Introduction
Osteosarcoma is the most common primary malignant
neoplasm of bone and is associated with rapid progression and poor
prognosis. Surgical amputation can achieve local tumor elimination,
yet 80% of osteosarcoma patients have a low 2-year survival rate
due to distant metastasis, particularly for patients receiving
single surgical treatment (1–3).
Consequently, lesionectomy combined with assistant
post/pre-operative chemotherapy has been considered significant and
necessary (4). Currently,
chemotherapeutic drugs include cisplatin, epirubicin, etopside,
methotrexate and cyclophosphamide (3). These drugs are known to cause serious
systemic toxicity, when used either as a single agent or in
combination with other drugs. Therefore, there is an urgent need to
develop additional available chemotherapeutic strategies or seek
safer and more effective chemotherapeutic agents for the treatment
of osteosarcoma.
The mammalian target of rapamycin (mTOR) is a
serine/threonine kinase at the nexus between oncogenic
phosphoinositide 3-kinase (PI3K)/Akt signaling and critical
downstream pathways that drive cancer cell growth, survival and
resistance to therapeutic agents (5,6). The
functions of mTOR are elicited by the context of two multiprotein
complexes termed mTOR complex 1 (mTORC1) and mTORC2. mTORC1 is
sensitive to rapamycin while mTORC2 is insensitive to acute
rapamycin treatment. The major downstream effectors of mTORC1 are
the ribosomal subunit S6 kinase 1 (S6K1) and the eukaryotic
initiation factor 4E binding protein 1 (4E-BP1), two regulators of
protein translation initiation and cell growth. mTORC2
phosphorylates and activates protein kinase B/Akt (Ser473), an
important pro-survival kinase in cells. Consistent with its role as
a growth-promoting pathway, mTOR signaling is dysregulated in
>50% of all human cancers including osteosarcoma and is a major
cancer drug target (7).
As first-generation mTOR inhibitors, rapamycin and
rapalogs (everolimus, temsirolimus) can slow the proliferation of
cancer cell lines and have achieved some success in cancer
treatment (8). Unfortunately, their
overall efficacy as cancer therapeutics has been limited to a few
rare cancers, including mantle cell lymphoma, renal cell carcinoma
and endometrial cancer (7,9). Clinical trials have shown that
osteosarcoma patients are not sensitive to rapalogs when employed
in a monotherapy setting.
Second-generation mTOR ATP competitive inhibitors
have been recently developed and are able to completely suppress
both mTORC1/C2 complex-mediated signaling, thereby suppressing the
feedback activation of Akt (10–15).
Importantly, they have shown marked improvement in antitumor
activity in vivo and in vitro, and the effectiveness
of these drugs in cancer treatment is currently being tested in
clinical trials (7,16,17).
The effects of mTOR kinase inhibitors on osteosarcoma, however,
have not been reported. Furthermore, mTORC2 is emerging as a
promising therapeutic target as its activity is essential for the
transformation and vitality of various types of cancer cells.
Consequently, it is important to determine the efficacy of
targeting mTORC2 in osteosarcoma. In the present study, we compared
the inhibitory effects of the targeting of mTORC1 with mTORC2 on a
variety of osteosarcoma cell lines and demonstrated that targeted
inhibition of mTORC2, but not mTORC1, prevents osteosarcoma cell
migration and promotes cell apoptosis.
Materials and methods
Reagents and antibodies
PP242 was purchased from Active Biochemicals. Co.
(Hong Kong); rapamycin was from Sigma Chemical Co. (St. Louis, MO,
USA). PP242 and rapamycin were diluted in dimethyl sulfoxide
(DMSO). The following antibodies were used: phospho-S6 (S235/236),
raptor, cleaved poly(ADP-ribose) polymerase (PARP) and caspase 7
from Cell Signaling Technology, Inc. (Beverly, MA, USA); S6, actin,
P-Akt (S473), mTOR, rictor and Akt were from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell lines
Osteosarcoma cell lines, MG63, U2-OS and Saos-2,
were purchased from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). MG63 cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine
serum (FBS), and U2-OS and Saos-2 cells were grown in McCoy’s 5A
medium with 10% FBS. All media and FBS were from Gibco-BRL
(Rockville, MD, USA). Cells were cultured at 37°C in a humidified
atmosphere consisting of 5% CO2 and 95% air.
Western blotting
Western blotting was performed as previously
described (18,19). In brief, cells were lysed in a
buffer containing 1% SDS. Equal amounts of whole protein extract
were resolved on SDS-polyacrylamide gel, transferred to a
nitrocellulose membrane (Amersham Biosciences, Italy), probed
overnight at 4°C with antibodies and then revealed using the ECL
western blot analysis system.
Drug interaction analysis
Drug combination analysis was performed as
previously described (19). In
briefly, the method describe by Chou and Talalay (20), and multiple drug dose-effect
calculations and the combination index plots were generated using
CalcuSyn 2.1 software (Biosoft, Cambridge, UK). We assessed the
effects of drug interactions by combination index (CI) values.
CI<1 indicated synergism, whereas CI=1 and CI>1 indicated
additive effect and antagonism, respectively. The pro-apoptosis
studies were performed by using 31, 62, 125, 250 and 500 nM of
PP242 and rapamycin; both studies were combined with 31, 62, 125,
250 and 500 nM cisplatin treatment.
RNA interference
Human mTOR-, raptor- and rictor-specific siRNAs were
chemically synthesized by GenePharma Co., Ltd. (Shanghai, China).
Target sequences of these siRNAs were mTOR
(50-GAGCCUUGUUGAUCCUUAA-30), raptor (50-CGAGAUUGGACGACCAAAU-30) and
rictor (50-GACUAUCCAUAAUCCUUA-30). Cells were transfected with the
siRNAs at 60% confluency using Lipofectamine 2000 (Invitrogen,
Grand Island, NY, USA) according to the manufacturer’s
instructions.
