Introduction
Thyroid cancer is the most common malignancy of the
endocrine system and accounts for ~1% of newly diagnosed cancers,
and its incidence is rapidly increasing worldwide (1). Thyroid carcinoma can be divided into
papillary, follicular, medullary and anaplastic types according to
histological type (2). Papillary is
the most common thyroid carcinoma, accounting for more than 83% of
all such malignancies (3,4). Although the prognosis of patients with
thyroid malignancies has improved as a result of the
standardization of surgical techniques and advances in
chemotherapy, a subset of patients suffers from recurrent disease
that is refractory to surgical resection and radioactive iodine
ablation (5,6). Despite the fact that chemotherapy and
radiotherapy have been widely used in the treatment of these
patients, the outcome remains poor due to the intrinsic
chemoresistance and radioresistance of papillary thyroid carcinoma
(PTC) (5,7). Therefore, there is a need to search
for novel therapies to augment both systemic chemotherapy and
radiotherapy for the treatment of patients with advanced PTC.
The NIN1/RPN12 binding protein 1 homologue (NOB1),
an evolutionarily conserved protein, is a subunit of the 26S
proteasome and is composed of nine exons and eight introns 1,749-bp
long, containing a putative open reading frame of 1,239 bp, and is
located on chromosome 16q22.1 (8).
The NOB1 gene, encoding a 50-kDa protein consisting of a
PilT N terminus (PIN) domain and a C terminal zinc ribbon domain
(9,10), is mainly expressed in liver, lung
and spleen tissue (8). The PIN
domain was postulated as the enzymatic domain of NOB1 since
cells expressing the mutant PIN failed to cleave the 20S pre-rRNA,
strengthening the notion that NOB1 is the long-sought D-site
endonuclease (11,12). As a ribosome assembly factor, recent
biochemical and genetic studies have revealed that genetic
depletion of Nob1 strongly suppresses the processing of the 20S
pre-rRNA to the mature 18S rRNA, producing remarkably high levels
of the 20S pre-RNA with novel degradation intermediates (13). Additionally, a recent study also
found that NOB1 plays a role in the formation of the 26S proteasome
and the maturation of the 20S proteasome in eukaryotes (14). These above findings suggest that
NOB1 plays a crucial role in the process of ribosome synthesis and
the maturation and formation of the 26S proteasome.
A larger amount of evidence suggests that the
ribosome and the 26S proteasomes are involved in the protein
synthesis and ubiquitinated (ub) protein degradation pathways,
respectively (15,16). Numerous studies have demonstrated
that the ubiquitin (Ub) pathway plays a critical role in regulating
essential cellular processes, such as gene transcription and signal
transduction (17,18), which have close relationships with
the development of human diseases, especially cancer (18–20).
NOB1 is involved in the process of ribosome synthesis and the
maturation and formation of the 26S proteasome, which suggests a
role for NOB1 in the development of human malignancies. NOB1
has been found to be upregulated in a variety of cancers such as
ovarian cancer, prostate carcinoma, breast infiltrating ductal
carcinoma as well as in non-small lung cancer (21–24).
Consistent with these findings, recently, our previous study
demonstrated that the expression level of NOB1 protein was higher
in PTC tissues than that in normal thyroid tissues and benign
thyroid tumor tissues and there were significant associations
between NOB1 protein expression and UICC stage, tumor size and
lymph node metastasis (25). In
particular, recently, several studies demonstrated that RNA
interference (RNAi)-mediated downregulation of endogenous NOB1
inhibits tumor cell proliferation and survival, and induces cell
apoptosis (21,24,26,27).
Radiation might kill cancer cells by inducing apoptosis, or
programmed cell death, thus we hypothesized that reduction of NOB1
expression in PTC cells might enhance the radiosensitivity of cells
by facilitating apoptotic pathways. However, the question of
whether or not inhibition of NOB1 is an effective approach to
overcome the radioresistance of PTC has not yet been explored. In
addition, to our knowledge, whether NOB1 affects tumor cell
proliferation or tumor growth of PTC remains unclear. Therefore, to
solve these questions and explore the possibility of NOB1 as a
therapeutic target for the treatment of human PTC, we first
constructed an adenoviral vector plasmid to deliver small
interfering RNA molecules targeting the NOB1 gene by the RNA
interference (RNAi) technology. Then, cell proliferation, cell
cycle distribution, apoptosis, migration and invasion in
vitro and tumor growth in vivo were determined after
downregulation of NOB1 by RNAi. Finally, the in vitro and
in vivo radiosensitivity of PTC cells was evaluated.
Materials and methods
Cell culture
The human PTC cell lines (TPC-1) and HEK-293T,
provided by the Chinese Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) were cultured in RPMI-1640 (Gibco,
Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco),
100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2.
siRNA design and production of the
recombinant adenovirus
The siRNA target design tools from Ambion were used
to design NOB1-shRNA and negative control scramble-shRNA sequences.
shRNAs targeting NOB1 and the control sequence (Scramble) were
designed and synthesized as follows: sh-NOB1 sense,
5′-GATCCAAGGTTAAGGTGAGCTCATCGTCA AGAGAAGTTGTACTCCAGCTTGTGAGA-3′ and
antisense, 5′-AGCTTAAGGTTAAGGTGAGCTCATCGCTCTTGAA
AGTTGTACTCCAGCTTGTGG-3′; sh-Scramble sense,
5′-GATCCAATTCTCCGAACGTGTCACGTTCAAGAGAT CCGATTACGGCGTTTCTTAGA-3′ and
antisense, 5′-AGC TTAAGAAACGCCGTAATCGGATCTCTTGAATCCGAT
TACGGCGTTTCTTG-3′. The oligonucleotides were annealed and inserted
into the BamHI and HindIII sites of pSilencer 2.1-U6
neo (Ambion, Austin, TX, USA), and then subcloned into the
HindIII and BglII sites of the pAd-Track adenoviral
shuttle vector to get pAdTrack-U6/sh-NOB1 or
pAdTrack-U6/sh-Scramble, referred to as pAD-NOB1 and pAD-Scramble.
Then, pAD-NOB1 or pAD-Scramble vectors were linearized with
PmeI and cotransformed into BJ5183 cells with adenoviral
backbone vector pAdEasy-1. Positive clones were selected and
determined by DNA miniprep and PacI digestion. Plasmids from
the correct clones were amplified by transforming into DH5K cells.
Adenoviral DNA (pAD-NOB1 and pAD-Scramble) was prepared and
linearized with PacI and purified by ethanol precipitation.
The linearized recombinant plasmid was then transduced into the
packaging cell line 293 as previously described (28,29).
pAD-NOB1 and pAD-Scramble adenovirus vectors were amplified using
293 cells, and titered using the Adeno-X™ Rapid Titer kit (BD
Biosciences, San Jose, CA, USA) in 293 cells.
Adenovirus infection
On the day before virus infection, 3×105
TPC-1 cells were plated in each well of 6-well plates. When the
cells reached ~70–90% confluency, the culture medium was aspirated,
and the cell monolayer was washed with pre-warmed sterile PBS (pH
7.4). Cells were then incubated with the indicated virus (pAD-NOB1
and pAD-Scramble) at a multiplicity of infection (MOI) of 0, 25, 50
or 75 at 37°C, respectively. After adsorption for 2 h, 2 ml of
fresh growth medium was added, and the cells were placed in an
incubator for an additional 48–72 h. Cell analysis and other
experiments were performed. The following experiments were
performed using viruses at such MOIs except for special
indications.
Reverse transcription and real-time
PCR
Total RNA from the cell samples was isolated by the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Total RNA (2 mg) was
reverse-transcribed into cDNA using M-MLV Reverse Transcriptase kit
(Promega, Madison, WI, USA) according to the manufacturer’s
protocol. Real-time quantitative PCR analysis was performed using a
SYBR-Green Master Mix Kit on a Bio-Rad Connect Real-Time PCR
platform. In brief, each PCR reaction mixture, containing 10 μl of
2× SYBR-GreenMaster Mix, 1 μl of sense and antisense primers (5
μmol/μl) and 1 μl of cDNA (20 ng), was run for 40 cycles with
denaturation at 95°C for 15 sec, annealing at 60°C for 15 sec and
extension at 72°C for 30 sec in a total volume of 20 μl. For
relative quantification, 2−ΔΔCt was calculated and used
as an indication of the relative expression levels, which was
calculated by subtracting the Ct values of the control gene from
the Ct values of NOB1. The primer sequences for the PCR
amplification of the NOB1 gene were 5′-AAGTGAGGAGGAGGAGGAG-3′ and
5′-ACTTTCTTC AGGGTCTTGTTC-3′. The primers for housingkeeping gene
GAPDH were forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′.
