Introduction
The resistance of gastric cancer cells to multiple
chemotherapeutic agents remains a major therapeutic obstacle.
MGr1-Ag is an upregulated protein in drug-resistant SGC7901/VCR
cells. Using SGC7901/VCR cells as the immunogen, we prepared a
monoclonal antibody against MGr1-Ag named MGr1 (1), and obtained the gene MGr1-Ag (GenBank
AF503367) by screening a cDNA library with the MGr1 monoclonal
antibody. Sequence analysis revealed that MGr1-Ag is identical to
the human 37-kDa laminin receptor precursor protein (37LRP)
(2). Further study suggested that
MGr1-Ag/37LRP may promote MDR in gastric cancer cells by decreasing
intracellular drug accumulation and inhibiting drug-induced
apoptosis (3). However, the exact
mechanism of the contribution of MGr1-Ag/37LRP to MDR in gastric
cancer remains unknown.
The extracellular matrix (ECM) of cancer cells
profoundly influences major malignant phenotypes, including
oncogenesis, progression and apoptosis (4). Adhesion confers a novel acquired
chemotherapeutic drug-resistant phenotype referred to as cell
adhesion-mediated drug resistance (CAM-DR) (5). Laminin (LN) and collagen IV (COL IV)
are natural basement membrane components that constitute a specific
ECM that maintains malignant phenotypes in gastric adenocarcinoma
cells (6,7). MGr1-Ag/37LRP directly correlates with
tumor growth and proliferation as a laminin receptor (8). Therefore, we hypothesized that
MGr1-Ag/37LRP binding-induced adhesion and the binding-initiated
intracellular signaling pathways participate in protecting gastric
cancer cells from a number of apoptotic stimuli caused by
chemotherapeutic drugs.
In the present study, we investigated whether
MGr1-Ag/37LRP binding-induced adhesion participates in protecting
gastric cancer cells from apoptotic stimuli caused by
chemotherapeutic drugs. We found that MGr1-Ag/37LRP binding
decreased intracellular drug accumulation by inhibiting the
expression of P-glycoprotein (P-gp) and multidrug
resistance-associated protein (MRP), and inhibited drug-induced
apoptosis through regulation of Bcl-2 and Bax expression.
Sensitivity to chemotherapeutic drugs in xenografts was
significantly enhanced by inhibiting MGr1-Ag/37LRP expression.
These studies aimed to characterize the role, and the molecular
mechanisms of the effects of MGr1-Ag/37LRP on CAM-DR in gastric
cancer cells.
Materials and methods
Cell lines and cell culture
The human gastric adenocarcinoma cell line SGC7901
was a gift from the Academy of Military Medical Science (Beijing,
China). We previously generated and characterized the MDR gastric
cancer cell variants SGC7901/VCR and SGC7901/ADR (9). All cell lines were maintained in
RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum
(both from Gibco, Grand Island, NY, USA) and antibiotics, at 37°C
in a humidified atmosphere of 5% CO2 and 95% air. To
maintain the drug-resistant phenotype, SGC7901/VCR cells received
vincristine (VCR), and SGC7901/ADR cells received adriamycin (ADR)
at 1 μg/ml. Two weeks before assessing MDR in gastric cancer cells
and the transfected cells, VCR and ADR treatment was ceased, to
eliminate drug exposure effects.
Plasmids and transfection
The sense expression vector pcDNA3.1/MGr1 and the
siRNA vector of MGr1-Ag/37LRP were constructed previously in our
laboratory (3,10). Vectors pGL3-MGr1L and pGL3-MGr1S
contain promoter fragments for nucleotides −1600 to +964, and −292
to +964 relative to transcription start constructed previously in
our laboratory (11).
Drug-sensitive SGC7901 cells were transfected with the sense vector
pcDNA3.1/MGr1 to generate line SGC7901-MGr1, or with the control
vector pcDNA3.1 to generate SGC7901-pc. Drug-resistant SGC7901/VCR
cell line was transfected with the siRNA targeting MGr1-Ag/37LRP
and named SGC7901/VCR-siMGr1, or the control vector pSilenser and
named SGC7901/VCR-ps. Cell transfection was carried out with
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s protocol. For transient transfection, cells were
harvested after 48 h; for stable transfecion, G418 (400 μg/ml) was
added after 24 h, and mixed clones were screened and expanded for
an additional 6 weeks.
Western blotting and
immunohistochemistry
Whole cells pretreated as indicated, or harvested
tumor tissue, were lysed on ice for 30 min in lysis buffer (10 mM
Tris, pH 8.0, 1 mM EDTA, 400 mM NaCl, 10% glycerol, 0.5% NP-40, 5
mM sodium fluoride, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM
dithiothreitol). Equal amounts of protein (25 μg) were analyzed by
western blotting with anti-P-gp, anti-MRP, anti-Bcl-2, anti-Bax
(Santa Cruz Biotechnology Inc.), anti-MGr1-Ag [prepared by our
laboratory (12)], or anti-β-actin
(Sigma, USA) for a 3-h incubation. Blots were washed, and a
species-matched peroxidase-conjugated secondary antibody was added
(1:2,000). Labeled bands from the washed blots were detected with
an ECL kit (Amersham).
Tissues were dewaxed in xylene, washed in 96%
vol/vol ethanol, and incubated for 30 min in 0.3% hydrogen peroxide
before washing in phosphate-buffered saline (PBS). Blocking was for
30 min with 1% bovine serum albumin (BSA) at room temperature,
followed by incubation for 120 min at room temperature with the
MGr1-Ag/37LRP antibody in PBST (0.01%) supplemented with 10% milk
powder. Samples were washed three times with PBST (0.01% Tween) and
incubated with the secondary antibody for 30 min, before processing
with the Vectastain Elite ABC kit according to the manufacturer’s
instructions (Vector Laboratories) prior to digital photography on
a Nikon Eclipse E600 microscope with a Spot RT slider camera and
imaging software (Imsol Imaging Solutions).
Semi-quantitive reverse transcription
(RT)-PCR analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer’s recommendations.
RT-PCR analysis of mRNA levels was performed using primers specific
for MGr1-Ag (yielding 463 bp) forward primer,
5′-GCTGGACGATAGCTTGGA-3′ and reverse primer,
5′-GATGACAGATAGCTGGTG-3′; for β-actin (yielding 287 bp) forward
primer, 5′-AGCGGGAAATCGTGCGTG-3′ and reverse primer,
5′-CAGGGTACATGGTGGTGCC-3′. PCR consisted of 94°C for 4 min, 30
cycles of 94°C for 50 sec, 60°C for 50 sec and 70°C for 30 sec, in
a Touchgene Gradient thermal cycler (Techne, Cambridge, UK). PCR
products were analyzed by agarose gel electrophoresis to determine
PCR quality.