Cell migration and motility analysis
Migration ability was assessed by a wound healing
assay. Cells were seeded in 12-well plates and treated with PP242
(200 nM) or rapamycin (200 nM) or transfected with the negative
control (NC), mTOR, raptor or rictor siRNA, and grew until reaching
100% confluency. Cells were then treated with 2 μg/ml of mitomycin
C for 24 h in a minimum serum medium (containing 0.5% FBS). After
using a pipette tip to create a scratch in the confluent cells, the
wound distance was visualized at regular intervals of time (24/48
h) at ×100 magnifications using an inverted microscope (Nikon
Diaphot). The cell motility assay was performed on a Transwell
plate. Cells (1.0×105) were seeded in a Matrigel-coated
chamber (BD Biosciences). Cells were seeded in serum-free media and
translocated toward complete growth media. After 12 h of incubation
with PP242 (200 nM) and rapamycin (200 nM) at 37°C, invaded cell
were fixed and stained in dye solution containing 20% methanol and
0.1% crystal violet. The cells which had migrated or invaded were
imaged using a BH-2 inverted microscope (Olympus).
Apoptotic cell staining
Cells were treated with PP242 (500 nM) and rapamycin
(500 nM) or transfected with siRNA in medium without serum for 36
h. Apoptotic cells were assessed using a PI assay kit
(MultiSciences Biotech Co. Ltd., Beijing, China) according to the
manufacturer’s instructions. Briefly, the treated cells were
carefully washed three times with PBS, and then staining was
performed with a mixture containing 10 mg/ml PI and PBS in the
wells and stained for 15 min at 37°C in the dark. Photomicrographs
of the stained cells were then recorded under a fluorescence
microscope with digital equipment, and the apoptotic cells and
viable cells were counted visually. The apoptosis ratio = apoptotic
cells/total cells × 100%.
Statistical analysis
Data analysis was performed with SPSS 13.0.
Statistical analysis was performed by applying one-way analysis of
variance (ANOVA). Data are presented as means ± standard deviation
(SD), and p<0.05 was considered to indicate statistical
significance.
Results
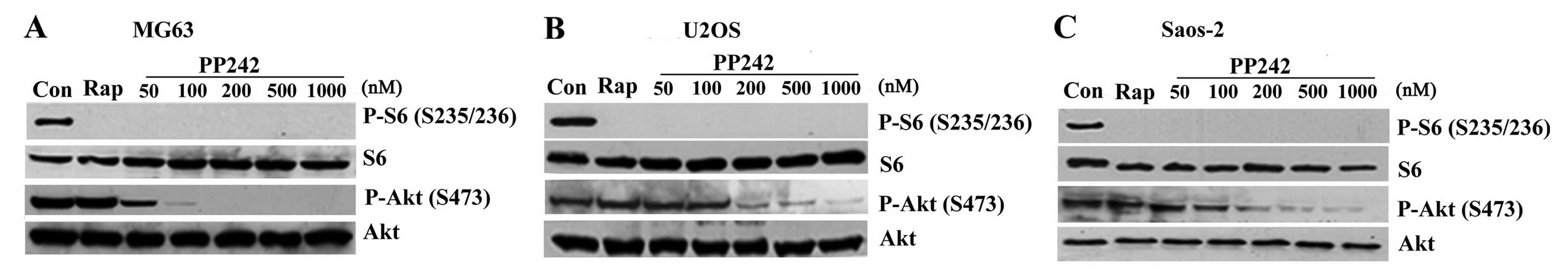
mTOR downstream kinases are deregulated
by mTORC1 inhibitor rapamycin and mTORC1/2 inhibitor PP242
We first examined the distinct effects of the
targeting of mTORC1 by rapamycin and the targeting of mTORC1/2 by
mTOR kinase inhibitor PP242 on their downstream signals in
osteosarcoma MG63, U2OS and Saos-2 cell lines. Although these two
drugs were able to effectively suppress phosphorylation of S6
(S235/236) in all tested cell lines, only PP242 dose dependently
(50–1000 nM) inhibited phosphorylation of Akt (S473), an mTORC2
phosphorylation site. Rapamycin treatment did not cause any
significant changes in the phosphorylation level of Akt (S473)
(Fig. 1A–C). This indicated that
mTOR kinase inhibitors profoundly diminished both mTORC1 and mTORC2
signaling, whereas rapamycin only suppressed mTORC1 in the
osteosarcoma cell lines.
