Introduction
Colorectal cancer (CRC) is the third most common
cancer type in men and women worldwide. CRC is thought to result
from an interaction between environmental and genetic factors
(1).
Currently, functional variation of DNA repair and
cell cycle control-related genes in the presence of
carcinogen-mediated cell damage is believed to be a mechanism for
explaining inter-individual variation in CRC susceptibility
(1).
Analysis of phenotype concordance in monozygotic
twin CRC cases suggest that inherited susceptibility underlies 35%
of all CRCs. However, only 6% of CRCs occur in the context of a
known high-penetrance cancer predisposition syndrome, such as
familial adenomatous polyposis or Lynch syndrome (2,3).
Therefore, most of the genetic risks for CRC remain unknown
(4).
Fearon and Vogelstein (5) proposed a model for the development of
CRC whereby colorectal carcinoma arises and progresses through
histological stages due to an accumulation of genetic and
epigenetic changes. A particular stage of progression of late
adenoma to adenocarcinoma involves mutations in TP53. p53
regulates many cellular functions including cell cycle progression,
DNA repair, senescence, apoptosis and cellular metabolism (6). In normal cells, the expression level
of p53 is extremely low. However, p53 protein levels increase in
response to various stress signals, such as DNA damaging agents,
oxidative stress, amino acid depletion and temperature change
(7).
In addition to the gene mutation, which represent
the most common TP53 genetic alteration, multiple single
nucleotide polymorphisms (SNPs) have been identified in this gene.
However, the relevance of the majority of the SNPs remains unclear.
The p53 codon 72 SNP (rs1042522), which is located in exon 4 within
the p53 transactivation domain in a proline-rich region, results in
the expression of either a proline or an arginine due to a
nucleotide substitution of the second base in the codon
(CCC>CGC), thus, changing from an amino acid with a non-polar
aliphatic side chain to an amino acid with a positively charged
basic side chain (1).
Several lines of evidence suggest that the resulting
two alleles confer different properties to the p53 protein. These
p53 variants are not biochemically equivalent, since they show
different transcriptional regulation activities, interactions with
p73 (a homologue of p53) and degradation rates mediated by the
proteasome (8). The allele with
proline (Pro72) is considered the wild-type one (9), and it appears less efficient than the
allele with arginine (Arg72) at suppression of cell transformation
and induction of apoptosis (10).
Structural and functional features of p53 might be useful as a
molecular prognostic marker (11).
An association between the genotyped p53 codon 72
SNP and human cancer risk has been reported in breast (12,13),
gastric (14,15), thyroid (16,17),
lung (18,19), vulval (20) and bladder cancers (21,22).
However, this SNP does not appear to affect the risk of cervical
(23,24), prostate (25,26)
and endometrial cancers (27,28)
and head and neck squamous cell carcinomas (29,30).
Previous studies have shown that the p53 codon 72
SNP is associated with the risk of CRC or its precursor lesion
adenoma (8,31–36),
while others have reported discordant results (37,38).
The p53 codon 72 SNP was not found to be associated with the
alteration of colorectal cancer risk in a meta-analysis with 20
case-control studies (1).
Mammano et al (8) demonstrated that the genotyped p53
codon 72 SNP is associated with a higher risk of CRC and with more
advanced and undifferentiated tumors, suggesting that this SNP may
play a role in the progression of CRC.
Most epidemiological studies have evaluated the
genotypes of polymorphic genes, searching for alterations in cancer
risk. However, it is imperative to evaluate which allele is
expressed in the tumor as there may be preferential expression of a
specific allele in heterozygotes, which may explain the discordance
data related to association of p53 codon 72 SNP with CRC. Moreover,
evaluation of the association of the expression of this SNP with
patient clinicopathological variables and prognosis can provide
relevant data not previously identified.
In order to shed light on the role of this SNP in
CRC, we conducted a p53 codon 72 SNP expression analysis searching
for associations with p53 protein expression, TP53
mutations, patient clinicopathological variables and prognosis.
Materials and methods
Study population
We examined mRNA from 101 patients with sporadic
origin colorectal tumors who were treated at the Hospital A.C.
Camargo (São Paulo, Brazil) and who underwent surgical resection
for colorectal adenocarcinoma between 1992 and 2006. Individuals
fulfilling any familial syndrome clinical criteria or with
inflammatory bowel diseases and those treated with preoperative
chemoradiotherapy were excluded from the present study which was
reviewed and approved by a duly appointed ethics committee
(1042/08).
Clinicopathological data
All clinical data were collected from patient
reports, and pathological data of the CRC cases were systematically
evaluated by an experienced gastrointestinal pathologist (R.A.C.).
The data collected include gender, age at diagnosis, smoking habit
(yes or no), CRC location, histological grade, TNM stage
(UICC/AJCC), dirty necrosis, desmoplasia, Crohn’s-like lymphocytes,
infiltrating lymphocytes, vascular and lymphatic invasion, budding,
tumor border pattern of growth (expanding or infiltrating), tumor
recurrence, use of post-chemoradiotherapy (radiotherapy only for
rectal tumors) and follow-up time. Tumor budding was defined as an
isolated single cancer cell or a cluster composed of fewer than 5
cancer cells observed in the stroma of the actively invasive area
(39).
Immunohistochemistry
The expression status of p53 was evaluated using
immunohistochemistry (IHC) technique. IHC staining was performed on
3-μm formalin paraffin-embedded (FFPE) tissues. The reactions were
performed using a p53 monoclonal antibody (DO7 clone, 1:100
dilution; Dako, Glostrup, Denmark) and a polymer-based detection
system (Advance HRP Link Polymer amplification system; Dako).
Positive staining was defined as an unequivocal nuclear staining of
neoplastic cells. The percentage of positively stained neoplastic
cells was quantified. A tumor was considered positive when >20%
of its cells were stained.
RNA extraction
Fresh samples were matched with FFPE tissues used
for IHC. Total RNA was extracted from manually microdissected
frozen tissues with at least 70% of tumor cells (10–100 mg) by
homogenizing each tissue sample using Precellys equipment (Bertin
Technologies, Villeurbanne, France) in 1 ml of TRIzol reagent
according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA,
USA). RNA integrity was assessed using an Agilent 2100 Bioanalyzer
(Agilent Technologies, Foster City, CA, USA), and RNA was stored at
−80°C prior to use.