Cell adhesion assay
Gastric cancer cell adherence to ECM components LN
(LAMB1; Sigma) and COL IV, or to the BSA control was determined in
24-well plates as previously described (13). The plate surface was covered with 1
μg/cm2 LN, 1 μg/cm2 COL IV or 0.4
μg/cm2 BSA, incubated for 2 h, and the supernatant was
removed. A suspension of tumor cells (1×105/ml, 0.5 ml)
was transferred into the covered wells. After 0.5, 1, 2 or 4 h of
incubation at 37°C, the adhesive cells were washed with PBS twice
and counted under a microscope at a ×200 magnification in 10 random
fields/well. Each experiment was performed in triplicate.
In vitro drug sensitivity assay
For the colony-formation assays, gastric cancer
cells in log phase were harvested and plated into 35-mm culture
plates (1×103 cells/well) covered with ECM components or
BSA. In some cases, cells were pre-incubated with different
concentrations of MGr1-AG/37LRP siRNA. After overnight incubation
at 37°C for adhesion, VCR or 5-fluorouracil (5-Fu) was added and
the incubation was continued for 24 h. Plates were washed twice
with serum-free RPMI-1640, and grown in complete culture medium
(RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum
and antibiotics), for 10 days. The resulting colonies were stained
with Coomassie Brilliant Blue, and visible colonies were counted.
The concentration of drug that caused a 50% reduction in the number
of colonies (IC50) was calculated using SPSS 11.0
software (Chicago, IL, USA).
Fluorescence intensity assay for
intracellular ADR
The fluorescence intensity of intracellular ADR was
determined by flow cytometry, as described previously (14). In brief, gastric cancer cells in
log-phase were seeded into 6-well plates (1×106
cells/well) coated with ECM components or BSA and cultured
overnight at 37°C. After addition of ADR to a final concentration
of 5 mg/l, cells were cultured for 1 h, then trypsinized and
harvested to detect ADR accumulation by flow cytometry (Coulter,
Miami, FL, USA) with an excitation wavelength of 488 nm and an
emission wavelength of 575 nm. The ADR release index was calculated
according to the formula: Release index = (accumulation value -
retention value)/accumulation value.
Annexin V/propidium iodide staining
The apoptotic index (AI) of gastric cancer cells was
calculated as the number of apoptotic cells detected by flow
cytometry. In brief, cells in log phase were plated into 6-well
plates (1×106 cells/well) coated with ECM components or
BSA and cultured overnight at 37°C. VCR was added to a final
concentration of 0.3–0.6 mg/l, and culturing was continued for
36–72 h. Annexin V-FITC (5 μl) was added to the cells, and the mean
fluorescence intensity of Annexin V-FITC/PI was determined by flow
cytometry as described in the Clontech protocol (Palo Alto, CA,
USA). AI was calculated as the mean fluorescence intensity.
Dual luciferase reporter assay
SGC7901 cells were plated at 3×105
cells/35-mm dish coated ~12 h before transfection with 0.25, 0.50,
0.75, 1.00 or 1.50 μg/cm2 of LN. Transient transfections
used Lipofectamine 2000 reagent (Invitrogen) and 1.0 μg of
pGL3-MGr1L or pGL3-MGr1S, co-transfected with 0.1 μg of phRL-TK
vector (Promega) as an internal control. After 48 h, the
transfected cells were harvested, lysed, centrifuged to pellet the
debri, and used for luciferase assays. Luciferase activity was
measured as chemiluminescence, using a luminometer (PerkinElmer)
and the Dual-Luciferase reporter assay system (Promega), according
to the manufacturer’s protocol. All transfections were performed in
triplicate.
Assessment of in vivo tumor growth
Approximately 1×106 SGC7901/VCR cells
were inoculated subcutaneously with 0.1 ml of Matrigel
(Sigma-Aldrich) in the flank region of 6- to 8-week-old male
athymic nude mice (Experimental Animal Center, FMMU, China) using a
27-gauge needle under halothane anesthesia. When tumors reached
100±20 mm3, usually 3–4 weeks after injection, mice were
randomly selected for treatment with MGr1-Ag/37LRP siRNA or
scrambled siRNA oligonucleotide, administered as 50 μl of 1 μg/μl
siRNA, or control PBS was administrated once every three days by
intratumoral injection for 36 days (15). From day 7 to 14, and 28 to 35, 0.6
mg/kg of VCR was administered via tail vein injection (16). Tumor volume measurements were
performed once every four days and calculated by the formula: Tumor
volume = length × width × depth × 0.5236 (17). Data are expressed as average tumor
volume levels ± standard error (SE). All animal procedures were
performed according to the guidelines of the Chinese Council on
Animal Care, and with appropriate institutional certification.
Half of the transplanted tumors in each group were
dissected and fixed in formalin for immunohistochemical studies,
and the other half were immediately harvested in cold isopentane,
frozen in liquid nitrogen, and kept at −80°C for western blot
analyses. Formalin-fixed tissues were processed into 5-μm sections
of formalin-fixed paraffin-embedded specimens for
immunohistochemistry.
Statistical analysis
Each experiment was repeated at least three times.
Bands from western blot analyses or RT-PCR were quantified by
Quantity One software (Bio-Rad). Relative protein or mRNA levels
were calculated relative to β-actin. Numerical data are presented
as the means ± standard error of the mean (SEM). Analysis of
variance (ANOVA) was used to compare differences between
experimental groups. LSD t-test was used for multiple comparisons.
All statistical analysis was carried out with SPSS 11.0 software
(Chicago, IL, USA). A p-value ≤0.05 was considered to indicate a
statistically significant result.
