Introduction
Melanoma is the most deadly form of skin cancer
arising from the melanocytes in the skin epidermis. It is
characterized by rapid tumor progression, metastasis and a poor
prognosis (1). Although melanoma
accounts for only 4% of all dermatological cancers, it is
responsible for 80% of deaths from skin cancer; less than 10% of
patients with metastatic melanoma survive for 5 years (1,2). The
discovery and application of biomarkers, in conjunction with
traditional cancer diagnosis, staging and prognosis, could be
useful in improving early diagnosis, screening and subsequent
management and treatment of melanoma patients (3–5).
However, at present, reliable markers are still lacking and the
prognosis of melanoma patients remains poor. Therefore, a better
understanding of the regulating factors contributing to melanoma
initiation, progression and metastasis is needed.
The Sox gene family was first identified by
homology to the high mobility group (HMG) box of the
sex-determining gene SRY (6). There are at least 30 members in the
Sox family, which are expressed in various types of cells and
tissues at different developmental stages (7). Sox17 encodes an HMG box transcription
factor and has been demonstrated to play critical roles in the
regulation of vascular development (8), differentiation of embryonic stem cells
(9), hematopoietic development
(10) and tumor progression
(11,12).
Accumulating evidence indicates that activation of
Wnt/β-catenin signaling is one of the direct causes of tumor
development (13–15). The nuclear accumulation of
β-catenin, a hallmark of Wnt activation, is particularly enhanced
in the invasive front of tumors and metastasized colon cancer
cells, suggesting that the activation of Wnt/β-catenin signaling is
important for colon cancer progression (16). Notably, some research has
demonstrated that Sox17 inhibits the canonical Wnt/β-catenin
signaling in malignant tumors (10,11,17).
Recombinant Sox17 not only promoted the level of the Wnt antagonist
SFRP1, but also decreased the expression level of Wnt/Frizzled and
endogenous β-catenin in HOG cells, leading to cell cycle exit and
differentiation (10). On the other
hand, Sox17 expression was found to be downregulated in colon
cancer cells and Sox17 overexpression inhibited colon cancer cell
proliferation and reduced the efficiency of colony formation
(18). Other research has
illustrated that Sox17 protects benign gastrointestinal tumors from
malignant progression at an early stage of tumorigenesis and
downregulation of Sox17 contributes to malignant progression
through promotion of Wnt activity (19). These results suggest that Sox17
plays a tumor suppressor role in gastric and colorectal cancer
development. However, to our knowledge, there is no report on the
influence of Sox17 involvement in the biological regulation of
human melanoma. Here, we investigated Sox17 expression in a large
set of melanocytic lesions at different stages by tissue
microarray, investigated the correlation of Sox17 expression to
melanoma progression and determined its prognostic value in
patients with melanoma.
The cyclin-dependent kinase (CDK) inhibitor p27,
also known as Kip1, is an atypical tumor suppressor that regulates
G0-S phase transitions and plays important roles in cell
proliferation, motility and apoptosis (20). Reduced nuclear p27 expression is
associated with a poor prognosis in several types of cancers
(20,21). Our previous study also demonstrated
that p27 expression is inversely associated with the survival of
melanoma patients (22).
Sox17-siRNA transfection of gastric cancer MKN45 cells led to
observably increased expression of cyclin D1 while p27 expression
was markedly decreased (12).
Furthermore, p27 expression was found to be significantly induced
in liver-specific β-catenin knockdown mice (23). Based on these findings, we further
examined the correlation between Sox17 and p27 expression in
melanoma biopsies and analyzed the combined effect of Sox17 and p27
expression in predicting patient outcome.
Materials and methods
Ethics statement
The use of human skin tissues in this study was
approved by the Clinical Research Ethics Board of the University of
British Columbia (24,25). The study was conducted according to
the principles expressed in the Declaration of Helsinki.
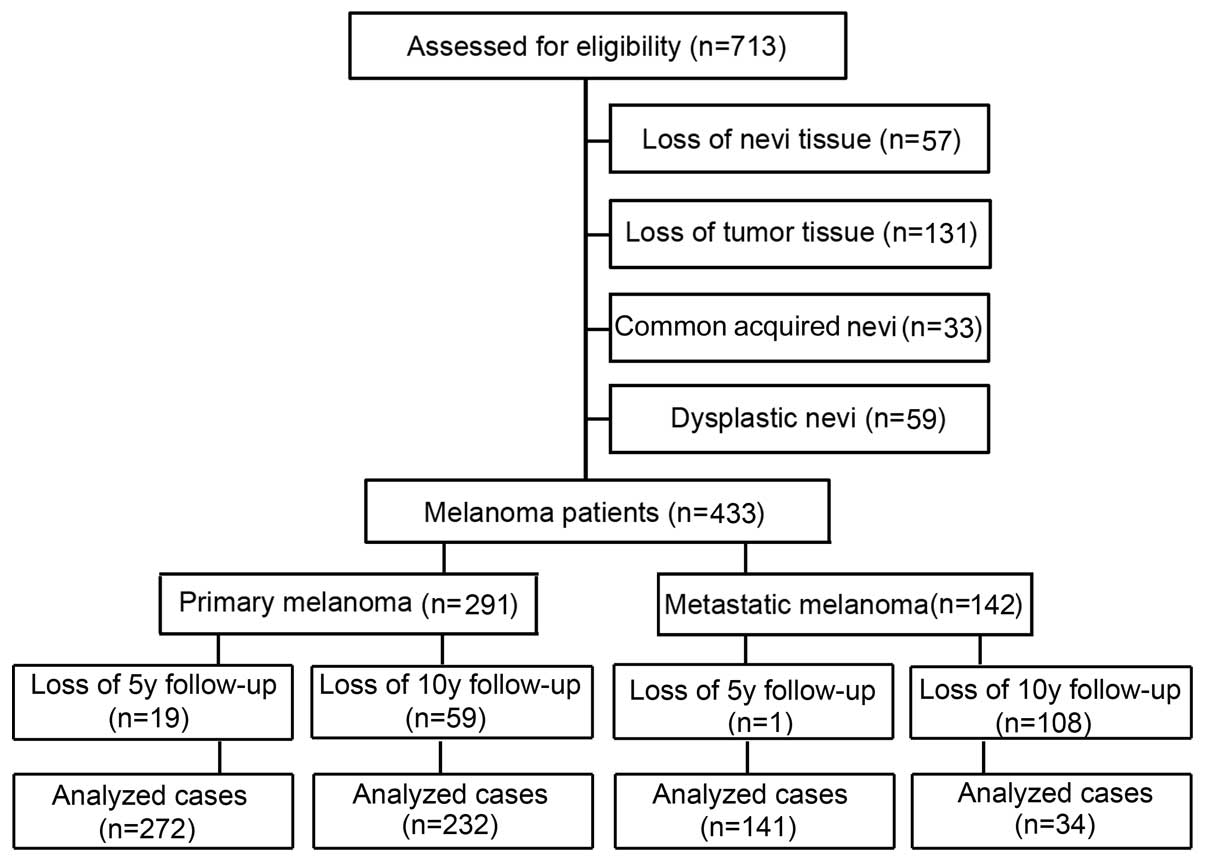
Tissue microarray (TMA) construction
The collection of melanoma patient specimens and the
construction of the TMA have been previously described (24,25).
