Introduction
Squamous cell carcinoma (SCC) accounts for ~90% of
oral cancers (1); it is the sixth
most common malignancy in the world (2) and one of the leading causes of
cancer-related mortality (3,4). In
Taiwan, based on a 2012 report from the Department of Health,
R.O.C. (Taiwan), ~11.0 individuals per 10,000 die annually from
oral cancer. In Taiwan, oral cancer is the fourth most common
cancer in men and the 16th in women. There are several different
treatments for oral cancer (including surgery, radiation and
chemotherapy); however, the overall survival rate is unsatisfactory
(4). A challenge to cancer
treatment is that cancer cells migrate to and invade other tissues
or organs. There is a need to find new agents to treat SCC.
Gallic acid (3,4,5-trihydroxybenzoic acid; GA)
exists in natural plants and has been shown to have anticancer
effects in human leukemia HL-60RG (5), lung cancer (6), stomach cancer, colon cancer (7), prostate cancer (8,9),
melanoma (10) and esophageal
cancer (11), PC12 rat
pheochromocytoma (12) and mouse
leukemia WEHI-3 cells (13). Our
laboratory previously reported that GA induces apoptosis in human
lung cancer NCI-H460 cells in vitro and in vivo
(14). Cancer metastasis is caused
by cell adhesion, migration and invasion. GA has anti-metastatic
effects on gastric cancer cells which is due to inhibition of NF-κB
activity and downregulation of PI3K/AKT/small GTPs signaling
(15). GA inhibits the migration
and invasion of A375.S2 human melanoma cells through the inhibition
of matrix metalloproteinase (MMP)-2 and Ras (16), and GA inhibits adhesion of melanoma
B16F10 cells (14). Recently, we
also found that GA-inhibited migration and invasion in U-2 OS cells
that may be due to downregulation of PKC, inhibition of
mitogen-activated protein kinase (MAPK) and PI3K/AKT, resulting in
inhibition of MMP-2 and MMP-9 expression (17). There are no reports on whether GA
blocks migration and invasion of human oral cancer cells. In the
present study, we determined whether GA inhibits migration and
invasion of human oral cancer SCC-4 cells in vitro.
Materials and methods
Materials and chemicals
GA, dimethyl sulfoxide (DMSO), pyruvate, penicillin
G, streptomycin, trypan blue and Triton X-100 were purchased from
Sigma Chemical. (St. Louis, MO, USA). Primary and secondary
antibodies for western blotting were purchased from Biotechnology
(Santa Cruz, CA, USA). Materials and chemicals for electrophoresis
were obtained from Bio-Rad (Hercules, CA, USA).
SCC-4 cell culture
The SCC-4 human oral cell line was purchased from
the Food Industry Research and Development Institute (Hsinchu,
Taiwan). Cells were cultured in 90% DMEM medium with 10% FBS and 2
mM L-glutamine containing antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin). Cells were cultured in 75-cm2
tissue culture flasks and incubated under a humidified 5%
CO2 atmosphere at 37°C as previously described (18).
Cell viability assay
SCC-4 cells (2×105 cells/well) were
maintained in 12-well plates for 24 h. Cells were then incubated
with 0, 5, 30 and 60 μM of GA for 24 and 48 h. Each treatment was
performed in triplicate. Cells were harvested and PI (5 μg/ml)
added to the cells and analyzed using a PI exclusion method by flow
cytometry (BD Biosciences, FACSCalibur, San Jose, CA, USA) as
previously described (18,19).
Cell-matrix adhesion assay
SCC-4 cells (2×105 cells/well) were
maintained in 12-well plates and were then incubated with 0, 5, 30
and 60 μM of GA for 24 and 48 h. Cells were then placed on 24-well
plates which were coated with 150 μl of 10 μg/ml type I collagen
for 2 h. Non-adherent cells were removed and adherent cells were
fixed with ethanol for 15 min and then stained with 0.2% crystal
violet for 10 min and washed with PBS. Cells were lysed in 0.2%
Triton X-100 for 30 min and 150 μl of the lysate was added to a
96-well ELISA plate. The absorbance (540 nm) was measured as
described previously (19).
Wound healing assay
SCC-4 cells (5×105 cells/well) were kept
in 10-cm Petri dishes for 24 h. Wounds were created with a sterile
yellow micropipette tip on cell monolayers. Unscraped cells were
washed with PBS three times and dead cells removed and fresh DMEM
medium supplemented with FBS containing 0, 5, 30 and 60 μM of GA
added for 24 h. The wound healing area was then examined and images
were captured using an inverted microscope as described previously
(18,19).
Cell migration and invasion assays
Matrigel Cell Migration Assay and Invasion System
were used for measuring cell migration and invasion, respectively,
as described previously (18). The
cell migration assay was performed using Transwell cell culture
chambers (8-mm pore size; Millipore, Billerica, MA, USA). SCC-4
cells (5×104 cells/well) were added to serum-free DMEM
medium and placed in the upper chamber of the Transwell insert and
incubated with 0, 30 and 60 μM of GA. DMEM medium (90%) containing
10% FBS was added to the lower chamber as a chemoattractant.
Following incubation for 24 and 48 h, non-migrating cells were
removed from the top chamber with a cotton swab. The cells on the
lower surface were fixed with 4% formaldehyde in PBS. At the end of
fixation, the chambers were rinsed with PBS and cells in the lower
chamber were stained with 2% crystal violet in 2% ethanol for 10
min. Images were counted and captured using a light microscope at
×200. The cell invasion assay was performed the same as the cell
migration assay except that the filter membrane was coated with
Matrigel from a BioCoat Matrigel invasion kit. Cells that invaded
to the underside of the filter were counted using a light
microscope at ×200 as described previously (18,19).
Gelatin gel zymographic assay for MMP-2
activity
Gelatin gel zymography was used to quantify MMP-2
activity according to the manufacturer’s instructions. Briefly,
SCC-4 cells (5×105 cells/well) were maintained in
12-well plates and then treated with GA (0, 5, 30 and 60 μM) for 24
and 48 h. Total protein (50 μg) was re-suspended in non-reducing
loading buffer and incubated at 37°C for 15 min. Electrophoresis
was performed on 10% SDS-PAGE cast with 0.1% gelatin and
electrophoresed on Novex® Zymogram gels (Life
Technologies). The specific MMP-2 bands were detected by staining
with Coomassie Brilliant Blue (Life Technologies) as described
previously (18,19).