Western blot analysis
TPC-1 cells were collected 5 days after infection
with the adenovirus constructs, and total protein was isolated.
Cells were harvested and washed with cold phosphate-buffered saline
solution, and total proteins were extracted in the extraction
buffer [150 mM sodium chloride, 50 mM Tris hydrochloride (pH 7.5),
1% glycerol, 1% Nonidet-40 substitute solution] and quantified
using the bicinchoninic acid (BCA) method. Equal amounts of protein
(20 μg per lane) from the treated cells were loaded and
electrophoresed on an 8–12% sodium dodecyl sulfate (SDS)
polyacrylamide gel and then electroblotted onto nitrocellulose
membranes, blocked by 5% skim milk, and probed with the antibodies
to NOB1 (Abcam, Cambridge, UK), and antibodies against
phosphorylated (p−)p38MAPK (Cell Signaling Technology, Danvers, MA,
USA), XIAP, Bcl-2, p38MAPK, p-ERK1/2, ERK1/2, JNK, p-JNK and GAPDH
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), respectively,
followed by treatment with the secondary antibody conjugated with
horseradish peroxidase (1:1,000; Santa Cruz Biotechnology). The
proteins were detected by the enhanced chemiluminescence system and
exposed to X-ray film. The absorbances of the positive bands in the
analysis were measured by densitometry using a GIS Analysis system
(Tannon, Shanghai, China).
Cell viability analysis
The MTT assay was used to determine the effect of
the downregulation of NOB1 on the proliferation of cells. In brief,
cells infected with Ad/sh-NOB1 or Ad/sh-Scramble (MOI of 50 each),
along with untreated cells were seeded in a 96-well plates at a
density of 5×103 cells/well. At indicated time points,
20 μl methylthiazol tetrazolium (MTT) solution (5 mg/ml) was added
into each well. After 4 h of incubation at 37°C, 150 μl dimethyl
sulfoxide (DMSO) was added to dissolve the crystals. After 10 min
at room temperature, the absorbance was recorded at 570 nm.
BrdU incorporation assay
Cells infected with Ad/sh-NOB1 or Ad/sh-Scramble
(MOI of 50 each), along with the untreated cells, were cultured in
96-well plates with 5×103 cells per well. A
5-bromodeoxyuridine (BrdU) incorporation assay was performed using
the BrdU cell proliferation assay kit (Chemicon, Temecula, CA, USA)
according to the manufacturer’s instructions. The growth rate of
cells was calculated as described previously (21).
Cell cycle analysis
Cells infected with Ad/sh-NOB1 or Ad/sh-Scramble
(MOI of 50 each), along with the untreated cells, were cultured in
6-well plates. At the indicated time point, cells were harvested by
centrifugation at 2,000 × g for 5 min, washed twice with PBS, and
then fixed in ethanol. Then, cells were rehydrated and resuspended
in PBS containing RNase A (100 μg/ml) on ice. After an additional
incubation at room temperature for 30 min, cells were stained with
propidium iodide (PI) and were then analyzed by using a BD FACS
Calibur Flow Cytometer (BD Biosciences, San Diego, CA, USA).
Apoptosis analysis
To measure the effect of adenovirus-mediated siRNA
targeting NOB1 on cell apoptosis of TPC1 cells, TUNEL assay was
performed. In briefly, cellular DNA fragmentation was measured with
the ApoTag Red in situ apoptosis detection kit (Chemicon)
according to the manufacturer’s instructions when TPC1 cells were
infected with Ad/sh-NOB1, or Ad/sh-Scramble for 48 h. To quantify
the apoptotic cells, the terminal deoxynucleotidyl
transferase-mediated nick end labeling (TUNEL)-positive cells were
counted using confocal microscopy (Olympus, Tokyo, Japan).
In addition, at the molecular level, we also
detected other anti-apoptotic molecules, such as XIAP and Bcl-2
protein expression by western blotting as an additional indicator
of apoptosis.
Cell migration assay
To assess the effect of the downregulation of NOB1
on cell migration, a wound-healing assay was performed. In brief,
cells infected with Ad/sh-NOB1 or Ad/sh-Scramble at an MOI of 50,
along with the untreated cells, were seeded into 24-well tissue
culture plates. Forty-eight hours later, an artificial homogenous
wound was scratched into the monolayer with a sterile plastic
100-μl micropipette tip. After wounding, the debris was removed by
washing the cells with serum-free medium. Migration of cells into
the wound was observed at different time points. Cells that
migrated into the wounded area or cells with extended protrusion
from the border of the wound were visualized and photographed under
an inverted phase-contrast microscope (Leica DMR, Germany).
Invasion analysis
The migration capacity of TPC1 cells was determined
in vitro using Transwell Chambers (Corning, Tewksbury, MA,
USA) in which the two chambers were separated by a Matrigel-coated
polycarbonate membrane (8-μm pore size). Cells infected with
Ad/sh-NOB1 or Ad/sh-Scramble at an MOI of 50, along with the
untreated cells, were seeded into cell culture inserts (8-μm pore
size; Falcon; BD Bioscience) with 1×105 cells/well,
precoated with 25 μl of 20% Matrigel (2–3 mg/ml protein), and then
placed in a 24-well plate (Falcon). After cells had been cultured
at 37°C for 40 h, they were fixed and stained with 0.5% crystal
violet. The cells on the top of the cell culture insert were
removed by wiping with a cotton swab, and cell invasion was
observed with an immunofluorescence microscope by counting the
cells that had invaded into the bottom of the cell culture insert.
All experiments were performed in triplicate.
Clonogenic cell survival assay
Cells infected with Ad/sh-NOB1 or Ad/sh-Scramble at
an MOI of 50, along with the untreated cells, were plated into each
well of 96-well plates with 5.0×103 cells/well.
Seventy-two hours after plating, the cells were trypsinized,
counted, and the appropriate number of cells were plated into
6-well plates and allowed to attach for 6 h. Then, three types of
TPC1 cells were irradiated with different doses of 6 MV X-ray
radiation by a 23-EX accelerator (Varian). The radiation doses were
0, 2, 4, 6, and 8 Gy, respectively (the dose efficiency was 1.6
Gy/min) at room temperature and incubated for 14 days. Colonies
were stained with crystal violet and colonies with more than 50
cells were counted. Plating efficiency was calculated as follows:
Plating efficiency = (clone number/total cell number) × 100%. All
experiments were repeated in triplicate. Finally, the cell survival
fraction was determined and the cell survival curve was
constructed.
Detection of caspase-3 activity
The caspase-3 activity was determined using the
ApoAlert caspase-3 assay kit (Clontech, Mountain View, CA, USA)
following the manufacturer’s instructions. In brief, cells infected
with Ad/sh-NOB1 or Ad/sh-Scramble at an MOI of 50, along with the
untreated cells, were seeded at a density of 1.0×107
cells/well in a 6-cm dish; 48 h later, after irradiation, the cells
were lysed in 50 μl lysis buffer provided in the assay kit and were
incubated at 4°C for 30 min. The enzyme activity in the supernatant
was measured by cleavage of the substrate
acetyl-Asp-Glu-Val-Asp-p-nitroanilidine (DEVD-pNA) to yield pNA.
The relative caspase-3 activity was measured as the absorbance at
405 nm via a microplate reader (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). The results were calibrated relative to known
concentrations of pNA and expressed as picomoles of substrate
cleaved per min and per microgram of protein.
Tumor xenograft assay
All animal experiments were performed in accordance
with institutional guidelines, following a protocol approved by the
Ethics Committees of the Disease Model Research Center, The First
Hospital of Jilin University (Changchun, China). Female BALB mice
~6 weeks old were provided by the Disease Model Research Center,
The First Hospital of Jilin University, and maintained under
specific pathogen-free conditions and provided with food and water
ad libitum.
The female BALB/c nude mice were inoculated
subcutaneously with a total of 1.0×107 TPC1 cells in 100
μl of PBS into the right flank. Three weeks after tumor inoculation
with the TCP1 cells, when the tumors grew to ~100 mm3,
60 tumor-bearing mice were randomly divided into the following 4
treatment groups (n=10/group): i) PBS group (Control group); ii)
Ad/sh-Scramble; iii) Ad/sh-NOB1; iv) irradiation; v) Ad/sh-Scramble
+ irradiation; vi) Ad/sh-NOB1 + irradiation. Three of the groups
were irradiated, and three groups remained non-irradiated.
