Introduction
Prostate cancer (PCa) is the most common type of
cancer and the second leading cause of cancer-related mortality in
men in North America (1). Since
many patients have already developed metastatic lesions at initial
clinical presentation, hormone therapy has become a standard
treatment with confirmed beneficial effects for hormone-sensitive
disease. However, castration-resistant prostate cancer (CRPC)
invariably recurs within 1 to 2 years, and few treatment options
are available once this aggressive prostate cancer has the capacity
to resist chemotherapy or radiotherapy (2–4).
Therefore, novel adjuvant agents with complete efficacy and low
toxicity are desired to treat metastatic CRPC and substantially
prolong patient survival.
Flavonoids, consisting of flavones, flavanones,
isoflavones and flavanols, are important for human nutrition and
plant biology as plant secondary metabolites. Several isolated
naturally existing flavanones have been determined for their
anti-inflammatory, anti-oxidative and anti-bacterial activities
(5). In addition,
structure-activity studies have demonstrated that flavanones with
more hydroxyl groups exhibit increased bioactivities (6). Moreover, hydroxyflavanones have also
been well characterized to have various antitumor properties via
distinct mechanisms of action (7,8). For
example, flavanones and 2′-hydroxyflavanone (2HF, chemical
structure as shown in Fig. 1A)
inhibit the metastasis of lung cancer cells via downregulation of
proteinase activities and the MAPK pathway (9). 2HF was also found to inhibit
proliferation, tumor vascularization and to promote normal
differentiation in VHL-mutant renal cell carcinoma (10). Moreover, 2HF induced apoptosis
through Egr-1 involving expression of Bax, p21 and NAG-1 in colon
cancer cells (11). However, few
studies have shown the therapeutic effects and potential mechanisms
of 2HF in regards to the growth of prostate cancer, particularly
CRPC.
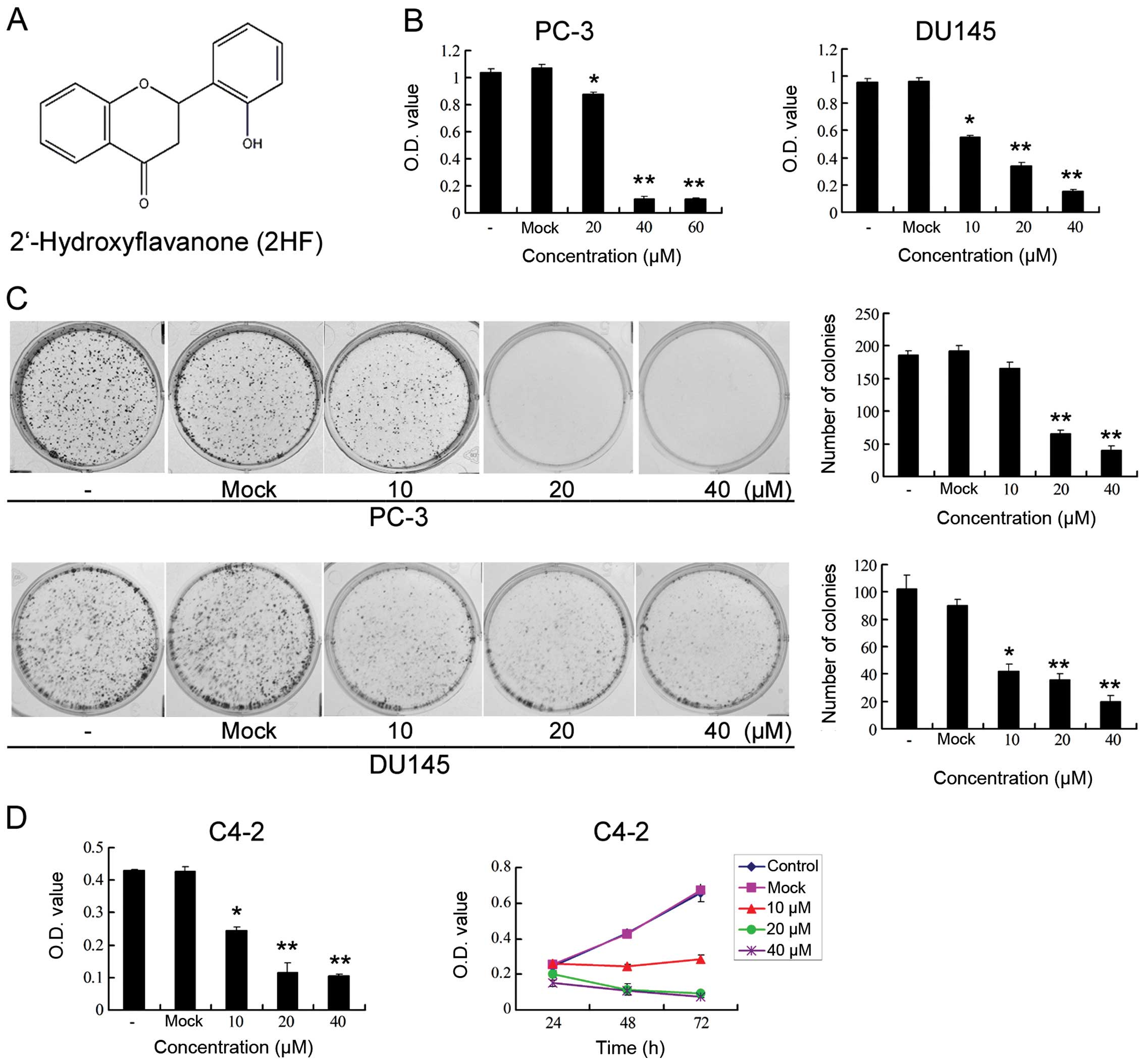 | Figure 12HF inhibits cell proliferation and
clonogenicity of metastatic CRPC cells in vitro. (A)
Chemical structure of 2HF. (B) PC-3 and DU145 cells were treated
with different doses of 2HF (0, 20 and 40 μmol/l) for 48 h and
processed for MTT assay to measure the changes in cell
proliferation. Compared with the control, *P<0.05,
**P<0.01. (C) PC-3 and DU145 cells were treated with
different doses of 2HF (0, 20 and 40 μmol/l) for 48 h and processed
for colony formation assay to measure the changes in cell
clonogenicity. Left panel, representative images of colony
formation after 2HF treatment are shown. Right panel, quantitative
analysis is shown. Compared with the control,
*P<0.05, **P<0.01. (D) Left panel, C4-2
cells were treated with different doses of 2HF (0, 20 and 40
μmol/l) for 48 h and processed for MTT assay to measure the changes
in cell proliferation. Compared with the control,
*P<0.05, **P<0.01. Right panel, C4-2
cells were treated with different doses of 2HF (0, 20 and 40
μmol/l) for the indicated times before MTT assay and the growth
curve was analyzed. |
In the present study, utilizing three different
metastatic and androgen-independent PCa cell models (PC-3, DU145
and C4-2), we showed that 2HF treatment significantly suppressed
cell proliferation and clonogenicity in a dose- and time-dependent
manner. It also markedly inhibited subcutaneous tumor growth in
vivo. Furthermore, 2HF enhanced cell apoptosis in a
dose-dependent manner, which was confirmed by Annexin V/propidium
iodide (PI) staining and cleavage of poly(ADP-ribose) polymerase
(PARP) and caspase-3. Mechanistically, 2HF induced AKT
dephosphorylation, inhibited the phosphorylation and
transactivation of signal transducer and activator of
transcription-3 (STAT3) and subsequently downregulated the
expression of its downstream target genes (i.e., Mcl-1, Bcl-2 and
Bax), which are probably involved in the regulation of apoptotic
and anti-apoptotic pathways.