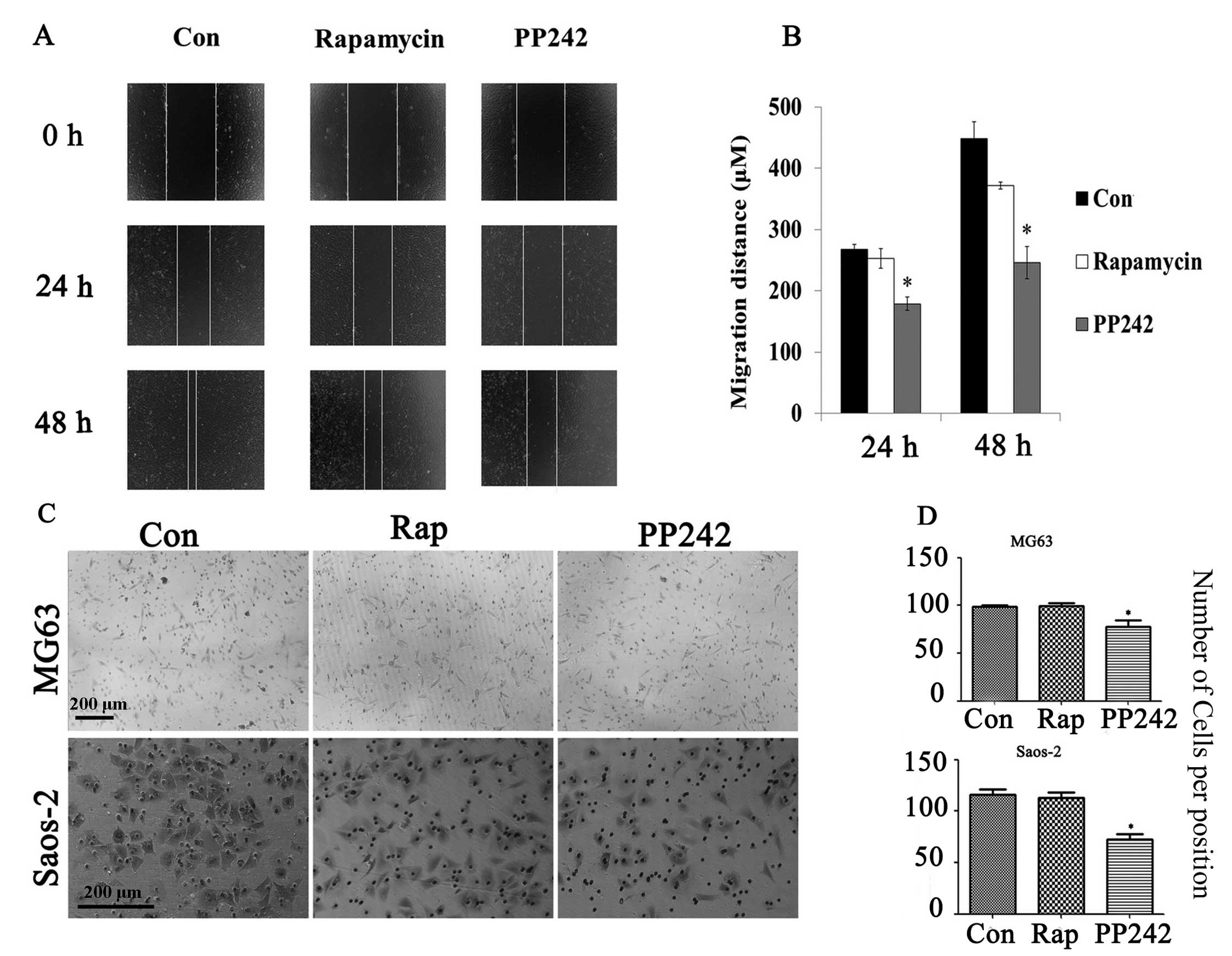
Inhibition of mTORC1/2 or mTORC2 prevents
osteosarcoma cell migration
Metastasis is the major cause of mortality and
morbidity of osteosarcoma patients. Invasion of cancer cells into
surrounding tissue and vasculature is an initial step in tumor
metastasis. This requires migration of cancer cells (21). The effects of targeted inhibition of
mTORC1 and/or mTORC2 on cell migration were examined by wound
healing assay. PP242-treated Saos-2 cells exhibited a slower
migration speed than the control and rapamycin-treated Saos-2 cells
(Fig. 2A and B). In addition, the
Transwell assay was performed to confirm the inhibitory effect of
PP242 on osteosarcoma cell motility. As expected, the number of
migrated cells markedly decreased in the PP242-treated but not in
the rapamycin-treated MG63 and Saos-2 cells (p<0.05) (Fig. 2C and D). These results indicated
that the inhibitor of mTORC1/2 prevented osteosarcoma cell
migration. To further identify the roles of mTORC1 and mTORC2 in
osteosarcoma cell migration, the effects of raptor, mTOR or rictor
knockdown on migration were examined. Raptor, rictor or mTOR siRNA
markedly decreased the protein levels of raptor, rictor or mTOR and
reduced the phosphorylation of their outputs S6 (S235/236) and Akt