Concerning the irradiated groups, intratumoral injections of 0.1 ml
of PBS, 6.0×108 pfu of pAd-sh-Scramble or pAd-sh-NOB1
were administered three times on days 1, 3 and 5, and subsequently
X-ray irradiation was performed at a clinically relevant dose of
5.0 Gy on days 2, 4 and 6. For the non-irradiated groups,
intratumoral injections of 0.1 ml of PBS, 6.0×108 pfu of
Ad/sh-Scramble or Ad/sh-NOB1 were conducted. Tumors were measured
using a caliper gauge once a week over a 5-week period following
the initial virus injections. Tumor volume was calculated according
to the formula: TV (mm3) = length × width2 ×
0.4. All mice were sacrificed and tumors were resected and weighed,
and part of the tumors was fixed in 10% PBS for the TUNEL assay. We
measured the primary tumors and performed western blot analyses for
NOB1 protein expression. Then, TUNEL staining was performed on 5-μm
sections of the excised tumors using Dead End™ Fluorometric TUNEL
system (Promega) according to the manufacturer’s protocol. The
number of apoptotic cells in 5 random high-power fields was counted
and expressed as a percentage of total cells (apoptotic
fraction).
Statistical analysis
All data are expressed as mean ± standard deviation
(SD) from three independent experiments. Statistical analysis
between two samples was performed using the Student’s t-test.
Statistical comparison of more than two groups was performed using
one-way ANOVA followed by a Tukey post hoc test. Graphpad Prism 6.0
software (GraphPad Software, San Diego, CA, USA), was used for
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Adenovirus-mediated siRNA targeting NOB1
inhibits the expression of NOB1 in TPC1 cells
To determine the optimal MOI for maximal transgene
expression, TPC1 cells were infected with Ad/sh-Scramble or
Ad/sh-NOB1 at different MOIs (0, 25, 50, 75) and examined by
fluorescence microscopy. Approximately 90% of GFP expression could
be observed in the TPC1 cells infected with Ad/sh-Scramble or
Ad/sh-NOB1 at an MOI of 50 (data not shown). Thus, an MOI of 50 was
selected as an optimal dose for infection of the TPC1 cells and
were used subsequently in all procedures. To determine the effect
of the adenovirus-mediated siRNA targeting NOB1 on the expression
of the NOB1 gene in the PTC cell line, real-time RT-PCR and
western blot assay were performed to detect the expression of NOB1
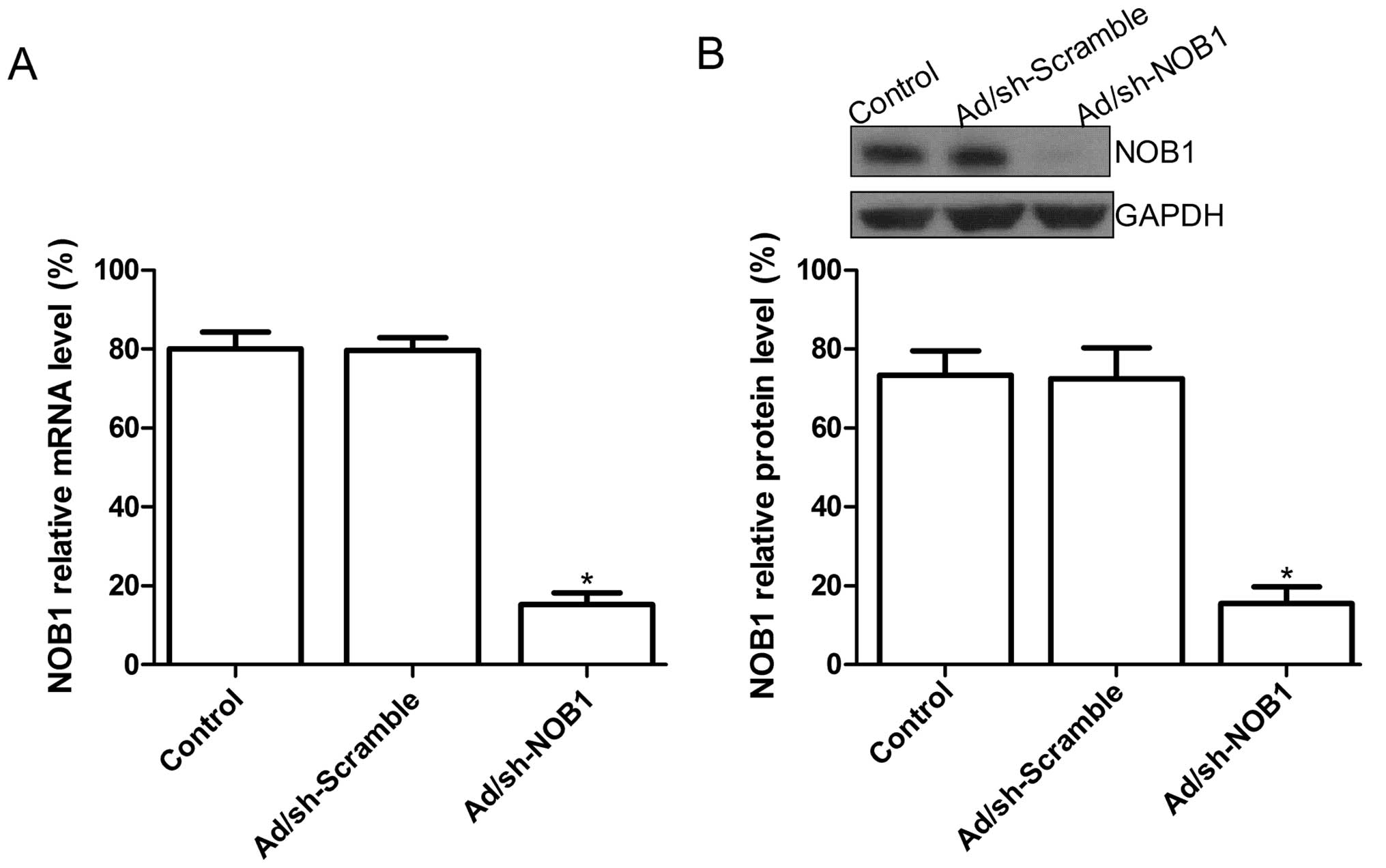
at the mRNA and protein levels, respectively. The expression level
of NOB1 mRNA in the Ad/sh-NOB-infected TPC1 cells was decreased
compared to that in the untreated cells or Ad/sh-Scramble-infected
cells (P<0.05; Fig. 1A).
Additionally, at the protein expression level, no significant
inhibition in NOB1 protein expression was found in the untreated
cell or Ad/sh-Scramble-infected cells (P>0.05), while the band
density decreased dramatically in the Ad/sh-NOB1-infected TPC1
cells as compared with the uninfected cell or the
Ad/sh-Scramble-infected cells (P<0.05; Fig. 1B). These data revealed that
adenovirus-mediated siRNA targeting NOB1 specifically and
significantly inhibited the expression of the NOB1 gene in
the TPC1 cells.