Materials and methods
Cell culture and 2HF treatment
Androgen receptor (AR)-negative PCa cell lines PC-3
(bone metastatic) and DU145 (brain metastatic) were maintained in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco, San Diego, CA,
USA) supplemented with 10% fetal bovine serum (FBS) at 37°C with 5%
CO2 in a humidified incubator. Androgen-sensitive but
independent AR-positive PCa cell line C4-2 (bone metastatic) was
maintained in T-medium supplemented with 5% FBS. 2HF was obtained
from Sigma (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide
(DMSO). For 2HF treatment, a stock solution (0.02 M in DMSO) was
added into the culture medium with 1% FBS to achieve the
appropriate concentration (10, 20 or 40 μmol/l) and then incubated
with the cells, whereas DMSO solution without 2HF was used as the
control.
Cell viability assay
To evaluate the sensitivities of different prostate
cancer cells to 2HF treatment,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma) proliferation assays were performed to determine cell
viability. Different PCa cells were plated at a density of
2×103 cells/well in 96-well plates for 24 h and then
treated with different concentrations of 2HF (0, 10, 20 and 40 μM)
for the indicated time. After the exposure period, 20 μl MTT was
added to each well for another 5 h incubation at 37°C in 5%
CO2. Thereafter, the medium containing MTT was removed,
and 150 μl DMSO was added to solubilize the formazan crystals. The
absorbance (OD) was then measured at a wavelength of 590 nm by a
microplate autoreader (Bio-Tek Instruments, Vinooski, VT, USA).
Independent experiments were repeated in triplicate.
Colony formation assays
The colony formation assay was used to determine the
clonogenicity capabilities in vitro as described previously
(12). The indicated cells were
treated with different concentrations of 2HF (0, 10, 20 and 40 μM)
for 36 h. Then an equal number of cells were seeded in 6-well
plates to form colonies for at least two weeks and fresh medium was
changed every 3–4 days. The plates were then washed with ice-cold
PBS, fixed with 4% paraformaldehyde, stained in crystal violet
solution for 15 min at room temperature and washed with distilled
water to remove excess dye. The number of colonies was counted for
each sample.
Flow cytometric analysis for cell
apoptosis
PC-3, DU145 or C4-2 cells (1–3×106) were
plated on a 6-cm dish for 24 h, and treated with different
concentrations of 2HF (0, 10, 20 and 40 μM) for another 24 h. After
that, cells were harvested, washed with PBS and then subjected to
Annexin V and PI staining using an Annexin V-FITC apoptosis
detection kit (Invitrogen, Eugene, OR, USA) according to the
manufacturer’s instructions and immediately analyzed by flow
cytometry (FACSCalibur, BD Biosciences, NJ, USA).
Western blot analyses
The indicated cells were treated with 2HF for 36 h
and then total cellular protein lysates were prepared with RIPA
buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% NP-40 and
0.5% sodium deoxycholate] containing proteinase inhibitors, 1%
Cocktail and 1 mmol/l PMSF (Sigma, St. Louis, MO, USA). A total of
20–40 μg of protein was separated by 10–12% SDS-PAGE and
transferred to nitrocellulose membranes. Following blocking of the
non-specific binding sites on the membranes with 5% skimmed milk in
Tris-buffered saline with 0.1% Tween-20 (pH 7.6, TBST) for 1 h at
room temperature, the membranes were then incubated with the
desired primary antibody overnight at 4°C. Antibodies for western
blotting were rabbit anti-PARP (CST-9532; Cell Signaling
Technology, Danvers, MA, USA) at a 1:500 dilution, rabbit
anti-phosphorylated-AKT (Ser473, CST-4060, 1:1,000), rabbit
anti-AKT (CST-9272, 1:1,000), mouse anti-phosphorylated-STAT3
(Tyr705, CST-4113, 1:1,000), rabbit anti-STAT3 (CST-8232, 1:1,000),
rabbit anti-Mcl-1 (CST-5453, 1:500), rabbit anti-Bcl-2 (SC-492;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:500 dilution,
rabbit anti-Bax (SC-493, 1:200) and rabbit anti-caspase-3
(CST-9665, 1:1,000). After being washed with TBST, membranes were
incubated with secondary antibodies coupled to horseradish
peroxidase at room temperature for 1 h and visualized with an ECL
chemiluminescent detection system (Pierce, Rockford, IL, USA).
Loading differences were normalized using monoclonal
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody.
Dual-luciferase reporter assay
For the reporter gene assay, cells seeded in 24-well
plates were transfected with 200 ng STAT3-responsive luciferase
reporter plasmid pLucTKS3 or the control plasmid pLucTK (13) and 1 ng of the pRL-SV40
Renilla luciferase construct (as an internal control) for 24
h and then subjected to 2HF, PI3K inhibitor LY 294002 or STAT3
inhibitor Stattic treatment. Cell extracts were prepared 36 h after
treatment and the luciferase activity was measured using the
Dual-Luciferase reporter assay system (Promega) as described
previously (14). Relative
luciferase activity is represented as mean ± SEM from each sample
after normalizing with the control.
In vivo subcutaneous xenograft model
Sixteen male BALB/c-nu mice at the age of 3–4 weeks
and weighing 15–20 g were purchased from the Shanghai Laboratory
Animal Center (SLAC, Shanghai, China) and were acclimated for one
week before beginning the experiment. All animal experiments were
carried out in accordance with a protocol approved by the
Institutional Animal Care and Use Committee. Single cells
(5×106) were subcutaneously inoculated into both flanks
of the mice. Once the tumors were established (100–150
mm3), the mice were randomly divided into two groups.