Briefly, formalin-fixed, paraffin-embedded tissues from 49 common
acquired nevi, 100 dysplastic nevi, 402 primary melanomas and 162
metastatic melanomas were used for this TMA construction. All
specimens were obtained from the 1990 to 2009 archives of the
Department of Pathology, Vancouver General Hospital. The most
representative tumor area was carefully selected and marked on the
hematoxylin and eosin stained slides, and the TMAs were assembled
using a tissue-array instrument (Beecher Instruments, Silver
Spring, MD, USA). Due to loss of biopsy cores or insufficient tumor
cells present in the cores, 33 common acquired nevi (CAN) which are
prototypical benign melanocytic nevi, 59 dysplastic nevi, 291
primary melanomas, and 142 metastatic melanomas were able to be
evaluated for Sox17 staining (Fig.
1).
Immunohistochemistry of TMA
Immunohistochemistry was performed as described
previously (24,25). TMA slides were dewaxed at 55°C for
30 min and then washed with xylene. Tissues were rehydrated by a
series of washes in 100, 95 and 80% ethanol, followed by two washes
in distilled water. Antigen retrieval was performed by heating the
samples at 95°C for 30 min in 10 mmol/l sodium citrate (pH 6.0).
After inactivating the endogenous peroxidase by incubation in 3%
H2O2 for 30 min and blocking with universal
blocking serum for 30 min, the slides were incubated with a primary
anti-Sox17 antibody (1:100; EMD Millipore Corporation, Billerica,
MA, USA) at 4°C overnight. Negative controls were constructed by
omitting the Sox17 antibody during the primary antibody incubation.
The slides were then incubated with biotin-labeled secondary
antibody and streptavidin-peroxidase for 30 min each, followed by
developing with a diaminobenzidine substrate kit (DAB; Dako,
Glostrup, Denmark) and counterstaining with hematoxylin.
Evaluation of immunostaining
Positive Sox17 immunostaining was identified as a
brown nuclear color and graded according to both intensity and
percentage of cells with positive staining to eliminate the
heterogeneous staining pattern. The evaluation of Sox17 staining
was carried out blindly by microscopic examination of the tissue
sections by two observers (including one pathologist). Sox17
staining intensity was scored as 0, 1+, 2+ and 3+. The percentage
of Sox17-positive cells was also scored into 4 categories: 1
(0–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). On the basis of
the immunoreactive score, the staining pattern was defined as:
negative (0), weak (1–4), moderate (6–8) and
strong (9–12). The optimal cutoff point for staining
score was calculated using MedCalc software for Windows, version
12.5 (MedCalc Software, Ostend, Belgium). The best area under the
ROC curves (AUC) was used to determine the optimal cutoff point of
staining. Based on the optimal value of the cutoff points for the
Sox17 scores that was identified as 4, we grouped negative and weak
staining as low expression and moderate and strong staining as high
expression. The correlation between Sox17 and p27 expression was
studied in 374 melanoma samples which were common to the present
study and the previously published study on the prognostic
significance of p27 expression in melanoma (22).
Statistical analysis
Differences in the patient demographic and clinical
characteristics and Sox17 expression were evaluated by
Kruskal-Wallis test and Chi-square (χ2) test between
patient subgroups. Survival time was calculated from the date of
melanoma diagnosis to the date of death or last follow-up. The
effect of Sox17 expression on overall and disease-specific survival
was evaluated by Kaplan-Meier analysis and log-rank test.
Univariate and multivariate Cox proportional hazard regression
models were performed to estimate the hazard ratios (HRs) or
adjusted HRs and their 95% confidential intervals (CIs). A P-value
of <0.05 was considered to indicate a statistically significant
result. SPSS version 16 (SPSS Inc., Chicago, IL, USA) software was
used for all analyses.
Results
Sox17 expression is inversely correlated
with melanoma progression
Sox17 staining was stronger in common acquired nevi
and dysplastic nevi biopsies than that in the primary and
metastatic melanoma cases (Fig.
2A). Kruskal-Wallis test on the Sox17 scoring pattern in the
patient samples revealed that Sox17 expression was significantly
decreased from common acquired nevi (mean 8.3) and dysplastic nevi
(mean 7.9) to primary melanoma (mean 5.8) and to metastatic
melanoma (mean 3.6) (n=551; P<0.0001; Fig. 2B). Furthermore, the Chi-square test
revealed that the percentage of high Sox17 staining was
significantly reduced in primary melanoma (60%) and metastatic
melanoma (28%) compared to the common acquired nevi (88%) and
dysplastic nevi (85%) (n=551; P=2.4×10−17; Fig. 2C).
