Introduction
Gastric cancer is a significant worldwide health
problem and accounts for more than 900,000 new cases of cancer
annually and caused ~740,000 cancer-related deaths in 2008
worldwide (1). Although the exact
etiology of gastric cancer remains to be defined, multiple genetic
and epigenetic alterations, such as activation of oncogenes,
silencing of tumor-suppressor genes and genetic instability, are
implicated in a multistep process of gastric cancer development
(2). Thus, further studies on the
gastric carcinogenesis process could support the development of
novel strategies for effectively controlling gastric cancer in
future clinical practice.
Towards this end, programmed cell death 2 protein
(PDCD2) has attracted attention since this protein was originally
identified during the apoptosis of rat thymocytes (3). Human PDCD2 is located at
chromosome 6q27, a region that is involved in both translocations
and deletions in leukemia and lymphoma (4,5). PDCD2
plays a critical role during embryonic development; for example, a
mutant of Drosophila PDCD2 (i.e., Zfrp8) resulted in
embryonic developmental delay, larval and pupal lethality (4,6).
Knockout mouse data indicate that PDCD2 is essential for embryonic
stem cell viability and self-renewal (7) and is enriched in three types of mouse
stem cells (embryonic, neural and hematopoietic) (8) and in human embryonic stem cells
compared to their differentiated derivatives (9). Thus, PDCD2 is thought to be a
potential auxiliary factor in stem cell maintenance and
differentiation. Alteration of PDCD2 expression could contribute to
human cancer development and progression. Fan et al
(10) showed that PDCD2 expression
is decreased in multidrug-resistant colon cancer cells. However,
subsequent studies failed to associate PDCD2 expression with tumor
cell apoptosis (5,6). Thus, in the present study, we first
analyzed the expression of PDCD protein and mRNA in gastric cancer
and matched normal tissues. We next investigated the anti-tumor
effects of PDCD2 on gastric cancer cell lines and the underlying
molecular events by confirmation of p53-dependent, PDCD2-mediated
apoptosis in gastric cancer cells. We also utilized p53-knockout
mice as an in vivo ultraviolet light (UV)-induced skin
carcinogenesis model to confirm the role of p53 in mediating the
effects of PDCD2 on the regulation of cell apoptosis. The objective
of the present study was to provide novel insight into the
molecular mechanisms of PDCD2′s action in gastric
tumorigenesis.
Materials and methods
Tissue specimens
Tissue specimens were obtained from 34 patients
without pre-surgical chemotherapy or radiotherapy at the Department
of Gastroenterological Surgery, Hangzhou First Hospital, School of
Clinical Medicine, Nanjing Medical University between January 2009
and December 2013. The study was approved by our hospital review
board, and each patient provided informed consent. Fresh tissue
specimens and paraffin blocks were used to assess gene
expression.
qRT-PCR
Total cellular RNA was isolated from tissue samples
or cells using an RNeasy Mini kit (Biomed, Beijing, China) and
reversely transcribed into first-strand cDNA using a Takara reverse
transcription kit (Takara, Dalian, China) and oligo (dT)15 primers
(Takara) according to the manufacturer’s instructions. The
resultant cDNA was then used for qPCR amplification of PDCD2 and
p53 expression, and GAPDH mRNA was used as a control. PDCD2
primers were 5′-CTGTGGAGCTGGGCTTCGCC-3′ and
5′-CAGCAGGAAGGAGAGCGGGC-3′. p53 primers were
5′-ACTCCAGCCACCTGTAGTCCAAAAAGGGTC-3′ and
5′-GACCCTTTTTGGACTACAGGTGGCTGGAGT-3′. GAPDH primers were
5′-AGAAGGCTGGGGCTCATTTG-3′ and 5′-AGGGGCCATCCACAGTCTTC-3′.
Amplification of PDCD2, p53 and GADPH mRNA was
performed with one cycle at 95°C for 10 min and 40 cycles of 95°C
for 15 sec and 60°C for 60 sec. Calculation of the relative
expression of each transcript was performed using the
2−ΔΔCt method.
Protein extraction and western
blotting
Tissues and cells were lysed in a lysis buffer (20
mM Tris-HCl, 150 mM NaCl, 2 mM EDTA and 1% Triton-X100) containing
a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA).
Cellular protein was then quantified using the BCA protein assay
kit (Beyotime, Beijing, China). Equivalent amounts of protein
samples (20 μg) were separated using 12% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore Corp.,
Billerica, MA, USA). Anti-PDCD2 (sc-377250) and anti-β-actin
(sc-130301) antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA), and cell cycle checkpoint-regulated
proteins, including anti-ataxia telangiectasia mutated (ATM),
anti-p53, anti-checkpoint kinase (Chk)1 and anti-Chk2 were
purchased from Santa Cruz Biotechnology. Anti-phospho-S345-Chk1 and
anti-phospho-T68-Chk2 antibodies were from Cell Signaling
Technology (Danvers, MA, USA). Each specific antibody binding was
detected with horseradish peroxidase (HRP)-conjugated respective
secondary antibodies (Amersham Biosciences, Amersham, UK) and
enhanced chemiluminescence (ECL) solutions (Amersham
Biosciences).
Immunohistochemistry
Tumor tissues were fixed in 4% para-formaldehyde for
24 h, embedded in paraffin and then sectioned into 4-μm sections
for immunohistochemistry. Briefly, endogenous peroxidase activity
was blocked by incubating sections in 3% hydrogen peroxide for 30
min. Antigen retrieval was performed in citrate buffer (10 mM, pH
6.0) for 30 min at 95°C in a pressure cooker. After that, the
sections were blocked in 1.5% blocking serum in phosphate-buffered
saline (PBS) for 2 h at room temperature and incubated with an
anti-PDCD2 antibody (1:200 dilution) or anti-p53 antibody (1:200
dilution; both from Santa Cruz Biotechnology) overnight at 4°C in a
moist chamber. The next day sections were washed with PBS and
incubated with a biotinylated secondary antibody at 37°C for 2 h
before exposure to a streptavidin complex (HRP; Beyotime). Positive
reaction was visualized using 3,3′-diami-nobenzidine
tetrahydrochloride (DAB; Beyotime) followed by counterstaining with
hematoxylin (Beyotime). The stained sections were reviewed and
scored independently under a light microscope by two researchers.