Western blot analysis of proteins
associated with cell migration and invasion
SCC-4 cells (1×106 cells/well) were
placed in 6-well plates for 24 h and were then incubated with 0, 5,
30 and 60 μM of GA for 0, 24 and 48 h. Cells were harvested, lysed,
and total protein determined by a Bio-Rad protein assay kit
(Hercules, CA, USA) with bovine serum albumin (BSA) as the standard
as described previously (18,19).
Samples (30 μg protein) were loaded onto 12% SDS-polyacrylamide
gels, electrophoresed and then electrotransferred to nitrocellulose
membranes, blotted with the relevant primary antibodies [anti-FAK,
MEKK3, p-PERK, p-p38, p-JNK1/2, p-ERK1/2, SOS1, RhoA, GRB2, Ras,
PKC, p-AKT (Thr308), PI3K, NF-κB p65, MMP-2 and MMP-9], washed, and
then stained with a secondary antibody. Protein bands were examined
by an enhanced chemiluminescence reagent (ECLTM; Amersham
Biosciences) and bands were quantified using an NIH Image analyzer
(NIH, Bethesda, MD, USA) as described previously (18,19).
Immunofluorescence staining and confocal
laser scanning microscopy
SCC-4 cells (3×105 cells/well) were
plated on 6-well chamber slides, treated with 0, 30 and 60 μM of GA
for 24 h, and fixed with formaldehyde (3%) in PBS for 15 min. The
cells were permeabilized using 0.1% Triton X-100 in PBS for 1 h and
washed three times with PBS followed by blocking of SCC-4 cells
(3×105 cells/well) using 2% BSA in non-specific binding
sites as described previously (18). Cells were stained with primary
antibodies such as anti-NF-κB p65 and RhoA (1:100 dilution)
overnight, and were then stained with secondary FITC-conjugated
goat anti-mouse IgG (1:200 dilution) (green fluorescence). Cell
nuclei were counterstained with PI (Molecular Probes; Invitrogen
Corp.) (red fluorescence). All samples were photomicrographed using
a Leica TCS SP2 confocal spectral microscope as described
previously (18,19).
Statistical analysis
All experiments were performed in triplicate and
data are expressed as mean ± SD. Statistical analysis was carried
out using Student’s t-test, with values of *P<0.05
considered to indicate statistically significant differences.
Results
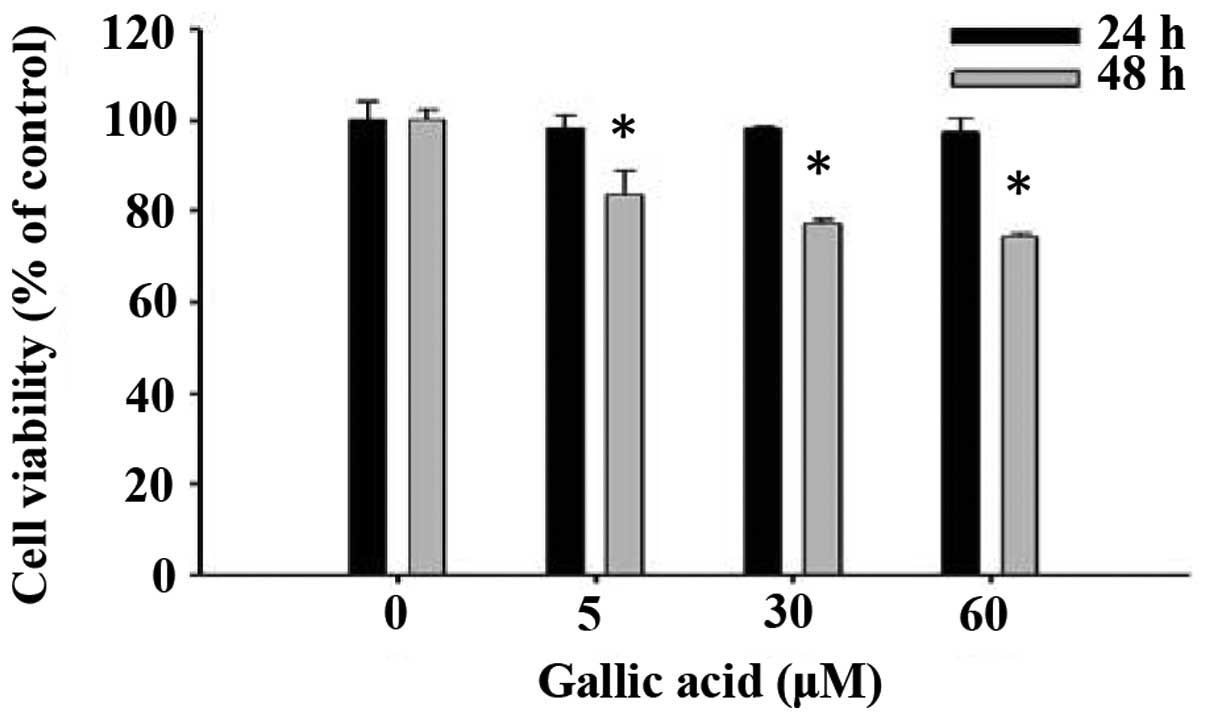
GA decreases the percentage of viable
SCC-4 human oral cancer cells
We evaluated the effects of GA on cell viability
using flow cytometry and results are shown in Fig. 1. GA at concentrations of 5–60 μM
significantly reduced cell viability in a dose and time-dependent
manner (Fig. 1).