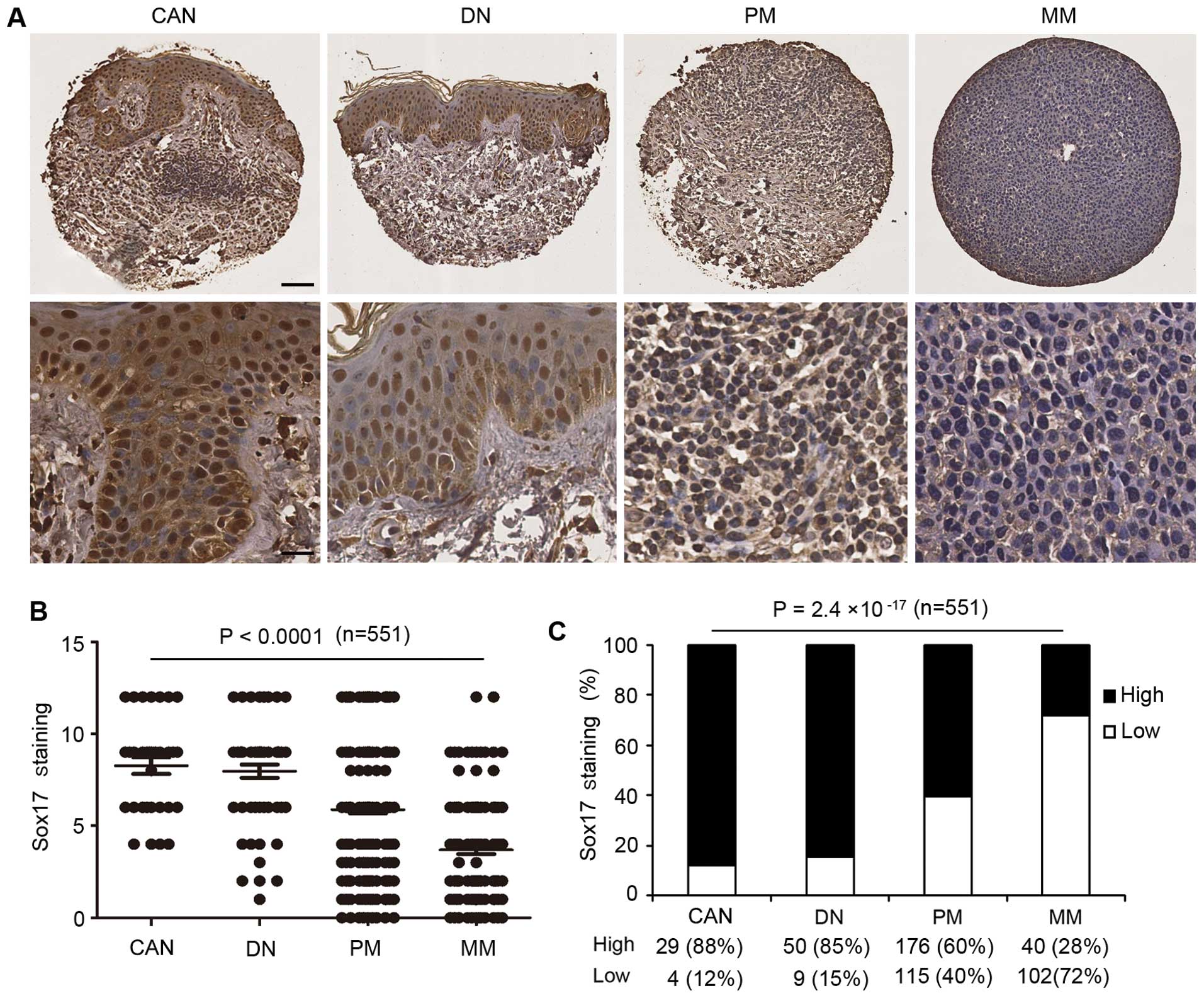 | Figure 2Reduced Sox17 expression inversely
correlates with melanoma progression. (A) Representative images of
CAN with strong Sox17 staining, DN with moderate Sox17 staining, PM
with weak Sox17 staining and MM with negative Sox17 staining (upper
panel: scale bar, 40 μm; lower panel: scale bar, 20 μm). (B)
Kruskal-Wallis test for differences in Sox17 staining among CAN,
DN, PM and MM. The mean is depicted as a horizontal line in each
group (n=525; P<0.0001). (C) High Sox17 staining was decreased
from CAN to DN, PM and MM (n=525; P=2.4×10−17,
χ2 test). Sox17, SRY-box containing gene 17; CAN, common
acquired nevi; DN, dysplastic nevi, PM, primary melanoma; MM,
metastatic melanoma. |
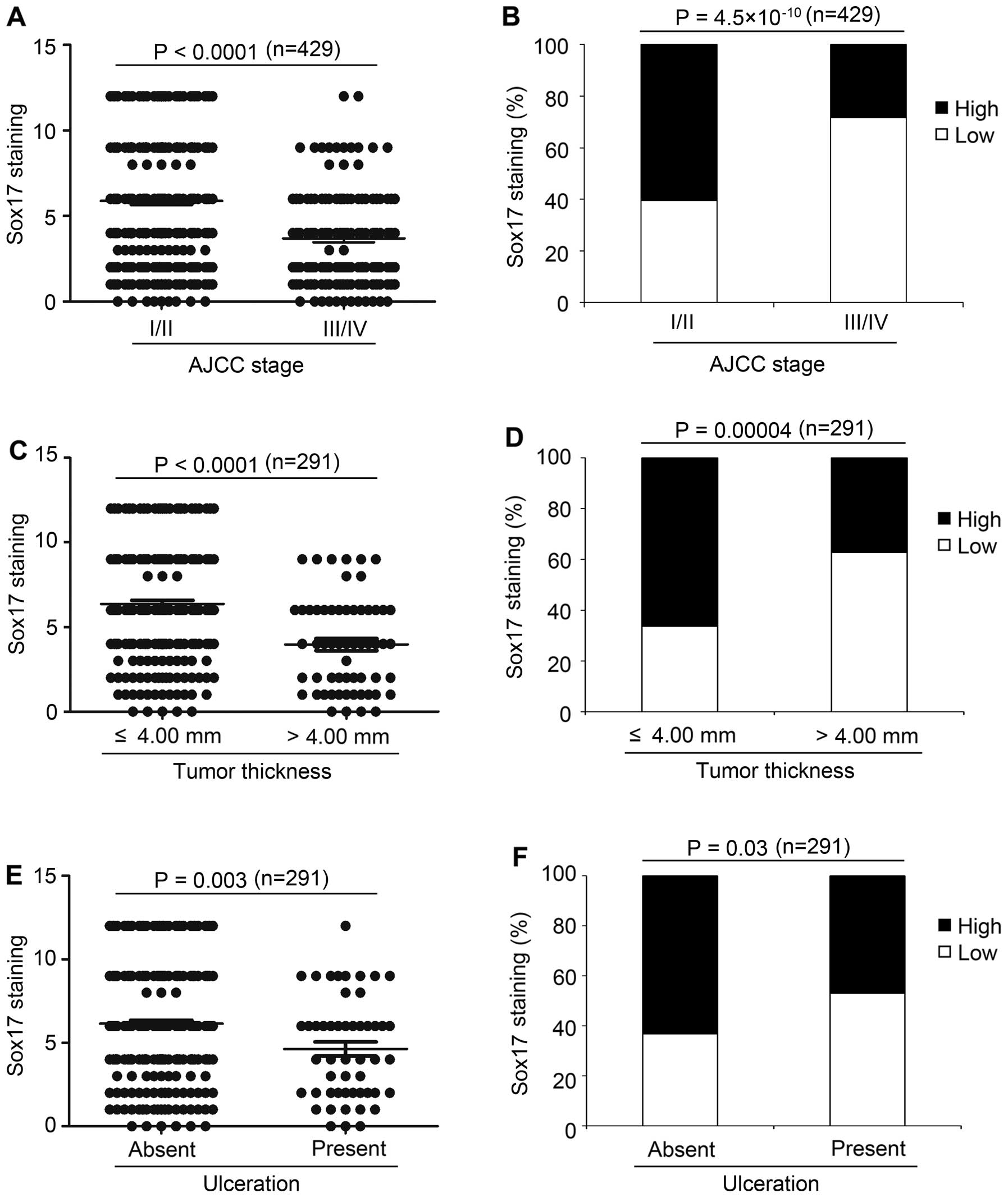
Sox17 expression inversely correlates
with American Joint Committee on Cancer (AJCC) stage, tumor
thickness and ulceration
The Kruskal-Wallis test on the Sox17 scoring pattern
in the melanoma samples revealed that Sox17 expression was
significantly decreased from early stage (AJCC I and II; mean 5.9)
to advanced stage (AJCC III and IV; mean 3.7) melanoma
(P<0.0001, Fig. 3A). As shown in
Fig. 3B and Table I, high expression of Sox17 was
detected in 61% of melanomas at AJCC stage I and II compared to 28%
of melanomas at AJCC III and IV (P=4.5×10−10),
indicating that decreased Sox17 expression may play an important
role in primary to metastatic melanoma transition. Next, we found
that in primary melanoma, Sox17 expression was decreased in tumors
with thickness >4.00 mm (mean 3.9), compared to melanomas with
thickness ≤4.00 mm (mean 6.3) (P<0.0001, Fig. 3C). Moreover, high Sox17 expression
was found in 66% of melanomas with thickness ≤4.00 mm, compared to
37% of tumors with thickness >4.00 mm (P=0.00004; Fig. 3D and Table I). Furthermore, Sox17 expression
decreased in the melanomas with ulceration (mean 4.6), compared to
the melanomas with no ulceration (mean 6.1) (P=0.003; Fig. 3E), which is consistent with the
