak D:
Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin
Oncol. 22:42–60. 1995.
|
|
3
|
Li L, Gao Y, Zhang L, Zeng J, He D and Sun
Y: Silibinin inhibits cell growth and induces apoptosis by caspase
activation, down-regulating survivin and blocking EGFR-ERK
activation in renal cell carcinoma. Cancer Lett. 272:61–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bex A, Kerst M, Mallo H, Meinhardt W,
Horenblas S and de Gast GC: Interferon alpha 2b as medical
selection for nephrectomy in patients with synchronous metastatic
renal cell carcinoma: a consecutive study. Eur Urol. 49:76–81.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344.
2011.PubMed/NCBI
|
|
6
|
Cui Y, Morgenstern H, Greenland S, et al:
Dietary flavonoid intake and lung cancer - a population-based
case-control study. Cancer. 112:2241–2248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nothlings U, Murphy SP, Wilkens LR,
Henderson BE and Kolonel LN: Flavonols and pancreatic cancer risk:
the multiethnic cohort study. Am J Epidemiol. 166:924–931. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gates MA, Tworoger SS, Hecht JL, De Vivo
I, Rosner B and Hankinson SE: A prospective study of dietary
flavonoid intake and incidence of epithelial ovarian cancer. Int J
Cancer. 121:2225–2232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo H, Daddysman MK, Rankin GO, Jiang BH
and Chen YC: Kaempferol enhances cisplatin’s effect on ovarian
cancer cells through promoting apoptosis caused by down regulation
of cMyc. Cancer Cell Int. 10:162010.PubMed/NCBI
|
|
10
|
Kang JW, Kim JH, Song K, Kim SH, Yoon JH
and Kim KS: Kaempferol and Kaempferol and quercetin, components of
Ginkgo biloba extract (EGb 761), induce caspase-3-dependent
apoptosis in oral cavity cancer cells. Phytother Res. 24(Suppl 1):
S77–S82. 2010.PubMed/NCBI
|
|
11
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Du B, Wang T, Wang S and Zhang J:
Kaempferol induces apoptosis in human HCT116 colon cancer cells via
the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement
of p53 Upregulated Modulator of Apoptosis. Chem Biol Interact.
177:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin W, Lei Y-H, Su M, Li D-J, Zhang N and
Shen Y-Q: Kaempferol inhibits proliferation of human prostate
cancer PC-3 cells via down-regulation of PCNA and VCAM-1. Zhongguo
Yaolixue Tongbao. 27:553–557. 2011.(In Chinese).
|
|
14
|
Ahn MR, Kunimasa K, Kumazawa S, et al:
Correlation between antiangiogenic activity and antioxidant
activity of various components from propolis. Mol Nutr Food Res.
53:643–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schindler R and Mentlein R: Flavonoids and
vitamin E reduce the release of the angiogenic peptide vascular
endothelial growth factor from human tumor cells. J Nutr.
136:1477–1482. 2006.PubMed/NCBI
|
|
16
|
Shin MS, Shinghirunnusorn P, Sugishima Y,
et al: Cross interference with TNF-alpha-induced TAK1 activation
via EGFR-mediated p38 phosphorylation of TAK1-binding protein 1.
Biochim Biophys Acta. 1793:1156–1164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen D, Lane B, Jin T, et al: The
prognostic significance of epidermal growth factor receptor
expression in clear-cell renal cell carcinoma: a call for
standardized methods for immunohistochemical evaluation. Clin
Genitourin Cancer. 5:264–270. 2007. View Article : Google Scholar
|
|
18
|
Badalian G, Derecskei K, Szendroi A,
Szendroi M and Timar J: EGFR and VEGFR2 protein expressions in bone
metastases of clear cell renal cancer. Anticancer Res. 27:889–894.
2007.PubMed/NCBI
|
|
19
|
Fanger GR, Johnson NL and Johnson GL: MEK
kinases are regulated by EGF and selectively interact with
Rac/Cdc42. EMBO J. 16:4961–4972. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marshall CJ: Specificity of receptor
tyrosine kinase signaling: transient versus sustained extracellular
signal-regulated kinase activation. Cell. 80:179–185. 1995.
View Article : Google Scholar
|
|
21
|
Han Y, Caday CG, Nanda A, Cavenee WK and
Huang HJ: Tyrphostin AG 1478 preferentially inhibits human glioma
cells expressing truncated rather than wild-type epidermal growth
factor receptors. Cancer Res. 56:3859–3861. 1996.PubMed/NCBI
|
|
22
|
Clerk A and Sugden PH: The p38-MAPK
inhibitor, SB203580, inhibits cardiac stress-activated protein
kinases/c-Jun N-terminal kinases (SAPKs/JNKs). FEBS Lett.
426:93–96. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Labbe D, Provencal M, Lamy S, Boivin D,
Gingras D and Beliveau R: The flavonols quercetin, kaempferol, and
myricetin inhibit hepatocyte growth factor-induced medulloblastoma
cell migration. J Nutr. 139:646–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen TTT, Tran E, Ong CK, et al:
Kaempferol-induced growth inhibition and apoptosis in A549 lung
cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol.
197:110–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang WW, Tsai SC, Peng SF, et al:
Kaempferol induces autophagy through AMPK and AKT signaling
molecules and causes G2/M arrest via downregulation of
CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol.
42:2069–2077. 2013.PubMed/NCBI
|
|
27
|
Naowaratwattana W, De-Eknamkul W and De
Mejia EG: Phenolic-containing organic extracts of mulberry
(Morus alba L.) leaves inhibit HepG2 hepatoma cells through
G2/M phase arrest, induction of apoptosis, and inhibition of
topoisomerase IIα activity. J Med Food. 13:1045–1056.
2010.PubMed/NCBI
|
|
28
|
Zhang Q, Zhao XH and Wang ZJ: Cytotoxicity
of flavones and flavonols to a human esophageal squamous cell
carcinoma cell line (KYSE-510) by induction of G2/M arrest and
apoptosis. Toxicol In Vitro. 23:797–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mu JJ, Zeng YY, Huang XY, Zhao XH and Song
B: Effects of Kaempferol on activation, proliferation and cell
cycle of mouse T lymphocytes in vitro. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 25:1106–1108. 2009.(In Chinese).
|
|
30
|
Eto M and Naito S: Molecular targeting
therapy for renal cell carcinoma. Int J Clin Oncol. 11:209–213.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Staehler M, Rohrmann K, Haseke N, Stief CG
and Siebels M: Targeted agents for the treatment of advanced renal
cell carcinoma. Curr Drug Targets. 6:835–846. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang LJ, Chen S, Wu P, et al: Inhibition
of MEK blocks GRP78 up-regulation and enhances apoptosis induced by
ER stress in gastric cancer cells. Cancer Lett. 274:40–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oda K, Matsuoka Y, Funahashi A and Kitano
H: A comprehensive pathway map of epidermal growth factor receptor
signaling. Mol Syst Biol. 1:2005.00102005.PubMed/NCBI
|