Positive staining was defined as 25% or more of tumor cells
staining positively.
Cell lines and culture
Gastric cancer MKN28 cells (p53 mutant) and MKN45
cells (p53 wild-type) were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and grown in RPMI-1640 medium
(HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Invitrogen, Carlsbad, CA, USA) and antibiotics (100 U/ml
penicillin and 100 μg/ml streptomycin) in a humidified incubator
with 5% CO2 at 37°C.
Gene transfection
Plasmids carrying PDCD2 or p53 cDNA, namely
pCDNA-3.1-PDCD2 or pCDNA-3.1-p53, respectively, were obtained from
Dr Cong Jie (China Medical University). p53 shRNA (cat #
sc-29435) plasmid was obtained from Santa Cruz Biotechnology. For
gene transfection, gastric cancer MKN28 and MKN45 cells were grown
overnight and transfected with pCDNA-3.1-PDCD2 using Lipofectamine
2000 (Invitrogen) according to the manufacturer’s instructions.
Plasmid pCDNA-3.1-p53 was transfected into MKN28-PDCD2 cells and
p53 shRNA was transfected into MKN45-PDCD2 cells using
Lipofectamine 2000.
[3H]thymidine incorporation
assay
[Methyl-3H]thymidine was obtained from
Sigma-Aldrich. Cells (1,000 cells/well) following gene transfection
were seeded at least in quadruplicate in 96-well plates, grown for
4 days and then incubated with 20 mCi/well [methyl-3H]
thymidine for 16 h. Subsequently, the cells were washed with
ice-cold PBS and lysed with ice-cold 10% trichloroacetic acid (TCA)
in PBS for 20 min at 4°C. Ice-cold 0.2 M NaOH was then added to the
cells and incubated for 10 min at −20°C. The cell lysates were then
collected, universal scintillation cocktail (2.5 ml) was added and
the incorporation of [3H]thymidine into DNA was assessed
using a liquid scintillation counter (Xi’an Nuclear Instrument
Factory, Xi’an, China) to determine the disintegrations per minute
(dpm) values.
Flow cytometric cell cycle and apoptosis
assays
To detect cell cycle distribution, the cells
following 48 h of gene transfection were harvested, suspended in
PBS and fixed with 70% ethanol at 4°C. After centrifugation (1,500
× g for 5 min), the supernatants were discarded, cellular DNA was
stained with 10 μM propidium iodide (PI; Keygen, Nanjing, China)
and samples were analyzed by a FACSCalibur flow cytometer (BD
Biosciences, Baltimore, MD, USA).
Apoptosis was determined using an apoptosis
detection kit (Keygen). Briefly, cells following 48 h of gene
transfection were collected, washed twice in ice-cold PBS and then
resuspended in binding buffer at a density of 1×106
cells/ml. The cells were simultaneously incubated with
fluorescein-labeled Annexin V and PI for 20 min. The mixture was
then analyzed using a FACSCalibur (BD Biosciences).
Labeling of cells with thymidine
analogs
Actively replicating cells at the beginning of each
hour of the S phase were first labeled with the thymidine analog,
5-iodo-2′-deoxyuridine (IdU; 50 μM, Sigma-Aldrich) for 40 min,
washed three times with PBS and then labeled with
5-chloro-2′-deoxyuridine (CldU; 100 μM, Sigma-Aldrich) for 40 min.
The IdU and CldU incorporated into replicating DNA were then
detected with red or green fluorescent antibodies, respectively.
Specifically, slides were treated with 70% ethanol, washed in PBS,
denatured in 2.5 M HCl for 30 min, permeabilized in 0.25% Triton
X-100 for 5 min and blocked with 1% bovine serum albumin. The
slides were incubated at room temperature with the following
antibodies: i) a mouse anti-bromodeoxyuridine antibody at a
dilution of 1:500 (detects IdU; Sigma-Aldrich); ii) an AlexaFluor
488-conjugated anti-mouse antibody at a dilution of 1:1,000
(Invitrogen); iii) a rat anti-bromodeoxyuridine antibody at a
dilution of 1:2,000 (detects CldU; Santa Cruz Biotechnology) and
iv) an AlexaFluor 633-conjugated anti-rat antibody at a dilution of
1:1,000 (Invitrogen). After counter-staining with DAPI (1 μg/ml,
KeyGen), images were captured under an Olympus CX71 fluorescence
microscope (Olympus, Tokyo, Japan).
UVB-induced skin carcinogenesis in
p53−/− and p53+/+ nude mice
The animal study was approved by the ethics
committee of our University. According to a previous study
(11), male haired
p53−/− mice with a C57BL/6J genetic background
from the Jackson Laboratory (Bar Harbor, ME, USA) were mated with
female hairless SKH-1 mice (p53 wild-type) to obtain male
and female hairless congenic p53-deficient mice. The
hairless p53−/− mice were intercrossed to obtain
homozygous p53-deficient mice and their wild-type littermates.
Then, UV lamps (FS72T12-UVB-HO, National Biological Corp.,
Twinsburg, OH, USA) were used to expose 30 p53−/−
and 30 p53+/+ SKH-1 mice to 180 mJ/cm2
UVB (280–320 nm; 75–80% of total energy) twice/week for 20 weeks.
This irradiation induced skin tumors in 100% of irradiated mice. At
the end of the experiment, tumor-bearing p53−/−
or p53+/+ SKH-1 mice were randomly divided into
two groups of 15 each for PDCD2 modulation. Specifically,
pCDNA3.1-PDCD2 carrying PDCD2 cDNA was injected into the tail vein
of each mouse. Two injections were administered at 8 a.m. and 8
p.m. for 3 days. Tumor number was recorded once every 2 weeks and
plotted against time (in weeks). At the end of 25 weeks, the
experiment was terminated. All of the animals were sacrificed, and
skin and tumor samples were harvested.