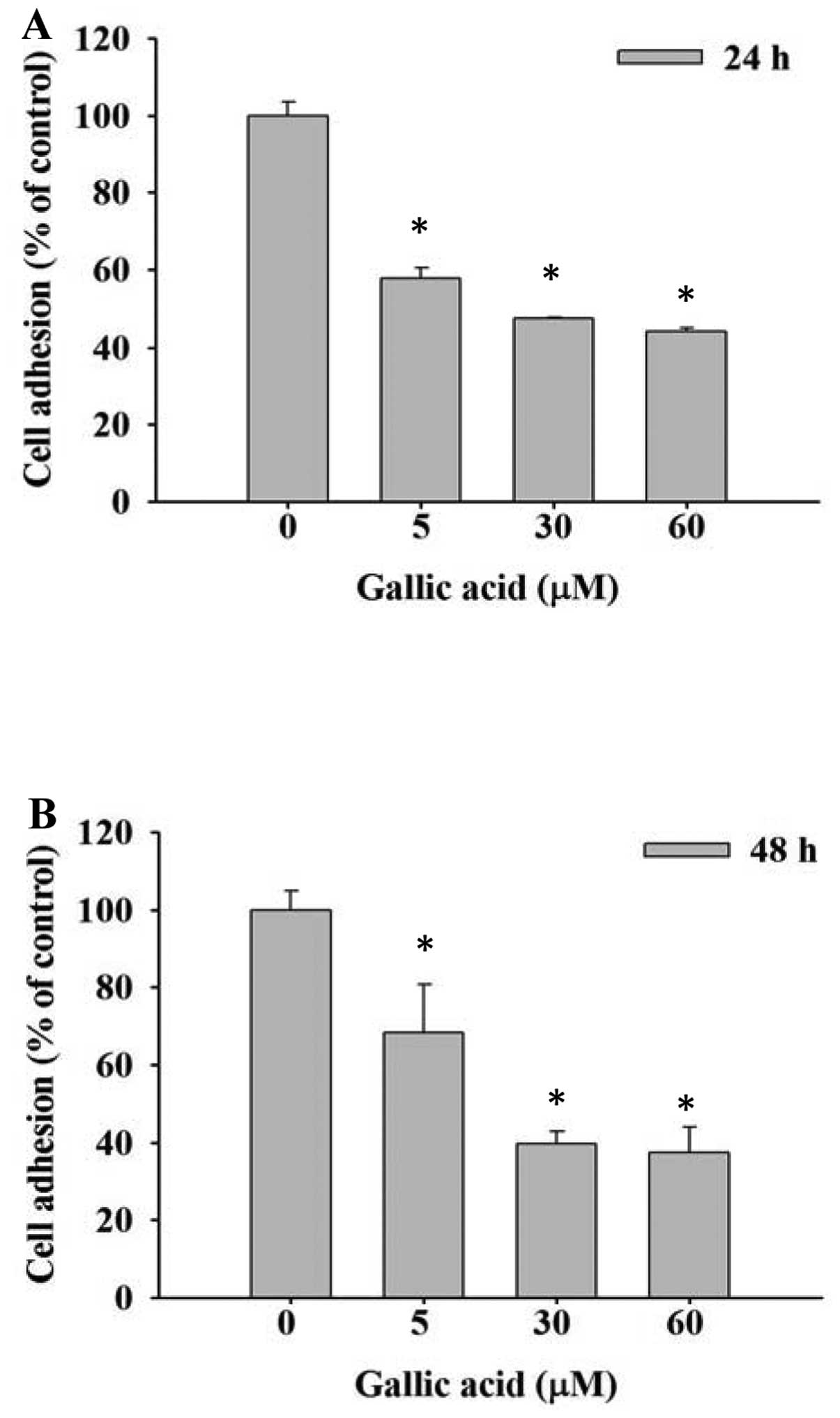
GA inhibits the adhesion of SCC-4 cells
in vitro
SCC-4 cells were treated with 0, 5, 30 and 60 μM GA
and inhibition of cell adhesion was determined using an ELISA
reader. Results are shown in Fig. 2A
and B. Cell adhesion was significantly reduced dose- and
time-independently by GA compared with control groups.
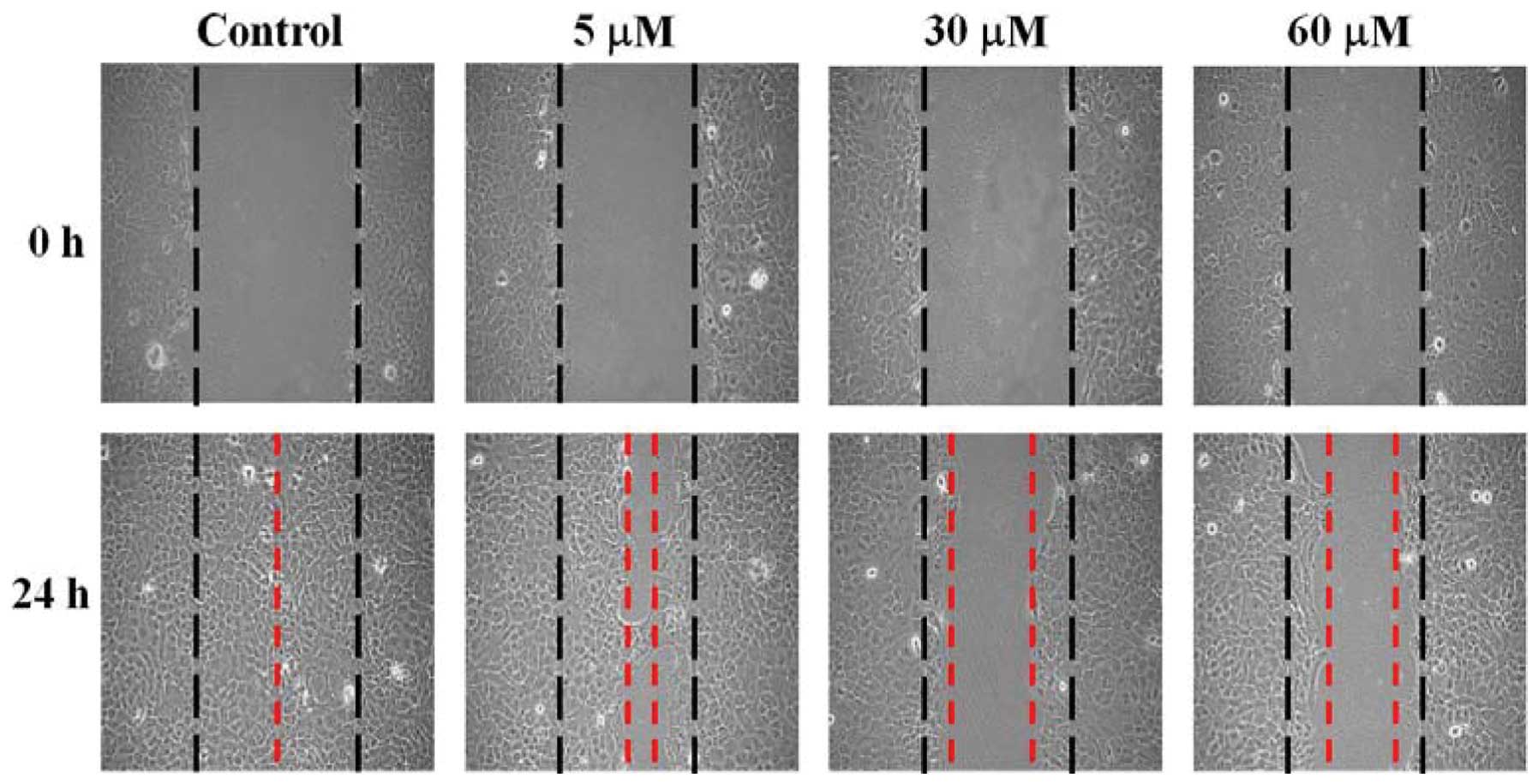
GA suppresses the migration of SCC-4
cells in vitro
SCC-4 cells were treated with 0, 5, 30 and 60 μM of
GA and wound healing assay performed. Data in Fig. 3 show that GA inhibited the migration
of SCC-4 cells based on the extent of wound closure. These effects
were dose- and time-dependent.