Adenovirus-mediated siRNA targeting NOB1
inhibits cell growth in TPC1 cells
To further assess the role of NOB1 in regulating PTC
cell proliferation, MTT assays were performed on TPC1 cells
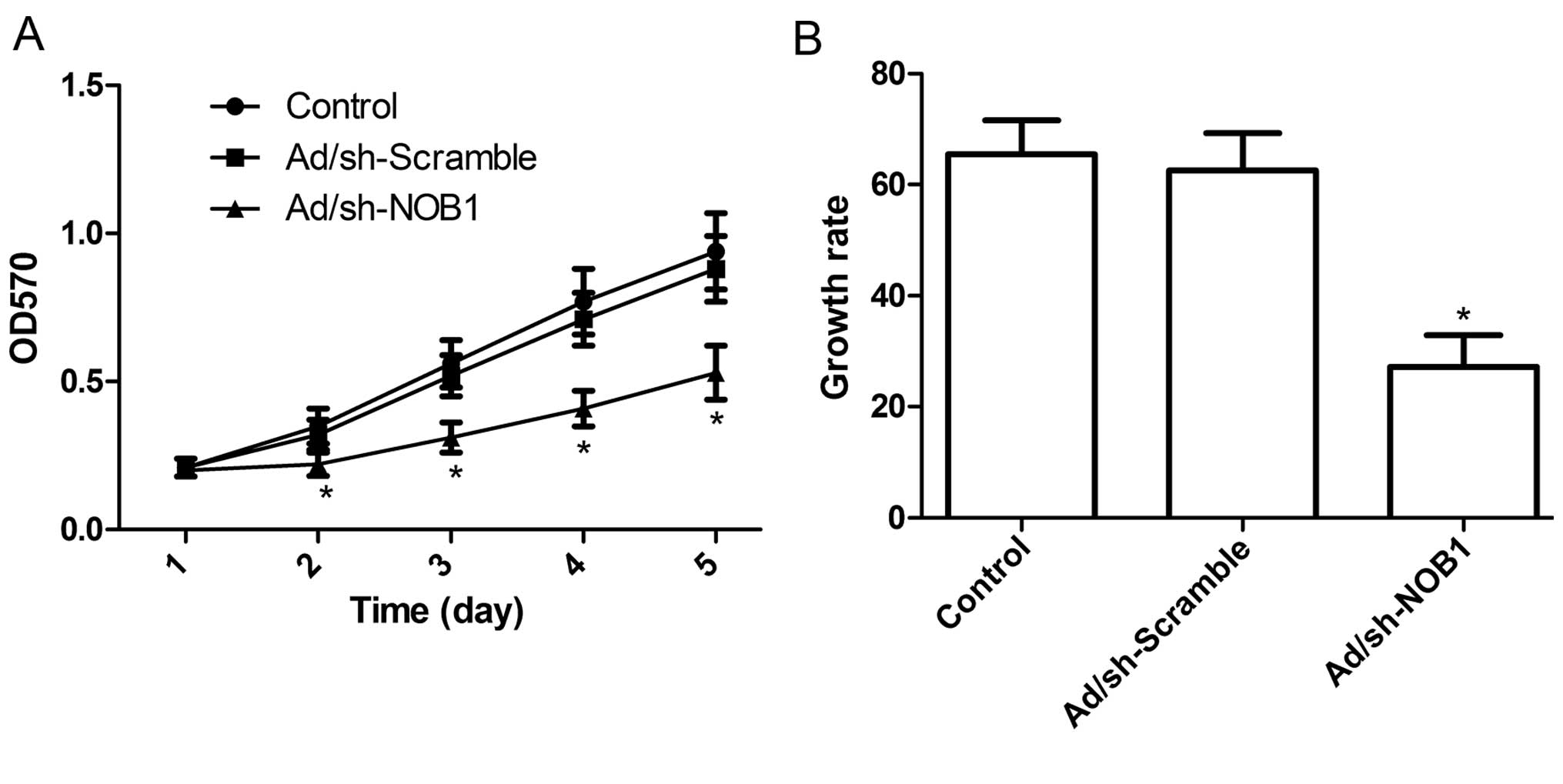
following adenoviral infection for 5 days. Fig. 2A shows that there was no
statistically significant difference in viability between the
non-infected cells and the cells infected with Ad/sh-Scramble,
indicating that the adenoviral system itself had no cytotoxic
effect on cells. In contrast, the viability of the TPC1 cells was
markedly inhibited following NOB1 knockdown (P<0.05 compared to
control), and the inhibitory effect of Ad/sh-NOB1 on cell
proliferation was observed beginning on day 2; it became more
obvious on days 4 and 5 (P<0.05; Fig. 2A). Moreover, BrdU incorporation
assays also revealed that the inhibition of NOB1 expression
significantly reduced the growth rate of TPC1 cells during the 48-h
incubation period (P<0.05; Fig.
2B). These findings suggest that the knockdown of NOB1 greatly
diminished the cell proliferative ability in the TPC1 cells.
Adenovirus-mediated siRNA targeting NOB1
induces cell G0/G1 phase and apoptosis
Knowing that the inhibition of NOB1 in TPC1 cells
markedly slows cell proliferation, we further employed cell-cycle
analysis to reveal the mechanism governing the inhibitory effect of
the downregulation of NOB1 on cell proliferation. As shown in
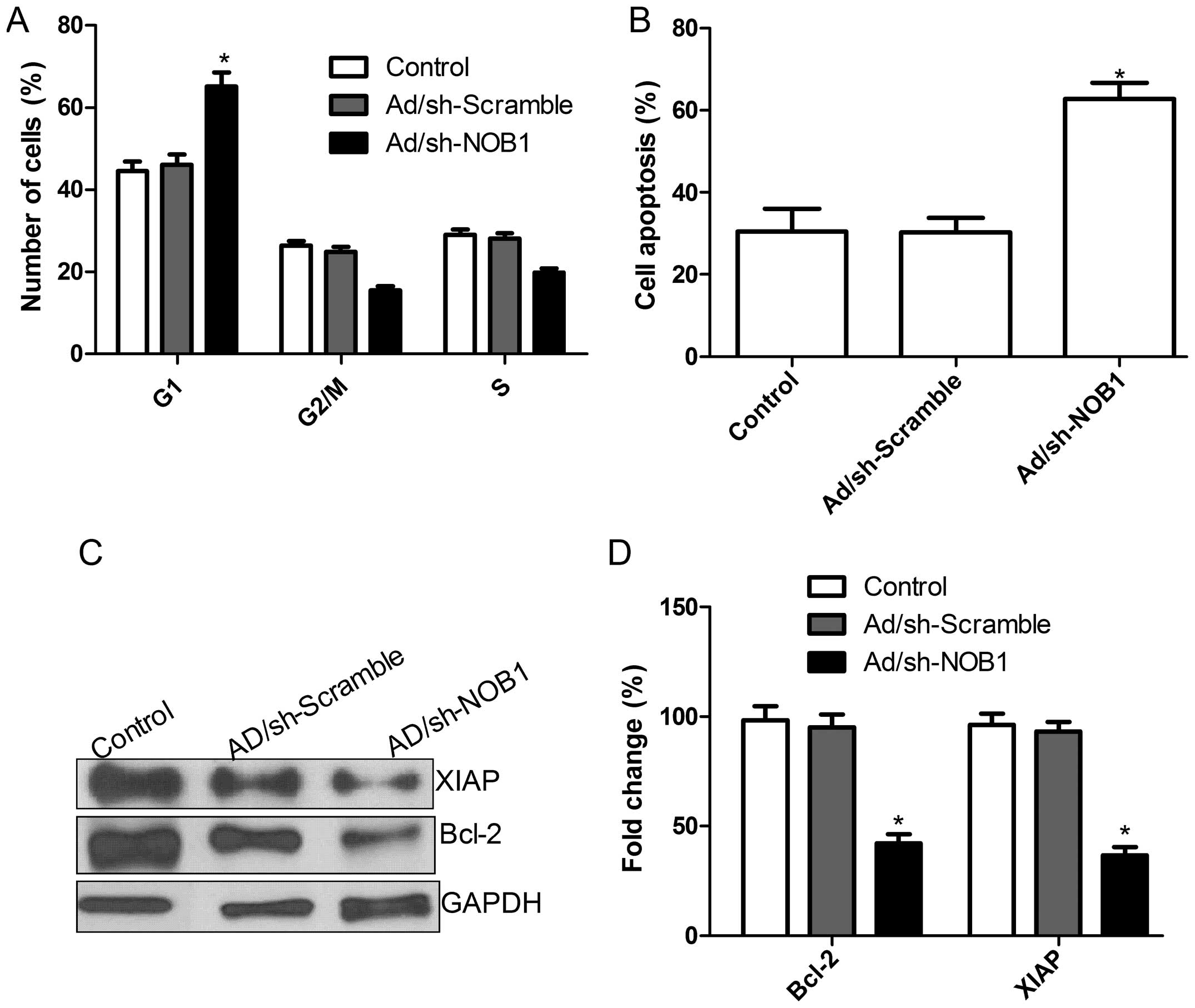
Fig. 3A, in TPC1 cells, an obvious
increase in the G1-phase cell population was observed in
the Ad/sh-NOB1 group as compared with this population in the
Ad/sh-Scramble and control groups (P<0.05). Our results suggest
that Ad/sh-NOB1 exerted an inhibitory effect on TPC1 cell
proliferation via G0/G1.
Next, to further investigate the effect of the
adenovirus-mediated siRNA targeting NOB1 on cell apoptosis in TPC1
cells, cell apoptosis was analyzed by TUNEL. Compared with the
control group and Ad/sh-Scramble group, the Ad/sh-NOB1 group
underwent significantly induced cell apoptosis (P<0.05; Fig. 3B). Next, we analyzed the effects of
adenovirus-mediated siRNA targeting NOB1 on the expression of other
apoptosis relevant proteins including Bcl-2 and XIAP. As shown in
Fig. 3C and D, the levels of Bcl-2
and XIAP protein expression in the cells infected with Ad/sh-NOB1
showed a significant decrease compared with the levels in the
control group and Ad/sh-Scramble cells (P<0.05; Fig. 3D). These results suggest that the
adenovirus-mediated siRNA targeting NOB1 can induce cell
G0/G1 arrest and apoptosis in TPC1 cells.