Results
MDR of gastric cancer cells is enhanced
by ECM adhesion
To gain insight into the relationship between the
adhesive properties and drug resistance of gastric cancer, we
compared the adhesive potential between the MDR variants
SGC7901/VCR and SGC7901/ADR with the parental cell line SGC7901,
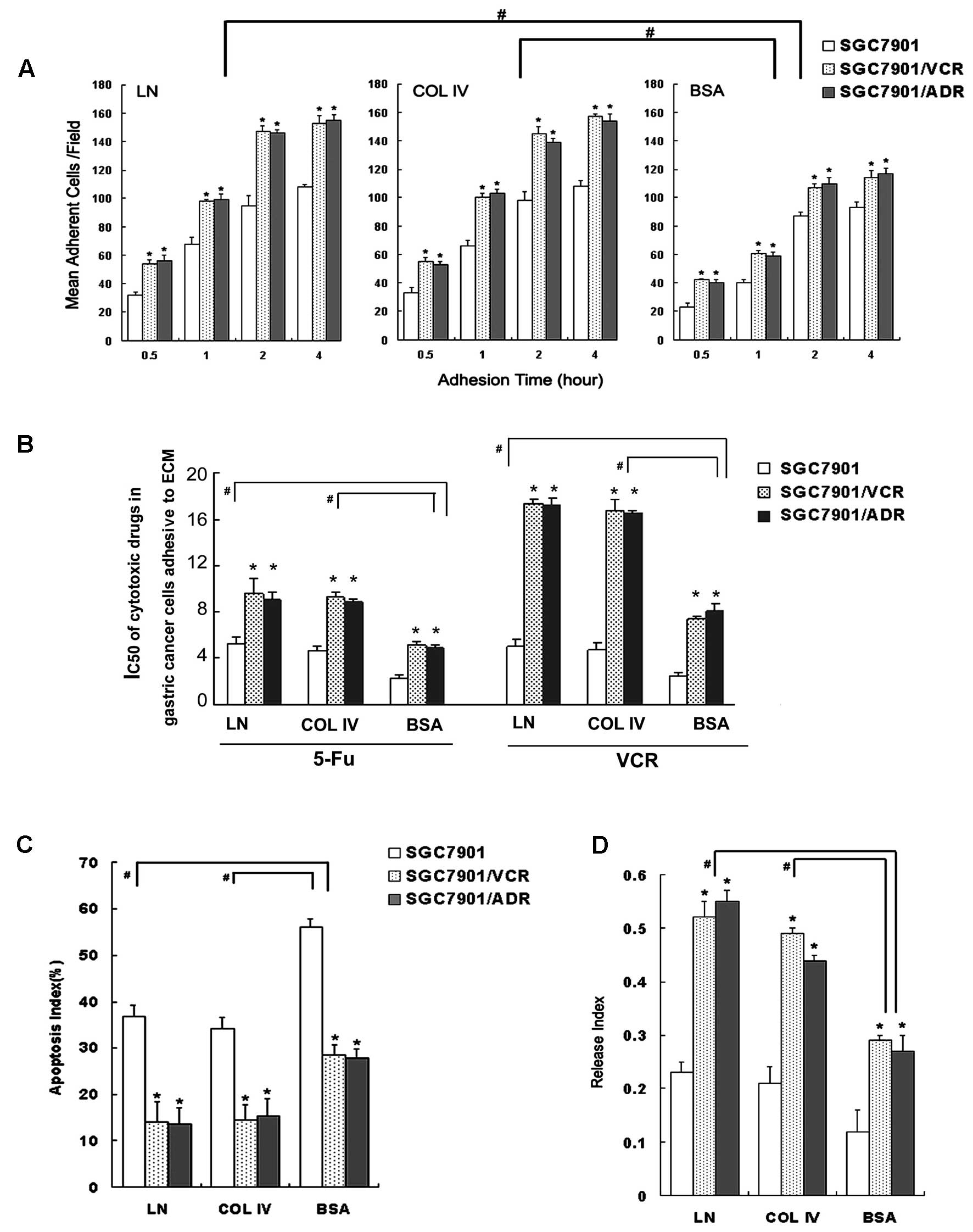
after adhesion to the ECM components. As shown in Fig. 1, The MDR cells showed a
significantly increased mean adhesion cell number than the SGC7901
cells after adhesion to both ECM and the BSA (Fig. 1A). This indicated that SGC7901/VCR
and SGC7901/ADR cells exhibited a relatively high adhesive
potential to ECM.
As shown by colony-forming assays, SGC7901/VCR and
SGC7901/ADR cells had significantly increased IC50
values for VCR or 5-Fu after adhesion to ECM components, compared
to IC50 values for these agents in cells following
adhesion to BSA (Fig. 1B).
Similarly, after adhesion to ECM components, SGC7901/VCR and
SGC7901/ADR cells showed significantly decreased AI values compared
to the control, and had decreased ADR accumulation and retention,
as well as increased release indices (Fig. 1C and D).
We next examined the expression of P-gp, MRP, Bcl-2
and Bax in both MDR and drug-sensitive gastric cancer cells after
adhesion to ECM components. Western blot analysis showed that in
the MDR gastric cancer SGC7901/VCR and SGC7901/ADR cells following
adhesion to laminin, significantly increased expression of P-gp,
MRP and Bcl-2 and decreased Bax expression were observed when
compared to the drug-sensitive SGC7901 cells (Fig. 2). These results indicated that cell
adhesion to ECM enhanced the MDR phenotype of the gastric cancer
cell line SGC7901 by decreasing intracellular drug accumulation and
by inhibiting drug-induced apoptosis, by regulating P-gp, MRP,
Bcl-2 and Bax expression.
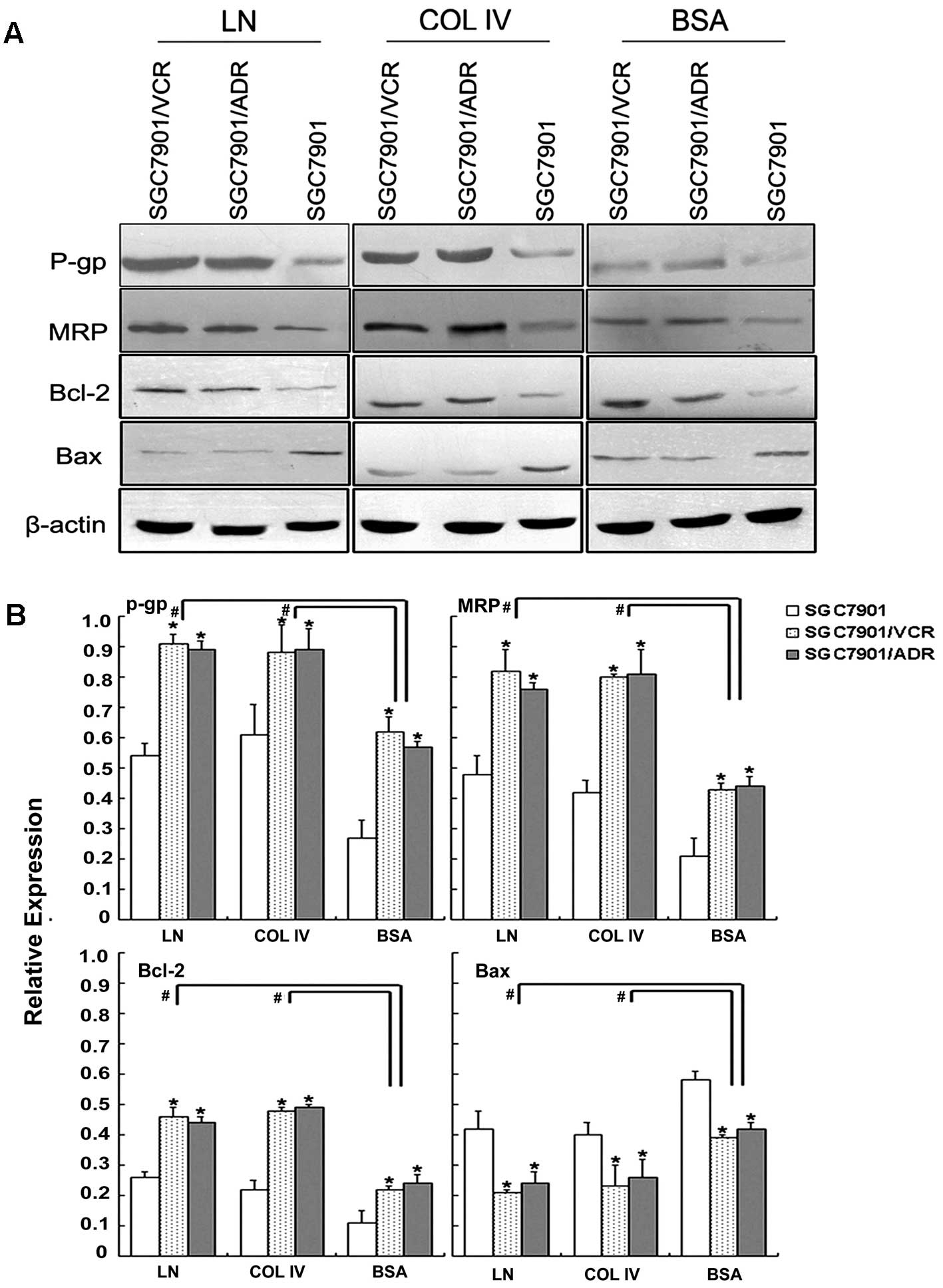 | Figure 2P-gp, MRP, Bcl-2 and Bax expression of
gastric cancer cells adhering to ECM. (A) Expression of P-gp, MRP,
Bcl-2 and Bax evaluated by western blotting. (B) Relative
expression of P-gp, MRP, Bcl-2 and Bax. Signals were quantified by
densitometric scanning. Data are expression relative to β-actin.