Immunofluorescence staining of p53 and
PDCD2 proteins
To assess expression of p53 and PDCD2 proteins in
mouse tissues, the double-immunofluorescence technique with
specific antibodies against p53 (rabbit IgG, sc-6243, Santa Cruz
Biotechnology) and PDCD2 (mouse IgG1, sc-377250, Santa Cruz
Biotechnology) was performed. Anti-rabbit AlexaFluor®
488 IgG or anti-mouse AlexaFluor® 594 IgG (Invitrogen)
was used as the secondary antibody. Photographic images were
captured using an Olympus CX71 fluorescence microscope
(Olympus).
Terminal deoxynucleotidyl
transferase-mediated nick end labeling (TUNEL)
The TUNEL assay was performed using a kit from Roche
Applied Science (no. 1684795; Indianapolis, IN, USA) according to
the manufacturer’s protocol. Briefly, 6-μm frozen tissue sections
were rinsed three times in PBS and were incubated in 0.3% Triton
X-100 (v/v) in 0.01 M PBS (pH 7.4) for 20 min at room temperature.
Subsequently, the TUNEL reaction mixture was applied for 60 min at
37°C. The fluorescence signal was detected with an Olympus
microscope (model CX71) at excitation/emission wavelengths of
492/520 nm.
Statistical analysis
Statistical analysis was performed using a
one-tailed Student’s t-test (unilateral and unpaired). Kaplan-Meier
survival plots were generated, and comparisons between survival
curves were assessed using the log-rank statistical analysis. Data
were analyzed using GraphPad Prism 5 software (San Diego, CA, USA),
and a P-value <0.05 was considered to indicate a statistical
significant difference.
Results
Reduced PDCD2 expression in gastric
cancer tissue specimens is associated with poor survival of
patients
In the present study, we collected 34 tissue
specimens from gastric cancer patients and analyzed PDCD2
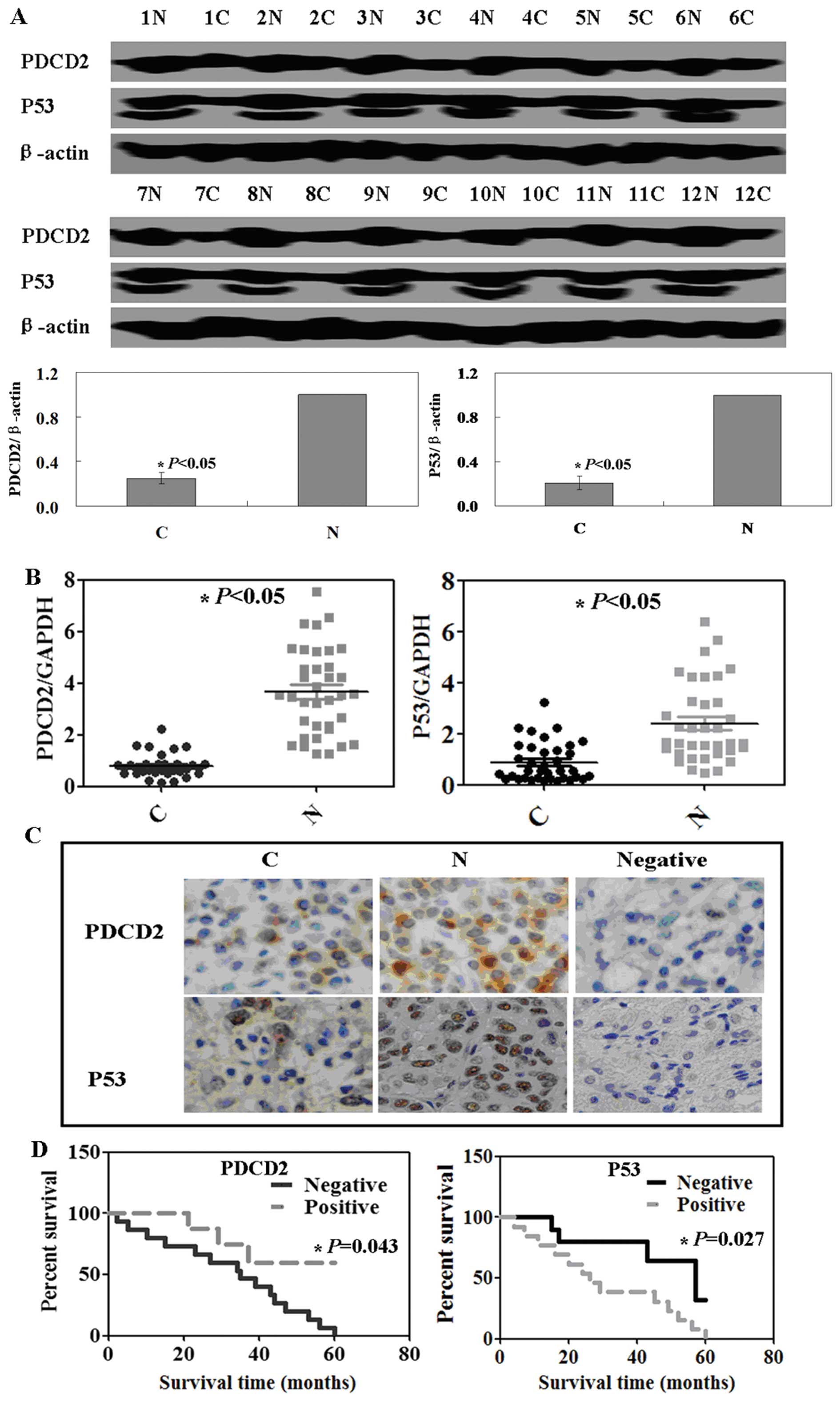
expression. The western blotting data showed that PDCD2 protein
expression was significantly lower in the tumor tissues than that
in the adjacent normal mucosa. We then analyzed expression of p53
protein and similar data were obtained (P<0.05; Fig. 1A). qRT-PCR data confirmed the
western blotting data (P<0.05; Fig.
1B). To assess why p53 protein was more highly expressed in
normal vs. tumor tissues, we performed immunohistochemistry and
found that PDCD2 protein was localized in the cytoplasm, whereas
p53 protein was localized in the nuclei of cells (Fig. 1C).