Adenovirus-mediated siRNA targeting NOB1
inhibits cell migration and invasion in TPC1 cells
To ascertain the inhibitory effect of the
adenovirus-mediated siRNA targeting NOB1 on thyroid cancer cell
motility in vitro, a wound-healing assay was performed to
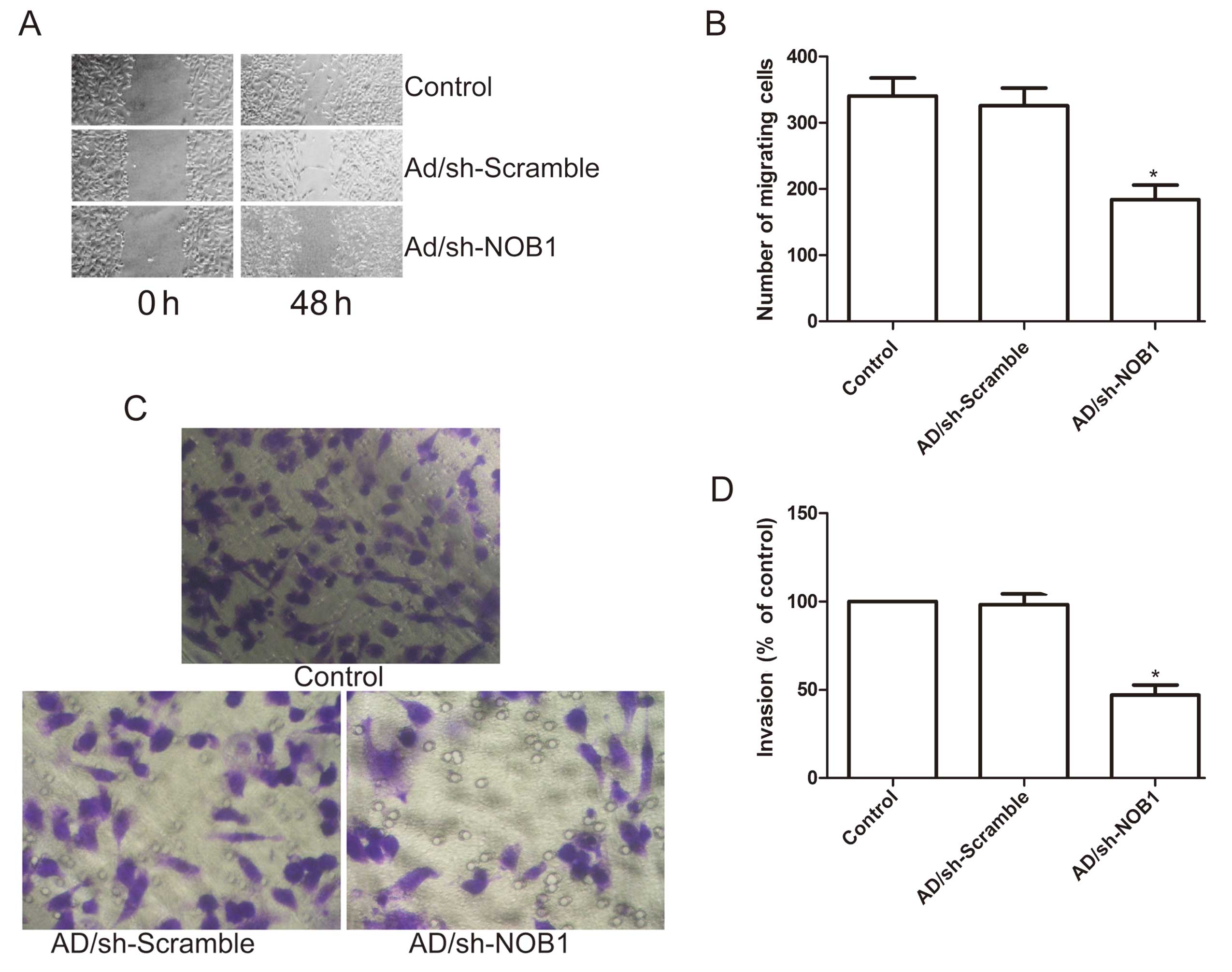
investigate the effects on the migration potential of PTC cells. A
scratch was introduced into confluent monolayers of the cells
expressing the different treatment plasmids, and the time-dependent
movement of cells into the injured area was monitored
microscopically. Cells in the control and Ad/sh-Scramble groups
began migrating 8 h after scratching. After 48 h, cells in the
Ad/sh-NOB1 group migrated significantly less rapidly than those in
the control and the Ad/sh-Scramble groups (P<0.05; Fig. 4A and B).
Next, the ability of adenovirus-mediated siRNA
targeting NOB1 to reduce the invasiveness of the PTC cells was
further investigated using the Transwell system assay. Invasion was
also decreased significantly in the Ad/sh-NOB1 treatment group
compared to the control and the Ad/sh-Scramble groups (P<0.05;
Fig. 4C and D).
Adenovirus-mediated siRNA targeting NOB1
activates the MAPK signaling pathway
To clarify the molecular mechanisms involved in the
inhibition of cell proliferation and survival of PTC cells
following downregulation of NOB1, in the present study, we
investigated the effects of adenovirus-mediated siRNA targeting
NOB1 on the activation of the MAPK pathway, which participates in
the main intracellular signaling required for cell proliferation
and survival. Measurements of the phosphorylation/activation
pattern of p38 MAPK, ERK1/2 and JNK were performed by western
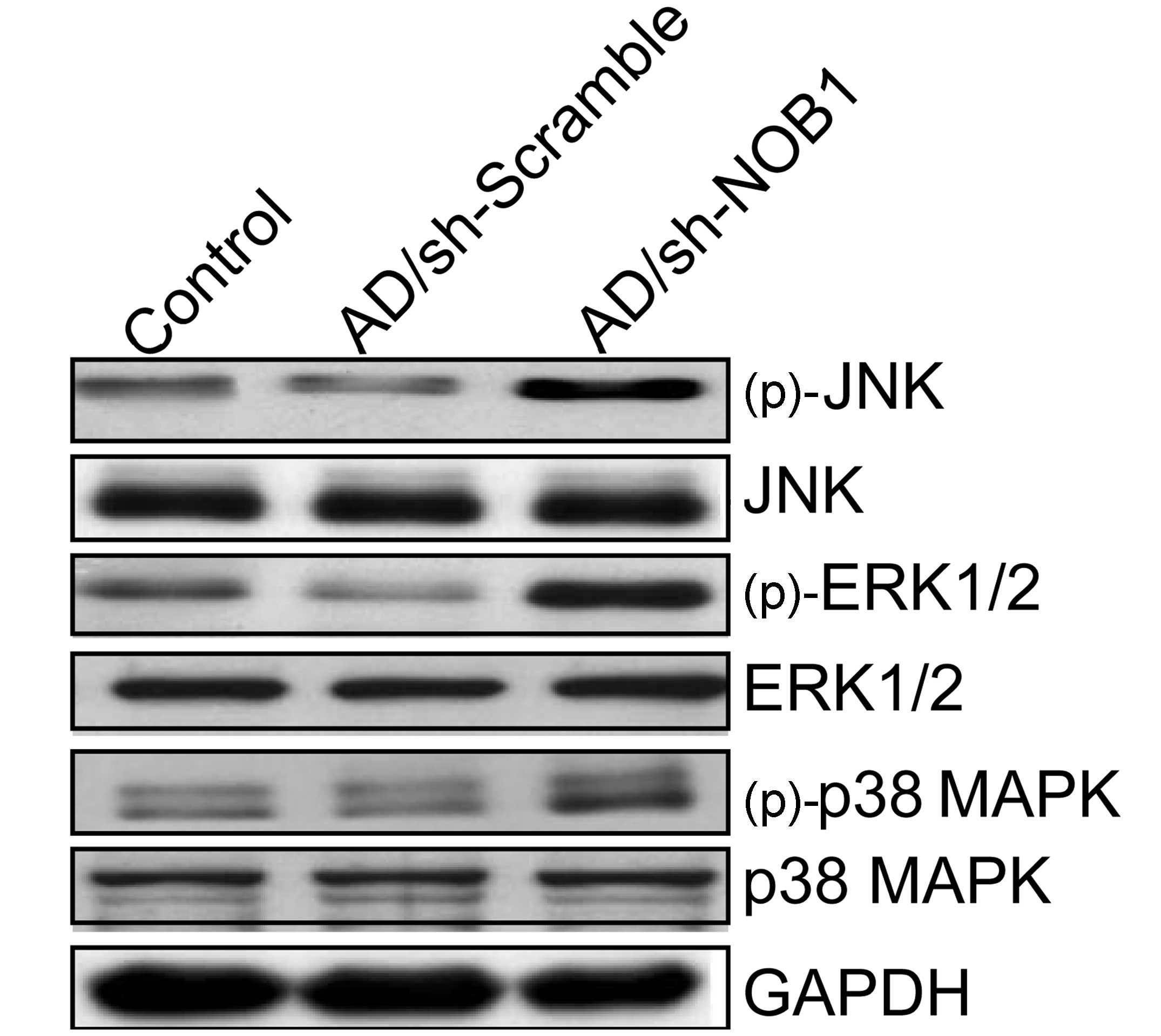
blotting. Our results showed that adenovirus-mediated siRNA
targeting NOB1 resulted in a marked increase in phosphorylated
(p)-p38 MAPK, (p)-ERK1/2 and (p)-JNK relative to the levels in the
control group and Ad/sh-Scramble group, without altering the total
protein levels of p38 MAPK, ERK1/2 and JNK in each group (Fig. 5). These results indicate that
adenovirus-mediated siRNA targeting NOB1 inhibits TPC1 cell
proliferation and induces apoptosis, to some extent, by activating
the MAPK signaling pathway.