The mice (eight in each group) received 2HF (100 mg/kg body weight,
daily) in 100 μl corn oil by oral gavage once per day. Control
groups were treated with corn oil only. Body weight and tumor
volume were measured two times per week. Calipers were used to
measure the two dimensions of the tumors. Thirty days after
treatment, mice were sacrificed by cervical dislocation, and tumors
were removed and weighed.
Immunohistochemical (IHC) staining
Xenografts were harvested for staining and IHC was
carried out with Dako Autostainer Plus system (Dako, Carpinteria,
CA, USA) as previously described (14). Briefly, sections were
deparaffinized, rehydrated and subjected to antigen retrieval in
citrate buffer (10 mM, pH 6.0) for 5 min and then endogenous
peroxidase and alkaline phosphatase activities were blocked with
Dual Block for 10 min. The slides were then incubated overnight at
4°C with p-AKT (Ser473, CST-4060), p-STAT3 (Tyr705, CST-4113),
Bcl-2 (SC-492) and cleaved caspase-3 (CST-9661) antibodies (1:75
dilution). After washing, this was followed by incubation with
EnVision secondary antibody for 30 min at room temperature. Signals
were detected by adding substrate hydrogen peroxide using
diaminobenzidine (DAB) as a chromogen followed by hematoxylin
counterstaining.
Statistical analysis
All assays were repeated in triplicate in three
independent experiments and quantitative data are presented as
means ± SEM. The differences between two groups were compared by
the 2-tailed Student’s t-test. In all cases, a P-value of <0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SPSS 18.0 (SPSS Inc,
Chicago, IL, USA).
Results
2HF inhibits cell proliferation and
clonogenicity of prostate cancer cells in vitro
The progression to androgen independence is a
multi-factorial process by which cells acquire the ability to both
survive in the absence of androgens and proliferate using
non-androgenic stimuli for mitogenesis. Therefore, targeting tumor
cell growth may still play an important role in CRPC therapy. As
shown in Fig. 1B, we initially
showed that 2HF inhibited in vitro cell proliferation in a
dose-dependent manner in PC-3 cells, which were derived from bone
metastatic lesion and are androgen-independent. Furthermore, we
performed a colony formation assay to analyze the potential effects
of 2HF on the viability of PC-3 cells. Indeed, 2HF significantly
inhibited the clonogenicity of PC-3 cells with a decreased number
of colonies, in a dose-dependent manner (Fig. 1C). To rule out cell-specific
effects, we repeated our MTT and colony formation assays in another
PCa cell line, DU145, which was also androgen-independent and brain
metastatic. Consistently, 2HF suppressed in vitro cell
proliferation and clonogenicity of DU145 cells in a dose-dependent
manner (Fig. 1B and C). Moreover,
similar results were also observed in another bone metastatic,
androgen-independent but androgen receptor (AR)-positive PCa cell
line C4-2 (Fig. 1D), since the PC-3
and DU145 cells were AR-negative. All of these findings suggest
that 2HF targets androgen-independent CRPC tumor growth in
vitro, which does not depend on the status of AR
expression.
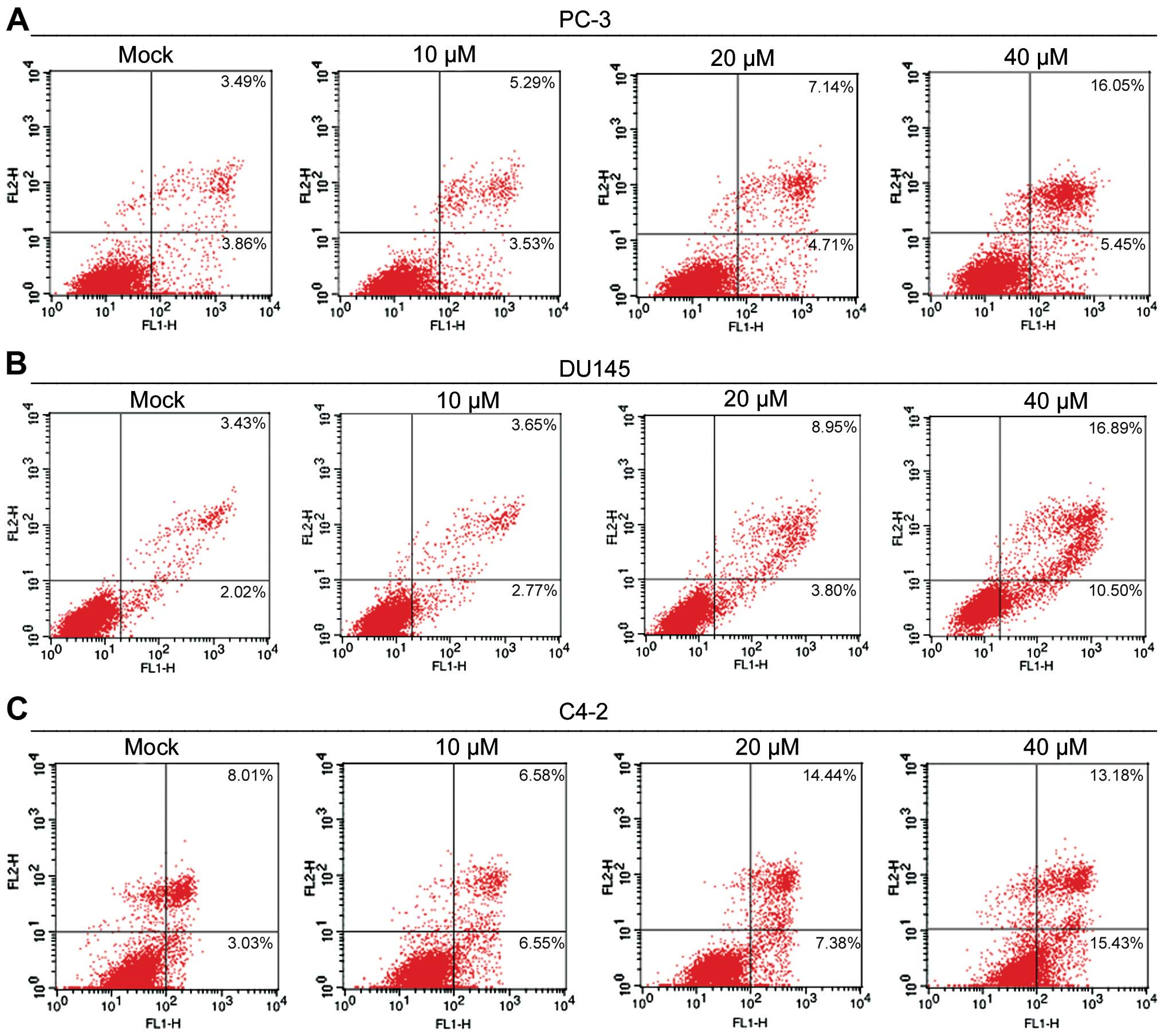
2HF induces cell apoptosis in prostate
cancer cells
Apoptosis is one of the important mechanisms by
which anticancer agents inhibit the growth of cancer cells. To
understand the mechanism by which 2HF inhibits the viability of
prostate cancer cells, PC-3 and DU145 cells were exposed to various
concentrations of 2HF for 24 h, and the population of apopototic
cells after Annexin V/PI staining was determined by flow cytometry.