We then collected follow-up data from these patients
and examined the association of PDCD2 expression with patient
survival. Specifically, the 34 patients were followed up between 1
month and 5 years with a median time of 28 months. Kaplan-Meier
analysis showed that expression of PDCD2 protein was associated
with favorable prognosis, whereas p53 expression was associated
with poor prognosis of the gastric cancer patients (P<0.05;
Fig. 1D).
Antitumor effects of PDCD2 expression in
gastric cancer cells is dependent on p53 expression in vitro
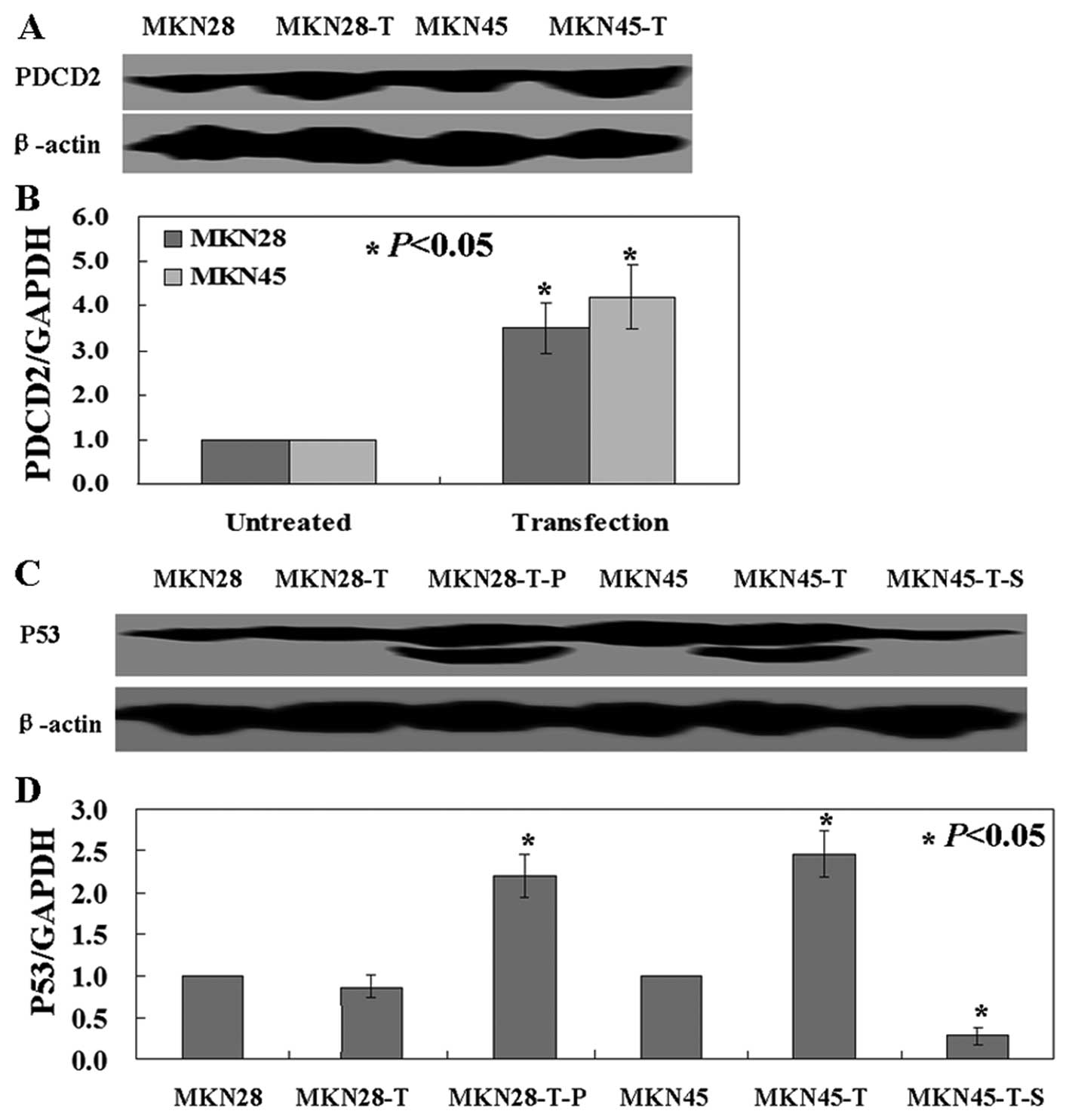
We next investigated the effects of exogenous PDCD2
expression in gastric cancer cell lines by transfecting PDCD2 cDNA
into MKN28 and MKN45 cells. qRT-PCR and western blotting data
confirmed the exogenous expression of PDCD2 in MKN28 and MKN45
cells after transfection (Fig. 2A and
B). Similarly, we transfected p53 cDNA and shRNA into these two
cell lines, respectively, and qRT-PCR and western blotting data
confirmed p53 expression in MKN28 cells and p53 knockdown in MKN45
cells (Fig. 2C and D).
We then assessed phenotypic changes in these cells
and found slower growth in the PDCD2-expressing MKN45 cells when
compared with the growth of the untreated cells by using an
[3H]thymidine incorporation assay (P<0.05; Fig. 3A). In addition, the cells showed a
high level of apoptosis after PDCD2 cDNA transfection, as evidenced
by Annexin V/PI double staining (P<0.05, Fig. 3B). PI staining of the cells revealed
that PDCD2-expressing MKN45 cells were arrested in the S phase of
the cell cycle (P<0.05, Fig.
3C), and CldU and IdU staining confirmed that the MKN45 cells
were arrested at the early S phase (Fig. 3D). However, there was no effect of
PDCD2 expression on the regulation of cell growth, apoptosis and
cell cycle arrest in MKN45 cells transfected with p53 shRNA
or parental cells (Fig. 3).
Similarly, PDCD2 expression was able to inhibit proliferation, but
induced apoptosis and early S phase arrest in p53-overexpressing
MKN28 cells (Fig. 3). These results
indicate that the antitumor activities of PDCD2 in gastric cancer
cells are p53-dependent.