*p<0.05 vs. SGC7901 adhering to ECM components or BSA
control. #p<0.05 vs. adhering to BSA. P-gp,
P-glycoprotein; MRP, multidrug resistance-associated protein; ECM,
extracellular matrix; BSA, bovine serum albumin. |
Cell adhesion to LN upregulates
MGr1-Ag/37LRP expression in vitro
It has been reported that binding of LN by
cell-surface LN receptors induces synthesis of 37LRP in melanoma
cells, resulting in increased delivery of LN-binding proteins to
the cell surface, and potentiating attachment to the basement
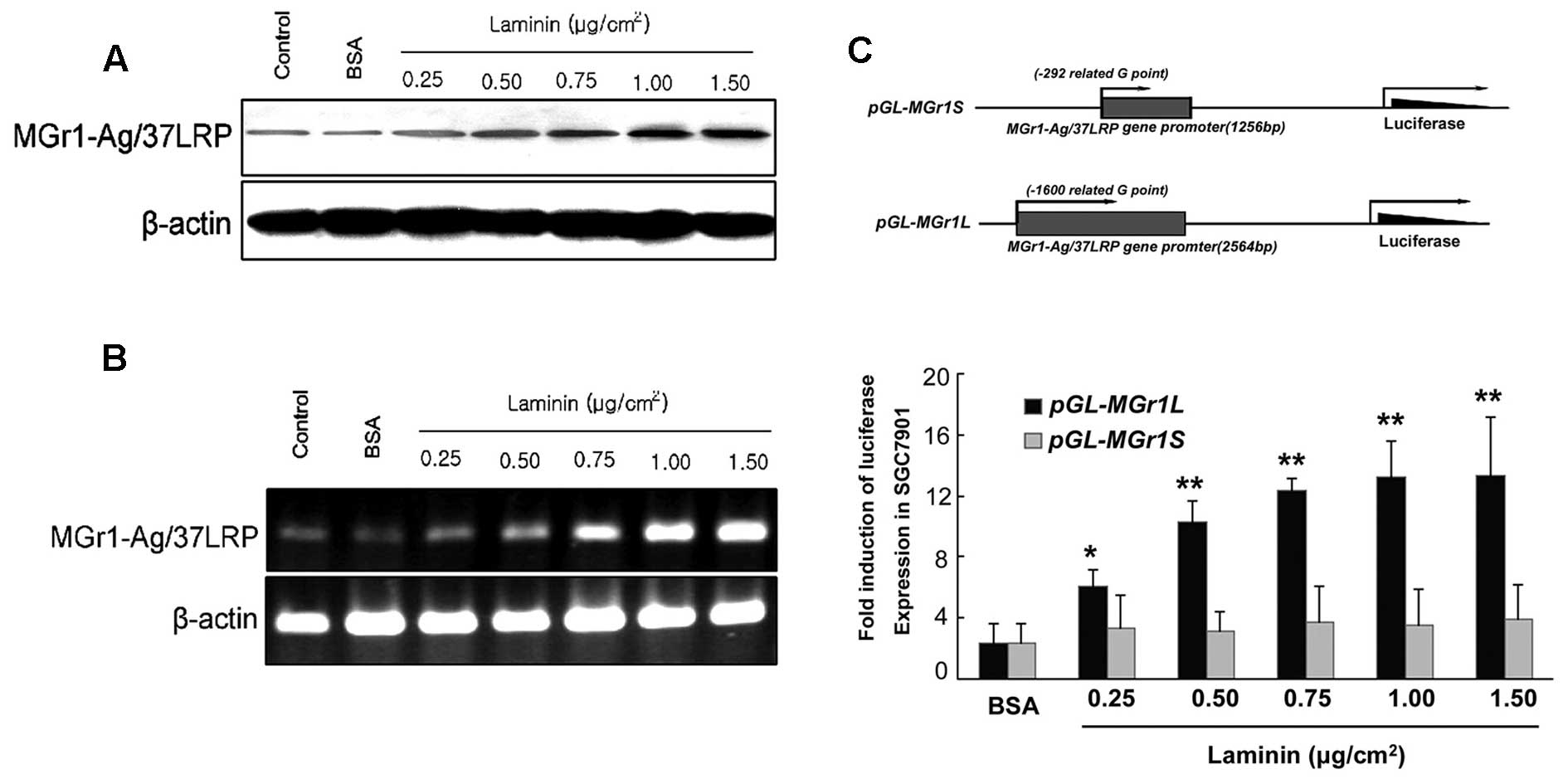
membrane during invasion and metastasis (18). We investigated whether LN
upregulates MGr1-Ag/37LRP expression by examining mRNA and protein
levels and transcriptional activity by using reporter assays with
MGr1 promoter-containing plasmids in gastric cancer cells. As shown
in Fig. 3, using LN as an adhesion
substrate significantly increased mRNA and protein in a
dose-dependent manner. Luciferase reporter constructs with MGr1
promoter fragments of 2564 bp (pGL3-2564-Luc, nucleotides −1600 to
+964), or 1256 bp (pGL3-1256-Luc, nucleotides −292 to +964) were
used to determine induction by LN. As shown in Fig. 3C, SGC7901 cells treated with LN and
transfected with pGL3-2564-Luc showed a significant, dose-dependent
increase in luciferase activity over cells treated with the BSA
control (p<0.05). The plasmid pGL3-1256-Luc showed no alteration
in luciferase activity over the BSA control. These results revealed
that LN induced MGr1-Ag expression at the transcriptional level,
and the promoter nucleotides −1600 to −292 were essential for this
effect.