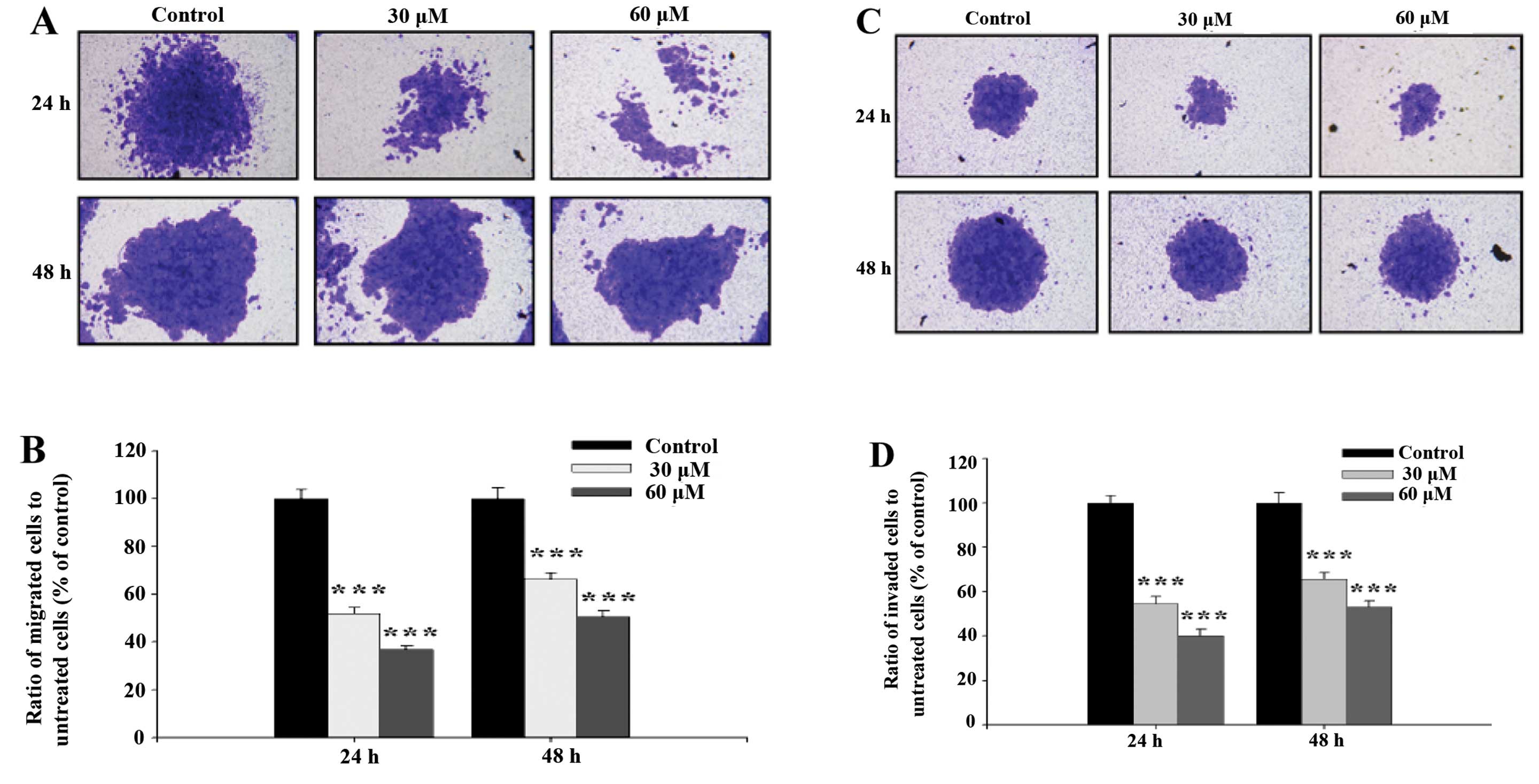
GA inhibits the migration and invasion of
SCC-4 cells in vitro
Cell migration activity and invasion potential of
SCC-4 cells were examined and photographed and the representative
figures and evaluated inhibitions are shown in Fig. 4. Fig. 4A
and B indicate that GA significantly inhibited migration of
SCC-4 cells, ratio of migrated cells went from 50.56 to 36.81%
treated with 30 and 60 μM respectively at 24 h, to 66.40 and 51.99%
at 48 h. This was in agreement with results from the healing assay
(Fig. 3). Fig. 4C and D indicate that GA
significantly inhibited the invasion of SCC-4 cells, ratio of
invaded cells went from 53.18 to 40.17% treated with 30 and 60 μM
respectively at 24 h, to 65.67 and 54.74 % at 48 h.
GA inhibits MMP-2 activity of SCC-4
cells
Effects of GA on MMP-2 activity were determined
using gelatin zymography. As shown in Fig. 5, SCC-4 cells constitutively secreted
high levels of MMP-2 but when incubated with GA at 5, 30 and 60 μM
for 24 and 48 h, MMP-2 activity was significantly reduced. This
effect of GA was dose-dependent (Fig.