RT-PCR
Total mRNA was employed to synthesize cDNA for
TP53 allele expression and mutation analyses using a High
Capacity cDNA reverse transcription kit (Applied Biosystems, Foster
City, CA, USA). The TP53 transcript was amplified as two
overlapping fragments, from exon 2 to 6 and from exon 6 to 11,
covering the entire coding region. PCR was performed in 25-μl
reactions containing 20 ng of template DNA, 1.5 mM
MgCl2, 0.2 mM dNTP, 0.3 μM of forward and reverse
primers and 1.5 U of Platinum® Taq Polymerase
(Invitrogen). PCR products were analyzed on an agarose gel
containing SYBR Safe (Invitrogen). PCR primers are described in
Table I.
 | Table IAmplification and sequencing
primers. |
Table I
Amplification and sequencing
primers.
| Use | Primer | Sequence | Amplicon (bp) |
|---|
| PCR fragment 1 | E2ForE
RNAm
E6RevE RNAm |
GACGGTGACACGCTTCCCTG
CACCACCACACTATGTCG | 708 |
| PCR fragment 2 | E6ForE
RNAm
E11RevE RNAm |
CCTCAGCATCTTATCCGAG
AGGCTGTCAGTGGGGAAC | 645 |
| Sequencing fragment
1 | E2ForI
RNAm
E6RevI RNAm |
CAGCCAGACTGCCTTCCGGGTC
CTGTCATCCAAATACTCCACACG | 654 |
| Sequencing fragment
2 | E6ForI
RNAm
E11RevI RNAm |
GGAAATTTGCGTGTGGAG
CAAGAAGTGGAGAATGTC | 604 |
Sequencing analysis
Samples were screened for mutations over the entire
coding region of TP53 and for the presence of polymorphic
variants at codon 72. ExoSAP-IT (1 μl, USB; Affymetrix, Cleveland,
OH, USA) was used to purify 7 μl of the PCR product. Sequencing
reactions were performed using the Big Dye Terminator v3.1 Cycle
sequencing kit (Applied Biosystems) with specific primers that
overlapped the region amplified (Table
I) and an ABI PRISM 3730xl Automatic Genetic analyzer (Applied
Biosystems), according to the manufacturer’s recommendations.
The TP53 polymorphic and mutation status was
analyzed using CLC Main Workbench software (CLCbio version 4.6)
with p53 NM_000546 reference sequence. Allele expression was
determined using electropherogram data from the sequencing
analysis. Double peaks of C and G nucleotides were considered to
indicate heterozygosity.
Statistical analysis
To test the distribution of genotypes and the
relationship between the p53 codon 72 SNP and clinical variables,
data were analyzed using the 2-sided Pearson’s Chi-square or
Fisher’s exact tests in the SPSS v17.0 program (SPSS, Inc.,
Chicago, IL, USA). A P<0.05 was considered to indicate a
statistically significant result.
To identify the variables associated with
recurrence, univariate analysis was performed. Variables with
P<0.20 were selected for multiple logistic regression model. In
this model we considered variables with P<0.05 and present the
OR and the 95% CI. To determine the variables associated with
survival, univariate analysis was performed using the Kaplan-Meier
and log rank test. Variables with P<0.20 were selected for the
Cox proportional hazards regression model and the OR and 95% CI are
presented. An α error of 5% was considered.
Results
Clinicopathological characteristics
The present study was performed using samples from
101 patients consisting of 49 men and 52 women with a mean age of
62.4 years (median age 63 years; age range 27–88 years). There were
52 (51.5%) tumors with negative and 49 (48.5%) with positive
p53-protein nuclear accumulation. The tumor was located in the
proximal colon in 36 cases (35.6%), in the distal colon in 42 cases
(41.6%) and in the rectum in 23 cases (22.8%). Regarding the
histological grade, 9 (8.9%) were well differentiated, 80 (79.2%)
were moderately differentiated and 12 (11.9%) were poorly
differentiated. Based on TNM staging criteria, 20 (19.8%) tumors
were stage I, 34 (33.7%) were stage II, 26 (25.7%) were stage III
and 21 (20.8%) were stage IV; 58 (57.4%) tumors were N0, 21 (20.8%)
were N1 and 22 (21.8%) were N2; and 80 (79.2%) tumors were M0 and
21 (20.8%) were M1 (Table II).
 | Table IIAssociation between p53 codon 72
polymorphism and clinicopathological variables. |
Table II
Association between p53 codon 72
polymorphism and clinicopathological variables.
| | Allele
expression | | Arginine allele
expression |
|---|
| |
| |
|
|---|
| Variables | N (%) | Arginine (%) | Arg/Pro (%) | Proline (%) | P-value | Expressers (%) | No expressers