As the representative data show in Fig.
2A, 2HF treatments at 10, 20 and 40 μM resulted in 8.82, 11.85
and 21.5% apoptotic cells in the PC-3 cells, respectively, while
the baseline of the control cells was 7.35%. In addition, 2HF
treatment at 10, 20 and 40 μM induced 6.42, 12.75 and 27.39%
apoptosis in the DU145 cells, respectively, while the baseline of
the control cells was 5.45% (Fig.
2B). Moreover, similar results were observed in C4-2 cells
after 2HF treatment (Fig. 2C).
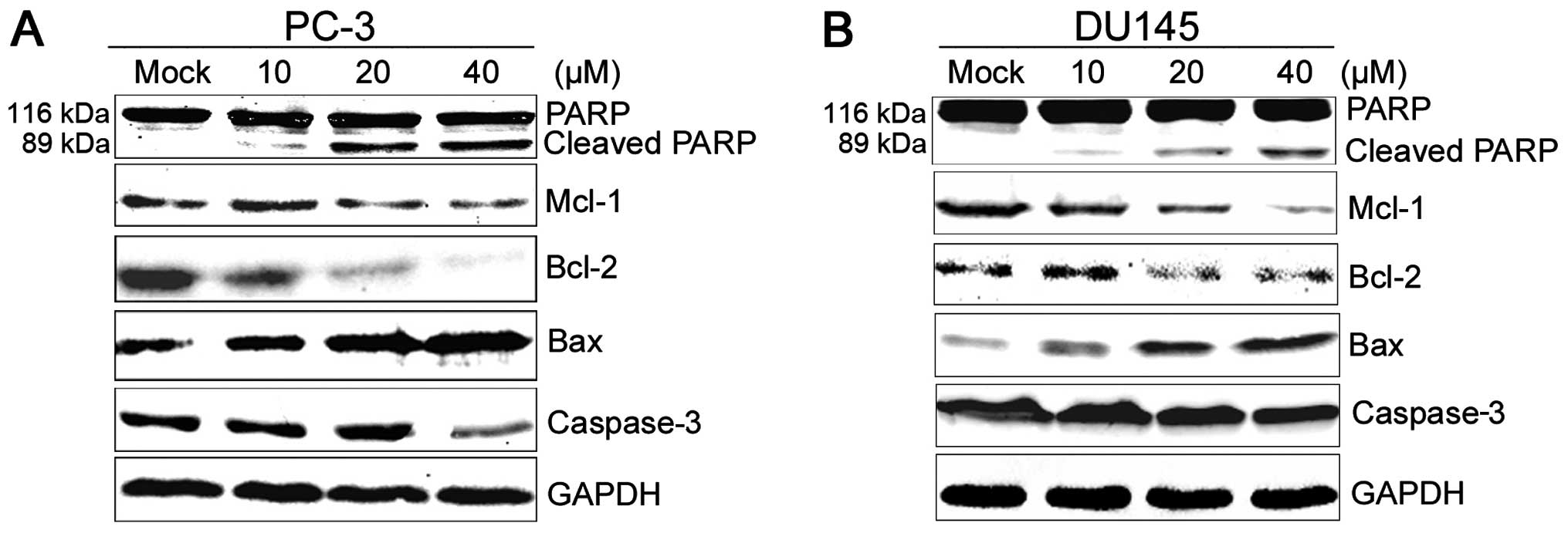
Correspondingly, 2HF gradually increased the cleavage of poly-PARP,
a chromatin-associated enzyme that plays an important role in DNA
repair and the recovery of cells from DNA damage, in a
dose-dependent manner in both PC-3 and DU145 cells (Fig. 3A and B). Consistent with the
results, total caspase-3 proteins decreased accordingly after 2HF
treatment, indicating the cleavage of caspase-3. Collectively,
these results indicate that the induction of apoptotic cell death
by 2HF probably occurs through a caspase-dependent pathway.
2HF suppresses AKT/STAT3 signaling and
the expression of the BCL-2 family in prostate cancer cells
To further investigate the mechanisms of 2HF
targeting prostate tumor growth, we performed western blot analyses
to detect the expression of proteins related to apoptosis. Indeed,
2HF treatment modulated the expression of members of the
BCL-2 gene family (15),
which include protective proteins involved in the mitochondrial
apoptotic pathway. As shown in Fig.
3A, following the treatment of PC-3 cells with 10, 20 and 40 μM
2HF for 48 h, the expression levels of Mcl-1 and Bcl-2 proteins
were significantly downregulated in a dose-dependent manner, while
Bax protein was gradually upregulated after treatment. Similar
results were also observed in the DU145 cells (Fig. 3B).