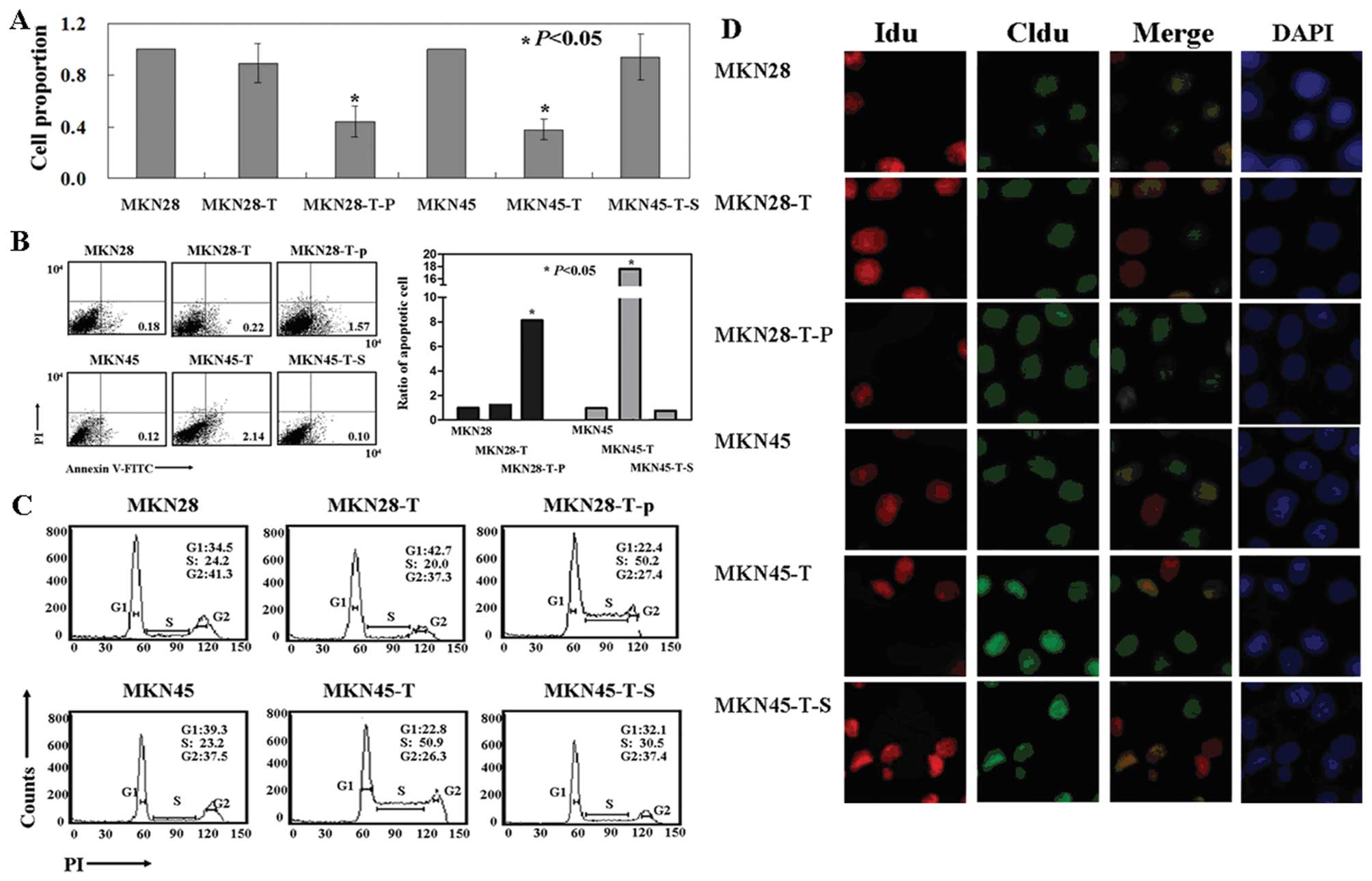 | Figure 3Effects of the antitumor activity of
PDCD2 on gastric cancer cell lines in vitro. (A)
(Methyl-3H) thymidine incorporation assays. (B) Flow
cytometric apoptosis assay. (C) Flow cytometric cell cycle assay.
(D) Thymidine analog labeling assay. Representative images show
that cells were labeled with IdU (AlexaFluor 488) only, CldU
(AlexaFluor 633) only, both, or neither. PDCD2, programmed cell
death 2 protein. MKN28, parental MKN28 cells; MKN28-T, MKN28 cells
transfected with pcDNA3.1-PDCD2; MKN28-T-P, MKN28 cells transfected
with pcDNA3.1-p53 and pcDNA3.1-PDCD2; MKN45, parental MKN45 cells;
MKN45-T, MKN45 cells transfected with pcDNA3.1-PDCD2; MKN45-T-S,
MKN28 cells transfected with p53 shRNA and pcDNA3.1-PDCD2. PDCD2,
programmed cell death 2 protein. |
Effects of PDCD2 expressionon the
regulation of UVB-induced skin carcinogenesis in p53−/−
and p53+/+ nude mice
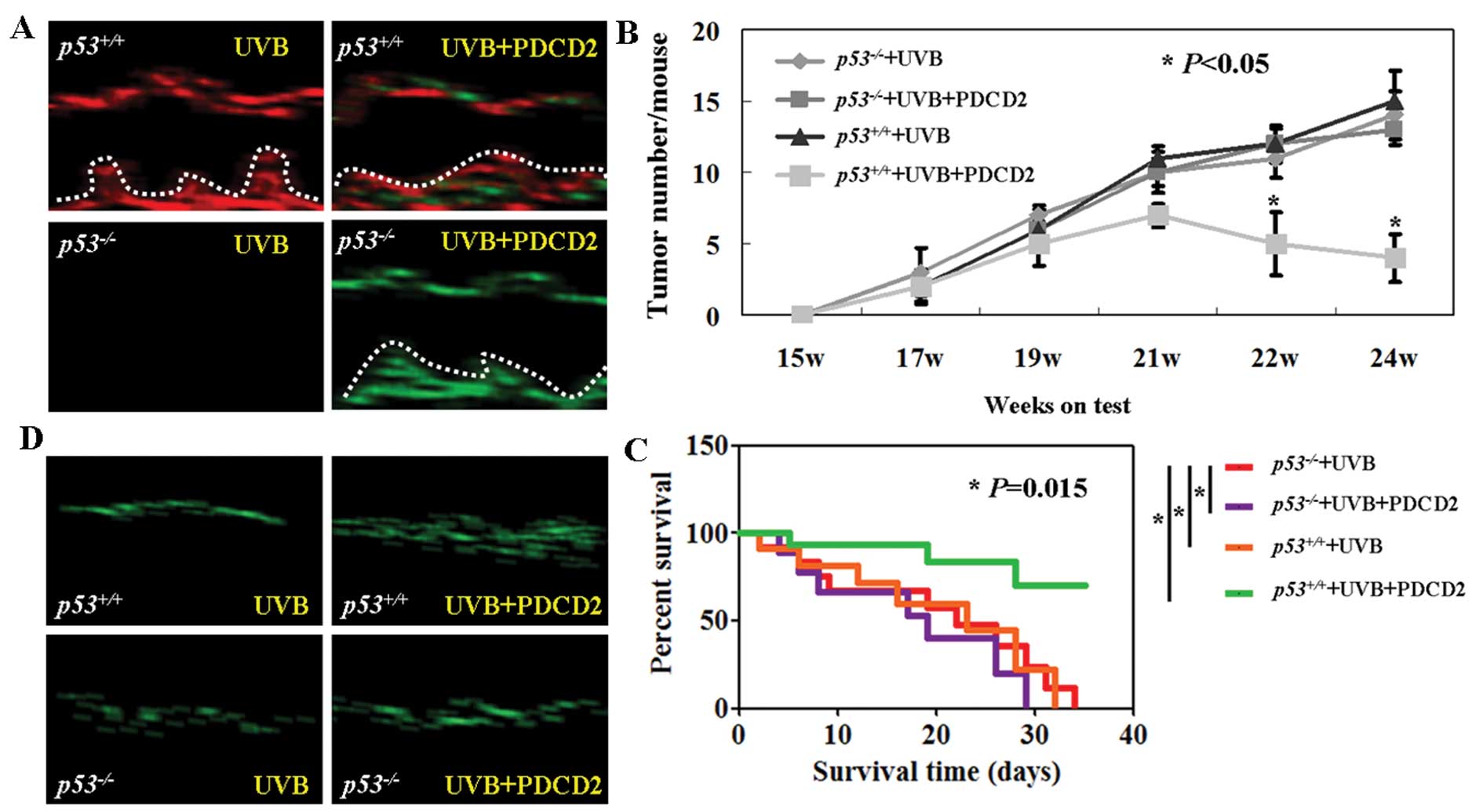
To determine whether p53 is required for the
antitumor effects of PDCD2, we utilized p53+/+
and p53−/− nude mice and induced skin
carcinogenesis with UVB irradiation. Expression and localization of
p53 and PDCD2 proteins were assessed using immunofluorescence. The
data revealed that PDCD2 protein was expressed in the skin tissues
of both the p53−/− and p53+/+