Chi-square test results that high Sox17 expression was found in 47%
of melanomas with ulceration, compared to 63% of melanomas with no
ulceration (P=0.03; Fig. 3F and
Table I). Moreover, Sox17
expression was significantly different among the different subtypes
of melanoma (P=0.00003; Table I).
We did not find significant correlations between Sox17 expression
and other variables (Table I).
 | Table ISox17 staining and clinicopathologic
characteristics of the 433 melanoma cases. |
Table I
Sox17 staining and clinicopathologic
characteristics of the 433 melanoma cases.
| Sox17 staining | | |
|---|
|
| | |
|---|
| Variables | Low n (%) | High n (%) | Total n (%) | P-value |
|---|
| All melanoma
(n=433) |
| Age, years |
| ≤60 | 112 (50.5) | 110 (49.8) | 222 (51.3) | 0.89 |
| >60 | 105 (49.5) | 106 (50.2) | 211 (48.7) | |
| Gender |
| Male | 133 (52.0) | 123 (48.0) | 256 (59.1) | 0.36 |
| Female | 84 (47.5) | 93 (52.5) | 177 (40.9) | |
| AJCC stage |
| I | 47 (27.5) | 124 (72.5) | 171 (39.9) |
4.6×10−15a |
| II | 68 (56.7) | 52 (43.3) | 120 (28.0) | |
| III | 46 (79.3) | 12 (20.7) | 58 (13.5) | |
| IV | 53 (66.3) | 27 (33.7) | 80 (18.6) | |
| Primary melanoma
(n=291) |
| Age, years |
| ≤60 | 57 (40.1) | 85 (59.9) | 142 (48.8) | 0.83 |
| >60 | 58 (38.9) | 91 (61.1) | 149 (51.2) | |
| Gender |
| Male | 62 (39.5) | 95 (60.5) | 157 (54.0) | 1.0 |
| Female | 53 (39.6) | 81 (60.4) | 134 (46.0) | |
| Tumor thickness
(mm) |
| ≤4 | 78 (33.6) | 154 (66.4) | 232 (79.7) | 0.00004 |
| >4 | 37 (62.7) | 22 (37.3) | 59 (20.3) | |
| Ulceration |
| Absent | 88 (36.7) | 152 (63.3) | 240 (82.5) | 0.03 |
| Present | 27 (52.9) | 24 (47.1) | 51 (17.5) | |
| Subtype |
| Lentigo
maligna | 18 (27.7) | 47 (72.3) | 65 (24.3) | 0.0003 |
| Superficial
spreading | 32 (30.5) | 73 (69.5) | 105 (39.2) | |
| Nodular | 28 (60.9) | 18 (39.1) | 46 (17.2) | |
| Unspecified | 26 (50.0) | 26 (50.0) | 52 (19.3) | |
| Siteb |
|
Sun-protected | 84 (39.6) | 128 (60.4) | 212 (72.9) | 0.95 |
| Sun-exposed | 31 (39.2) | 48 (60.8) | 79 (27.1) | |
| Metastatic melanoma
(n=142) |