(%)a | P-value |
|---|
| CRC cases | 101 (100) | 67 (66.4) | 16 (15.8) | 18 (17.8) | - | 83 (82.2) | 18 (17.8) | - |
| Gender |
| Males | 49 (48.5) | 29 (43.3) | 7 (43.8) | 13 (72.2) | NS | 36 (43.4) | 13 (72.2) | 0.037 |
| Females | 52 (51.5) | 38 (56.7) | 9 (56.3) | 5 (27.8) | | 47 (56.6) | 5 (27.8) | |
| Age at onset
(years) |
| <50 | 19 (18.8) | 10 (14.9) | 5 (31.3) | 4 (22.2) | NS | 15 (18.1) | 4 (22.2) | NS |
| ≥50 | 82 (81.2) | 57 (85.1) | 11 (68.8) | 14 (77.8) | | 68 (81.9) | 14 (77.8) | |
| Smoking habit |
| No | 82 (85.4) | 52 (82.5) | 13 (86.7) | 17 (94.4) | NS | 65 (83.3) | 17 (94.4) | NS |
| Yes | 14 (14.6) | 11 (17.5) | 2 (13.3) | 1 (5.6) | | 13 (16.7) | 1 (5.6) | |
| Tumor location |
| Proximal | 36 (35.6) | 26 (38.8) | 5 (31.3) | 5 (27.8) | NS | 31 (37.3) | 5 (27.8) | NS |
| Distal | 42 (41.6) | 26 (38.8) | 9 (56.3) | 7 (38.9) | | 35 (42.2) | 7 (38.9) | |
| Rectum | 23 (22.8) | 15 (22.4) | 2 (12.5) | 6 (33.3) | | 17 (20.5) | 6 (33.3) | |
| Histological
grade |
| Well
differentiated | 9 (8.9) | 8 (11.9) | 1 (6.3) | 0 | NS | 9 (10.8) | 0 | NS |
| Moderately
differentiated | 80 (79.2) | 52 (77.6) | 14 (87.5) | 14 (77.8) | | 66 (79.5) | 14 (77.8) | |
| Poorly
differentiated | 12 (11.9) | 7 (10.4) | 1 (6.3) | 4 (22.2) | | 8 (9.6) | 4 (22.2) | |
| Tumor infiltration
(T) |
| T1+T2 | 24 (23.8) | 19 (28.4) | 4 (25) | 1 (5.6) | NS | 23 (27.7) | 1 (5.6) | NS |
| T3+T4 | 77 (76.2) | 48 (71.6) | 12 (75) | 17 (94.4) | | 60 (72.3) | 17 (94.4) | |
| Nodal status
(N) |
| N0 | 58 (57.4) | 41 (61.2) | 9 (56.3) | 8 (44.4) | NS | 50 (60.2) | 8 (44.4) | NS |
| N1+N2 | 43 (42.6) | 26 (38.8) | 7 (43.8) | 10 (55.6) | | 33 (39.8) | 10 (55.6) | |
| Metastasis (M) |
| M0 | 80 (79.2) | 55 (82.1) | 12 (75) | 13 (72.2) | NS | 67 (80.7) | 13 (72.2) | NS |
| M1 | 21 (20.8) | 12 (17.9) | 4 (25) | 5 (27.8) | | 16 (19.3) | 5 (27.8) | |
| TNM stage |
| I–II | 54 (53.5) | 39 (58.2) | 9 (56.3) | 6 (33.3) | NS | 48 (57.8) | 6 (33.3) | NS |
| III–IV | 47 (46.5) | 28 (41.8) | 7 (43.8) | 12 (66.7) | | 35 (46.5) | 12 (66.7) | |
| Dirty necrosis |
| Not observed | 51 (51) | 36 (54.5) | 9 (53.6) | 6 (33.3) | NS | 45 (54.9) | 6 (33.3) | NS |
| Present | 49 (49) | 30 (45.5) | 7 (43.8) | 12 (66.7) | | 37 (45.1) | 12 (66.7) | |
| Desmoplasia |
| Not observed | 29 (28.7) | 20 (29.9) | 5 (31.3) | 4 (22.2) | NS | 25 (30.1) | 4 (22.2) | NS |
| Present | 72 (71.3) | 47 (70.1) | 11 (68.8) | 14 (77.8) | | 58 (69.9) | 14 (77.8) | |
| Crohn’s-like
lymphocytes |
| Not observed | 100 (99) | 66 (98.5) | 16 (100) | 18 (100) | NS | 82 (98.8) | 18 (100) | NS |
| Present | 1 (1) | 1 (1.5) | 0 | 0 | | 1 (1.2) | 0 | |
| Infiltrating
lymphocytes |
| Not observed | 3 (3) | 3 (4.5) | 0 | 0 | NS | 3 (3.6) | 0 | NS |
| Present | 98 (97) | 64 (95.5) | 16 (100) | 18 (100) | | 80 (96.4) | 18 (100) | |
| Vascular
invasion |
| Not observed | 91 (90.1) | 62 (92.5) | 14 (87.5) | 15 (83.3) | NS | 76 (91.6) | 15 (83.3) | NS |
| Present | 10 (9.9) | 5 (7.5) | 2 (12.5) | 3 (16.7) | | 7 (8.4) | 3 (16.7) | |
| Lymphatic
invasion |
| Not observed | 88 (87.1) | 57 (85.1) | 14 (87.5) | 17 (94.4) | NS | 71 (85.5) | 17 (94.4) | NS |
| Present | 13 (12.9) | 10 (14.9) | 2 (12.5) | 1 (5.6) | | 12 (14.5) | 1 (5.6) | |
| Budding |
| Not observed | 70 (69.3) | 48 (71.6) | 11 (68.8) | 11 (61.1) | NS | 59 (71.1) | 11 (61.1) | NS |
| Present | 31 (30.7) | 19 (28.4) | 5 (31.3) | 7 (38.9) | | 24 (28.9) | 7 (38.9) | |
| Tumor border |
| Infiltrating | 69 (68.3) | 42 (62.7) | 11 (68.8) | 16 (88.9) | NS | 53 (63.9) | 16 (88.9) | 0.05 |
| Expanding | 32 (31.7) | 25 (37.3) | 5 (31.3) | 2 (11.1) | | 30 (36.1) | 2 (11.1) | |
| Recurrence |
| Negative | 85 (86.7) | 61 (93.8) | 12 (80) | 12 (66.7) | 0.008 | 73 (91.3) | 12 (66.7) | 0.013 |
| Positive | 13 (13.3) | 4 (6.2) | 3 (20) | 6 (33.3) | | 7 (8.8) | 6 (33.3) | |
|
Chemoradiotherapy |
| No | 52 (53.1) | 41 (62.1) | 8 (53.3) | 3 (17.6) | 0.005 | 49 (60.5) | 3 (17.6) | 0.002 |
| Yes | 46 (46.9) | 25 (37.9) | 7 (46.7) | 14 (82.4) | | 32 (39.5) | 14 (82.4) | |
| p53 IHC
expression |
| Negative | 52 (51.5) | 31 (46.3) | 13 (81.3) | 8 (44.4) | 0.034 | 45 (54.2) | 8 (44.4) | NS |