nude mice after tail vein injection of pCDNA3.1-PDCD2 (Fig. 4A). In contrast, there was no p53
expression observed in the p53−/− nude mice
(Fig. 4A). After establishing
UVB-induced skin tumorigenesis, PDCD2 expression resulted in a
significant reduction in tumor number in the
p53+/+ nude mice. In contrast, there was no
change in tumor number in the p53−/− nude mice
(P<0.05; Fig. 4B). Furthermore,
we also found that PDCD2-transfected p53+/+ mice
had a prolonged survival rate when compared with the survival of
the PDCD2-transfected p53−/− mice (P<0.05;
Fig. 4C). In addition, TUNEL
staining of skin tissues showed that PDCD2 expression induced
apoptosis in UVB-induced skin carcinoma of the
p53+/+ mice but not in the
p53−/− mice (Fig.
4D).
PDCD2 expression inhibits activity of the
ATM/Chk1/2/p53 signaling pathway
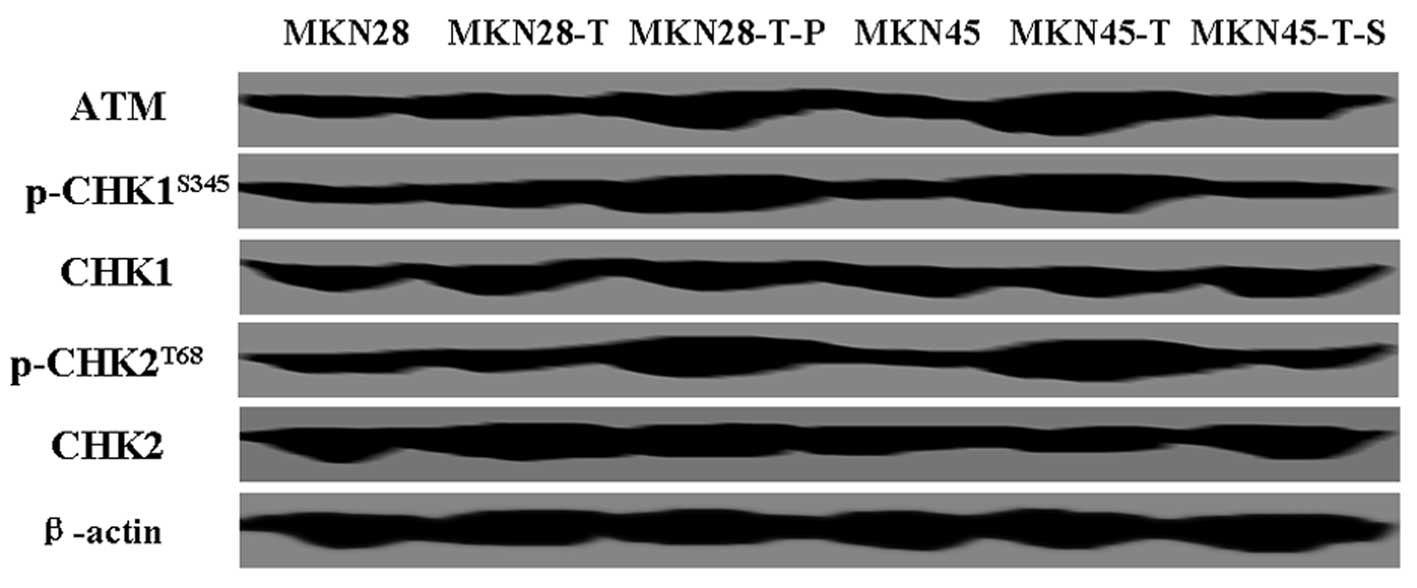
To explore the underlying mechanism responsible for
PDCD2-induced apoptosis and cell cycle arrest in gastric cancer
cells, we assessed the expression of cell cycle checkpoint
proteins, including the protein kinase ATM and checkpoint kinase 1
and 2 (Chk1/Chk2), which are major components of the mechanisms
that oversee the control of DNA replication and genomic integrity
(10). We found that levels of Chk1
and Chk2 were not significantly changed in the MKN28 and MKN45
cells after PDCD2 transfection (Fig.