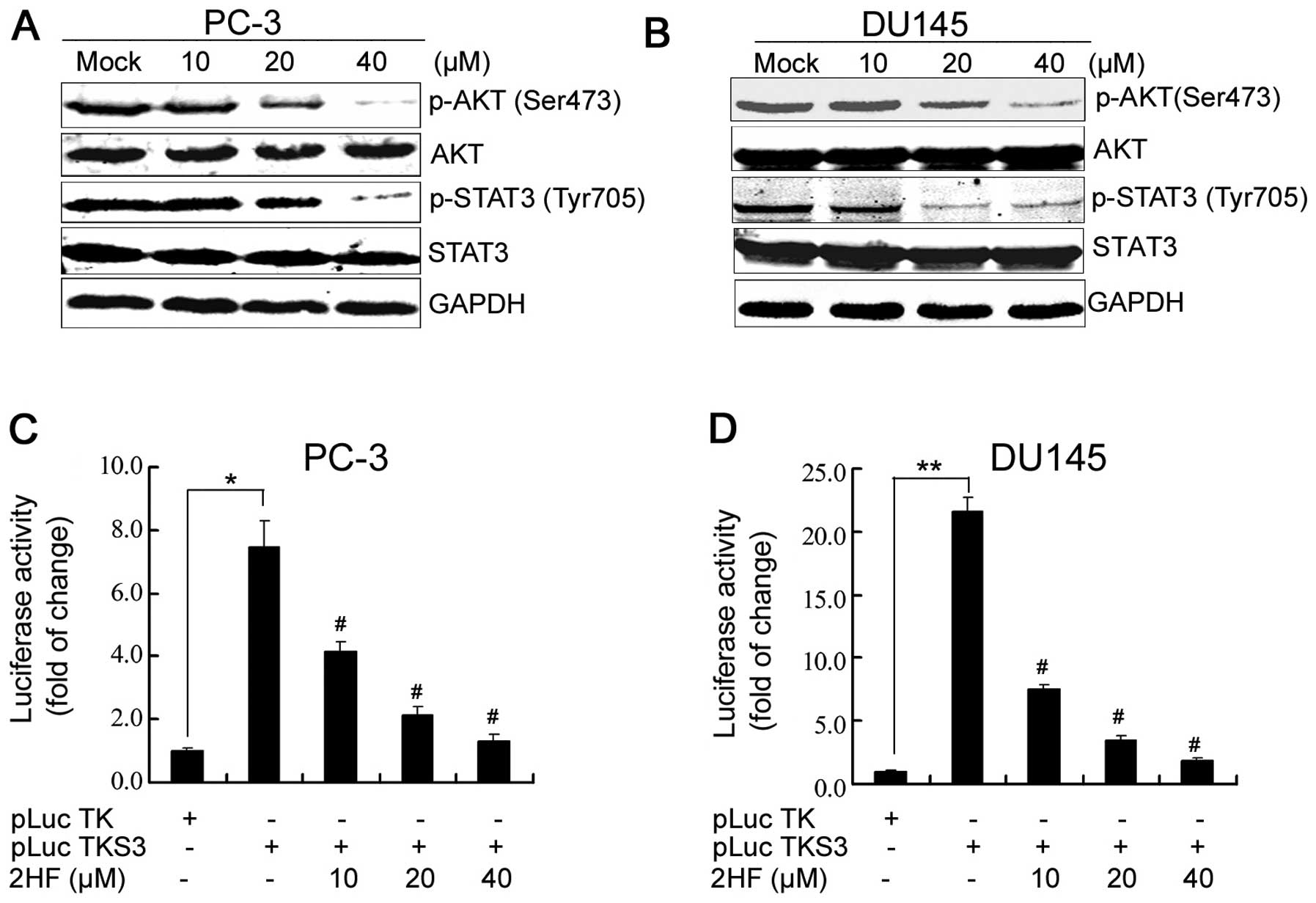
PTEN deletions and mutations lead to aberrant
activation of the PI3K/AKT pathway in prostate cancer (16), which is involved in the regulation
of cancer cell growth, motility, survival and metabolism. Thus
inactivation or inhibition of PI3K/AKT signaling pathways provide a
new substantial strategy to target CRPC using small molecules or
plant extracts (17,18). In the present study, we observed
that 2HF treatment markedly inhibited the phosphorylation of AKT on
the site of serine 473 in both PC-3 and DU145 cells (Fig. 4A and B). 2HF treatment also
inhibited the phosphorylation of STAT3 (Fig. 4A and B), another critical signaling
transduction protein, which is constitutively activated in prostate
cancer by phosphorylation of its tyrosine 705 residue (19). After phosphorylation, STAT3
homodimerizes and translocates to the nucleus where it binds to
specific STAT3 response elements of target gene promoters to
regulate transcription. Indeed, using a specific STAT3-responsive
luciferase reporter (13), we
further demonstrated that 2HF suppressed STAT3 transactivation in
both PC-3 and DU145 cells based on a luciferase reporter gene
assay, which was also dose-dependent (Fig. 4C and D). Therefore, 2HF inhibited
AKT/STAT3 signaling and the expression of the BCL-2 family in the
prostate cancer cells.
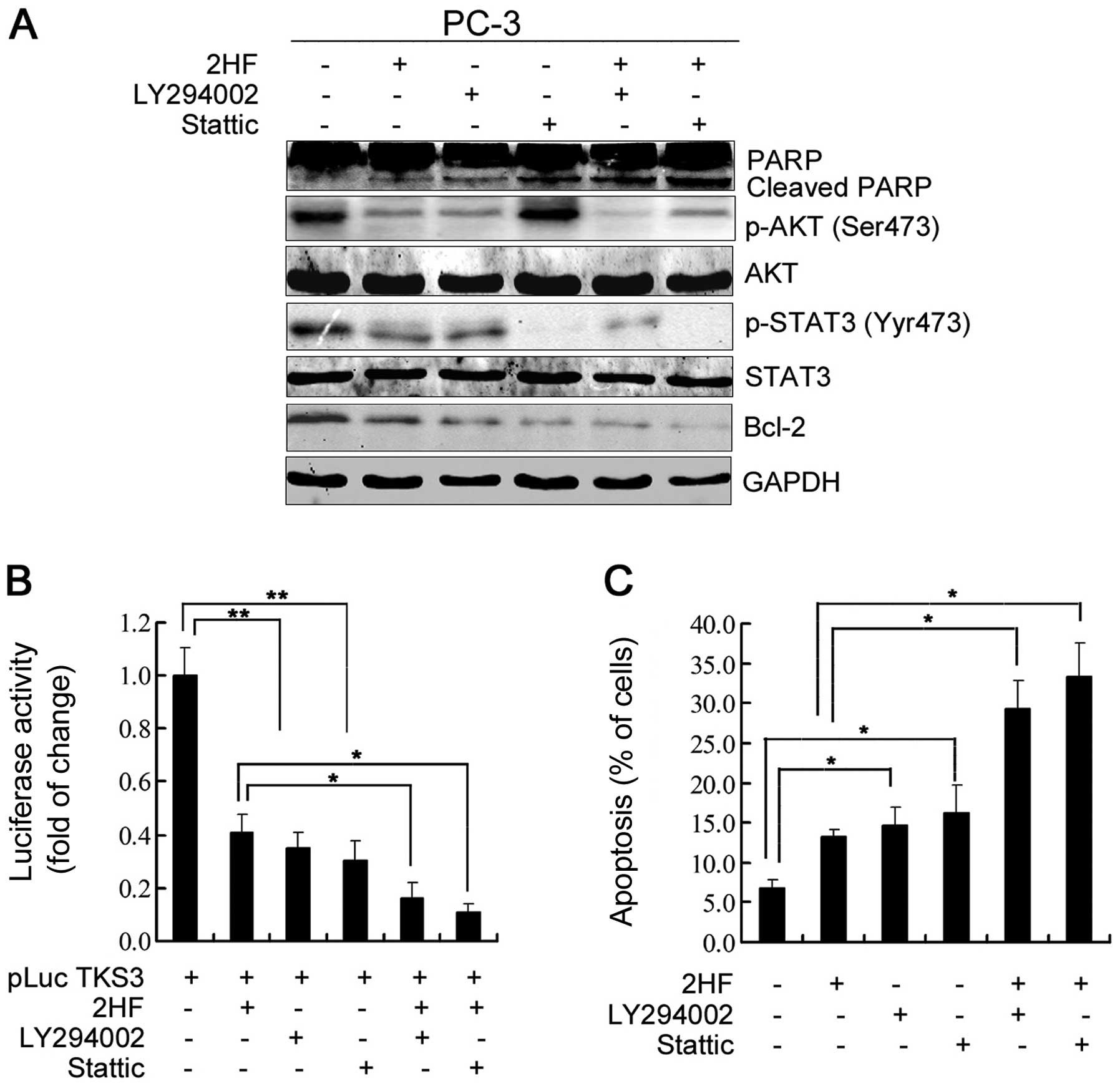
AKT/STAT3 signaling regulates Bcl-2
expression and cell apoptosis after 2HF treatment
To further dissect the roles of AKT and STAT3
signaling in the inhibitory effects of 2HF on PCa cells, we applied
the PI3K inhibitor LY294002 or STAT3 inhibitor Stattic to pretreat
PC-3 cells and analyzed the changes in Bcl-2 protein expression and
cell apoptosis. As shown in Fig.