| Positive | 49 (48.5) | 36 (53.7) | 3 (18.8) | 10 (55.6) | | 38 (45.8) | 10 (55.6) | |
| p53 mutation |
| Not observed | 46 (45.5) | 28 (41.8) | 13 (81.3) | 5 (27.8) | 0.004 | 41 (49.4) | 5 (27.8) | NS |
| Positive | 55 (54.5) | 39 (58.2) | 3 (18.8) | 13 (72.2) | | 42 (50.6) | 13 (72.2) | |
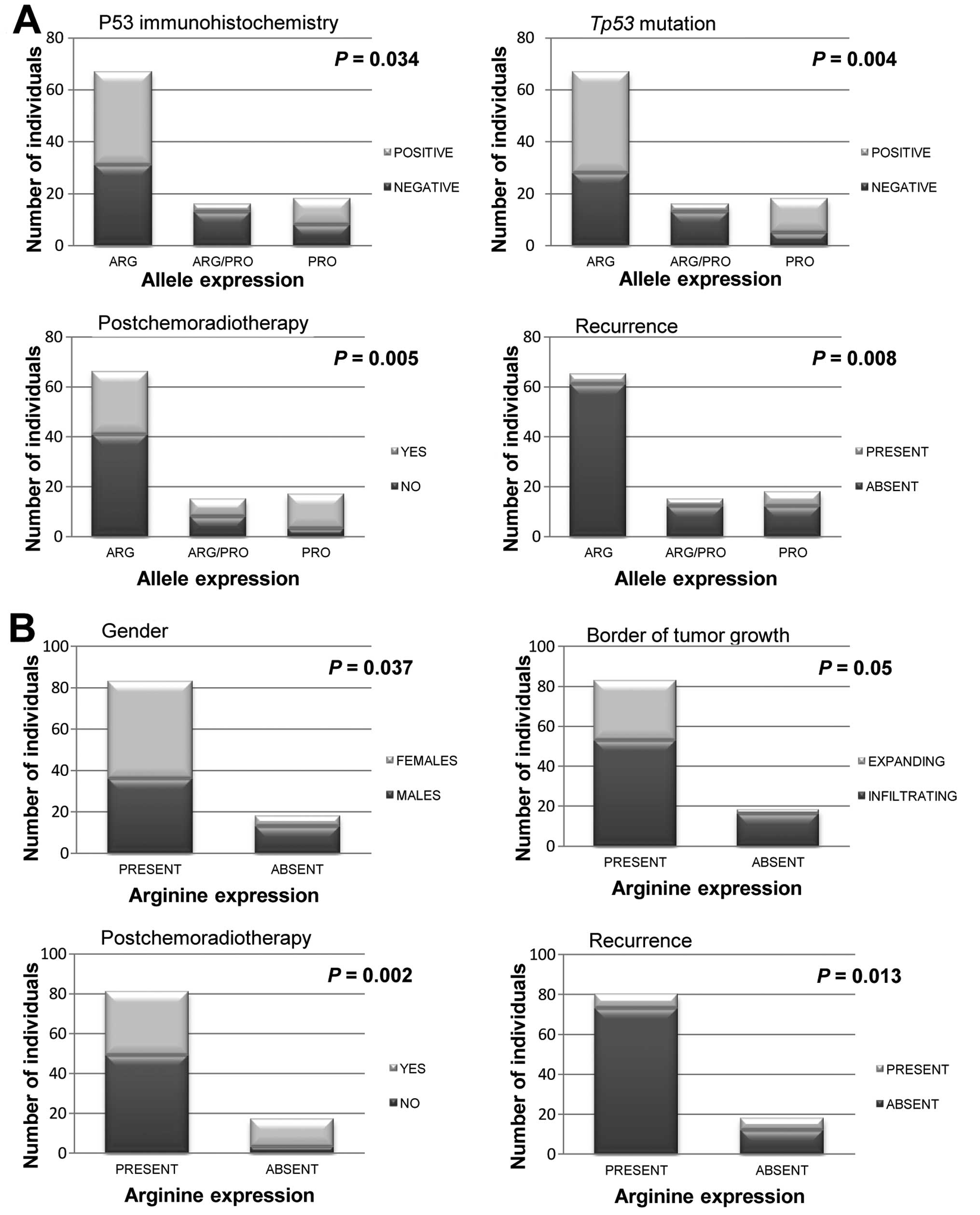
Allelic expression associations
All significant associations are shown in Fig. 1. The expression allelic frequencies
were 0.26 for Pro72 and 0.74 for Arg72. With regard to allele
expression, 66.4% (n=67) of individuals were homozygote for Arg72,
15.8% (n=16) were heterozygote and 17.8% (n=18) were homozygote for
Pro72. The analysis also considered the presence of expression of
the Arg72 allele: 83 samples (82.2%) expressed Arg72 and 18 (17.8%)
did not express it. When Arg72 allele expression was correlated
with gender, we found that among the CRC tissues from women, 90.4%
expressed Arg72 (at least one allele) and 72.2% of the CRC tissues
from men expressed Pro72 exclusively (P=0.037). Of the declared
smokers, 64.3% were men.
Correlations between the alleles and the IHC-derived
p53 monoclonal antibody staining revealed that the presence of
either the Pro72 or Agr72 allele did not affect protein expression.
However, the presence of both alleles was associated with normal
cellular conditions since 81.3% of the heterozygotes showed the
normal absence of expression of the p53 protein (P=0.034).
The association between the SNP and tumor stage
revealed that 94.4% of those patients not expressing the Arg72
allele presented tumors with T3 and T4 stages, whereas 95.8% of the
tumors in T1 and T2 stages were Arg72 expressers (P=0.064).
Correlations between the polymorphism and the tumor
characteristics showed that the pattern border of tumor growth
varied with the allele expressed. An infiltrating border of tumor
growth was present in 88.9% of those tumors not expressing the
Arg72 allele, and among all expanding-border tumors, 93.8%
expressed Arg72 (P=0.05).
Another finding from the present study was the
relationship between tumor recurrence and this SNP since 93.8% of
the Arg72-exclusive expresser tumors did not show tumor recurrence
(P=0.008). Similarly, 91.3% of those tumors with Arg72 expression
did not show recurrence (P=0.013). The average time for tumor
recurrence was 17 months post surgery.
Further analysis showed a statistically significant
relationship between the p53 codon 72 SNP and the use of
postoperative adjuvant chemoradiotherapy. Of the tumors expressing
Pro72 exclusively, 82.4% of individuals underwent some type of
post-chemoradiotherapy (P=0.002). Of those who did not receive
post-chemoradiotherapy, 78.8% of the tumors expressed Arg72
exclusively (P=0.005).
The present study did not evidence significant
difference between this SNP and the relative CRC-free survival
rates. In the Cox proportional survival model, the independent
variables were TNM stage (TNM I–II and III–IV; OR, 4.38; CI
1.84–10.43; P=0.001) and perineural invasion (OR, 3.21; CI
1.26–8.19; P=0.014) (Table
III).
 | Table IIIMultivariate analysis. |
Table III
Multivariate analysis.
| Variables | OR | CI 95% | P-value |
|---|
| Survival |
| TNM stages |
| I–II | 1 | 1.84–10.43 | 0.001 |
| III–IV | 4.38 | | |
| Perineural
invasion |
| Not observed | 1 | 1.26–8.19 | 0.014 |
| Observed | 3.21 | | |
| Recurrence |
| Arg 72 |
| Expressers | 1 | 1.02–14.35 | 0.046 |
| No
expressers | 3.83 | | |
| TNM stages |
| I–II | 1 | 1.45–35.29 | 0.016 |
| III–IV | 7.15 | | |
In the multiple logistic regression model, the
variables that were independent predictors for recurrence were the
expression of Arg72 allele (OR 3.83; CI 1.02–14.35; P=0.046) and
the TNM stage (TNM I–II and III–IV; OR, 7.15; CI 1.45–35.29;
P=0.016) (Table III). This model
explained 24.5% of the variation of recurrence.
Mutation analysis
We also evaluated the mutation status of the
TP53 gene in the CRC samples, published in detail (40). Relative to the alleles expressed,
pathogenic mutations were detected in 72.2% (n=13) of the
Pro72-exclusive expressers, in 18.8% (n=3) of the heterozygotes and
in 58.2% (n=39) of the Arg72-exclusive expressers (P=0.004).