Establishment of forced and siRNA
expression of MGr1-Ag by stable transfection
We previously reported that overexpression of
MGr1-Ag could prompt drug resistance in gastric cancer. To study
the functional role of MGr1-Ag/37LRP in MDR in gastric cancer after
adhesion to ECM, an MGr1-Ag/37LRP sense vector and an siRNA vector
were used to upregulate and downregulate MGr1-Ag/37LRP. As shown by
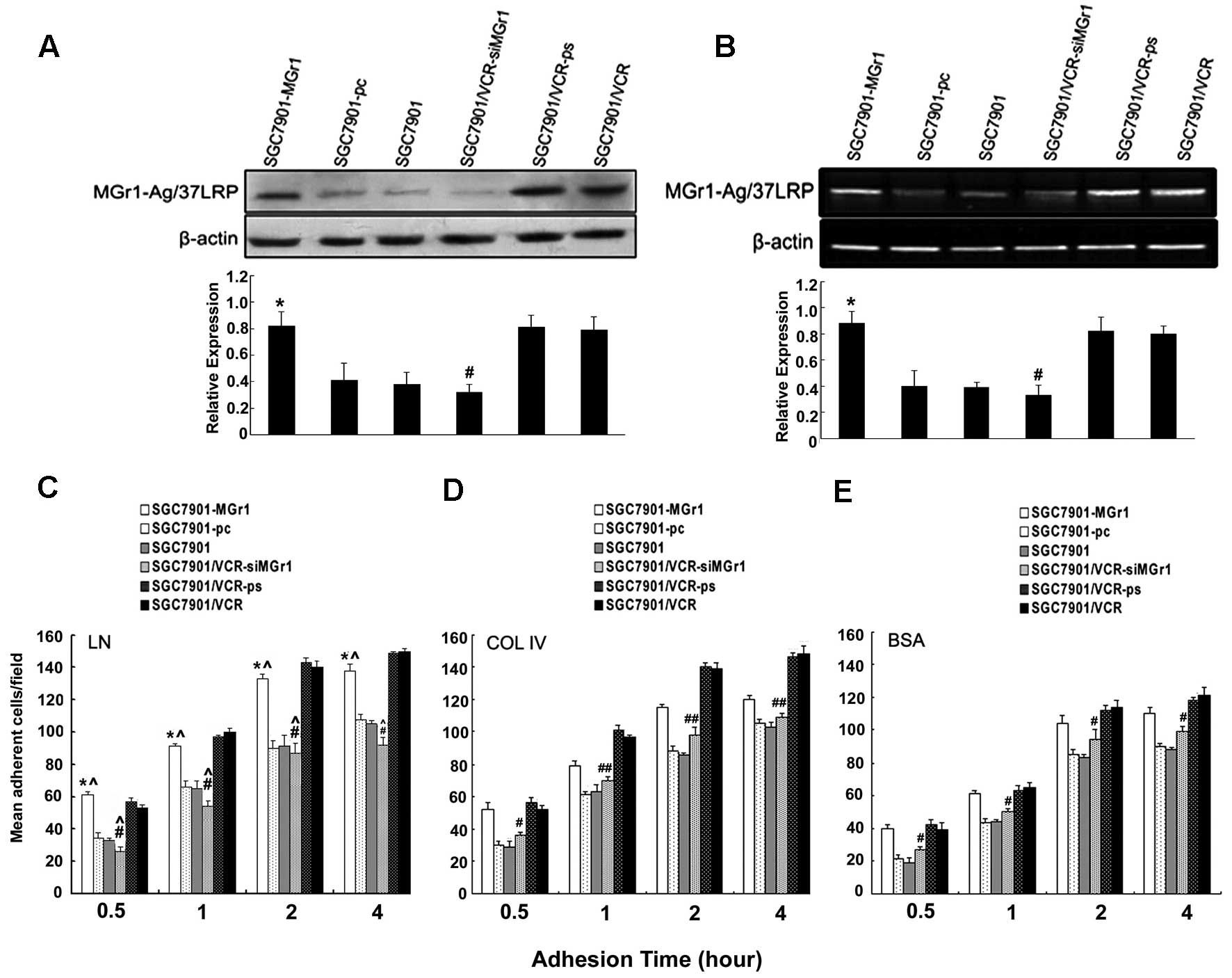
western blotting and RT-PCR analysis (Fig. 4), treatment of SGC7901 cells with
the MGr1-Ag/37LRP expression vector significantly induced
MGr1-Ag/37LRP expression at the protein and mRNA levels (Fig. 4A and B, lane 1). SGC7901/VCR cells
transfected with the MGr1-Ag/37LRP siRNA vector had significantly
reduced protein and mRNA levels (Fig.
4A and B, lane 4), while a control vector with a scrambled
oligonucleotide did not significantly suppress expression.
CAM-DR phenotype of gastric cancer cells
is enhanced by MGr1-Ag/37LRP upregulation in vitro
To investigate the role of MGr1-Ag/37LRP in gastric
cancer CAM-DR, cell adhesion assays, in vitro drug
sensitivity assays, fluorescence intensity assays for intracellular
ADR, Annexin V/PI staining, and western blotting were utilized to
determine the MDR phenotype of gastric cancer cells with
upregulation or downregulation of MGr1-Ag/37LRP, after adhesion to
LN, COL IV, or control BSA (Fig.
4C–E). Under the same conditions, SGC7901/VCR-siMGr1 cells
showed significantly decreased mean adhesion cell numbers compared
to SGC7901/VCR or SGC7901/VCR-ps cells. Adhesion to LN caused a
significant increase in SGC7901-MGr1 cell adhesion, over adhesion
to COL IV (p<0.05, Fig.
4C–E).
Significantly increased IC50 values for
VCR and 5-Fu were noted for SGC7901-MGr1 cells after adhesion to
either ECM components or control (p<0.05, Fig. 5A). Decreased ADR accumulation and
retention, and increased release indices (p<0.05, Fig. 5B), were observed, along with
decreased AI values (p<0.05, Fig.
5C), increased expression of P-gp, MRP and Bcl-2, and decreased
expression of Bax (p<0.05, Fig. 5D
and E), all relative to SGC7901 and SGC7901-pc cells under the
same conditions. SGC7901/VCR-siMGr1 cells showed significantly
decreased IC50 values for VCR and 5-Fu, decreased
release indices, increased AI values, decreased expression of P-gp,
MRP and Bcl-2, and increased expression of Bax compared to
SGC7901/VCR and SGC7901/VCR-ps cells under the same conditions
(p<0.05). In addition, after adhesion to LN, SGC7901-MGr1 cells
showed significantly increased VCR and 5-Fu IC50 values,
increased release indices, decreased AI values, increased
expression of P-gp, MRP and Bcl-2, and decreased expression of Bax
compared to the same cells after COL IV adhesion (p<0.05). These
data indicated that overexpression of MGr1-Ag/37LRP partially
promoted the MDR phenotype of gastric cancer cells by decreasing
intracellular drug accumulation and inhibiting drug-induced
apoptosis.