5).
GA alters levels of proteins associated
with migration and invasion of SCC-4 cells
MMP-2 and MMP-9 are potential target molecules for
anti-metastatic activity, and we investigated the effects of GA on
MMP-2 and MMP-9 and associated signal protein levels in SCC-4
cells. GA markedly reduced protein levels of FAK, MEKK3, p-PERK
(Fig. 6A), p-p38, p-JNK1/2,
p-ERK1/2 (Fig. 6B) and SOS1, RhoA,
Ras (Fig. 6C). It was also observed
that protein levels of PKC, p-AKT (Thr308), PI3K, (Fig. 6D) were decreased in GA-treated cells
compared with control cells.
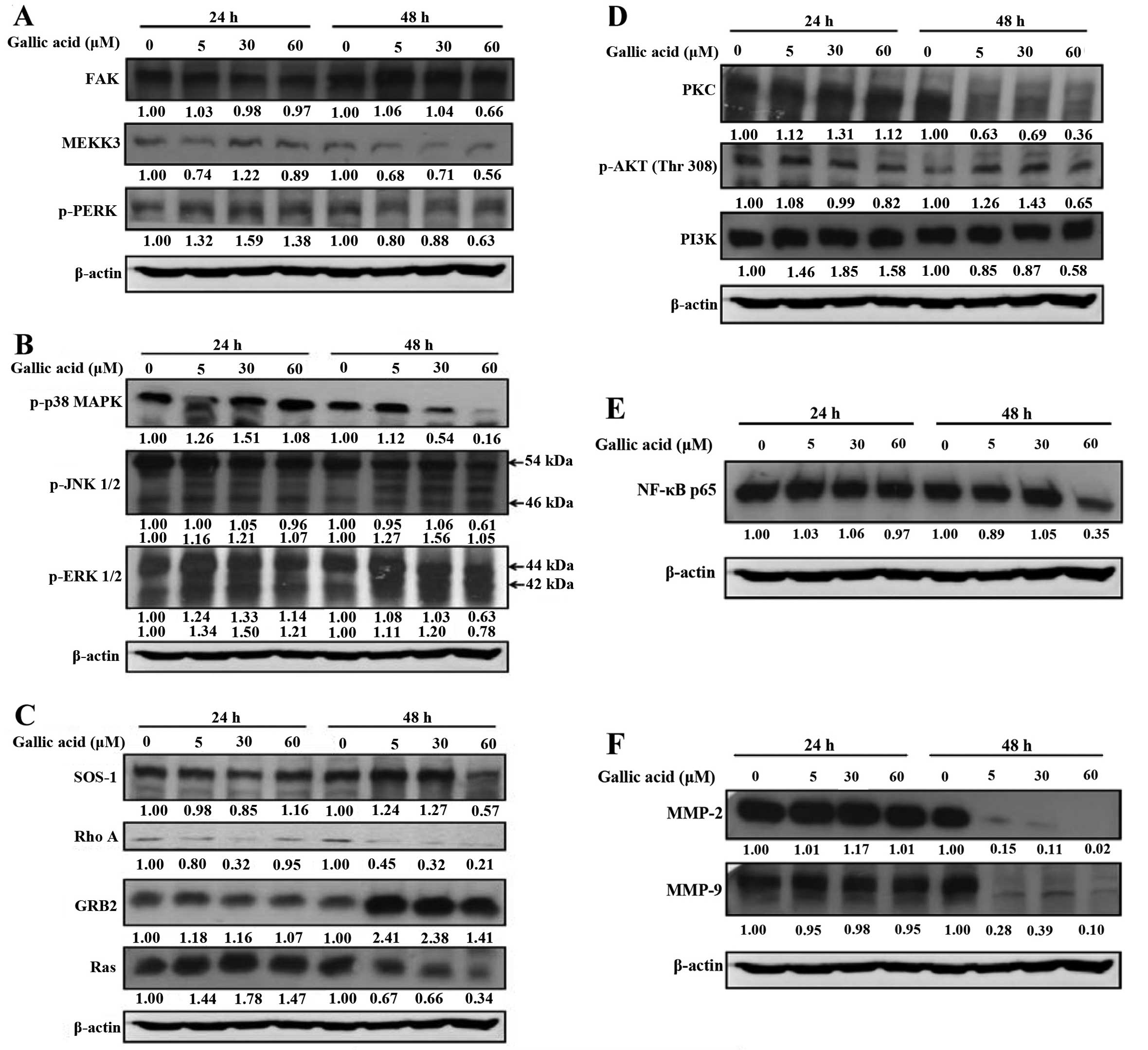 | Figure 6Gallic acid (GA) affects the levels
of associated proteins in migration and invasion of SCC-4 cells.