Detailed analyses revealed that among the tumors showing no
mutations, 89.1% were Arg72 expressers.
Discussion
mRNA analysis
Genome-wide association studies (GWAS) assaying
hundreds of thousands of SNPs have successfully identified a large
number of genetic variants associated with complex traits, but each
of those variant often confers only a modest increase in risk. One
consequence of these small effects is that even combined, these
discoveries only explain a small proportion of the entire genetic
contribution to risk of disease, thus, leading to the ‘missing
heritability’ question (41).
Many reasons for the missing heritability have been
discussed (42,43). Complex patterns of inheritance
(44), epigenetic modifications of
the genome, common copy-number variants (CNVs) (45), analysis of gene-environment and
gene-gene interactions (epistasis) (46) and the recently proposed ‘synthetic
association’ signals created by rare variants in GWAS (47) can contribute to the missing
heritability. However, we propose that there may be differences in
the expression of different polymorphic alleles in large
heterozygote populations that may explain cancer behavior and that
the real associations can be missed looking only for genomic
alterations.
Studies have shown that variations exist in the
relative allelic expression levels of specific genes in
heterozygotes that contribute to phenotypic variation between
individuals. (48–53). Monoallelic expression with random
choice between paternal and maternal alleles has also been shown to
affect hundreds of autosomal genes and, thus, contribute to
individual cell variability (54).
The results presented in the present study highlight
a major limitation in comparing our results with other reports
since the majority of studies looking for relationships between
polymorphisms and CRC examine the genotype of the individuals
rather than the allele expressed in tumors. Siddique et al
(13) showed that breast tumors
from heterozygous Chinese women preferentially expressed the Pro72
allele compared to health ones. Thus, the expression status of the
p53 alleles in tumors, rather than the genotype, may be a
determining factor to better understand the tumor process.
Pro72 vs. Arg72
The p53 codon 72 SNP occurs in the proline-rich
domain of p53, which is necessary for the protein to fully induce
apoptosis. The polymorphic forms of the p53 protein result in
marked alterations in the protein primary structure (55). Data from Marin et al
(10) suggest that the Pro72 allele
displays decreased efficiency in binding p73 and consequently
inhibits p73-dependent apoptosis in p53 mutants. Dumont et
al (56) found that in cell
lines containing inducible versions of alleles encoding the Pro72
and Arg72 variants, and in cells with endogenous p53, the Arg72
variant induced much greater levels of apoptosis than the Pro72
variant. The higher induction of apoptosis by the Arg72 allele
results from the increased localization of p53 Arg72 to the
mitochondria, which is accompanied by the release of cytochrome
c into the cytosol (56).
Although further studies are required, differences in the
mitochondrial localization of the isoforms may also indicate that
the p53 codon 72 SNP affects the ability of p53 to regulate
mitochondrial respiration and other metabolic factors (7). Oseki et al (57) showed that these two polymorphic
variants differed particularly within the N-terminal region and
consequently, they differ in post-translational modifications at
this portion. The Arg72 variant shows significantly enhanced
phosphorylation at Ser-6 and Ser-20 compared with the Pro72
variant.
Allelic expression associations
We investigated whether the expression of the
Pro/Arg alleles of p53 in CRC correlates with cancer behavior and
progression. In studies evaluating genetic polymorphisms and
cancer, typically only the association with cancer risk is
investigated. However, the relationship between polymorphisms and
clinicopathological characteristics of the tumor must be elucidated
to enable understanding of the tumor pathogenesis and the tumor
course.
Our data revealed that there was a high number of
tumors expressing Arg72 in the CRC cohort although our results also
showed that the expression of the Arg72 allele may exert a
protective effect in this population. The expression of the Pro72
allele in CRC tissues may confer a poorer prognosis since
expression of this allele was associated with tumor recurrence.
Individuals who were Arg72 allele expressers presented a low number
of cancer recurrences, lower grade tumors, expanding tumor borders
in the majority of the cases and less frequent TP53
mutations.
The relationship between gender and the SNP showed
an apparent advantage for women as fewer tumors expressed the Pro72
allele in women. A common environmental source of DNA damage is
cigarette smoke, which contains many mutagenic compounds. If p53
protects cells from DNA damage caused by exposure to these
mutagens, the degree of protection should vary with the strength
and nature of the p53 response. Thus, individuals with a weaker p53
response may be less capable of responding appropriately to
cigarette smoke, which in turn will affect the ability to promote
an apoptotic function (58).
Indeed, epidemiological evidence reveals an association between
this SNP and cigarette smoking in lung and bladder cancer patients
(58,59). Thus, one possible explanation for
our finding is that men typically initiate smoking earlier and
smoke more frequently than women (60,61).
These findings were replicated in the present study (64.3% of
smokers were men) although we did not find a statistically
significant correlation between smoking, gender and the expression
of a specific allele. Furthermore, it is important to note that
data of this type of habit are derived from self-reported ‘yes or
no’ questionnaires, which may underestimate the true extent of
smoking (62).
Several studies have indicated that individual
susceptibility factors, including DNA repair capacity, metabolic
capacity and variation in genes involved in these processes, may
modulate the genotoxicity of xenobiotics (63,64).
Hanova et al (65) showed a
possible relationship between styrene exposure, DNA damage and the
transcript levels of TP53.
Tumor-host interaction at the invasive front of
colorectal cancer represents a critical interface where tumor
progression and tumor cell dissemination arise. The expanding tumor
border, identified as presenting margins reasonably
well-circumscribed, is often associated with a well-developed
inflammatory infiltrate (66,67).
In contrast, the infiltrative tumor border is characterized by
widespread dissection of normal tissue structures with a loss in
the clear boundary between tumor and host tissues. The infiltrating
tumor border configuration promotes progression and dissemination
of tumor cells by penetrating the vascular and lymphatic vessels
(66,67). Studies have revealed that the
infiltrative pattern of growth is an adverse prognostic factor and
may predict local recurrence (68),
whereas the expanding pattern was related with improved survival
(67), which is consistent with our
results.
The p53 pathway is critical in mediating the
response of commonly used cancer therapies. There is evidence that
the TP53 gene has functional SNPs that affect p53 signaling,
thus, possibly altering cancer risk and clinical outcome (69). How the functional p53 SNPs interact
with known cancer risk factors and therapeutics remains to be
answered. The present study provides evidence for the protective
effect of Arg72 expression on the requirement for postoperative
adjuvant chemoradiotherapy.