5). However, the levels of p-Chk1 and p-Chk2 were significantly
increased in the MKN45 cells after PDCD2 transfection and in MKN28
cells after transfection with PDCD2 and p53 (Fig. 5). Furthermore, we found that the
levels of ATM, the upstream protein of Chk1 and Chk2, were also
increased (Fig. 5).
Discussion
PDCD2 protein plays a role in embryonic development
and tissue remodeling by inducing apoptosis, and altered PDCD2
expression could contribute to the development of human cancers.
Indeed, in the present study, we detected reduced levels of PDCD2
mRNA and protein in gastric cancer tissues when compared to these
levels in paired normal tissues. Our study is the first report to
show the loss of PDCD2 expression in gastric cancer tissues.
Previous studies have mainly focused on the role of PDCD2 in
hematological tumors. Baron et al (12) showed that PDCD2 expression induces
apoptosis of human erythroleukemia cells via activation of
caspases. Barboza et al (13) reported that knockdown of PDCD2
expression in two different cancer cell lines (leukemia Jurkat
cells and lung cancer A549 cells) significantly impairs cell
proliferation and cell progression to S phase of the cell cycle. In
the present study, we confirmed that PDCD2 expression induced
apoptosis and S phase arrest in p53-expressing gastric cancer
cells. Furthermore, our data showed that p53 knockdown blocked the
effects of PDCD2 on gastric cancer cells, but p53 activity was
enhanced in PDCD2-transfected gastric cancer cells compared with
the control cells.
However, our ex vivo data on p53 expression
in gastric cancer tissues vs. paired normal mucosa conflict with
our present knowledge on p53 protein in cells, i.e., the half-life
of p53 protein is usually short and immunohistochemistry would not
be able to detect p53 expression in normal tissues. However,
certain p53 mutations will prolong the half-life of p53 protein and
thus, immunohistochemistry can easily detect p53 expression in
cells (14). In our present study,
we showed that normal gastric mucosa expressed more p53 protein
than did gastric cancer tissue. One explanation could be ‘the field
carcinogenesis’ and the adjacent normal mucosae are not ‘real’
normal cells and that ‘the field carcinogenesis’ has altered gene
expression and is committed to tumorigenesis (15). Indeed, gastric cancer development is
usually involved in premalignant diseases, such as dysplasia or
chronic gastritis and H. pylori infection is a single most
important risk factor in developing gastric cancer (16,17).
In this context, the adjacent normal tissue may not be really
‘normal’. Furthermore, our present data revealed that PDCD2
expression was associated with prolonged survival of patients with
gastric cancer, whereas p53 expression was associated with poor
prognosis of gastric cancer. The latter data were consistent with
those of previous literature reports (18,19).
The former data are novel and have not been reported by any study
in the present PubMed database.
Moreover, we further investigated the role of p53 in
mediating the effects of PDCD2 on cells by producing a mouse model
of UVB-induced skin carcinogenesis in p53−/− and
p53+/+ nude mice. Our data showed that PDCD2
expression inhibited UVB-induced skin carcinogenesis and tumor
growth by inducing apoptosis in skin cancer cells in
p53+/+ nude mice, but this did not occur in the
p53−/− nude mice. Taken together, our in
vivo and in vitro data indicate that the antitumor
activities of PDCD2 are dependent on p53 expression.
In addition, we showed that activity of the
ATM/Chk2/p53 pathway also mediated the antitumor effects of PDCD2
in the regulation of gastric cancer cell apoptosis and S phase
arrest. There are four cell cycle checkpoints, i.e., the
G1, G2/M, intra-S and replication checkpoints
during DNA damage (20,21). If DNA damage is beyond repair, then
the cells will commit to apoptosis through induction of apoptosis
pathways (22). ATM kinase could
transduce DNA damage signals to checkpoint proteins. Activation of
ATM induces phosphorylation and activation of downstream targets,
including Chk1 and Chk2 (23);
thus, both Chk1 and Chk2 play important roles in the DNA damage
response and in cell cycle checkpoints, including the S phase
checkpoint (24,25). The present study provides evidence
that PDCD2 expression induces expression of ATM, p-Chk1 and p-Chk2
proteins and S phase arrest and apoptosis in gastric cancer cells
that express p53, although a previous study provides compelling
evidence supporting the role of PDCD2 in cell differentiation. For
example, Kokorina et al (26) demonstrated that PDCD2 knockdown
regulates the stemness of hematopoietic cells via controlling the
expression of two hematopoietic progenitor cell markers, c-MYB and
GATA-2, whereas loss of PDCD2 function(s) in zebra fish perturbed
hematopoietic stem cell differentiation due to mitotic defects
during cell cycle progression and p53-independent apoptosis
(27). Scarr and Sharp (28) found that PDCD2 is a negative
regulator of host cell factor-1 in a hamster cell line. These
studies on PDCD2 were all conducted in normal cells, but only a few
reports have shown the role of PDCD2 in cancer cells. Baron et
al (12) indeed revealed that
PDCD2 expression induced the apoptosis of Burkitt lymphoma cells by
activation of caspases. However, to date, there has been no report
showing that the antitumor activity of PDCD2 is p53 dependent.
However, it remains to be determined whether loss of PDCD2
expression impacts the ability of p53 to induce apoptosis in cancer
cells. Furthermore, p53 may not be the only protein with which
PDCD2 associates and thus, a number of pathways may need to be
altered after loss of PDCD2 expression.