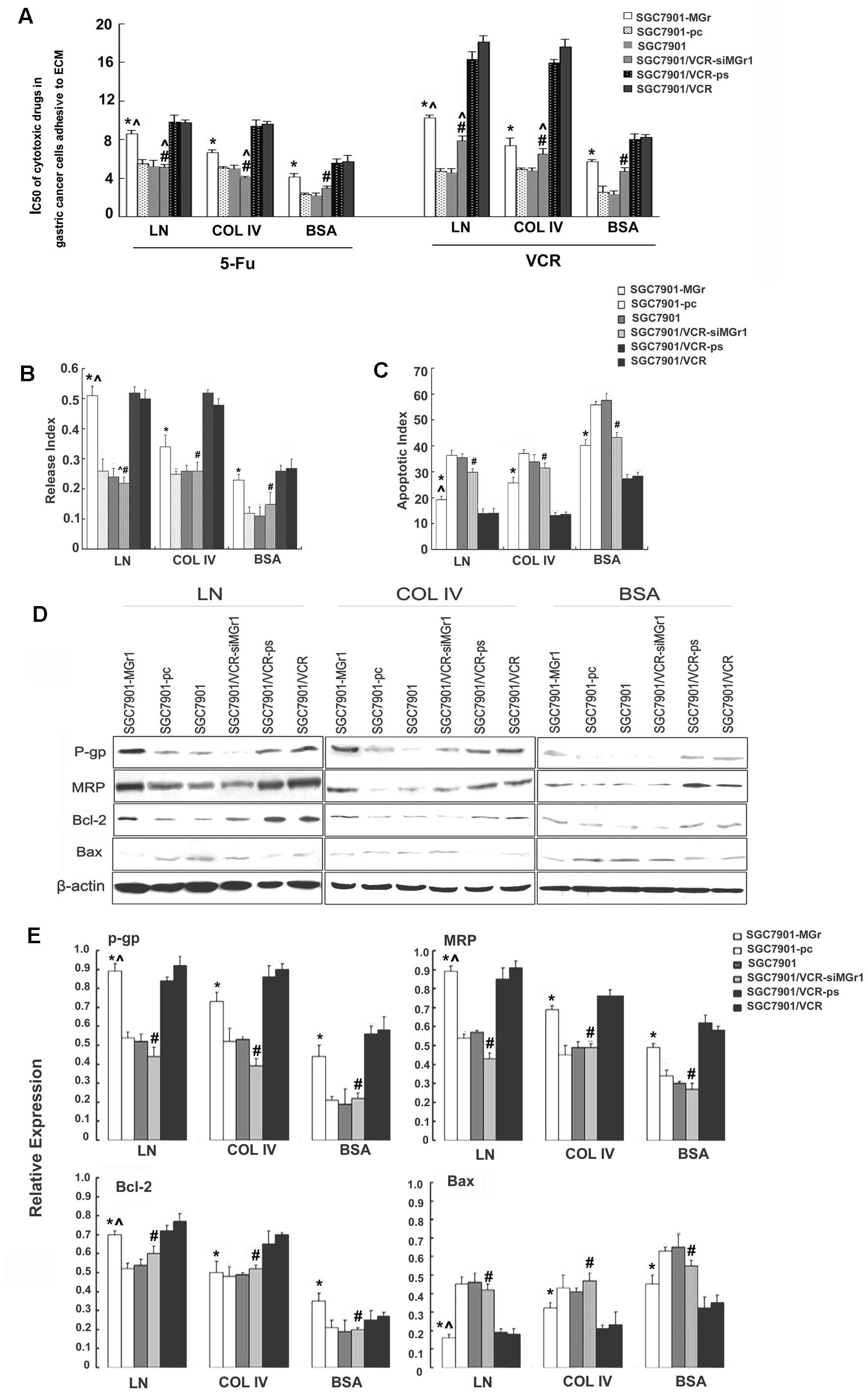 | Figure 5Characterization of the MDR phenotype
of gastric cancer cells transfected to upregulate or downregulate
MGr1-Ag/37LRP following adhesion to ECM components. (A) Sensitivity
of the transfected gastric cancer cell lines to chemotherapeutic
drugs, evaluated using colony-forming assay and shown as
IC50. (B) ADR release indices of gastric cancer cells
calculated by ADR accumulation and retention, as detected by flow
cytometry. (C) Apoptotic indices of the gastric cancer cells
treated by VCR, as detected by flow cytometry. (D) Expression of
P-gp, MRP, Bcl-2 and Bax, evaluated by western blotting with
β-actin as an internal control. (E) Relative expression of P-gp,
MRP, Bcl-2 and Bax. Signals were quantified by densitometric
scanning. Data are expression relative to β-actin.
*p<0.05 vs. SGC7901 and SGC7901-pc adhering to ECM
components and BSA control. #p<0.05 vs. SGC7901/VCR
and SGC7901/VCR-ps adhering to ECM components and BSA control.
^p<0.05 vs. SGC7901-MGr1 adhering to COL IV. ECM,
extracellular matrix; ADR, adriamycin; P-gp, P-glycoprotein; MRP,
multidrug resistance-associated protein; BSA, bovine serum albumin;
COL IV, collagen IV. |
siRNA of MGr1-Ag/37LRP partly reverses
the MDR phenotype of SGC7901/VCR cells in vivo
To address the potential effects of MGr1-Ag/37LRP
siRNA in vivo, equal numbers of MGr1-Ag/37LRP siRNA,
scrambled sequence oligonucleotide, or PBS with VCR were injected
into nude mouse subcutaneously transplanted tumors derived from
SGC7901/VCR cell lines. As shown in Fig. 6A, MGr1-Ag/37LRP siRNA with VCR
monotherapy significantly reduced SGC7901/VCR tumor volume by 50%
from days 12 to 36, relative to treatment with PBS alone, VCR
alone, VCR plus scrambled sequence, or the untreated control
(p<0.01). We examined expression levels of MGr1-Ag/37LRP, the
drug transporter proteins P-pg and MRP1 and the apoptosis-related
proteins Bcl-2 and Bax in the transplanted tumor tissues.
Immunohistochemistry staining and western blot analysis were
performed on lysates from tumor tissues after MGr1-Ag/37LRP siRNA
plus VCR, scrambled siRNA plus VCR, VCR or PBS alone. A marked
decrease in expression of MGr1-Ag/37LRP protein was observed in the
SGC7901/VCR tumors treated with MGr1-Ag/37LRP siRNA compared to the
four controls (Fig. 6B and C).
Western blot analyses showed that blocking MGr1-Ag/37LRP expression
with siRNA significantly decreased Bcl-2, P-gp and MRP1 expression,
while increasing expression of pro-apoptotic Bax (Fig. 6C).