Adjuvant therapy for colorectal cancer forms an
essential component of an effective treatment strategy. Initially,
adjuvant chemotherapy for colorectal cancer was delivered in the
post-operative setting following ‘curative’ surgery to destroy any
residual or micrometastatic disease. Today, the effects of
chemotherapy for colorectal cancer include delaying and possibly
preventing recurrences following ‘curative’ surgery, downsizing
incurable disease in the pre-operative setting and significantly
expanding the median survival in the advanced metastatic setting
(70). Despite the large number of
factors involved in predicting clinical outcome in patients with
colorectal cancer, the histologic stage at surgical diagnosis
remains the most important prognostic variable (71). Thus, the selection of appropriate
patients to receive adjuvant therapy has been based on their risk
of recurrence after surgery only and on disease variables known to
adversely affect prognosis, and the selection of systemic agents
has been typically based on antitumor activity in patients with
advanced disease of similar histology (72).
In particular, 5-fluorouracil (5-FU) is widely used
in the treatment of a range of cancers and has demonstrated the
largest impact on CRC. TP53 can be activated by 5-FU through
more than one mechanism including incorporation of fluorouridine
triphosphate into RNA, fluorodeoxyuridine triphosphate into DNA and
inhibition of thymidylate synthase with resultant DNA damage
(73). TP53 status
expectedly appears to have predictive value for the survival of CRC
patients receiving 5-FU chemotherapy (74).
One study suggest that cells from individuals that
carry the Pro72 allele may undergo less apoptosis in response to
DNA damage-inducing therapies when compared with individuals
carrying the Arg72 allele. This effect has been suggested to be
caused by reduced transcriptional activation of apoptotic effectors
(75). In this study, Arg72
expression in presence of chemotherapeutic treatment was shown to
induce up to 8-fold more apoptosis than the Pro72 with
chemotherapeutics. Studies using p53-inducible isogenic cell lines
also noted the greater apoptotic potential of the Arg72 both in the
presence (75) and absence
(76,77) of chemotherapeutics. Patients,
homozygote for the Arg72 allele, with breast or lung cancers have
been shown to survive and respond more favorably to chemotherapy
and radiotherapy (78–80). Further studies of p53 variants could
help to define patient populations by their abilities to respond to
stress, suppress tumor formation and respond to DNA damaging
therapies (69).
In the present study, we showed that 54.5% of
individuals harbor a pathogenic TP53 mutation. The total
number of mutations found in this population is consistent with the
literature. Petitjean et al (81) stated that TP53 appeared to be
mutated in ~50% of cases in the majority of human tumors. The
simultaneous presence of Arg72 allele in the mutated form of
TP53 may serve as a predictor of enhanced tumor development
due to inactivation of p73. On the other hand, Arg72 allele over
wild-type background may potentially increase apoptotic ability
(74). A modifier effect of this
SNP has been also reported in germline TP53 mutation
carriers, where Arg72 was found to be associated with an earlier
age at the initial cancer diagnosis (82).
In most studies, this SNP has been identified by
amplifying the exon 4 followed by digestion using the AccII
restriction enzyme. However, partial digestion of the Arg72
homozygote leads to the same pattern as that derived from a
heterozygote, causing erroneous conclusions. In the present study,
analyses were conducted using direct sequencing, considered the
gold standard for mutation/SNP detection. The method used here is
appropriate for determining the quantity of C or G nucleotides in
RNA samples, as described by Siddique et al (13).
Although we have not used more robust techniques for
quantifying allelic expression, the mRNA sequencing of a gene
allows for the direct verification of which relation exists between
the alleles being expressed and tumor characteristics.
The discrepancies between the present study and
others are most likely due to differences in population
stratification and the methods used to ascertain the polymorphism.
Further studies using larger samples and a more detailed analysis
of genetic variations within TP53 are required to examine
the role of Pro/Arg alleles in carcinogenesis and to determine
whether the proposed association is in linkage disequilibrium with
other alleles.
In summary, the data presented here demonstrated
that there is a strong correlation between expression of the p53
Pro allele and the aggressiveness of CRC. Thus, we propose that the
expression status, rather than the conventionally analyzed genomic
status, of p53 variants should be used in studies searching for
associations between exonic SNPs and cancers.
Allelic variation of gene expression is of
particular interest due to its potential contribution to variation
in heritable traits. Therefore, understanding the degree of,
structure of, and patterns of variations in gene expression is of
central importance to determine its role in the pathogenesis of
CRC.
Acknowledgements
The present study was supported by the ‘Fundação de
Amparo à Pesquisa do Estado de São Paulo’ - FAPESP (grant no.
2008/01241-3). The investigators thank the study participants and
the collaborators who contributed to this research.
References
|
1
|
Tang NP, Wu YM, Wang B, et al: Systematic
review and meta-analysis of the association between P53 codon 72
polymorphism and colorectal cancer. Eur J Surg Oncol. 36:431–438.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valentin MD, da Silva FC, dos Santos EM,
et al: Characterization of germline mutations of MLH1 and MSH2 in
unrelated South American suspected Lynch syndrome individuals. Fam
Cancer. 10:641–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
da Silva FC, de Oliveira LP, Santos EM, et
al: Frequency of extracolonic tumors in Brazilian families with
Lynch syndrome: analysis of a hereditary colorectal cancer
institutional registry. Fam Cancer. 9:563–570. 2010.PubMed/NCBI
|
|
4
|
Stadler ZK, Vijai J, Thom P, et al:
Genome-wide association studies of cancer predisposition. Hematol
Oncol Clin North Am. 24:973–996. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hollstein M and Hainaut P: Massively
regulated genes: the example of TP53. J Pathol. 220:164–173.
2010.PubMed/NCBI
|
|
7
|
Shi H, Tan SJ, Zhong H, et al: Winter
temperature and UV are tightly linked to genetic changes in the p53
tumor suppressor pathway in Eastern Asia. Am J Hum Genet.
84:534–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mammano E, Belluco C, Bonafé M, et al:
Association of p53 polymorphisms and colorectal cancer: modulation
of risk and progression. Eur J Surg Oncol. 35:415–419. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
The International HapMap Consortium. The
International HapMap Project. Nature. 426:789–796. 2003. View Article : Google Scholar
|
|
10
|
Marin MC, Jost CA, Brooks LA, et al: A
common polymorphism acts as an intragenic modifier of mutant p53
behaviour. Nat Genet. 25:47–54. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Millau JF, Bastien N and Drouin R: P53
transcriptional activities: a general overview and some thoughts.
Mutat Res. 681:118–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Li X, Yuan R, et al: Three common
TP53 polymorphisms in susceptibility to breast cancer,
evidence from meta-analysis. Breast Cancer Res Treat. 120:705–714.