(S473), respectively in Saos-2 cells (Fig. 3A). Knockdown of rictor and mTOR, but
not raptor significantly repressed cell migration (p<0.01)
(Fig. 3B and C). These results
further confirmed the critical role of mTORC2 in osteosarcoma cell
migration.
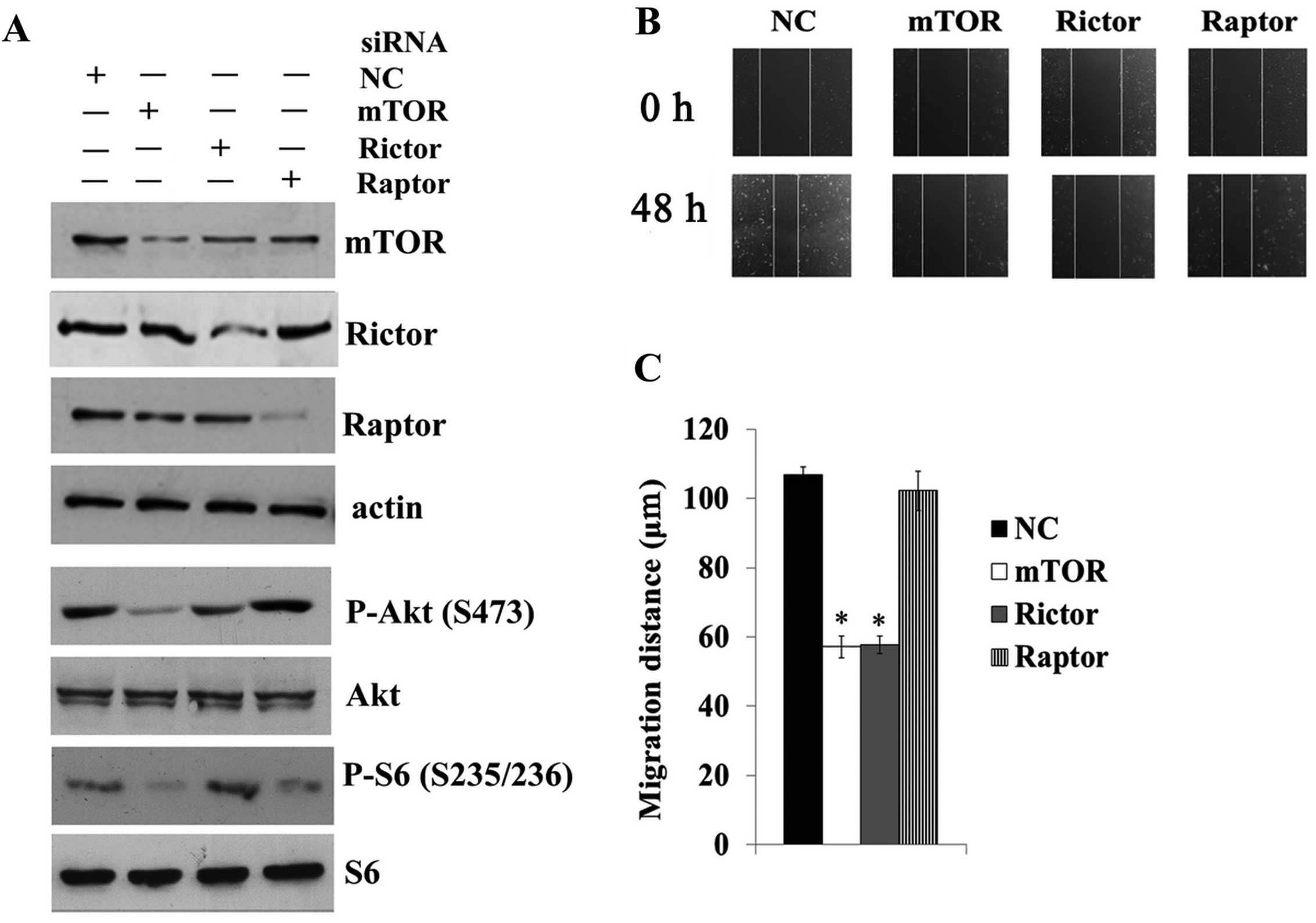 | Figure 3Targeted inhibition of mTORC2 prevents
osteosarcoma cell migration. (A) Saos-2 cells were transfected with
negative control (NC), mTOR, raptor or rictor siRNA for 48 h, and
cell lysates were subjected to immunoblotting for levels of mTOR,
raptor, rictor, phospho-Akt (S473), Akt, phospho-S6 (S235/236) and
S6. (B) Saos-2 cells were transfected with NC, mTOR, raptor, or
rictor siRNA for 48 h, and subsequently subjected to a wound
healing assay as described in Materials and methods. (C) The wound
distances were measured under a light microscope.
*p<0.01 compared with NC and raptor siRNA treatment.
mTORC2, mammalian target of rapamycin complex 2. |
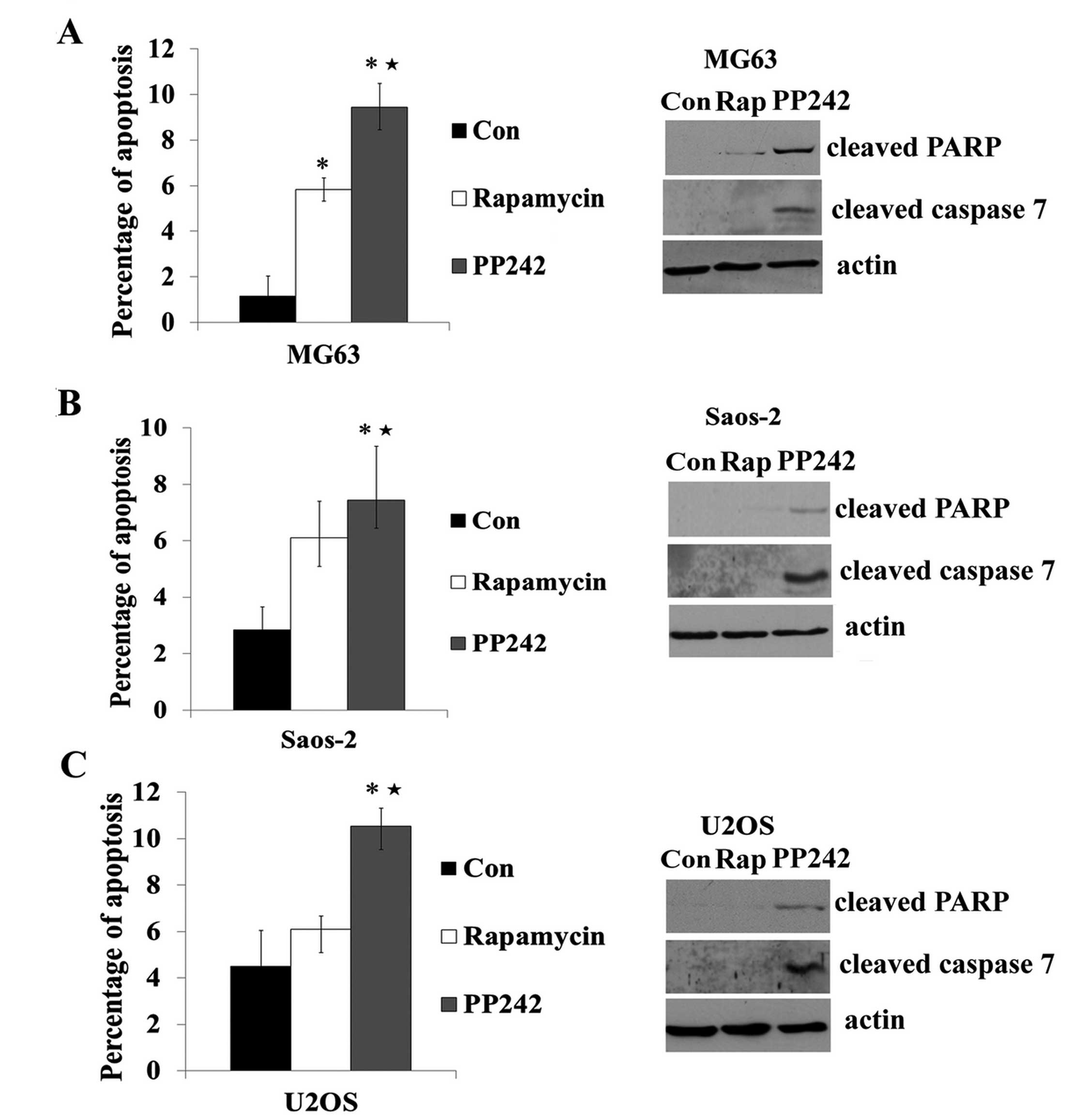
Inhibition of mTORC2 but not mTORC1
promotes apoptosis in osteosarcoma cells
Akt represents an important intracellular survival
signaling under a variety of conditions (22). Rapamycin did not inhibit mTORC2/Akt,
and accordingly it did not promote apoptosis in osteosarcoma MG63,
U2OS and Saos-2 cells (Fig. 4A–C).