In conclusion, our present study provides
proof-of-principle data, and future studies will explore whether
PDCD2 can be used as a biomarker to predict the outcomes of gastric
cancer patients and whether the target of PDCD2 can be used as a
novel strategy in the treatment of gastric cancer patients.
Acknowledgements
This study was supported in part by grants from the
Science and Technology Plan for Social Development of the Zhejiang
Provincial Science Department (2014C33236).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tahara E: Genetic pathways of two types of
gastric cancer. IARC Sci Publ. 157:327–349. 2004.PubMed/NCBI
|
|
3
|
Owens GP, Hahn WE and Cohen JJ:
Identification of mRNAs associated with programmed cell death in
immature thymocytes. Mol Cell Biol. 11:4177–4188. 1991.PubMed/NCBI
|
|
4
|
Kawakami T, Furukawa Y, Sudo K, et al:
Isolation and mapping of a human gene (PDCD2) that is highly
homologous to Rp8, a rat gene associated with programmed cell
death. Cytogenet Cell Genet. 71:41–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merup M, Moreno TC, Heyman M, et al: 6q
deletions in acute lymphoblastic leukemia and non-Hodgkin’s
lymphomas. Blood. 91:3397–3400. 1998.PubMed/NCBI
|
|
6
|
Minakhina S, Druzhinina M and Steward R:
Zfrp8, the Drosophila ortholog of PDCD2, functions in lymph gland
development and controls cell proliferation. Development.
134:2387–2396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mu W, Munroe RJ, Barker AK and Schimenti
JC: PDCD2 is essential for inner cell mass development and
embryonic stem cell maintenance. Dev Biol. 347:279–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramalho-Santos M, Yoon S, Matsuzaki Y,
Mulligan RC and Melton DA: ‘Stemness’: transcriptional profiling of
embryonic and adult stem cells. Science. 298:597–600. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skottman H, Mikkola M, Lundin K, et al:
Gene expression signatures of seven individual human embryonic stem
cell lines. Stem Cells. 23:1343–1356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan CW, Chan CC, Chao CC, Fan HA, Sheu DL
and Chan EC: Expression patterns of cell cycle and
apoptosis-related genes in a multidrug-resistant human colon
carcinoma cell line. Scand J Gastroenterol. 39:464–469. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lou Y, Peng Q, Nolan B, Wagner GC and Lu
Y: Oral administration of caffeine during voluntary exercise
markedly decreases tissue fat and stimulates apoptosis and cyclin
B1 in UVB-treated skin of hairless p53-knockout mice.
Carcinogenesis. 31:671–678. 2010. View Article : Google Scholar :
|
|
12
|
Baron BW, Hyjek E, Gladstone B, Thirman MJ
and Baron JM: PDCD2, a protein whose expression is repressed by
BCL6, induces apoptosis in human cells by activation of the caspase
cascade. Blood Cells Mol Dis. 45:169–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barboza N, Minakhina S, Medina DJ, et al:
PDCD2 functions in cancer cell proliferation and predicts relapsed
leukemia. Cancer Biol Ther. 14:546–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andreotti V, Ciribilli Y, Monti P, et al:
p53 transactivation and the impact of mutations, cofactors and
small molecules using a simplified yeast-based screening system.
PLoS One. 6:e206432011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yellapa A, Bitterman P, Sharma S, et al:
Interleukin 16 expression changes in association with ovarian
malignant transformation. Am J Obstet Gynecol. 210:272.e1–10. 2014.
View Article : Google Scholar
|
|
16
|
Everett SM, White KL, Drake IM, et al: The
effect of Helicobacter pylori infection on levels of DNA damage in
gastric epithelial cells. Helicobacter. 7:271–280. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canzian F, Franceschi S, Plummer M, et al:
Genetic polymorphisms in mediators of inflammation and gastric
precancerous lesions. Eur J Cancer Prev. 17:178–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lazăr D, Tăban S, Sporea I, et al: The
immunohistochemical expression of the p53-protein in gastric
carcinomas. Correlation with clinicopathological factors and
survival of patients. Rom J Morphol Embryol. 51:249–257. 2010.
|
|
19
|
Liu X, Wang S, Xia X, et al: Synergistic
role between p53 and JWA: prognostic and predictive biomarkers in
gastric cancer. PLoS One. 7:e523482012. View Article : Google Scholar
|
|
20
|
Harrison JC and Haber JE: Surviving the
breakup: the DNA damage checkpoint. Annu Rev Genet. 40:209–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Jaarsveld MT, Wouters MD, Boersma AW,
et al: DNA damage responsive microRNAs misexpressed in human cancer
modulate therapy sensitivity. Mol Oncol. 8:458–468. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith GC, Cary RB, Lakin ND, et al:
Purification and DNA binding properties of the
ataxia-telangiectasia gene product ATM. Proc Natl Acad Sci USA.
96:11134–11139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stracker TH, Usui T and Petrini JH: Taking
the time to make important decisions: the checkpoint effector
kinases Chk1 and Chk2 and the DNA damage response. DNA Repair
(Amst). 8:1047–1054. 2009. View Article : Google Scholar
|
|
25
|
Sun X, Liu B, Wang J, Li J and Ji WY:
Inhibition of p21-activated kinase 4 expression suppresses the
proliferation of Hep-2 laryngeal carcinoma cells via activation of
the ATM/Chk1/2/p53 pathway. Int J Oncol. 42:683–689. 2013.
|
|
26
|
Kokorina NA, Granier CJ, Zakharkin SO,
Davis S, Rabson AB and Sabaawy HE: PDCD2 knockdown inhibits
erythroid but not megakaryocytic lineage differentiation of human
hematopoietic stem/progenitor cells. Exp Hematol. 40:1028–1042.e3.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kramer J, Granier CJ, Davis S, et al:
PDCD2 controls hematopoietic stem cell differentiation during
development. Stem Cells Dev. 22:58–72. 2013. View Article : Google Scholar
|
|
28
|
Scarr RB and Sharp PA: PDCD2 is a negative
regulator of HCF-1 (C1). Oncogene. 21:5245–5254. 2002. View Article : Google Scholar : PubMed/NCBI
|