2010.
|
|
13
|
Siddique MM, Balram C, Fiszer-Maliszewska
L, et al: Evidence for selective expression of the p53 codon 72
polymorphs: implications in cancer development. Cancer Epidemiol
Biomarkers Prev. 14:2245–2252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song HR, Kweon SS, Kim HN, et al: p53
codon 72 polymorphism in patients with gastric and colorectal
cancer in a Korean population. Gastric Cancer. 14:242–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cañas M, Morán Y, Camargo ME, et al: TP53
codon 72 polymorphism and gastric cancer risk: a case-control study
in individuals from the central-western region of Venezuela. Invest
Clin. 50:153–161. 2009.(In Spanish).
|
|
16
|
Rogounovitch TI, Saenko VA, Ashizawa K, et
al: TP53 codon 72 polymorphism in radiation-associated human
papillary thyroid cancer. Oncol Rep. 15:949–956. 2006.
|
|
17
|
Granja F, Morari J, Morari EC, et al:
Proline homozygosity in codon 72 of p53 is a factor of
susceptibility for thyroid cancer. Cancer Lett. 210:151–157. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piao JM, Kim HN, Song HR, et al: p53 codon
72 polymorphism and the risk of lung cancer in a Korean population.
Lung Cancer. 73:264–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai S, Mao C, Jiang L, et al: P53
polymorphism and lung cancer susceptibility: a pooled analysis of
32 case-control studies. Hum Genet. 125:633–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenthal AN, Ryan A, Hopster D, et al:
p53 codon 72 polymorphism in vulval cancer and vulval
intraepithelial neoplasia. Br J Cancer. 83:1287–1290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin HY, Huang CH, Yu TJ, et al: p53 codon
72 polymorphism as a progression index for bladder cancer. Oncol
Rep. 27:1193–1199. 2012.PubMed/NCBI
|
|
22
|
Li DB, Wei X, Jiang LH, et al:
Meta-analysis of epidemiological studies of association of P53
codon 72 polymorphism with bladder cancer. Genet Mol Res.
9:1599–1605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sousa H, Santos AM, Pinto D, et al: Is
there a biological plausability for p53 codon 72 polymorphism
influence on cervical cancer development? Acta Med Port.
24:127–134. 2011.PubMed/NCBI
|
|
24
|
Klug SJ, Ressing M, Koenig J, et al: TP53
codon 72 polymorphism and cervical cancer: a pooled analysis of
individual data from 49 studies. Lancet Oncol. 10:772–784. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li MS, Liu JL, Wu Y, et al: Meta-analysis
demonstrates no association between p53 codon 72 polymorphism and
prostate cancer risk. Genet Mol Res. 10:2924–2933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Wang J, He Q, et al: Association of
p53 codon 72 polymorphism with prostate cancer: a meta-analysis.
Mol Biol Rep. 38:1603–1607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang W, He X, Chan Y, et al: Lack of
association between p53 codon 72 polymorphism and endometrial
cancer: a meta-analysis. Cancer Epidemiol. 36:153–157. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zubor P, Stanclova A, Kajo K, et al: The
p53 codon 72 exon 4 BstUI polymorphism and endometrial cancer in
Caucasian women. Oncology. 76:173–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suresh K, Chandirasekar R, Kumar BL, et
al: No association between the Trp53 codon 72 polymorphism and head
and neck cancer: a case-control study in a South Indian population.
Asian Pac J Cancer Prev. 11:1749–1753. 2010.PubMed/NCBI
|
|
30
|
Mojtahedi Z, Hashemi SB, Khademi B, et al:
p53 codon 72 polymorphism association with head and neck squamous
cell carcinoma. Braz J Otorhinolaryngol. 76:316–320.
2010.PubMed/NCBI
|
|
31
|
Själander A, Birgander R, Athlin L, et al:
P53 germ line haplotypes associated with increased risk for
colorectal cancer. Carcinogenesis. 16:1461–1464. 1995.PubMed/NCBI
|
|
32
|
Gemignani F, Moreno V, Landi S, et al: A
TP53 polymorphism is associated with increased risk of
colorectal cancer and with reduced levels of TP53 mRNA.
Oncogene. 23:1954–1956. 2004.
|
|
33
|
Goodman JE, Mechanic LE, Luke BT, et al:
Exploring SNP-SNP interactions and colon cancer risk using
polymorphism interaction analysis. Int J Cancer. 118:1790–1797.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pérez LO, Abba MC, Dulout FN, et al:
Evaluation of p53 codon 72 polymorphism in adenocarcinomas of the
colon and rectum in La Plata, Argentina. World J Gastroenterol.
12:1426–1429. 2006.PubMed/NCBI
|
|
35
|
Zhu ZZ, Wang AZ, Jia HR, et al:
Association of the TP53 codon 72 polymorphism with
colorectal cancer in a Chinese population. Jpn J Clin Oncol.
37:385–390. 2007.
|
|
36
|
Dakouras A, Nikiteas N, Papadakis E, et
al: p53Arg72 homozygosity and its increased incidence in
left-sided sporadic colorectal adenocarcinomas, in a
Greek-Caucasian population. Anticancer Res. 28:1039–1043. 2008.
|
|
37
|
Koushik A, Tranah GJ, Ma J, et al: p53
Arg72Pro polymorphism and risk of colorectal adenoma and cancer.
Int J Cancer. 119:1863–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tan XL, Nieters A, Hoffmeister M, et al:
Genetic polymorphisms in TP53, nonsteroidal anti-inflammatory drugs
and the risk of colorectal cancer: evidence for gene-environment
interaction? Pharmacogenet Genomics. 17:639–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hase K, Shatney C, Johnson D, et al:
Prognostic value of tumor ‘budding’ in patients with colorectal
cancer. Dis Colon Rectum. 36:627–635. 1993.
|
|
40
|
López I, Oliveira PL, Tucci P, et al:
Different mutation profiles associated to P53 accumulation in
colorectal cancer. Gene. 499:81–87. 2012.PubMed/NCBI
|
|
41
|
Goldstein DB: The importance of synthetic
associations will only be resolved empirically. PLoS Biol.
9:e10010082011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anderson CA, Soranzo N, Zeggini E, et al:
Synthetic associations are unlikely to account for many common
disease genome-wide association signals. PLoS Biol. 9:e10005802011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wray NR, Purcell SM and Visscher PM:
Synthetic associations created by rare variants do not explain most
GWAS results. PLoS Biol. 9:e10005792011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Orozco G, Barrett JC and Zeggini E:
Synthetic associations in the context of genome-wide association
scan signals. Hum Mol Genet. 19:137–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shields R: Common disease: are causative
alleles common or rare? PLoS Biol. 9:e10010092011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ritchie MD: Using biological knowledge to
uncover the mystery in the search for epistasis in genome-wide
association studies. Ann Hum Genet. 75:172–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dickson SP, Wang K, Krantz I, et al: Rare
variants create synthetic genome-wide associations. PLoS Biol.