Consistent with the inhibitory activities of PP242 on mTORC2/Akt
(S473) phosphorylation (Fig. 1),
the drug significantly enhanced cleavage of PARP, caspase 7 and the
number of apoptotic cells in the serum-starved MG63, U2OS and
Saos-2 cells (Fig. 4A–C).
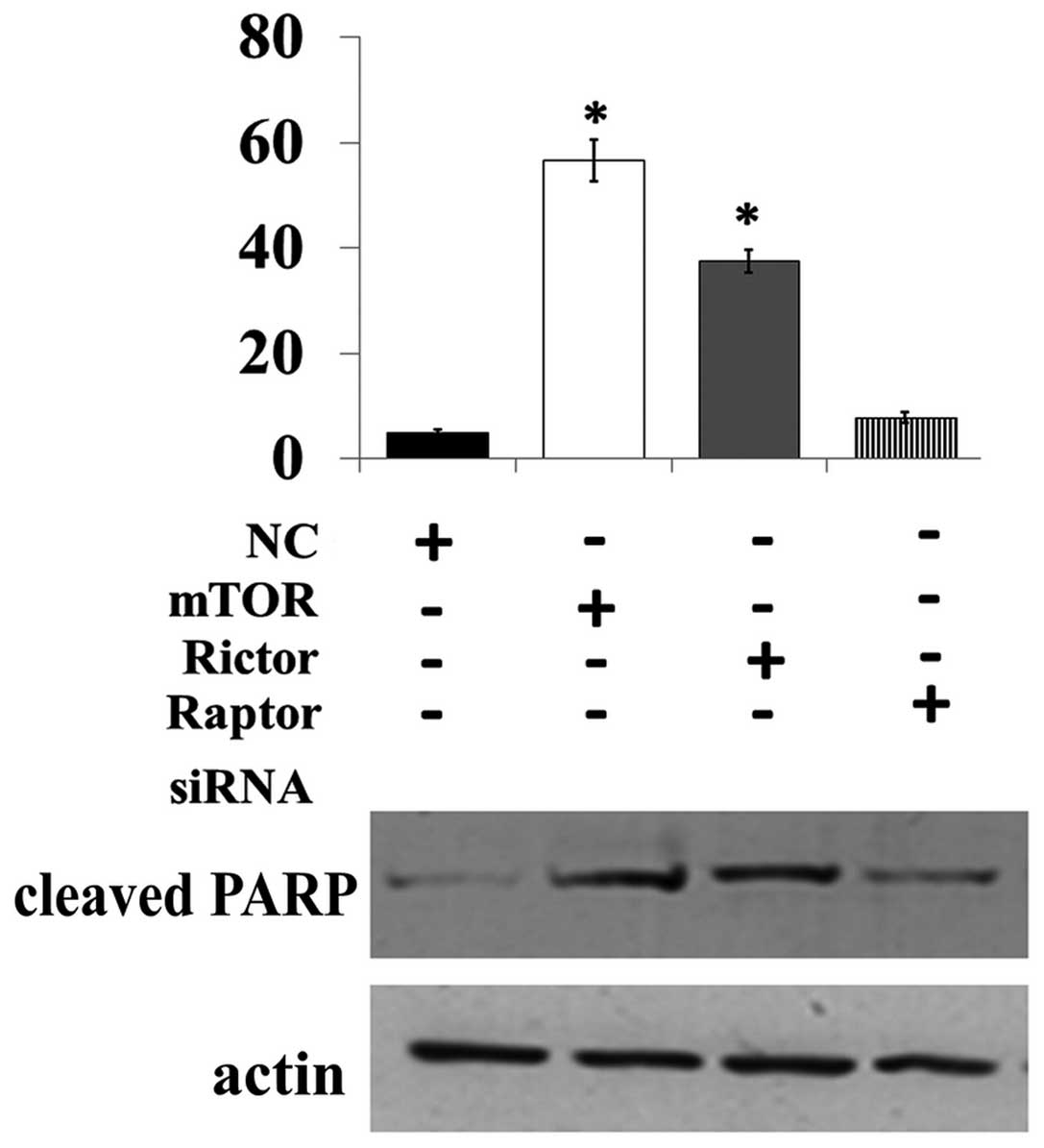
To further identify the different role of mTORC1 and
mTORC2 in osteosarcoma cell apoptosis, the raptor, rictor or mTOR
siRNA was transfected into Saos-2 cells. The results showed that
knockdown of mTOR and rictor but not raptor were able to increase
the number of apoptotic cells and the level of cleaved-PARP in the
Saos-2 cells (Fig. 5). Hence, we
demonstrated that targeting of mTORC2 but not mTORC1 promoted
apoptosis in the osteosarcoma cells.