| Age, years |
| ≤60 | 55 (68.8) | 25 (31.2) | 80 (56.3) | 0.35 |
| >60 | 47 (75.8) | 15 (24.2) | 62 (43.7) | |
| Gender |
| Male | 71 (71.7) | 28 (28.3) | 99 (69.7) | 0.96 |
| Female | 31 (72.1) | 12 (27.9) | 43 (30.3) | |
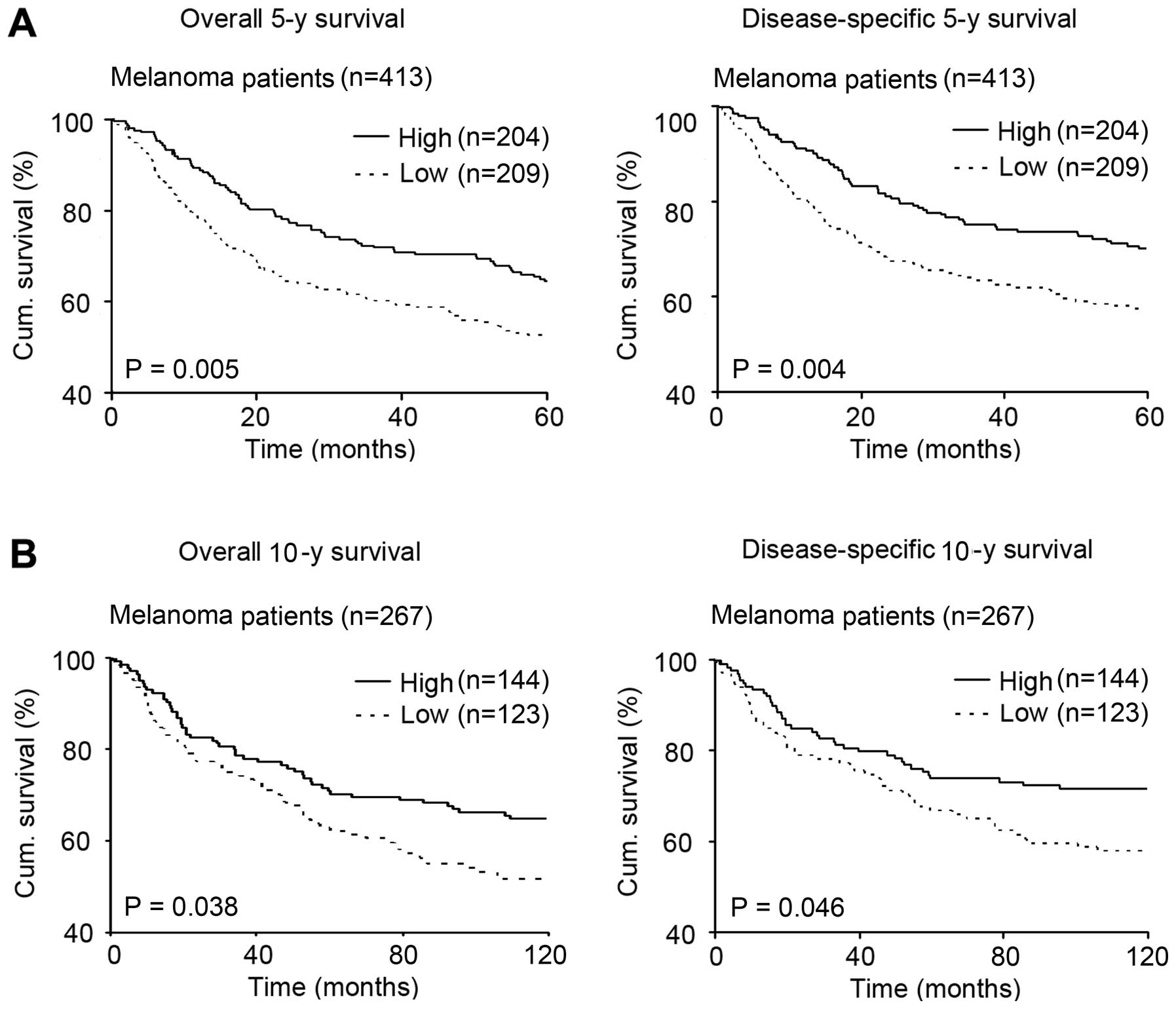
Reduced Sox17 expression is associated
with poor melanoma patient survival
In the 413 melanoma patients, Kaplan-Meier survival
curve revealed that the patients with high Sox17 expression had a
better overall and disease-specific 5-year survival than patients
with low Sox17 expression (P=0.005 and 0.004, respectively;
Fig. 4A). More meaningful, we
further investigated whether Sox17 expression was associated with
melanoma patient 10-year survival. We analyzed the 267 melanoma
patients and found that the patients with high Sox17 expression
also had a better overall and disease-specific 10-year survival
than patients with low Sox17 expression (P=0.038 and 0.046,
respectively; Fig. 4B).
Sox17 expression is an independent
prognostic marker for melanoma
To further estimate the prognostic value of Sox17
expression in melanoma patients, univariate and multivariate Cox
proportional hazard analyses were performed. When analyzed using
univariate Cox regressive analysis, patients with high Sox17
expression had significantly better overall survival (HR, 0.65; 95%
CI, 0.48–0.89; P=0.006) and disease-specific (HR, 0.63; 95% CI,
0.45–0.87; P=0.005; Table II)
5-year survival among the 413 melanoma patients. Concerning 10-year
survival, univariate Cox regressive analysis further confirmed that
patients with high Sox17 expression had significantly better
overall survival (HR, 1.48; 95% CI, 1.02–2.14; P=0.04) and
disease-specific (HR, 1.51; 95% CI, 1.00–2.28; P=0.05; Table II) 10-year survival in the 267
melanoma patients.
 | Table IIUnivariate and multivariate Cox
regression analyses of the 5- or 10-year survival of the melanoma
patients. |
Table II
Univariate and multivariate Cox
regression analyses of the 5- or 10-year survival of the melanoma
patients.