8:e10002942010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yan H, Yuan W, Velculescu VE, et al:
Allelic variation in human gene expression. Science. 297:11432002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bray NJ, Buckland PR, Owen MJ, et al:
Cis-acting variation in the expression of a high proportion
of genes in human brain. Hum Genet. 113:149–153. 2003.
|
|
50
|
Lo HS, Wang Z, Hu Y, et al: Allelic
variation in gene expression is common in the human genome. Genome
Res. 13:1855–1862. 2003.PubMed/NCBI
|
|
51
|
Schadt EE, Monks SA, Drake TA, et al:
Genetics of gene expression surveyed in maize, mouse and man.
Nature. 422:297–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pastinen T, Sladek R, Gurd S, et al: A
survey of genetic and epigenetic variation affecting human gene
expression. Physiol Genomics. 16:184–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheung VG, Bruzel A, Burdick JT, et al:
Monozygotic twins reveal germline contribution to allelic
expression differences. Am J Hum Genet. 82:1357–1360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gimelbrant A, Hutchinson JN, Thompson BR,
et al: Widespread monoallelic expression on human autosomes.
Science. 318:1136–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thomas M, Kalita A, Labrecque S, et al:
Two polymorphic variants of wild-type p53 differ biochemically and
biologically. Mol Cell Biol. 19:1092–1100. 1999.PubMed/NCBI
|
|
56
|
Dumont P, Leu JI, Della Pietra AC III, et
al: The codon 72 polymorphic variants of p53 have markedly
different apoptotic potential. Nat Genet. 33:357–365. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ozeki C, Sawai Y, Shibata T, et al: Cancer
susceptibility polymorphism of p53 at codon 72 affects
phosphorylation and degradation of p53 protein. J Biol Chem.
286:18251–18260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hancox RJ, Poulton R, Welch D, et al:
Accelerated decline in lung function in cigarette smokers is
associated with TP53/MDM2 polymorphisms. Hum Genet.
126:559–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pandith AA, Shah ZA, Khan NP, et al: Role
of TP53 Arg72Pro polymorphism in urinary bladder cancer
predisposition and predictive impact of proline related genotype in
advanced tumors in an ethnic Kashmiri population. Cancer Genet
Cytogenet. 203:263–268. 2010.
|
|
60
|
Tobacco Free Initiative (TFI). WHO report
on the global tobacco epidemic, 2011: warning about the dangers of
tobacco. World Health Organization; 2011, Available at http://www.who.int/tobacco/global_report/2011/en/.
|
|
61
|
Marqueta A, Nerín I, Jiménez-Muro A, et
al: Predictors of outcome of a smoking cessation treatment by
gender. Gac Sanit. 27:23–31. 2012.(In Spanish).
|
|
62
|
Hiscock R, Bauld L, Amos A, et al:
Socioeconomic status and smoking: a review. Ann NY Acad Sci.
1248:107–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Norppa H: Cytogenetic biomarkers and
genetic polymorphisms. Toxicol Lett. 149:309–334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vodicka P, Kumar R, Stetina R, et al:
Genetic polymorphisms in DNA repair genes and possible links with
DNA repair rates, chromosomal aberrations and single-strand breaks
in DNA. Carcinogenesis. 25:757–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hanova M, Vodickova L, Vaclavikova R, et
al: DNA damage, DNA repair rates and mRNA expression levels of cell
cycle genes (TP53, p21CDKN1A,
BCL2 and BAX) with respect to occupational exposure
to styrene. Carcinogenesis. 32:74–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jass JR, Love SB and Northover JM: A new
prognostic classification of rectal cancer. Lancet. 1:1303–1306.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zlobec I, Baker K, Minoo P, et al: Tumor
border configuration added to TNM staging better stratifies stage
II colorectal cancer patients into prognostic subgroups. Cancer.
115:4021–4029. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zlobec I, Terracciano LM and Lugli A:
Local recurrence in mismatch repair-proficient colon cancer
predicted by an infiltrative tumor border and lack of
CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res.
14:3792–3797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grochola LF, Zeron-Medina J, Mériaux S, et
al: Single-nucleotide polymorphisms in the p53 signaling pathway.
Cold Spring Harb Perspect Biol. 2:a0010322010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wilkinson NW: Adjuvant chemotherapy for
colorectal cancer-new perspectives for effective treatment. US
Gastroenterol Hepatol Rev. 1:91–93. 2007.
|
|
71
|
Casillas S, Pelley RJ and Milsom JW:
Adjuvant therapy for colorectal cancer: present and future
perspectives. Dis Colon Rectum. 40:977–992. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fuchs CS and Mayer RJ: Adjuvant
chemotherapy for colon and rectal cancer. Semin Radiat Oncol.
3:29–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Naccarati A, Polakova V, Pardini B, et al:
Mutations and polymorphisms in TP53 gene: an overview on the
role in colorectal cancer. Mutagenesis. 27:211–218. 2012.
|
|
75
|
Sullivan A, Syed N, Gasco M, et al:
Polymorphism in wild-type p53 modulates response to chemotherapy
in vitro and in vivo. Oncogene. 23:3328–3337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pim D and Banks L: p53 polymorphic
variants at codon 72 exert different effects on cell cycle
progression. Int J Cancer. 108:196–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bergamaschi D, Samuels Y, Sullivan A, et
al: iASPP preferentially binds p53 proline-rich region and
modulates apoptotic function of codon 72-polymorphic p53. Nat
Genet. 38:1133–1141. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nelson HH, Wilkojmen M, Marsit CJ, et al:
TP53 mutation, allelism and survival in non-small cell lung
cancer. Carcinogenesis. 26:1770–1773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tommiska J, Eerola H, Heinonen M, et al:
Breast cancer patients with p53 Pro72 homozygous genotype have a
poorer survival. Clin Cancer Res. 11:5098–5103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu Y, Yao L, Ouyang T, et al: p53 codon 72
polymorphism predicts the pathologic response to neoadjuvant
chemotherapy in patients with breast cancer. Clin Cancer Res.
11:7328–7333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Petitjean A, Mathe E, Kato S, et al:
Impact of mutant p53 functional properties on TP53 mutation
patterns and tumor phenotype: lessons from recent developments in
the IARC TP53 database. Hum Mutat. 28:622–629. 2007.PubMed/NCBI
|
|
82
|
Bougeard G, Baert-Desurmont S, Tournier I,
et al: Impact of the MDM2 SNP309 and p53 Arg72Pro
polymorphism on age of tumour onset in Li-Fraumeni syndrome. J Med
Genet. 43:531–533. 2006.
|