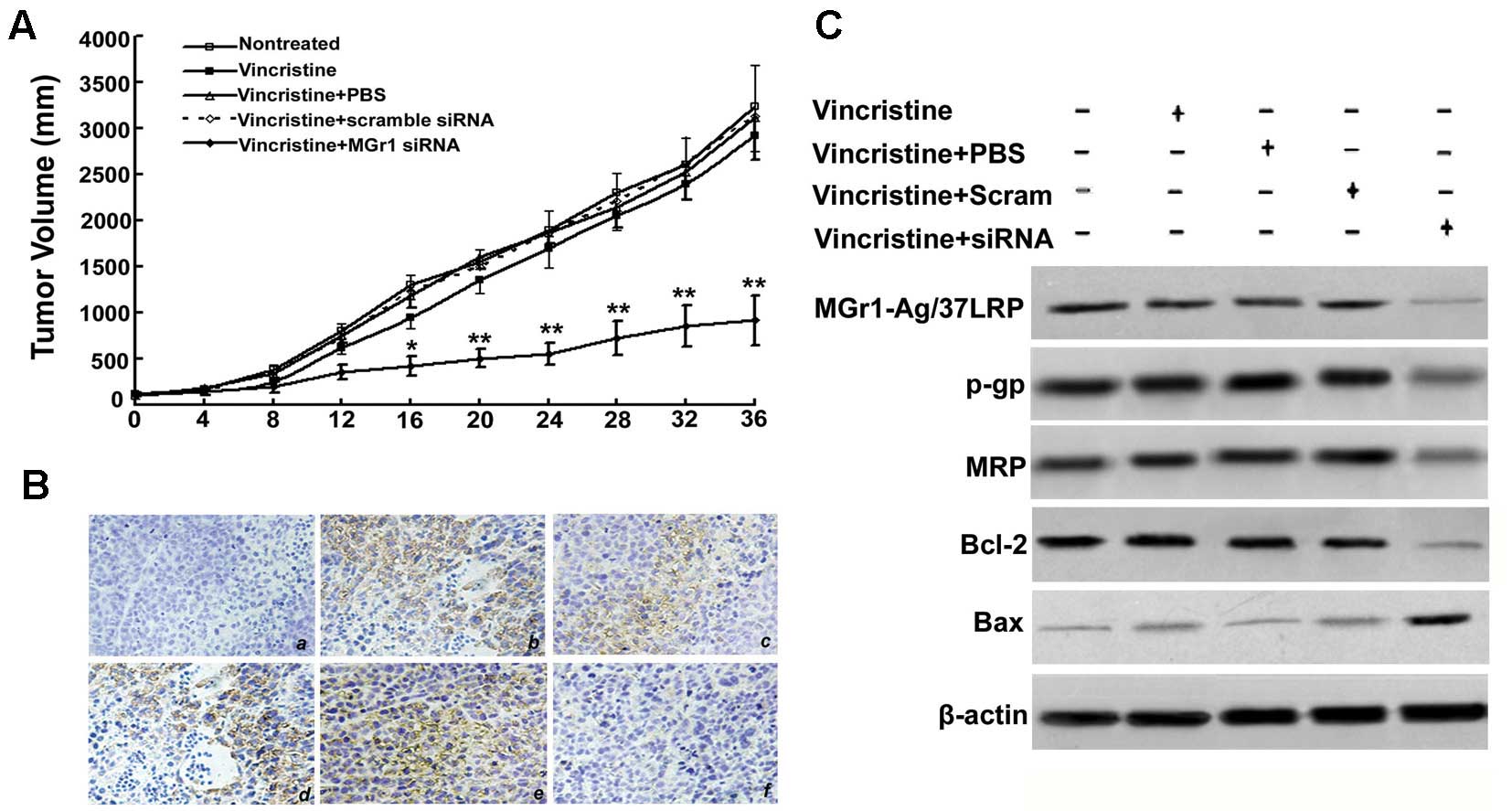 | Figure 6Effect of MGr1-Ag/37LRP siRNA on
SGC7901/VCR tumor growth and chemosensitivity in vivo. (A)
Mice bearing SGC7901/VCR cell-derived tumors were randomly selected
for different treatments (see details in Materials and methods).
*p<0.05 compared to control. (B) Immunohistochemical
staining for MGr1-Ag/37LRP in paraffin-embedded sections from
subcutaneous tumors: (a) negative control group (non-treated tumor
with mouse IgG staining); (b) non-treatment with VCR group; (c) VCR
treatment group; (d) VCR treatment plus scrambled siRNA group; (e)
positive control group (non-treatment tumor); (f) VCR treatment
plus MGr1-Ag siRNA group (original magnification, ×200). (C)
Protein expression of MGr1-Ag/37LRP, P-gp, MRP, Bax and Bcl-2 in
vivo. MGr1-Ag/37LRP, P-gp, MRP, Bax, Bcl-2 and β-actin were
assessed by western blotting. P-gp, P-glycoprotein; MRP, multidrug
resistance-associated protein. |
Discussion
Emerging evidence has shown that the main mechanism
underlying drug resistance in myeloma cells is adhesion to the ECM
(19,20). In the present study, we present
initial evidence that the laminin receptor MGr1-Ag/37LRP increased
the adhesive ability of gastric cancer cells to the ECM,
subsequently leading to CAM-DR.
The ECM is a complicated network of multifunctional
molecules that provides a sophisticated microenvironment for cell
survival, metabolism, migration, proliferation and differentiation
(21,22). LN and COL IV are the major
components of the basement membrane, and are implicated in
carcinogenesis and progression in gastric cancer cells (23). In the present study, we concluded
that the adhesive ability of MDR gastric cancer cells was
significantly increased over the parental cells that were sensitive
to chemotherapeutic drugs. After adhesion to the ECM components LN
or COL IV, resistance to VCR and ADR increased, suggesting that the
MDR phenotype of gastric cancer cells was associated with the cell
adhesion state. We also found that exogenous overexpression of
MGr1-Ag/37LRP upregulated the adhesive ability and drug resistance
of both drug-sensitive and MDR gastric cancer cell lines. Stably
transfected gastric cancer cells (SGC7901-MGr1) that overexpressed
MGr1-Ag/37LRP had a higher ability to adhere to ECM components, and
a greater resistance to chemotherapeutic drugs. The ability of
SGC7901-MGr1 cells to adhere to LN was stronger than adherence to
COL IV, and binding of MGr1-Ag/37LRP to LN may be associated with
gastric cancer cell adhesion. The drug resistance of SGC7901-MGr1
cells adhering to LN was significantly increased over cells
adhering to COL IV, thus binding of MGr1-Ag/37LRP to LN may be
involved in CAM-DR in gastric cancer. Further study indicated that
binding of MGr1-Ag/37LRP to LN promoted the CAM-DR phenotype of
gastric cancer cells by decreasing intracellular drug accumulation,
and inhibiting drug-induced apoptosis through regulation of P-gp,
MRP, Bcl-2 and Bax expression. These results indicated that the
binding of MGr1-Ag/37LRP to its ligand LN upregulated two major
drug transporters, and regulated two apoptosis-related molecules,
leading to the chemoresistance in gastric cancer cells. Notably, we
found that MGr1-Ag/37LRP protein and mRNA levels were upregulated
significantly in gastric cancer cells adhering to LN. Dual
luciferase assays showed increased transcription of the MGr1
promoter (−1600 and −292) in SGC7901 cells adhering to LN. We
propose that MGr1-Ag/37LRP aggravates MDR with the binding to its
ligand LN, at the same time, LN induces MGr1-Ag/37LRP expression by
enhancing transcription. The above data suggest that MGr1-Ag/37LRP
interaction with laminin induces CAM-DR which may be reinforced by
MGr1-LN binding by a positive feedback loop.
In vivo, siRNA of MGr1-Ag/37LRP significantly
enhanced xenograft sensitivity to chemotherapeutic drugs,
decreasing the volume of transplanted tumors. The expression of
P-gp, MRP, Bcl-2 and MGr1-Ag/37LRP in the xenograft tissues
decreased significantly after treatment with MGr1-Ag siRNA, while
pro-apoptotic Bax increased.