Targeting of mTORC2 but not mTORC1
promotes cisplatin-induced apoptosis in osteosarcoma cells
Cisplatin is a common chemotherapeutic agent that
induces apoptosis in a variety of cancer cell lines including
osteosarcoma cells (23). Drug CI
analysis is a generalized method for analyzing the effects of
multiple drugs and for determining summation, synergism and
antagonism.
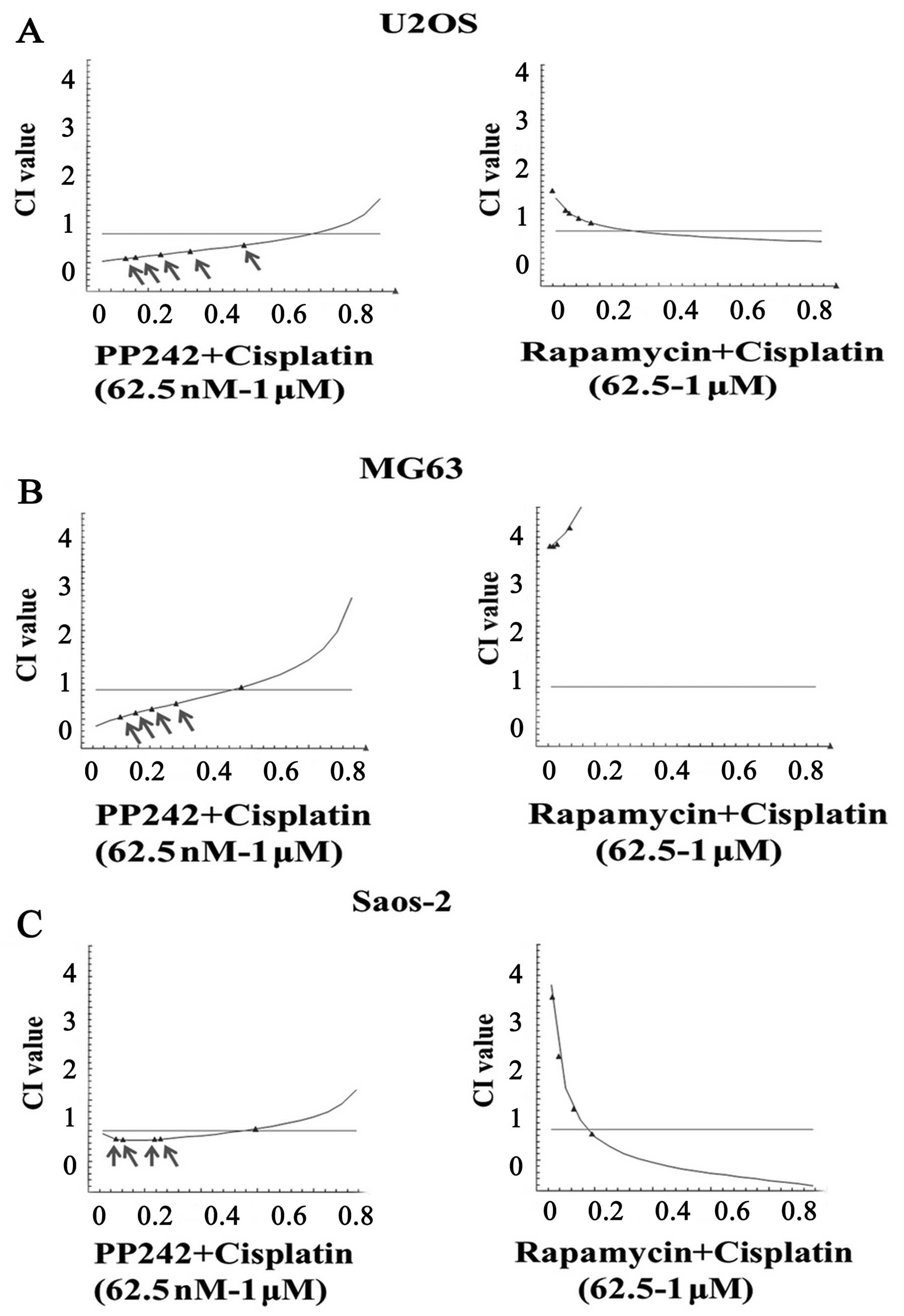
We used the drug CI analysis to examine the
interaction of mTORC1 inhibitor rapamycin or mTORC1/2 inhibitor
PP242 with cisplatin. We performed the dose-response studies in
osteosarcoma cell lines to obtain the apoptotic rate, and we
calculated the CI values by the CalcuSyn program. When PP242 was
applied at most concentrations in all tested cell lines, the CI
values were <1, indicating synergistic pro-apoptotic
interactions between PP242 and cisplatin (Fig. 6). In contrast, rapamycin did not
show any synergistic interactions with cisplatin, as the CI values
were >1 (Fig. 6). Most
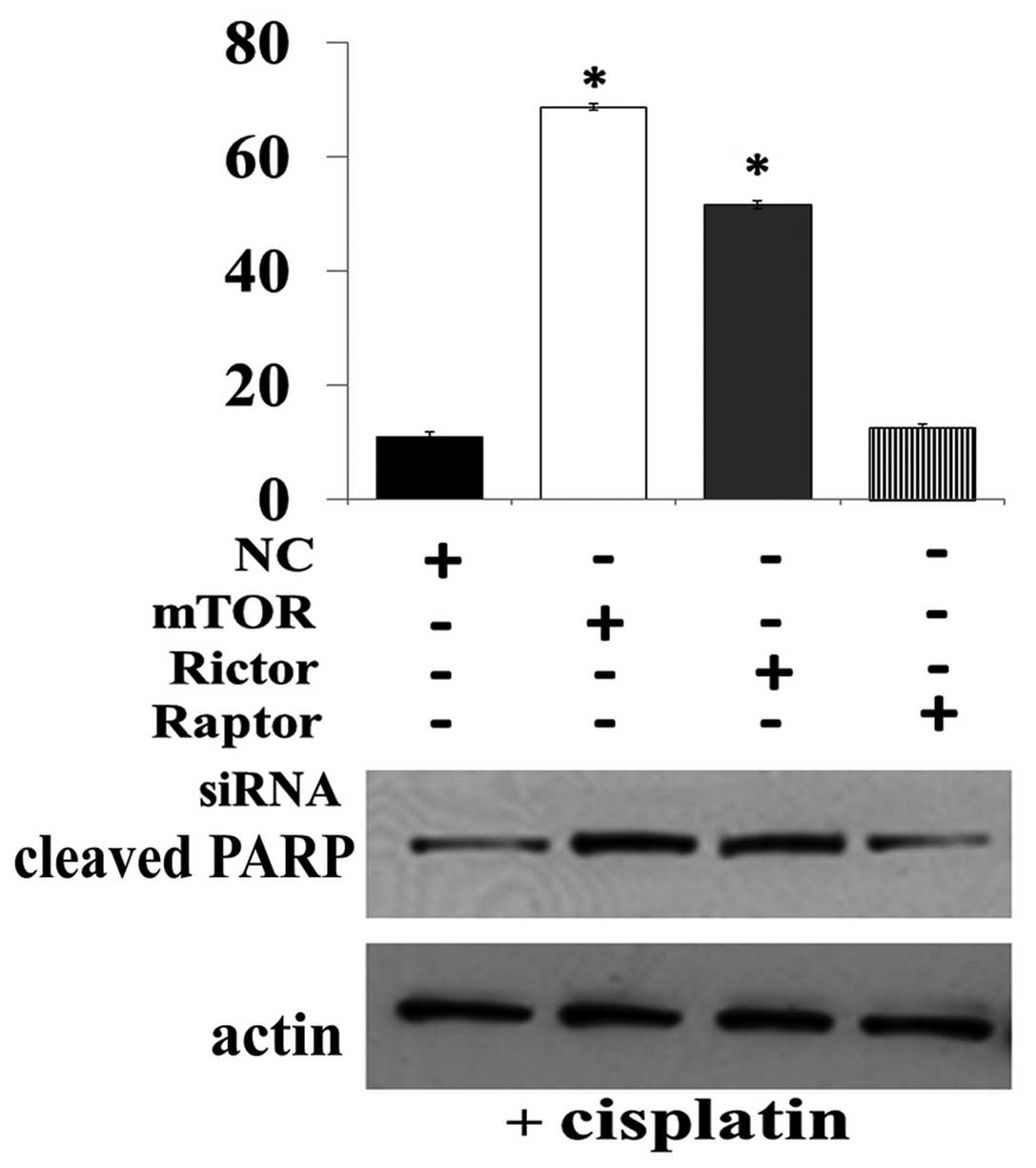
importantly, rictor or mTOR knockdown, but not raptor knockdown,
markedly enhanced cisplatin-induced cleavage of PARP and the number
of apoptotic cells in Saos-2 cells (Fig. 7). Taken together, our results
indicate that targeted inhibition of mTORC2 but not mTORC1 markedly
enhanced cisplatin-induced apoptosis in osteosarcoma cells.
Discussion
In the present study, we demonstrated that either
inhibition of mTORC1/2 by PP242 or knockdown of mTOR or rictor by
siRNA prevents migration and promotes apoptosis in osteosarcoma
cell lines. We also demonstrated the synergistic antitumor activity
achieved by combining mTOR kinase inhibitor PP242 with cisplatin.
Our findings suggest that agents that inhibit either mTORC2 or
mTORC1/2 may have advantages over selective mTORC1 inhibitors in
the treatment of osteosarcoma. Given that mTOR kinase inhibitors
(e.g., OSI-027) are undergoing clinical trials, this study provides
a strong rationale for testing the use of mTOR kinase inhibitors or
the combination of mTOR kinase inhibitors with traditional
chemotherapeutic drug cisplatin for the treatment of
osteosarcoma.