5A, western blotting data clearly indicated that LY294002
treatment alone significantly decreased p-AKT (Ser473), p-STAT3
(Tyr705) and Bcl-2 protein expression in the PC-3 cells and it also
enhanced the inhibitory effects of 2HF on AKT phosphorylation,
STAT3 phosphorylation and Bcl-2 protein expression (Fig. 5A). Consistently, it also increased
the inhibitory effects of 2HF on STAT3-responsive luciferase
activity (Fig. 5B). Moreover,
inhibition of PI3K/AKT also significantly enhanced the induction of
PC-3 cell apoptosis after 2HF treatment (Fig. 5C). In contrast, Stattic treatment
had no effects on AKT phosphorylation in PC-3 cells (Fig. 5A), indicating that the STAT3 pathway
was activated as downstream signaling of PI3K/AKT. However,
inhibition of STAT3 activity also decreased Bcl-2 protein
expression and increased cell apoptosis (Fig. 5A and C). Moreover, we observed that
additional Stattic treatment further decreased Bcl-2 protein
expression and increased cell apoptosis of PC-3 cells induced by
2HF treatment (Fig. 5A and C).
2HF inhibits prostate tumor growth and
AKT/STAT3/Bcl-2 signaling in vivo
We also generated xenograft models to show the
inhibitory effects of 2HF on prostate tumor growth in vivo.
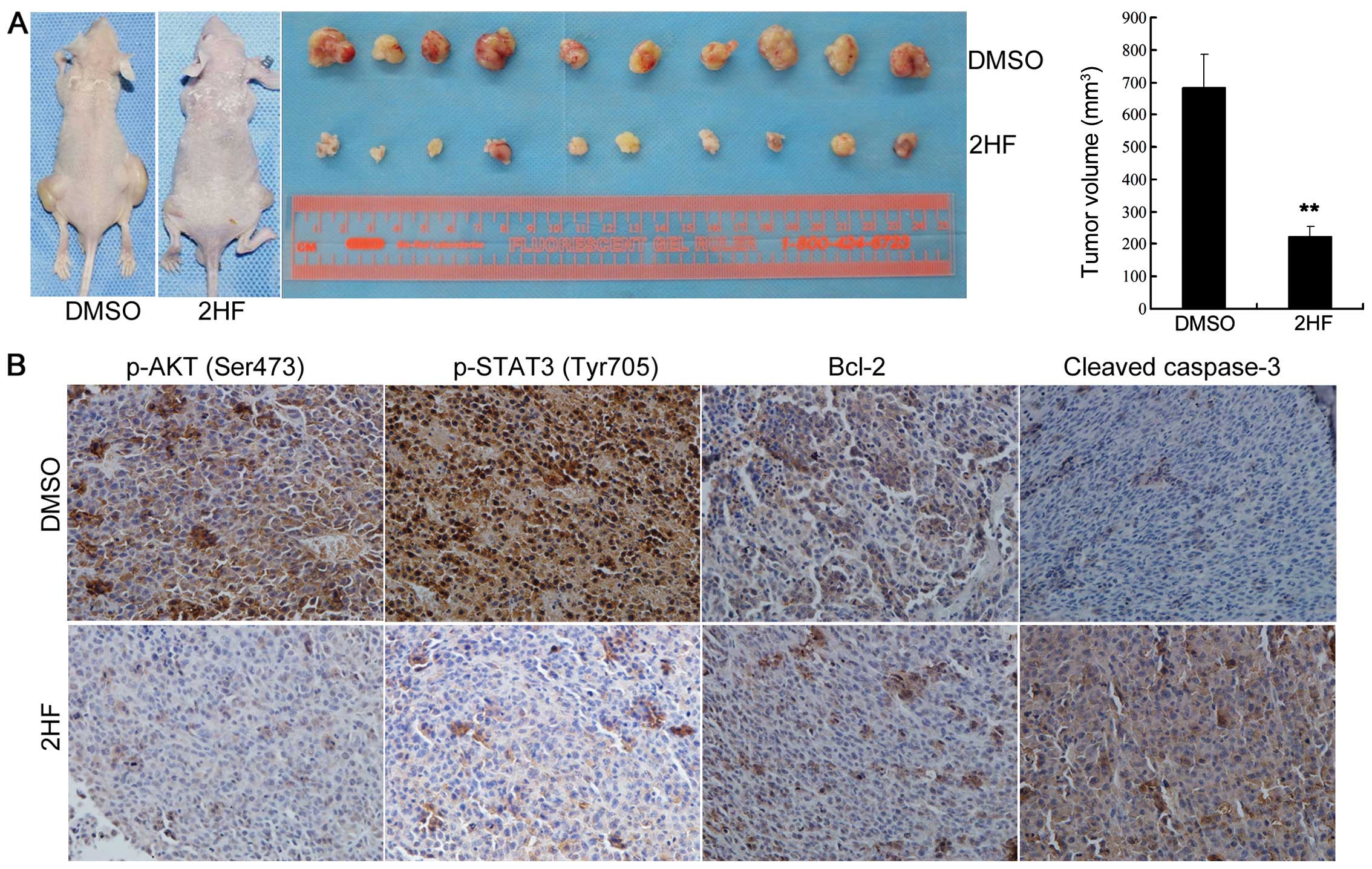
As shown in Fig. 6A, oral 2HF
treatment significantly decreased the tumor burden of subcutaneous
PC-3 xenografts (left and middle panel), and the average tumor
volume was much smaller (right panel, P<0.05). Furthermore, IHC
staining data also supported the observation in the cell lines
in vitro. p-AKT (Ser473), p-STAT3 (Tyr705) and Bcl-2 protein
expression levels in the subcutaneous PC-3 xenograft tissues with
or without 2HF treatment were detected by IHC staining. Consistent
with our findings in vitro, higher expression levels of
p-AKT (Ser473), p-STAT3 (Tyr705) and Bcl-2 were detected in the
PC-3 xenograft samples, indicating hyperactivation of
AKT/STAT3/Bcl-2 signaling in metastatic CRPC, while 2HF treatment
suppressed the phosphorylation of AKT and STAT3 and potentially
inactivated STAT3 transcriptional activity and decreased the
expression of its target gene Bcl-2 (Fig. 6B). We also observed that 2HF
treatment increased the cleavage of caspase-3 in the PC-3
xenografts using an IHC-specific cleaved-caspase-3 antibody
(Fig. 6B), indicating the incidence
of cell apoptosis in vivo after 2HF treatment.