Cells (5×104 cells/well) were treated with 5, 30 and 60
μM of GA for 24 and 48 h and then cells were collected for the
total protein extracted and determined as described in Materials
and methods. The levels of FAK, MEKK3, PERK (A); p38, JNK1/2,
ERK1/2 (B); SOS1, RhoA, GRB2, Ras (C); PKC, p-AKT(Thr308), PI3K
(D); NF-κB p65 (E); MMP-2, MMP-9 (F) expression were estimated by
western blotting as described in Materials and methods. |
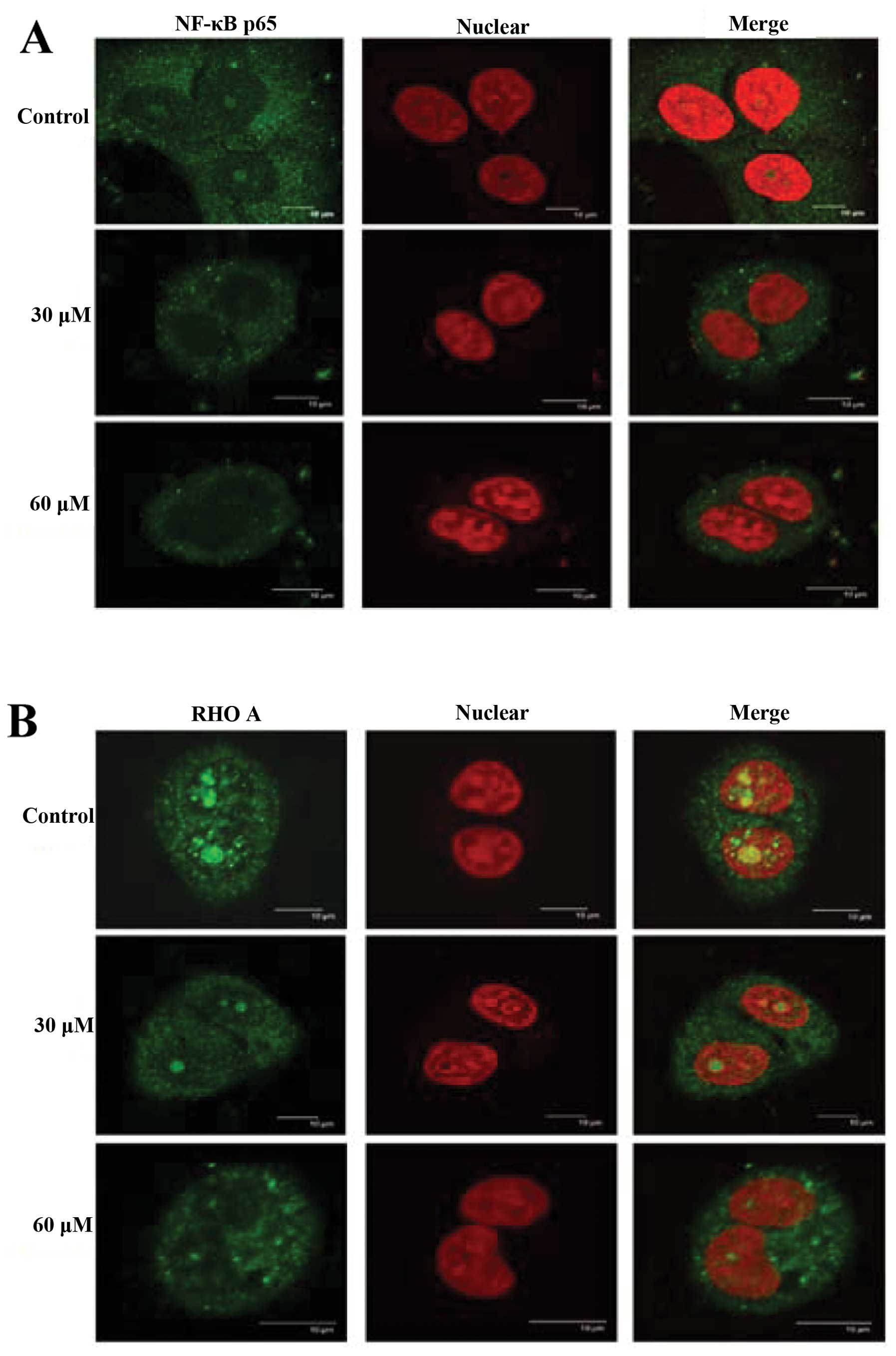
GA alters translocation of NF-κB and RhoA
in SCC-4 cells
Effects of GA on distribution of NF-κB p65 and RhoA
in SCC-4 cells were examined by confocal laser system microscopy
and results can be seen in Fig. 7A and
B. GA inhibited the NF-κB p65 (Fig.
7A) and RhoA (Fig. 7B) protein
levels in cytosol but increased the protein levels in nuclei.
Discussion
Numerous studies have demonstrated that gallic acid
(GA) induces cytotoxic effects through cell cycle arrest and
induction of apoptosis in many human cancer cell lines. There are a
few reports showing GA inhibited metastasis of cancer cells
(15–18). However, the effects of GA on the
cell motility of human oral cancer SCC-4 cells have not been
examined. We investigated the effects of GA on the migration and
invasion of SCC-4 cells in vitro. GA inhibited migration and
invasion and these effects were associated with MMP-2 and MMP-9
activity and other specific proteins (NF-κB p65, RhoA). MMP-2 and
MMP-9 play a critical role in cancer cell migration and invasion
(20,21) and overexpression of both enzymes
increases migration and invasion of cancer cells (22,23).
In the present study, we showed that GA markedly inhibited
activation status of MMP-2 and MMP-9 using gelatin zymography
(Fig. 5). These findings were
consistent with our data showing that GA decreased the protein
levels of MMP-2 and MMP-9 (Fig.
7F). GA also reduced protein levels of PI-3K and downstream
kinases JNK and p-p38 (Fig. 7B),
and NF-κB (Fig. 7E) in SCC-4 cells.
These findings are in agreement with another report which found
that GA blocked cell migration and invasion which was associated
with inhibition of NF-κB and downregulation of PI3K and AKT
signaling (15). It is well
documented that NF-κB acts on downstream proteins such as MMP-2 and
MMP-9 (24,25).
GA inhibited the translocation of NF-κB from the
cytosol to the nucleus (Fig. 7A)
and significantly reduced protein levels of FAK, MEEK3 and p-PERK
in SCC-4 cells. Earlier reports indicated that FAK/Scr was
associated with tumor cell migration and invasion (24,25).
Activated FAK (Tyr 397)/Src (Tyr 416) may act on PI-3K/AKT and
Ras/ERK1/2 cascades impacting signaling (26,27).
It was reported that FAK/Src complex allows Src to phosphorylate
FAK and then to interact with GRB2 and activation of the Ras-ERK
signaling pathway (28).
GA in the present study inhibited the expression of
RhoA in SCC-4 cells. RhoA belongs to the prototype protein of the
Rho GTPase superfamily that is associated with cell migration among
its many effects (28). Both RhoA
and ROCK1 are upregulated in tumors and are predicative of cancer
progression, metastasis and poor prognosis (29,30).
Inhibition of the RhoA/ROCK1 pathway leads to tumor cell death and
reduced metastasis (31,32). The RhoA/ROCK1 pathway may be a
promising target for cancer therapy in the future and further
investigation is required.