It has been well established that mTOR signaling
plays key roles in the pathogenesis and progression of osteosarcoma
and is a major cancer drug target. However, clinical trials have
shown that osteosarcoma patients are not sensitive to rapalogs when
employed in a monotherapy setting. The known mechanisms for rapalog
resistance are as follows. i) S6K is exquisitely inhibited, yet the
control of 4E-BP1 and mRNA translation is not sensitive (24); ii) mTORC2 activity is not acutely
blocked and iii) there is a feedback loop between mTORC1 and Akt.
Treatment with rapalogs results in elevated Akt activity, which
serves as a mechanism to enhance cell survival when mTORC1 is
inhibited (25).
In the past decade, much research has focused on the
antitumor effect of second-generation mTOR kinase inhibitors. This
research has not only furthered the understanding of the mTOR
signaling network, but has also contributed to to the development
of many mTORC1/2 inhibitors including Torin1, PP242, PP300 and
OSI-027. The antitumor effects of these mTOR inhibitors have been
assessed in many types of cancers (26). Some of the results demonstrated high
effectiveness, resulting in the move to clinical trials.
Nevertheless, the mechanism involved in the mTOR kinase regulation
of cancer cells and the specific target of mTOR inhibitors remain
unclear. Their effects on osteosarcoma, and how the therapeutic
function is regulated require further research. In the present
study, we compared the antitumor activity of the targeted
inhibition of mTORC1 and mTORC2 in osteosarcoma. Our results
demonstrated that although rapamycin or PP242 suppressed
proliferation in a variety of osteosarcoma cell lines, inhibition
of mTORC2 by PP242 or rictor knockdown effectively prevented
osteosarcoma cell migration and promoted cell apoptosis, while
inhibition of mTORC1 by rapamycin or raptor knockdown neither
prevented osteosarcoma cell migration nor promoted cell apoptosis.
These data suggest that mTOR kinase inhibitors are more effective
than rapalogs to suppress osteosarcoma and that promotion of
apoptosis and suppression of cell migration may contribute to the
therapeutic effects of mTORC1/2 inhibitors.