Adenovirus-mediated siRNA targeting NOB1
enhances the radiosensitivity of TPC1 cells
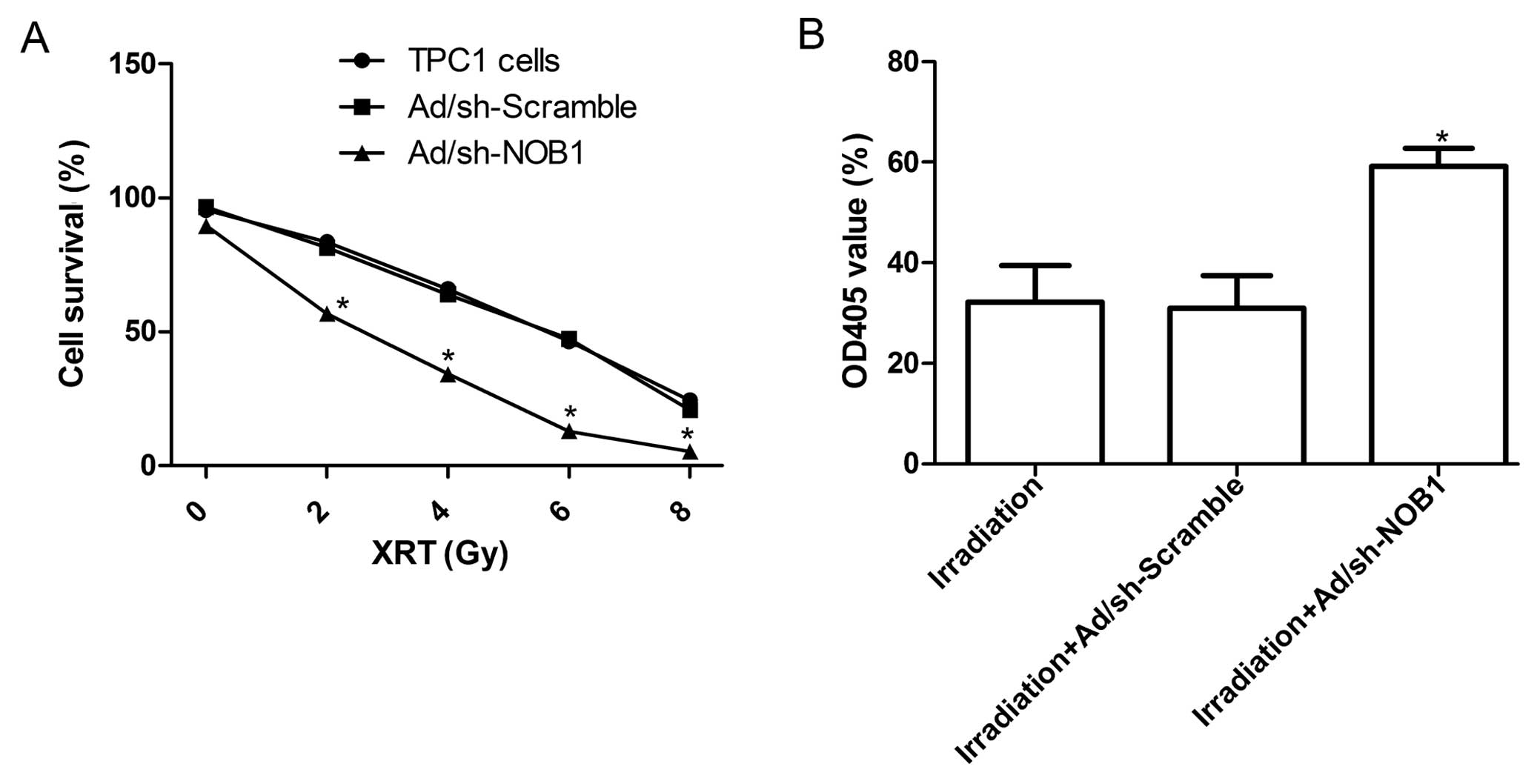
To explore the effect of adenovirus-mediated siRNA
targeting NOB1 on the radio-sensitivity of TPC1 cells, a clonogenic
cell survival assay was performed. As shown in Table I, the plating efficiencies of
Ad/sh-NOB1 cells at the same doses of radiation were significantly
decreased compared with those of the control cells and the
Ad/sh-Scramble group. Compared with the uninfected TPC1 cells, the
cell survival curve obviously decreased in the Ad/sh-NOB1 group
(P<0.05; Fig. 6A), whereas the
cell survival curve in the Ad/sh-Scramble group decreased but
without significance (Fig. 6A).
 | Table IPlating efficiencies at different
radiation doses. |
Table I
Plating efficiencies at different
radiation doses.
| Plating
efficiency |
|---|
|
|
|---|
| Cell | 0 Gy | 2 Gy | 4 Gy | 6 Gy | 8 Gy |
|---|
| TPC1 cell | 95.49±4.16 | 83.45±3.06 | 65.88±2.41 | 46.42±1.58 | 24.42±1.06 |
| Ad/sh-Scramble | 96.54±3.68 | 81.46±3.01 | 63.90±1.78 | 47.28±1.29 | 20.84±0.76 |
| Ad/sh-NOB1 | 89.58±3.38 | 56.66±1.84a | 34.21±1.76a | 12.89±0.58a | 5.34±0.41a |
To determine the activation of the caspase cascade
during the process of apoptosis, we detected the changes in
caspase-3 activity in the TPC1 cells treated with radiation alone,
radiation combination with Ad/sh-Scramble or radiation combined
with Ad/sh-NOB1. Results from the colorimetric assay showed that
the caspase-3 activity in TPC1 cells treated with radiation
combined with the Ad/sh-NOB1 was increased compared with the TPC1
cells treated with radiation alone or radiation combined with
Ad/sh-Scramble (Fig. 6B;
P<0.05). These results imply that the downregulation of NOB1
expression leads to radiosensitivity of thyroid cancer cells by
activating celluar caspase-3, but the exact mechanism needs to be
further investigated.