We found that GA inhibited protein levels of Ras and
SOS-1 but increased GRB2 abundance (Fig. 7C) in SCC-4 cells. Numerous studies
have demonstrated that Grb2 (33),
Ras (34), and PKC (35,36)
are involved in cell mobility. GA inhibition of cell migration and
invasion of SCC-4 cells may also involve inhibition of SOS-1, Ras
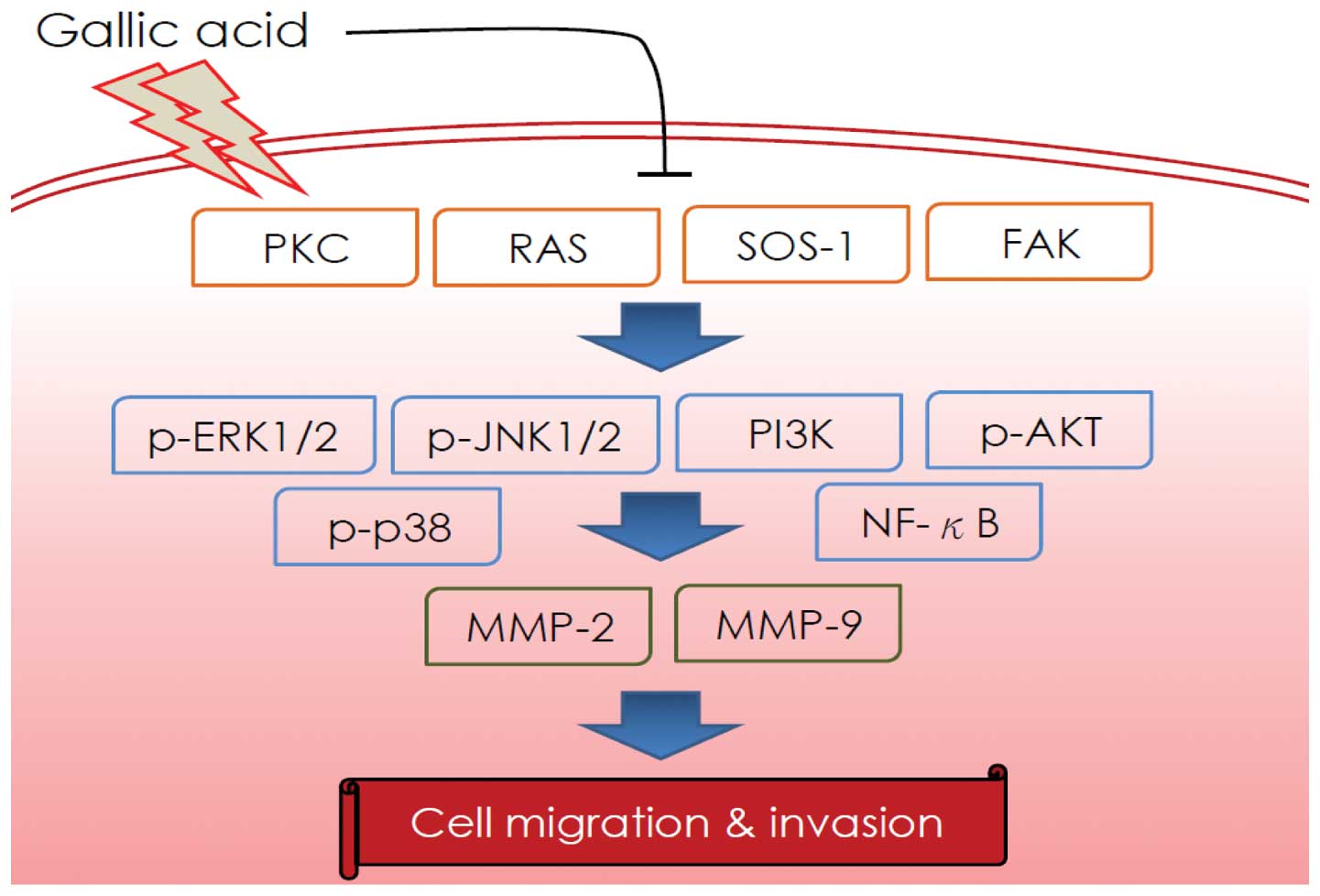
and RhoA. As depicted in Fig. 8, we
propose that GA inhibition of SCC-4 cell migration and invasion may
occur via regulation of PKC, Ras, SOS1, FAK, p-JNK1/2, p-ERK1/2,
p-p38, PI3K, p-AKT and NF-κB expression. A major consequence of
these effects is inhibition of MMP-2 and MMP-9 activity and protein
signaling pathways.
Acknowledgements
This study was supported by grant DOH99-TD-C-111-005
from Department of Health, Executive Yuan (Taiwan, R.O.C).
Experiments and data analysis were performed in part through the
use of the Medical Research Core Facilities Center, Office of
Research and Development at China Medical University, Taichung,
Taiwan, R.O.C.
References
|
1
|
Castro TP and Bussoloti Filho I:
Prevalence of human papillomavirus (HPV) in oral cavity and
oropharynx. Braz J Otorhinolaryngol. 72:272–282. 2006.PubMed/NCBI
|
|
2
|
Lingen MW, Pinto A, Mendes RA, et al:
Genetics/epigenetics of oral premalignancy: current status and
future research. Oral Dis. 17(Suppl 1): 7–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
5
|
Inoue M, Suzuki R, Koide T, Sakaguchi N,
Ogihara Y and Yabu Y: Antioxidant, gallic acid, induces apoptosis
in HL-60RG cells. Biochem Biophys Res Commun. 204:898–904. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You BR, Kim SZ, Kim SH and Park WH: Gallic
acid-induced lung cancer cell death is accompanied by ROS increase
and glutathione depletion. Mol Cell Biochem. 357:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshioka K, Kataoka T, Hayashi T, Hasegawa
M, Ishi Y and Hibasami H: Induction of apoptosis by gallic acid in
human stomach cancer KATO III and colon adenocarcinoma COLO 205
cell lines. Oncol Rep. 7:1221–1223. 2000.PubMed/NCBI
|
|
8
|
Veluri R, Singh RP, Liu Z, Thompson JA,
Agarwal R and Agarwal C: Fractionation of grape seed extract and
identification of gallic acid as one of the major active
constituents causing growth inhibition and apoptotic death of DU145
human prostate carcinoma cells. Carcinogenesis. 27:1445–1453. 2006.