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|---|
| Variable | βa | SE | HR | 95% CI | P-value | βa | SE | HR | 95% CI | P-value |
|---|
| 5-year overall
survival (n=413) |
| Age | −0.17 | 0.15 | 0.85 | 0.63–1.14 | 0.27 | −0.16 | 0.15 | 0.85 | 0.63–1.15 | 0.28 |
| Gender | 0.07 | 0.08 | 1.07 | 0.92–1.25 | 0.39 | −0.12 | 0.16 | 1.12 | 0.83–1.53 | 0.46 |
| Sox17 | −0.42 | 0.15 | 0.65 | 0.48–0.89 | 0.006 | 0.42 | 0.15 | 1.52 | 1.13–2.06 | 0.006 |
| 5-year
disease-specific survival (n=413) |
| Age | −0.05 | 0.16 | 0.95 | 0.69–1.31 | 0.76 | −0.05 | 0.16 | 0.95 | 0.69–1.31 | 0.77 |
| Gender | 0.06 | 0.08 | 1.06 | 0.90–1.25 | 0.47 | 0.10 | 0.17 | 1.11 | 0.80–1.54 | 0.54 |
| Sox17 | −0.47 | 0.17 | 0.63 | 0.45–0.87 | 0.005 | 0.46 | 0.17 | 1.59 | 1.15–2.20 | 0.005 |
| 10-year overall
survival (n=267) |
| Age | −0.78 | 0.20 | 0.46 | 0.31–0.68 |
7×10−5 | −0.78 | 0.20 | 0.46 | 0.31–0.68 |
8×10−5 |
| Gender | 0.17 | 0.19 | 1.19 | 0.82–1.73 | 0.37 | 0.11 | 0.19 | 1.12 | 0.77–1.63 | 0.56 |
| Sox17 | 0.39 | 0.19 | 1.48 | 1.02–2.14 | 0.04 | 0.41 | 0.19 | 1.51 | 1.04–2.19 | 0.03 |
| 10-year
disease-specific survival (n=267) |
| Age | −0.48 | 0.21 | 0.62 | 0.41–0.94 | 0.02 | −0.47 | 0.21 | 0.63 | 0.41–0.95 | 0.03 |
| Gender | 0.24 | 0.21 | 1.27 | 0.84–1.93 | 0.25 | 0.21 | 0.21 | 1.23 | 0.81–1.87 | 0.33 |
| Sox17 | 0.42 | 0.21 | 1.51 | 1.00–2.28 | 0.05 | 0.43 | 0.21 | 1.54 | 1.02–2.32 | 0.04 |
Furthermore, multivariate Cox regressive analysis
was carried out with age, gender and Sox17 expression together as
the variables. The results indicated that Sox17 expression is an
independent prognostic factor for both overall (HR, 1.52; 95% CI,
1.13–2.06; P=0.006) and disease-specific (HR, 1.59; 95% CI,
1.15–2.20; P=0.005; Table II)
5-year survival in the 413 melanoma patients. Concerning 10-year
survival, multivariate Cox regressive analysis further demonstrated
that Sox17 expression was also correlated with both overall (HR,
1.51; 95% CI, 1.04–2.19; P=0.03) and disease specific (HR, 1.54;
95% CI, 1.02–2.32; P=0.04; Table
II) 10-year survival in the 267 melanoma patients.
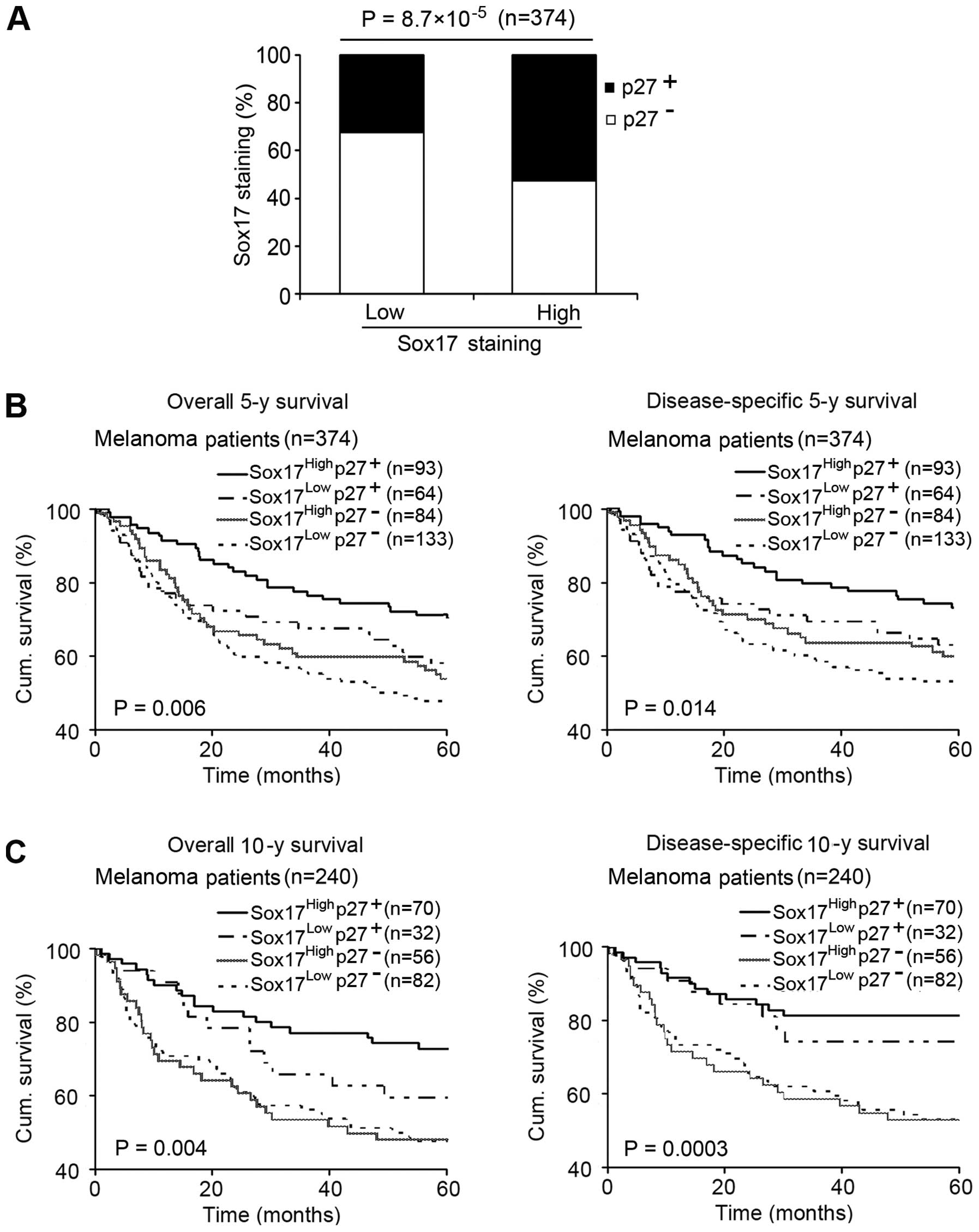
Sox17 expression positively correlates
with p27 expression, and their concomitant expression influences
melanoma patient survival
Reduced p27 expression has been shown to be
associated with increased invasion and unfavorable patient survival
(20, 21). Since the TMA we used for Sox17
staining in this study was the same as that previously stained for
p27 (22), we were able to analyze
the correlation between Sox17 and p27 expression. As shown in
Fig. 5A, high Sox17 was positively
correlated with positive p27 expression (P=8.7×10−5). We
then analyzed the effect of combined Sox17 and p27 expression on
patient survival and found that patients with both high Sox17 and
positive p27 expression showed a significantly increased 5-year
overall and disease-specific survival, compared with the patients
with other expression patterns of Sox17 and p27 using Kaplan-Meier
survival curves (P=0.006 and 0.014, respectively; Fig. 5B). More meaningful, concerning
10-year survival, the analysis also demonstrated that a high Sox17
and positive p27 expression pattern exerted a more significant
influence on melanoma patient survival (P=0.004 and 0.0003,
respectively; Fig. 5C).