Discussion
Treatment paradigms for men with metastatic CRPC
have changed dramatically in the last three years due to the
approval of agents such as the CYP-inhibitor abiraterone and
second-generation AR antagonist enzalutamide, as well as other
agents. These novel therapeutics have recently been proven to
extend survival via distinct mechanisms of action (20). However, drug resistance eventually
develops; thus, further exploration into new adjuvant agents and
their potential therapeutic mechanisms in prostate cancer are
necessary.
Flavanones richly exist in citrus and have been well
characterized to have various bioactive properties. In our previous
reports (21–24), we extensively studied the
chemopreventive and chemotherapeutic effects of silibinin, a
flavanone isolated from the fruits of milk thistle, in prostate and
bladder cancer cells. Here, we further observed that another
nontoxic natural flavanone 2HF also significantly inhibited the
cell proliferation and xenograft tumor growth of different
metastatic CRPC cells (PC-3, DU145 and C4-2), in which the
inhibitory effects of 2HF on cell growth and colony formation were
dose- and time-dependent.
Previous research has shown that AR is highly
expressed and transcriptionally active in CRPC and has indicated
that steroids from the adrenal glands contribute to this AR
activity (25). More recent studies
revealed that CRPC cells have increased expression of enzymes
mediating androgen synthesis from adrenal steroids and are also
able to synthesize androgens de novo from cholesterol
(26). Therefore, investigation of
novel agents targeting these critical enzymes involved in de
novo androgen synthesis is promising. A previous study in
particular screened 2HF from several dietary flavonoids as a
powerful selective inhibitor of AKR1C3, a critical aldo-keto
reductase converting the weak androgen Δ4-androstene-3,17-dione
into the potent androgen testosterone, and as a novel potential
target for CRPC treatment (27).
Indeed, Ofude et al also further demonstrated that 2HF
inhibited AR transcription activity, PSA expression and
androgen-induced cell proliferation in LNCaP cells (28). However, most of our CRPC cell models
(PC-3 and DU145) had no AR expression, suggesting that other
androgen-AR bypass signaling may be involved in the inhibitory
effects of 2HF on CRPC tumor growth.
CRPC cells maintain resistance to castration and
recur after chemotherapy, which depends on the ratio of
proliferating cells versus apoptotic cells. Therefore, the
alteration of cell death signaling may still play an important role
in novel PCa therapy (29). Indeed,
our present study demonstrated that 2HF treatment significantly
increased the cell apoptotic rate and cleavage of PARP and
caspase-3 in PC-3 and DU145 cells in a dose-dependent manner,
indicating that 2HF induces the incidence of caspase-mediated cell
apoptosis in CRPC cells. A similar phenomenon has also been
observed in other cancer cells, such as renal and colon carcinoma
cells (10,11). Moreover, a study by Ofude et
al also demonstrated the induction of cell apoptosis in PC-3
and DU145 cells after 2HF treatment (28), yet the mechanisms remain largely
unknown.
As known, the PI3K pathway is aberrantly activated
in prostate cancers due to PTEN deletions and mutations and may
also be involved in CRPC progression, which leads to the
recruitment and activation of the AKT serine/threonine kinase (34)
and subsequently regulates tumor cell proliferation, growth,
survival and metastasis. Therefore, inactivation or inhibition of
the PI3K/AKT signaling pathways provide a new substantial strategy
for CRPC treatment. In the present study, we further demonstrated
that 2HF significantly suppressed the phosphorylation of AKT on the
site of serine473. Furthermore, inactivation of AKT after 2HF
treatment in turn inhibited the phosphorylation and transactivation
of STAT3, indicating that 2HF targets distinct signaling cascades
for CRPC treatment.
As a constitutively active transcription factor in
CRPC, STAT3 becomes active by phosphorylation of a specific
tyrosine residue in the carboxy-terminal domain by a tyrosine
kinase (Tyr705) and then homodimerizes and translocates to the
nucleus where it binds to specific STAT3 response elements of
target gene promoters to regulate transcription (13). Indeed, our results also revealed
that 2HF modulated the expression of several apoptotic and
anti-apoptotic genes, Mcl-1, Bcl-2 and Bax, which have been
reported as typical downstream target genes of STAT3 (30). Therefore, consistent with our
previous study reporting the cross-talk of PI3K/AKT and STAT3
signaling regulating Bcl-2 expression in CRPC development (31), 2HF suppressed the activation of AKT
and STAT3 and then modulated the expression of members of the
BCL-2 gene family in vitro and in vivo, which
are critical for regulating mitochondrial apoptosis.