Adenovirus-mediated siRNA targeting NOB1
inhibits tumor growth and enhances the radiosensitivity of TPC1
cells in vivo
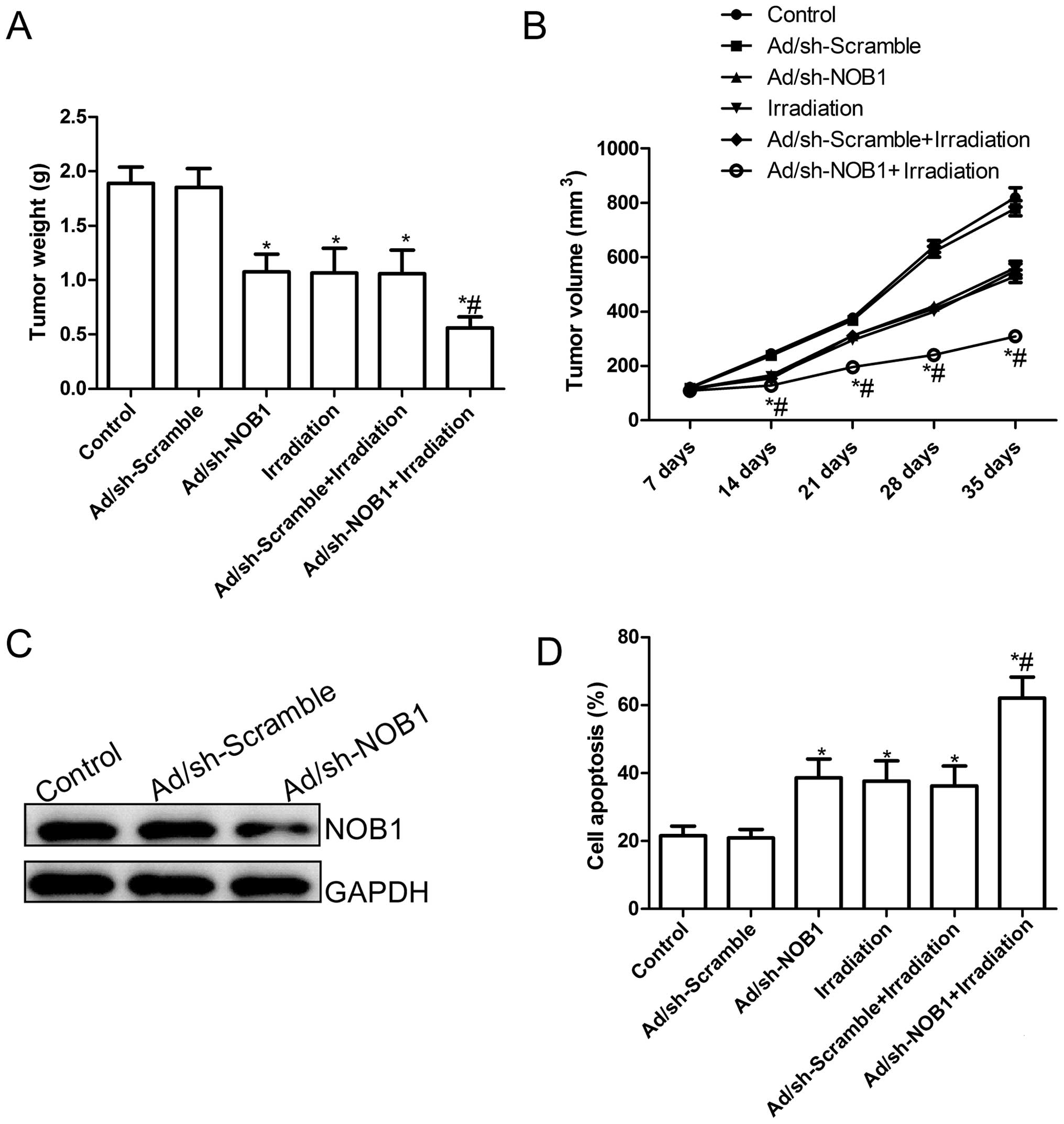
Finally, we assessed the in vivo therapeutic
efficacy of adenovirus-mediated siRNA targeting NOB1 on female BALB
mice bearing TPC1 cell tumors. Tumor growth was monitored for 36
days. On day 36, the animals were sacrificed, and then tumors were
excised, weighed and measured. Tumor weights were significantly
lower in the various treatment groups than those in the control
group (PBS group) and Ad/sh-Scramble group (P<0.05; Fig. 7A). Compared to the other groups, the
Ad/sh-NOB1 in combination with irradiation group showed a maximally
reduced weight. In addition, tumor volume was also determined at
different times. The tumor volumes in the various treatment groups
were significantly (P<0.05) diminished when compared with the
Ad/sh-Scramble group and control group (PBS group) at different
times (P<0.05; Fig. 7B).
Importantly, Ad/sh-NOB1 combined with irradiation led to a
significant inhibition of tumor volume compared with the other
treatment groups (P<0.05; Fig.
7B). At the same times, we also examined the expression of NOB1
in grafted tumor tissues by western blot analysis. As shown in
Fig. 7C, the expression of NOB1
protein in the tumors in the Ad/sh-NOB1-treated group was
significantly decreased compared with the PBS group or
Ad/sh-Scramble group. Finally, we also determined the synergistic
effects on tumor tissue cell apoptosis in vivo by TUNEL. The
results showed that Ad/sh-NOB1 and irradiation alone or the
combination treatment significantly induced cell apoptosis compared
to that noted in the control group and the Ad/sh-Scramble groups
(P<0.05; Fig. 7D). The
combination of Ad/sh-NOB1 and irradiation greatly induced tumor
cell apoptosis in vivo compared to the single treatment
groups (P<0.05; Fig. 7D). These
experimental data suggest that adenovirus-mediated siRNA targeting
NOB1 could increase the in vivo radiosensitivity of PTC
cells, which might imply that Ad/sh-NOB1 combined with radiotherapy
leads to a stronger antitumor effect on human PTC.
Discussion
The majority of patients with papillary thyroid
cancer (PTC) have a favorable prognosis, yet a subgroup of patients
suffer from recurrent disease that is refractory to surgical
resection and radioactive iodine ablation (6). Unfortunately, no effective systemic
treatment exists for these patients. The conventional chemotherapy
and radiotherapy regimens are more of a hindrance due to their
limited efficacy, significant toxicity, the development of
resistance and high relapse rates (30). Newer therapeutic approaches to solve
this issue are required. Therefore, further studies investigating
multiple treatment modalities and/or the inhibition of multiple
signaling pathways are required in our efforts to develop a novel
therapeutic strategy to enhance both systemic chemotherapy and
radiotherapy.
NOB1, identified as an interacting partner with
RPN12p by a yeast two-hybrid screening, is a nuclear protein that
forms a complex with the 19S regulatory particle of the 26S
proteasome, and takes part in processing the 20S pre-rRNA to the
mature 18S rRNA (31). It has been
shown that the 26S proteasome catalyzes protein degradation via the
ubiquitin-proteasome system (UPS), which is required for the
degradation of cyclic proteins and regulates multiple aspects of
the cell cycle progression in eukaryotes (32,33).
UPS plays a critical role in cancer development and progression. In
light of the important role of NOB1 and the critical function of
ubiquitin-dependent proteolysis in universal biological processes,
recently, several studies have focused on the role of NOB1 in tumor
development and progression (21–27).
For example, Li et al showed that downregulation of NOB1
expression using an RNA silencing approach in A549 tumor cells
significantly suppressed the proliferation and colony formation
ability, and induced tumor apoptosis in vitro, and
suppressed tumor growth in vivo (24). Lin et al demonstrated that
RNA interference (RNAi)-mediated downregulation of NOB1 expression
markedly reduced the proliferative and colony-formation ability of
ovarian cancer cells (21). Wang
et al found that the expression level of the NOB1 protein
was significantly higher in high-grade gliomas than in low-grade
gliomas, and that knockdown of the NOB1 gene resulted in
suppression of the proliferation and the colony forming abilities
of U251 and U87-MG cells, cell cycle arrest during the
G0/G1 phase, and a significant enhancement of
cell apoptosis (34). Consistent
with these results, our results showed that downregulation of NOB1
expression using adenovirus-mediated siRNA targeting NOB1 in TPC-1
cells significantly inhibited cell proliferation, migration and
invasion and induced cell apoptosis in vitro, as well as
suppressed tumor growth in a mouse model. These studies and our
results suggest that NOB1 plays critical roles in tumor progression
and development.
The MAPKs are serine/threonine protein kinases that
are involved in intracellular signaling during proliferation,
differentiation, cellular stress responses and apoptosis (35). MAPK signaling is mediated by
extracellular signal regulated kinase 1 and 2 (ERK1/2), p38 MAPK,
and the stress activated protein kinase (SAPK)/c-Jun NH2-terminal
kinase (JNK), which are important in the control of cell
proliferation, differentiation and apoptosis (36). The activation of the MAPK pathway
has been implicated in the activity of numerous chemotherapy and
genotoxic drugs (37). In addition,
activation of the MAPK pathway plays an important role in
radiotherapy by mediation or regulation of cellular responses such
as proliferation, migration and even transformation/carcinogenesis
(38). Importantly, it was recently
shown that silencing of NOB1 expression increased the
phosphorylation of ERK1/2, JNK and p38 MAPK proteins, and that the
anti-glioma effect of NOB1 might be mediated by MAPK activation
(39). However, Chen et al
found that NOB1 expression in prostate cancer was independently
associated with p38 MAPK activation, and that p38 MAPK expression
was completely suppressed by NOB1 interference in the prostate
cancer cell lines DU-145 and PC-3 (22). These controversial findings were
found since the role of MAPKs in certain functions is controversial
and complicated, and depends on the stimuli, intensity, and
duration, as well as cell type (40). In the present study, our results
showed that treatment with an adenovirus-mediated siRNA targeting
NOB1 resulted in a marked increase in phosphorylated (p)-p38 MAPK,
ERK1/2 and JNK relative to the control group and Ad/sh-Scramble
group, without altering the total protein levels of p38 MAPK,
ERK1/2 and JNK in each group, suggesting that activation of the
MAPK pathway may play an important role in PTC cell proliferation,
apoptosis and migration. In addition, our experimental data showed
that adenovirus-mediated siRNA targeting NOB1 could increase the
in vitro and in vivo radiosensitivity of PTC cells.