Discussion
A number of studies have demonstrated that Sox17
expression is downregulated in different types of human cancers and
it appears to act as a tumor suppressor (17–19).
However, a recent report demonstrated that Sox17 expressed in tumor
endothelial cells promoted tumor angiogenesis and vascular
destabilization by upregulation of VEGFR2 (26). To understand the role of Sox17 in
melanoma progression, we used TMA technology and
immunohistochemistry to investigate Sox17 expression in 525 cases
of pigmented skin lesions at different stages.
Our results revealed that Sox17 expression was
markedly decreased in primary melanoma when compared with the
expression in common acquired nevi and dysplastic nevi and was
further downregulated in metastatic melanoma compared with primary
melanoma. This indicates that reduced Sox17 activity may have a
relationship with the transformation from benign neoplasia to
malignancy, as well as in tumor progression from primary to
metastatic melanoma. This finding is consistent with previous
research that found that Sox17 was rarely detected by
immunohistochemistry in gastric and colon cancers, whereas strong
nuclear staining of Sox17 was found in 70% of benign gastric and
intestinal tumors (19). It is
therefore conceivable that Sox17 protects benign tumors from
malignant progression at an early stage of tumorigenesis, and
downregulation of Sox17 contributes to malignant progression.
It was also reported that Sox17 methylation was
found in 60.2% of primary human lung cancer samples. The promoter
region methylation silenced the expression of Sox17, and
re-expression of Sox17 inhibited Wnt signaling in lung cancer cells
(27). The global analysis of CpG
island hypermethylation and gene expression in colorectal cancer
cell lines has revealed that Sox17 gene silencing is associated
with DNA hypermethylation (18).
Recent research demonstrated that loss of Sox17 expression is
correlated to promoter region hypermethylation in esophageal
cancer. Restoration of Sox17 expression suppresses
TCF/β-catenin-dependent transcription and colony formation
(28). Furthermore, Sox17
expression is often epigenetically silenced by DNA methylation in
early gastric cancer and the introduction of Sox17 into cancer
cells suppresses cancer cell growth (29).
In addition, we found that Sox17 expression was
inversely correlated with AJCC stage, ulceration and tumor
thickness (≤4.00 vs. >4.00 mm) of melanomas. A reflection of the
intense metastatic propensity of melanomas is the fact that the
metastatic potential is measured on a scale of millimeters, where a
tumor thickness of 4.00 mm predicts a high risk of cancer
dissemination and death (30). By
constructing Kaplan-Meier survival curves, we found that reduced
Sox17 was associated with the poor survival of melanoma patients
and this correlation was further confirmed by univariate Cox
regression analyses. Furthermore, after adjustment for age and
gender in multivariate Cox regression models, the analysis further
indicated that Sox17 expression is an independent prognostic marker
for melanoma patients. To our knowledge, this is the first study to
reveal the prognostic value of Sox17 expression in tumor patients
by TMA technology.
p27, a cell-cycle inhibitory molecule, inhibits the
catalytic activity of CDK4. Increased levels of the p27 protein
typically cause cells to arrest in the G1 phase of the cell cycle
(20). It has been reported that
Sox17 overexpression in human colon cancer cell lines suppressed
the hyperactive β-catenin activity as well as reduced cyclin D1
expression and repressed cell proliferation (18,31).
Colony formation assays revealed that Sox17 suppressed lung cancer
cell proliferation (27). Sox17
knockdown in gastric cancer cells enhanced cyclin D1 expression
levels and reduced p27 expression levels (12). The expression of Sox17 and p27
increased in parallel before myelin basic protein expression in
oligodendrocytes (32). In
addition, it has been demonstrated that reduced nuclear p27
expression is associated with a worse cancer patient survival
(20–22).
Here, our results revealed that in 374 melanoma
samples, melanomas which had high Sox17 expression also had a
significantly higher percentage of positive p27 staining. Notably,
patients with both high Sox17 and positive p27 expression had an
extended 5- and 10-year overall and disease-specific survival as
compared to patients with both low Sox17 and negative p27
expression. Based on this, we speculate that Sox17 may be a
positive regulator of p27 and therefore, reduced Sox17 expression
has an inhibitory effect on melanoma progression.
In conclusion, this study shows that Sox17
expression is significantly decreased in association with
progression of human melanoma. Strikingly, reduced Sox17 expression
correlates with a worse 5- and 10-year survival of melanoma
patients and is an independent prognostic factor for melanoma
patients. Moreover, there is a significant positive correlation
between Sox17 and p27 expression in melanoma biopsies, and their
concomitant reduced expression is inversely correlated with
melanoma patient survival. These data suggest that Sox17 plays an
important role in melanoma pathogenesis and it may serve as a
promising prognostic marker for melanoma patients.
Acknowledgements
We acknowledge Dr Guangdi Chen for his previous
research on p27. This study was supported by the Canadian
Institutes of Health Research (CCI-117958), the Cancer Research
Society and the Canadian Dermatology Foundation. Dr Jing Lu is
supported by the National Natural Science Foundation of China
(81101731).