View Article : Google Scholar
|
|
9
|
Raina K, Rajamanickam S, Deep G, Singh M,
Agarwal R and Agarwal C: Chemopreventive effects of oral gallic
acid feeding on tumor growth and progression in TRAMP mice. Mol
Cancer Ther. 7:1258–1267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Locatelli C, Leal PC, Yunes RA, Nunes RJ
and Creczynski-Pasa TB: Gallic acid ester derivatives induce
apoptosis and cell adhesion inhibition in melanoma cells: the
relationship between free radical generation, glutathione depletion
and cell death. Chem Biol Interact. 181:175–184. 2009. View Article : Google Scholar
|
|
11
|
Faried A, Kurnia D, Faried LS, et al:
Anticancer effects of gallic acid isolated from Indonesian herbal
medicine, Phaleria macrocarpa (Scheff.) Boerl, on human
cancer cell lines. Int J Oncol. 30:605–613. 2007.PubMed/NCBI
|
|
12
|
Kang MK, Kang NJ, Jang YJ, Lee KW and Lee
HJ: Gallic acid induces neuronal cell death through activation of
c-Jun N-terminal kinase and downregulation of Bcl-2. Ann NY Acad
Sci. 1171:514–520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serrano A, Palacios C, Roy G, et al:
Derivatives of gallic acid induce apoptosis in tumoral cell lines
and inhibit lymphocyte proliferation. Arch Biochem Biophys.
350:49–54. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji BC, Hsu WH, Yang JS, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth in
vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho HH, Chang CS, Ho WC, Liao SY, Wu CH and
Wang CJ: Anti-metastasis effects of gallic acid on gastric cancer
cells involves inhibition of NF-kappaB activity and downregulation
of PI3K/AKT/small GTPase signals. Food Chem Toxicol. 48:2508–2516.
2010. View Article : Google Scholar
|
|
16
|
Lo C, Lai TY, Yang JS, et al: Gallic acid
inhibits the migration and invasion of A375.S2 human melanoma cells
through the inhibition of matrix metalloproteinase-2 and Ras.
Melanoma Res. 21:267–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao CL, Lai KC, Huang AC, et al: Gallic
acid inhibits migration and invasion in human osteosarcoma U-2 OS
cells through suppressing the matrix metalloproteinase-2/-9,
protein kinase B (PKB) and PKC signaling pathways. Food Chem
Toxicol. 50:1734–1740. 2012. View Article : Google Scholar
|
|
18
|
Lai WW, Hsu SC, Chueh FS, et al: Quercetin
inhibits migration and invasion of SAS human oral cancer cells
through inhibition of NF-κB and matrix metalloproteinase-2/-9
signaling pathways. Anticancer Res. 33:1941–1950. 2013.PubMed/NCBI
|
|
19
|
Liu KC, Huang AC, Wu PP, et al: Gallic
acid suppresses the migration and invasion of PC-3 human prostate
cancer cells via inhibition of matrix metalloproteinase-2 and -9
signaling pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
20
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sangle NA and Layfield LJ: Telangiectatic
osteosarcoma. Arch Pathol Lab Med. 136:572–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi GZ, Yuan Y, Jiang GJ, et al: PRAF3
induces apoptosis and inhibits migration and invasion in human
esophageal squamous cell carcinoma. BMC Cancer. 12:972012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen K, Zhang S, Ji Y, et al: Baicalein
inhibits the invasion and metastatic capabilities of hepatocellular
carcinoma cells via down-regulation of the ERK pathway. PLoS One.
8:e729272013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen J, Xu L, Owonikoko TK, et al: NNK
promotes migration and invasion of lung cancer cells through
activation of c-Src/PKCι/FAK loop. Cancer Lett. 318:106–113.
2012.PubMed/NCBI
|
|
25
|
Bianchi-Smiraglia A, Paesante S and Bakin
AV: Integrin β5 contributes to the tumorigenic potential of breast
cancer cells through the Src-FAK and MEK-ERK signaling pathways.
Oncogene. 32:3049–3058. 2013.
|
|
26
|
Mamali I, Tatari MN, Micheva I,
Lampropoulou M and Marmaras VJ: Apoptosis in medfly hemocytes is
regulated during pupariation through FAK, Src, ERK, PI-3K p85a, and
Akt survival signaling. J Cell Biochem. 101:331–347. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arpaia E, Blaser H, Quintela-Fandino M, et
al: The interaction between caveolin-1 and Rho-GTPases promotes
metastasis by controlling the expression of alpha5-integrin and the
activation of Src, Ras and Erk. Oncogene. 31:884–896. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bolos V, Gasent JM, Lopez-Tarruella S and
Grande E: The dual kinase complex FAK-Src as a promising
therapeutic target in cancer. Onco Targets Ther. 3:83–97. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: Rho GTPase function in tumorigenesis. Biochim Biophys
Acta. 1796:91–98. 2009.PubMed/NCBI
|
|
30
|
Li F, Jiang Q, Shi KJ, Luo H, Yang Y and
Xu CM: RhoA modulates functional and physical interaction between
ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells.
Cell Death Dis. 4:e7082013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fromigue O, Haÿ E, Modrowski D, et al:
RhoA GTPase inactivation by statins induces osteosarcoma cell
apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently
of BMP-2 and cell differentiation. Cell Death Differ. 13:1845–1856.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lochhead PA, Wickman G, Mezna M and Olson
MF: Activating ROCK1 somatic mutations in human cancer. Oncogene.
29:2591–2598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai NP and Wei LN: RhoA/ROCK1 signaling
regulates stress granule formation and apoptosis. Cell Signal.
22:668–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SH, Jeong EG, Nam SW, Lee JY, Yoo NJ
and Lee SH: Increased expression of Gab2, a scaffolding adaptor of
the tyrosine kinase signalling, in gastric carcinomas. Pathology.
39:326–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Anastasiadis PZ, Liu Y, Thompson
EA and Fields AP: Protein kinase C (PKC) betaII induces cell
invasion through a Ras/Mek-, PKC iota/Rac 1-dependent signaling
pathway. J Biol Chem. 279:22118–22123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lawler K, Foran E, O’Sullivan G, Long A
and Kenny D: Mobility and invasiveness of metastatic esophageal
cancer are potentiated by shear stress in a ROCK- and Ras-dependent
manner. Am J Physiol Cell Physiol. 291:C668–C677. 2006. View Article : Google Scholar : PubMed/NCBI
|