In conclusion, the present study revealed that
binding of MGr1-Ag/37LRP with laminin conferred CAM-DR in gastric
cancer by decreasing intracellular drug accumulation and by
inhibiting drug-induced apoptosis. Regulation of P-gp, MRP and
apoptosis-relate genes (Bcl-2 and Bax expression) may be the
underlying mechanisms, and a positive feedback of MGr1-Ag/37LRP
transcription may be involved. Inhibition of the expression of
MGr1-Ag/37LRP enhanced sensitivity of gastric cancer cells to
chemotherapeutic drugs both in vitro and in vivo.
MGr1-Ag/37LRP may serve as an effective potential target for
reversing the MDR of gastric cancer. However, further studies are
needed before a final conclusion is drawn. The present study was
based on a single type of cancer cell line. The MDR gastric cancer
cells and their parental cells were well characterized and
rigorously studied, which provided an ideal cell model. Although
well-established drug-resistant cells of other tissue origins may
not be available, the findings of this study need to be verified
using other types of cancers. In addition, how interaction of
MGr1-Ag/37LRP with cell adhesion ligands regulates the expression
of various genes remains to be clarified. Various pathways have
been implicated in adhesion-induced apoptosis resistance in cancer
including MAPK (mitogen-activated protein kinase)/ERK
(extracellular regulated kinase) kinase (MEK) and
phosphatidylinositol-3-kinase (PI3-K)-mediated survival cascades
(24). The regulatory mechanisms of
gene expression by MGr1-Ag/37LRP warrant further research.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (no. 81272349), and the 973 National
Key Scientific Research Project (no. 2010CB732400).
References
|
1
|
Fan DM, Xiao B, Shi YQ, Ming-Feng, Qiao
TD, Chen BJ and Chen Z: A novel cDNA fragment associated with
gastric cancer drug resistance was screened out from a library by
monoclonal antibody MGr1. World J Gastroenterol. 4:110–111.
1998.
|
|
2
|
Shi Y, Zhai H, Wang X, et al:
Multidrug-resistance-associated protein MGr1-Ag is identical to the
human 37-kDa laminin receptor precursor. Cell Mol Life Sci.
59:1577–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun L, Shi Y, Guo C, et al: Regulation of
multidrug resistance by MGr1-antigen in gastric cancer cells.
Tumour Biol. 27:27–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boudreau NJ and Jones PL: Extracellular
matrix and integrin signalling: the shape of things to come.
Biochem J. 339:481–488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Damiano JS, Cress AE, Hazlehurst LA, Shtil
AA and Dalton WS: Cell adhesion mediated drug resistance (CAM-DR):
role of integrins and resistance to apoptosis in human myeloma cell
lines. Blood. 93:1658–1667. 1999.PubMed/NCBI
|
|
6
|
Nakamura K, Mori M and Enjoji M:
Distribution of basement membrane antigens in clinical gastric
adenocarcinomas: an immunohistochemical study. J Clin Pathol.
40:1418–1423. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawaguchi M, Akagi M, Gray MJ, Liu W, Fan
F and Ellis LM: Regulation of vascular endothelial growth factor
expression in human gastric cancer cells by interleukin-1β.
Surgery. 136:686–692. 2004.
|
|
8
|
Jaseja M, Mergen L, Gillette K, Forbes K,
Sehgal I and Copié V: Structure-function studies of the functional
and binding epitope of the human 37 kDa laminin receptor precursor
protein. J Pept Res. 66:9–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai X, Zhang X and Fan D: Establishment of
multidrug resistant gastric cancer cell line and its biological
characteristics. Chin J Clin Oncol. 2S:67–71. 1994.(in Chinese with
English abstract).
|
|
10
|
Liu L, Zhang H, Sun L, et al: ERK/MAPK
activation involves hypoxia-induced MGr1-Ag/37LRP expression and
contributes to apoptosis resistance in gastric cancer. Int J
Cancer. 127:820–829. 2010.PubMed/NCBI
|
|
11
|
Liu L, Sun L, Zhang H, et al:
Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers
occurs via hypoxia-inducible-factor 1-dependent mechanism and
contributes to drug resistance. Int J Cancer. 124:1707–1715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan K, Fan D, Cheng LF and Li C:
Expression of multidrug resistance-related markers in gastric
cancer. Anticancer Res. 20:4809–4814. 2000.PubMed/NCBI
|
|
13
|
Thamilselvan V and Basson MD: Pressure
activates colon cancer cell adhesion by inside-out focal adhesion
complex and actin cytoskeletal signaling. Gastroenterology.
126:8–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Xu LZ, He KL, et al: Reversal
effects of nomegestrol acetate on multidrug resistance in
adriamycin-resistant MCF7 breast cancer cell line. Breast Cancer
Res. 3:253–263. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Filleur S, Courtin A, Ait-Si-Ali S, et al:
SiRNA-mediated inhibition of vascular endothelial growth factor
severely limits tumor resistance to antiangiogenic thrombospondin-1
and slows tumor vascularization and growth. Cancer Res.
63:3919–3922. 2003.
|
|
16
|
Kuo CC, Hsieh HP, Pan WY, et al: BPR0L075,
a novel synthetic indole compound with antimitotic activity in
human cancer cells, exerts effective antitumoral activity in vivo.
Cancer Res. 64:4621–4628. 2004. View Article : Google Scholar
|
|
17
|
Gleave M, Tolcher A, Miyake H, et al:
Progression to androgen independence is delayed by adjuvant
treatment with antisense Bcl-2 oligodeoxynucleotides after
castration in the LNCaP prostate tumor model. Clin Cancer Res.
5:2891–2898. 1999.
|
|
18
|
Romanov VI, Wrathall LS, Simmons TD, Pinto
da Silva P and Sobel ME: Protein synthesis is required for
laminin-induced expression of the 67-kDa laminin receptor and its
37-kDa precursor. Biochem Biophys Res Commun. 208:637–643. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noborio-Hatano K, Kikuchi J, Takatoku M,
et al: Bortezomib overcomes cell-adhesion-mediated drug resistance
through downregulation of VLA-4 expression in multiple myeloma.
Oncogene. 28:231–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobune M, Chiba H, Kato J, et al:
Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated
drug resistance of multiple myeloma in an autocrine mechanism. Mol
Cancer Ther. 6:1774–1784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hohenester E and Engel J: Domain structure
and organisation in extracellular matrix proteins. Matrix Biol.
21:115–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Timpl R: Macromolecular organization of
basement membranes. Curr Opin Cell Biol. 8:618–624. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
David L, Nesland JM, Holm R and
Sobrinho-Simões M: Expression of laminin, collagen IV, fibronectin,
and type IV collagenase in gastric carcinoma. An
immunohistochemical study of 87 patients. Cancer. 73:518–527. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westhoff MA and Fulda S: Adhesion-mediated
apoptosis resistance in cancer. Drug Resist Updat. 12:127–136.
2009. View Article : Google Scholar : PubMed/NCBI
|