Recent studies have suggested that mTORC2 activity
is essential for the transformation and vitality of a number of
cancers driven by mutations of PI3K or loss of PTEN, which include
glioma and prostate cancers (27,28).
Yet, the roles of mTORC2 in carcinogenesis and progression of
osteosarcoma are not known. Although both mTORC1 and mTORC2 have
been reported to mediate epithelial-mesenchymal transition (EMT)
and cell motility in epithelial, colon cancer and podocytes
(29–31), we found that inhibition of mTORC2
but not mTORC1 promoted apoptosis and suppressed migration in
osteosarcoma cells, indicating the critical role of mTORC2 in
osteosarcoma cell survival and migration. Thus, mTORC2 is a
promising therapeutic target in the prevention and treatment of
osteosarcoma. mTOR kinase inhibitors may prevent mTORC2 activity.
However, concurrent inhibition of mTORC1 may introduce
hyperactivation of PI3K signaling and possible deleterious effects
to normal host tissues, which limits their therapeutic potential
(32). Thus, mTORC2-specific
inhibitors may be promising therapeutic agents for
osteosarcoma.
Acknowledgements
This study was supported by the National Natural
Sciences Foundation of China (31271271, 31000633 and 81260401), and
GDUPS (2011).
References
|
1
|
Biermann JS, Adkins D, Benjamin R, et al:
Bone cancer. J Natl Compr Cancer Netw. 5:420–437. 2007.
|
|
2
|
Demetri GD, Baker LH, Benjamin RS, et al:
Soft tissue sarcoma. J Natl Compr Cancer Netw. 5:364–399.
2007.PubMed/NCBI
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel SR, Vadhan-Raj S, Burgess MA, et al:
Results of two consecutive trials of dose-intensive chemotherapy
with doxorubicin and ifosfamide in patients with sarcomas. Am J
Clin Oncol. 21:317–321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
from growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guertin DA and Sabatini DM: An expanding
role for mTOR in cancer. Trends Mol Med. 11:353–361. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fasolo A and Sessa C: Targeting mTOR
pathways in human malignancies. Curr Pharm Des. 18:2766–2777. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benjamin D, Colombi M, Moroni C and Hall
MN: Rapamycin passes the torch: a new generation of mTOR
inhibitors. Nat Rev Drug Discov. 10:868–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Houghton PJ: Everolimus. Clin Cancer Res.
16:1368–1372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guertin DA and Sabatini DM: The
pharmacology of mTOR inhibition. Sci Signal. 2:pe242009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feldman ME, Apsel B, Uotila A, et al:
Active-site inhibitors of mTOR target rapamycin-resistant outputs
of mTORC1 and mTORC2. PLoS Biol. 7:e382009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu K, Shi C, Toral-Barza L, et al: Beyond
rapalog therapy: preclinical pharmacology and antitumor activity of
WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and
mTORC2. Cancer Res. 70:621–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thoreen CC, Kang SA, Chang JW, et al: An
ATP-competitive mammalian target of rapamycin inhibitor reveals
rapamycin-resistant functions of mTORC1. J Biol Chem.
284:8023–8032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janes MR, Limon JJ, So L, et al: Effective
and selective targeting of leukemia cells using a TORC1/2 kinase
inhibitor. Nat Med. 16:205–213. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schenone S, Brullo C, Musumeci F, et al:
ATP-competitive inhibitors of mTOR: an update. Curr Med Chem.
18:2995–3014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao H, Gao C, Tang H, et al: Dual
targeting of mTORC1/C2 complexes enhances histone deacetylase
inhibitor-mediated anti-tumor efficacy in primary HCC cancer in
vitro and in vivo. J Hepatol. 56:176–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YJ, Duan Y and Zheng XF: Targeting
the mTOR kinase domain: the second generation of mTOR inhibitors.
Drug Discov Today. 16:325–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai X, Ma D, Liu A, et al: Rheb activates
mTOR by antagonizing its endogenous inhibitor, FKBP38. Science.
318:977–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Lin J, Wang X, et al: Targeting of
mTORC2 prevents cell migration and promotes apoptosis in breast
cancer. Breast Cancer Res Treat. 134:1057–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li DM and Feng YM: Signaling mechanism of
cell adhesion molecules in breast cancer metastasis: potential
therapeutic targets. Breast Cancer Res Treat. 128:7–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen SM and Lippard SJ: Cisplatin: from
DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol.
67:93–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thoreen CC and Sabatini DM: Rapamycin
inhibits mTORC1, but not completely. Autophagy. 5:725–726. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carew JS, Kelly KR and Nawrocki ST:
Mechanisms of mTOR inhibitor resistance in cancer therapy. Target
Oncol. 6:17–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masri J, Bernath A, Martin J, et al:
mTORC2 activity is elevated in gliomas and promotes growth and cell
motility via overexpression of rictor. Cancer Res. 67:11712–11720.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guertin DA, Stevens DM, Saitoh M, et al:
mTOR complex 2 is required for the development of prostate cancer
induced by Pten loss in mice. Cancer Cell. 15:148–159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shorning BY, Griffiths D and Clarke AR:
Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and
epithelial-mesenchymal transition in the mouse bladder. PLoS One.
6:e162092011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gulhati P, Bowen KA, Liu J, et al: mTORC1
and mTORC2 regulate EMT, motility, and metastasis of colorectal
cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inoki K, Mori H, Wang J, et al: mTORC1
activation in podocytes is a critical step in the development of
diabetic nephropathy in mice. J Clin Invest. 121:2181–2196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Proud CG: mTOR signalling in health and
disease. Biochem Soc Trans. 39:431–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sparks CA and Guertin DA: Targeting mTOR:
prospects for mTOR complex 2 inhibitors in cancer therapy.
Oncogene. 29:3733–3744. 2010. View Article : Google Scholar : PubMed/NCBI
|