In light of the important role of the MAPK signaling pathway and
the critical function of radiotherapy, we assume that
downregulation of NOB1 may influence the radiosensitivity of PTC
cells through activation of the MAPK pathway.
In conclusion, we demonstrated for the first time
that an adenovirus-mediated siRNA targeting NOB1 in human PTC cells
inhibits cell proliferation, migration and invasion in
vitro, and suppresses tumor growth in a mouse model, as well as
it enhances in vitro and in vivo radiosensitivity of
PTC cells. Moreover, our results also showed that downregulation of
NOB1 was able to significantly activate the phosphorylation of p38
MAPK, which might contribute to the inhibition of papillary PTC
cell growth. These results suggest that NOB1 appears to have
therapeutic potential for the treatment of PTC.
Acknowledgements
This research was supported by the Science and
Technology Research and Innovation Team funded by Jilin Province
(JL20130518).
References
|
1
|
Brown RL, de Souza JA and Cohen EE:
Thyroid cancer: burden of illness and management of disease. J
Cancer. 2:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson L: World Health Organization
classification of tumours: pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
3
|
Nikiforova MN and Nikiforov YE: Molecular
genetics of thyroid cancer: implications for diagnosis, treatment
and prognosis. Expert Rev Mol Diagn. 8:83–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dal Maso L, Bosetti C, La Vecchia C and
Franceschi S: Risk factors for thyroid cancer: an epidemiological
review focused on nutritional factors. Cancer Causes Control.
20:75–86. 2009.PubMed/NCBI
|
|
5
|
Jeong SY, Kim HW, Lee SW, Ahn BC and Lee
J: Salivary gland function 5 years after radioactive iodine
ablation in patients with differentiated thyroid cancer: direct
comparison of pre- and postablation scintigraphies and their
relation to xerostomia symptoms. Thyroid. 23:609–616. 2013.
|
|
6
|
Matuszczyk A, Petersenn S, Bockisch A, et
al: Chemotherapy with doxorubicin in progressive medullary and
thyroid carcinoma of the follicular epithelium. Horm Metab Res.
40:210–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strasser JF, Raben A and Koprowski C: The
role of radiation therapy in the management of thyroid cancer. Surg
Oncol Clin N Am. 17:219–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Ni J, Zhou G, et al: Cloning,
expression and characterization of the human NOB1 gene. Mol Biol
Rep. 32:185–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makarova KS, Aravind L and Galperin MY:
Comparative genomics of the Archaea (Euryarchaeota): evolution of
conserved protein families, the stable core, and the variable
shell. Genome Res. 9:608–628. 1999.PubMed/NCBI
|
|
10
|
Arcus VL, Backbro K, Roos A, Daniel EL and
Baker EN: Distant structural homology leads to the functional
characterization of an archaeal PIN domain as an exonuclease. J
Biol Chem. 279:16471–16478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamanna AC and Karbstein K: Nob1 binds the
signal-stranded cleavege site D at the 3′-end of 18S rRNA with its
PIN domain. Proc Natl Acad Sci USA. 106:14259–14264.
2009.PubMed/NCBI
|
|
12
|
Fatica A, Tollervey D and Dlakic M: PIN
domain of Nob1p is required for D-site cleavage in 20S pre-rRNA.
RNA. 10:1698–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fatica A, Oeffinger M, Dlakic M and
Tollervey D: Nob1p is required for cleavage of the 3′ end of 18S
rRNA. Mol Cell Biol. 23:1798–1807. 2003.
|
|
14
|
Veith T, Martin R, Wurm JP, et al:
Structural and functional analysis of the archaeal endonuclease
Nob1. Nucleic Acids Res. 40:3259–3274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nazar RN: Ribosomal RNA processing and
ribosome biogenesis in eukaryotes. IUBMB Life. 56:457–465. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montanaro L, Trere D and Derenzini M:
Changes in ribosome biogenesis may induce cancer by down-regulating
the cell tumor suppressor potential. Biochim Biophys Acta.
1825:101–110. 2012.PubMed/NCBI
|
|
17
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar
|
|
18
|
Ferrell K, Wilkinson CR, Dubiel W and
Gordon C: Regulatory subunit interactions of the 26S proteasome, a
complex problem. Trends Biochem Sci. 25:83–88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Micel LN, Tentler JJ, Smith PG and
Eckhardt GS: Role of ubiquitin ligases and the proteasome in
oncogenesis: novel targets for anticancer therapies. J Clin Oncol.
31:1231–1238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mani A and Gelmann EP: The
ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol.
23:4776–4789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Y, Peng S, Yu H, Teng H and Cui M:
RNAi-mediated down-regulation of NOB1 suppresses the growth and
colony-formation ability of human ovarian cancer cells. Med Oncol.
29:311–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Che JP, Li W, Yan Y, et al: Expression and
clinical significance of the nin one binding protein and p38 MAPK
in prostate carcinoma. Int J Clin Exp Pathol. 6:2300–2311.
2013.PubMed/NCBI
|
|
23
|
Li XY, Luo QF, Li J, et al: Clinical
significance of NOB1 expression in breast infiltrating ductal
carcinoma. Int J Clin Exp Pathol. 6:2137–2144. 2013.PubMed/NCBI
|
|
24
|
Li Y, Ma C, Qian M, Wen Z, Jing H and Qian
D: Downregulation of NOB1 suppresses the proliferation and tumor
growth of non-small cell lung cancer in vitro and in
vivo. Oncol Rep. 31:1271–1276. 2014.PubMed/NCBI
|
|
25
|
Lin S, Meng W, Zhang W, et al: Expression
of the NOB1 gene and its clinical significance in papillary thyroid
carcinoma. J Int Med Res. 41:568–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang WY, Chen DH and Ning L: siRNA
mediated silencing of NIN1/RPN12 binding protein 1 homolog inhibits
proliferation and growth of breast cancer cells. Asian Pac J Cancer
Prev. 13:1823–1827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Z, Guo Q, Shi A, Xie F and Lu Q: Down
regulation of NIN/RPN12 binding protein inhibits the growth of
human hepatocellular carcinoma cells. Mol Biol Rep. 39:501–507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guoan X, Hanning W, Kaiyun C and Hao L:
Adenovirus-mediated siRNA targeting Mcl-1 gene increases
radiosensitivity of pancreatic carcinoma cells in vitro and in
vivo. Surgery. 147:553–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Sun M, Zhang A, Lv C, De W and
Wang Z: Adenovirus-mediated siRNA targeting Bcl-xL inhibits
proliferation, reduces invasion and enhances radiosensitivity of
human colorectal cancer cells. World J Surg Oncol. 9:1172011.
View Article : Google Scholar
|
|
30
|
Lin CI, Whang EE, Donner DB, et al:
Galectin-3 targeted therapy with a small molecule inhibitor
activates apoptosis and enhances both chemosensitivity and
radiosensitivity in papillary thyroid cancer. Mol Cancer Res.
7:1655–1662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tone Y, Tanahashi N, Tanaka K, Fujimuro M,
Yokosawa H and Toh-e A: Nob1p, a new essential protein, associates
with the 26S proteasome of growing saccharomyces cerevisiae cells.
Gene. 243:37–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu G, Bernaudo S, Fu G, Lee DY, Yang BB
and Peng C: Cyclin G2 is degraded through the ubiquitin-proteasome
pathway and mediates the antiproliferative effect of activin
receptor-like kinase 7. Mol Biol Cell. 19:4968–4979. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fasanaro P, Capogrossi MC and Martelli F:
Regulation of the endothelial cell cycle by the
ubiquitin-proteasome system. Cardiovasc Res. 85:272–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Li P and Zhao B: Knockdown of NOB1
expression by RNAi inhibits cellular proliferation and migration in
human gliomas. Gene. 528:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yacoub A, Mitchell C, Lebedeva IV, et al:
mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of
glioma cells in vitro via JNK signaling. Cancer Biol Ther.
2:347–353. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sofia Vala I, Martins LR, Imaizumi N, et
al: Low doses of ionizing radiation promote tumor growth and
metastasis by enhancing angiogenesis. PLoS One.
5:e112222010.PubMed/NCBI
|
|
39
|
Zhou J, Xu T, Yan Y, et al: MicroRNA-326
functions as a tumor suppressor in glioma by targeting the Nin one
binding protein (NOB1). PLoS One. 8:e684692013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|