References
|
1
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar
|
|
2
|
Trinh VA: Current management of metastatic
melanoma. Am J Health Syst Pharm. 65:S3–S8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geller AC, Swetter SM, Brooks K, Demierre
MF and Yaroch AL: Screening, early detection, and trends for
melanoma: current status (2000–2006) and future directions. J Am
Acad Dermatol. 57:555–576. 2007.PubMed/NCBI
|
|
4
|
Geller AC, Swetter SM, Oliveria S, Dusza S
and Halpern AC: Reducing mortality in individuals at high risk for
advanced melanoma through education and screening. J Am Acad
Dermatol. 65:S87–S94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turner RM, Bell KJ, Morton RL, et al:
Optimizing the frequency of follow-up visits for patients treated
for localized primary cutaneous melanoma. J Clin Oncol.
29:4641–4646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gubbay J, Collignon J, Koopman P, et al: A
gene mapping to the sex-determining region of the mouse Y
chromosome is a member of a novel family of embryonically expressed
genes. Nature. 346:245–250. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wegner M: From head to toes: the multiple
facets of Sox proteins. Nucleic Acids Res. 27:1409–1420. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burtscher I, Barkey W, Schwarzfischer M,
Theis FJ and Lickert H: The Sox17-mCherry fusion mouse line allows
visualization of endoderm and vascular endothelial development.
Genesis. 50:496–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niakan KK, Ji H, Maehr R, et al: Sox17
promotes differentiation in mouse embryonic stem cells by directly
regulating extraembryonic gene expression and indirectly
antagonizing self-renewal. Genes Dev. 24:312–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen HL, Chew LJ, Packer RJ and Gallo V:
Modulation of the Wnt/beta-catenin pathway in human
oligodendroglioma cells by Sox17 regulates proliferation and
differentiation. Cancer Lett. 335:361–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia Y, Yang Y, Liu S, Herman JG, Lu F and
Guo M: SOX17 antagonizes WNT/β-catenin signaling pathway in
hepatocellular carcinoma. Epigenetics. 5:743–749. 2010.
|
|
12
|
Ye YW, Wu JH, Wang CM, et al: Sox17
regulates proliferation and cell cycle during gastric cancer
progression. Cancer Lett. 307:124–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Beisswenger C, Herr C, et al:
Myeloid cell RelA/p65 promotes lung cancer proliferation through
Wnt/β-catenin signaling in murine and human tumor cells. Oncogene.
33:1239–1248. 2013.PubMed/NCBI
|
|
14
|
Yang L, Chen Y, Cui T, et al: Desmoplakin
acts as a tumor suppressor by inhibition of the Wnt/β-catenin
signaling pathway in human lung cancer. Carcinogenesis.
33:1863–1870. 2012.PubMed/NCBI
|
|
15
|
Zeitlin BD, Ellis LM and Nor JE:
Inhibition of vascular endothelial growth factor
receptor-1/Wnt/{beta}-catenin crosstalk leads to tumor cell death.
Clin Cancer Res. 15:7453–7455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fodde R and Brabletz T: Wnt/β-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007.
|
|
17
|
Fu DY, Wang ZM, Li C, et al: Sox17, the
canonical Wnt antagonist, is epigenetically inactivated by promoter
methylation in human breast cancer. Breast Cancer Res Treat.
119:601–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Glockner SC, Guo M, et al:
Epigenetic inactivation of the canonical Wnt antagonist SRY-box
containing gene 17 in colorectal cancer. Cancer Res. 68:2764–2772.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du YC, Oshima H, Oguma K, et al: Induction
and down-regulation of Sox17 and its possible roles during the
course of gastrointestinal tumorigenesis. Gastroenterology.
137:1346–1357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wander SA, Zhao D and Slingerland JM: p27:
a barometer of signaling deregulation and potential predictor of
response to targeted therapies. Clin Cancer Res. 17:12–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Cheng Y, Zhang Z, Martinka M and
Li G: Prognostic significance of cytoplasmic p27 expression in
human melanoma. Cancer Epidemiol Biomarkers Prev. 20:2212–2221.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao GZ, Lehwald N, Jang KY, et al:
Wnt/β-catenin signaling protects mouse liver against oxidative
stress-induced apoptosis through the inhibition of forkhead
transcription factor FoxO3. J Biol Chem. 288:17214–17224. 2013.
|
|
24
|
Dai DL, Martinka M and Li G: Prognostic
significance of activated Akt expression in melanoma: a
clinicopathologic study of 292 cases. J Clin Oncol. 23:1473–1482.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin H, Wong RP, Martinka M and Li G: Loss
of SNF5 expression correlates with poor patient survival in
melanoma. Clin Cancer Res. 15:6404–6411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Lee S, Kim K, et al: Sox17
promotes tumor angiogenesis and destabilizes tumor vessels in mice.
J Clin Invest. 123:418–431. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin D, Jia Y, Yu Y, et al: SOX17
methylation inhibits its antagonism of Wnt signaling pathway in
lung cancer. Discov Med. 14:33–40. 2012.PubMed/NCBI
|
|
28
|
Jia Y, Yang Y, Zhan Q, et al: Inhibition
of SOX17 by microRNA 141 and methylation activates the WNT
signaling pathway in esophageal cancer. J Mol Diagn. 14:577–585.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oishi Y, Watanabe Y, Yoshida Y, et al:
Hypermethylation of Sox17 gene is useful as a molecular diagnostic
application in early gastric cancer. Tumour Biol. 33:383–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarhini AA and Agarwala SS: Cutaneous
melanoma: available therapy for metastatic disease. Dermatol Ther.
19:19–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sinner D, Kordich JJ, Spence JR, et al:
Sox17 and Sox4 differentially regulate β-catenin/T-cell factor
activity and proliferation of colon carcinoma cells. Mol Cell Biol.
27:7802–7815. 2007.PubMed/NCBI
|
|
32
|
Sohn J, Natale J, Chew LJ, et al:
Identification of Sox17 as a transcription factor that regulates
oligodendrocyte development. J Neurosci. 26:9722–9735. 2006.
View Article : Google Scholar : PubMed/NCBI
|