Taken together, our research revealed a novel
mechanism for 2HF targeting metastatic CRPC tumor growth in
vitro and in vivo, in which inactivation of AKT/STAT3 by
2HF treatment led to a change in expression of the BCL-2 gene
family members and then induced cell apoptosis and growth
inhibition. Therefore, our findings demonstrated that 2HF may be a
novel potential agent for the prevention and therapy of metastatic
CRPC, not only working as a selective inhibitor of AKR1C3 but also
as a strong inducer of cell apoptosis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (NSFC 81202014 to K.W.) and the
Fundamental Research Funds for the Central Universities (to
K.W.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Heidenreich A and Pfister D: Treatment
decisions for metastatic castration-resistant prostate cancer
progressing after docetaxel chemotherapy: the role of cabazitaxel
in the continuum of care. Eur Urol. 62:1201–1204. 2012. View Article : Google Scholar
|
|
3
|
Beltran H, Beer TM, Carducci MA, et al:
New therapies for castration-resistant prostate cancer: efficacy
and safety. Eur Urol. 60:279–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mottet N, Bellmunt J, Bolla M, et al: EAU
guidelines on prostate cancer. Part II: Treatment of advanced,
relapsing, and castration-resistant prostate cancer. Eur Urol.
59:572–583. 2011. View Article : Google Scholar
|
|
5
|
Vitale DC, Piazza C, Melilli B, Drago F
and Salomone S: Isoflavones: estrogenic activity, biological effect
and bioavailability. Eur J Drug Metab Pharmacokinet. 38:15–25.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ko CH, Shen SC, Lin HY, et al: Flavanones
structure-related inhibition on TPA-induced tumor promotion through
suppression of extracellular signal-regulated protein kinases:
involvement of prostaglandin E2 in anti-promotive process. J Cell
Physiol. 193:93–102. 2002. View Article : Google Scholar
|
|
7
|
Romagnolo DF and Selmin OI: Flavonoids and
cancer prevention: a review of the evidence. J Nutr Gerontol
Geriatr. 31:206–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manthey JA, Grohmann K and Guthrie N:
Biological properties of citrus flavonoids pertaining to cancer and
inflammation. Curr Med Chem. 8:135–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao YC, Kuo WH, Chen PN, et al:
Flavanone and 2′-OH flavanone inhibit metastasis of lung cancer
cells via down-regulation of proteinases activities and MAPK
pathway. Chem Biol Interact. 167:193–206. 2007.
|
|
10
|
Nagaprashantha LD, Vatsyayan R, Singhal J,
et al: 2′-hydroxyflavanone inhibits proliferation, tumor
vascularization and promotes normal differentiation in VHL-mutant
renal cell carcinoma. Carcinogenesis. 32:568–575. 2011.
|
|
11
|
Shin SY, Kim JH, Lee JH, Lim Y and Lee YH:
2′-Hydroxyflavanone induces apoptosis through Egr-1 involving
expression of Bax, p21, and NAG-1 in colon cancer cells. Mol Nutr
Food Res. 56:761–774. 2012.
|
|
12
|
Wu K, Gore C, Yang L, et al: Slug, a
unique androgen-regulated transcription factor, coordinates
androgen receptor to facilitate castration resistance in prostate
cancer. Mol Endocrinol. 26:1496–1507. 2012. View Article : Google Scholar
|
|
13
|
Turkson J, Bowman T, Garcia R, Caldenhoven
E, De Groot RP and Jove R: Stat3 activation by Src induces specific
gene regulation and is required for cell transformation. Mol Cell
Biol. 18:2545–2552. 1998.PubMed/NCBI
|
|
14
|
Wu K, Fan J, Zhang L, et al: PI3K/Akt to
GSK3β/β-catenin signaling cascade coordinates cell colonization for
bladder cancer bone metastasis through regulating ZEB1
transcription. Cell Signal. 24:2273–2282. 2012.
|
|
15
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Majumder PK and Sellers WR: Akt-regulated
pathways in prostate cancer. Oncogene. 24:7465–7474. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nelson EC, Evans CP, Mack PC, Devere-White
RW and Lara PN Jr: Inhibition of Akt pathways in the treatment of
prostate cancer. Prostate Cancer Prostatic Dis. 10:331–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morgan TM, Koreckij TD and Corey E:
Targeted therapy for advanced prostate cancer: inhibition of the
PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 9:237–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mora LB, Buettner R, Seigne J, et al:
Constitutive activation of Stat3 in human prostate tumors and cell
lines: direct inhibition of Stat3 signaling induces apoptosis of
prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|
|
20
|
Gaya JM, Ahallal Y, Sanchez-Salas R, et
al: Current, new and novel therapy for castration-resistant
prostate cancer. Expert Rev Anticancer Ther. 13:819–827. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu KJ, Zeng J, Zhu GD, et al: Silibinin
inhibits prostate cancer invasion, motility and migration by
suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin.
30:1162–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu K, Zeng J, Li L, et al: Silibinin
reverses epithelial-to-mesenchymal transition in metastatic
prostate cancer cells by targeting transcription factors. Oncol
Rep. 23:1545–1552. 2010.PubMed/NCBI
|
|
23
|
Zeng J, Sun Y, Wu K, et al:
Chemopreventive and chemotherapeutic effects of intravesical
silibinin against bladder cancer by acting on mitochondria. Mol
Cancer Ther. 10:104–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu K, Ning Z, Zeng J, et al: Silibinin
inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer
metastasis via dual-blocking epithelial-mesenchymal transition and
stemness. Cell Signal. 25:2625–2633. 2013.
|
|
25
|
Stanbrough M, Bubley GJ, Ross K, et al:
Increased expression of genes converting adrenal androgens to
testosterone in androgen-independent prostate cancer. Cancer Res.
66:2815–2825. 2006. View Article : Google Scholar
|
|
26
|
Yuan X, Cai C, Chen S, Yu Z and Balk SP:
Androgen receptor functions in castration-resistant prostate cancer
and mechanisms of resistance to new agents targeting the androgen
axis. Oncogene. Jun 10–2013.(Epub ahead of print). View Article : Google Scholar
|
|
27
|
Adeniji AO, Chen M and Penning TM: AKR1C3
as a target in castrate resistant prostate cancer. J Steroid
Biochem Mol Biol. 37:136–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ofude M, Mizokami A, Kumaki M, et al:
Repression of cell proliferation and androgen receptor activity in
prostate cancer cells by 2′-hydroxyflavanone. Anticancer Res.
33:4453–4461. 2013.
|
|
29
|
Zielinski RR, Eigl BJ and Chi KN:
Targeting the apoptosis pathway in prostate cancer. Cancer J.
19:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Real PJ, Sierra A, De Juan A, Segovia JC,
Lopez-Vega JM and Fernandez-Luna JL: Resistance to chemotherapy via
Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer
cells. Oncogene. 21:7611–7618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, He D, Xue Y, et al: PrLZ protects
prostate cancer cells from apoptosis induced by androgen
deprivation via the activation of Stat3/Bcl-2 pathway. Cancer Res.
71:2193–2202. 2011. View Article : Google Scholar : PubMed/NCBI
|