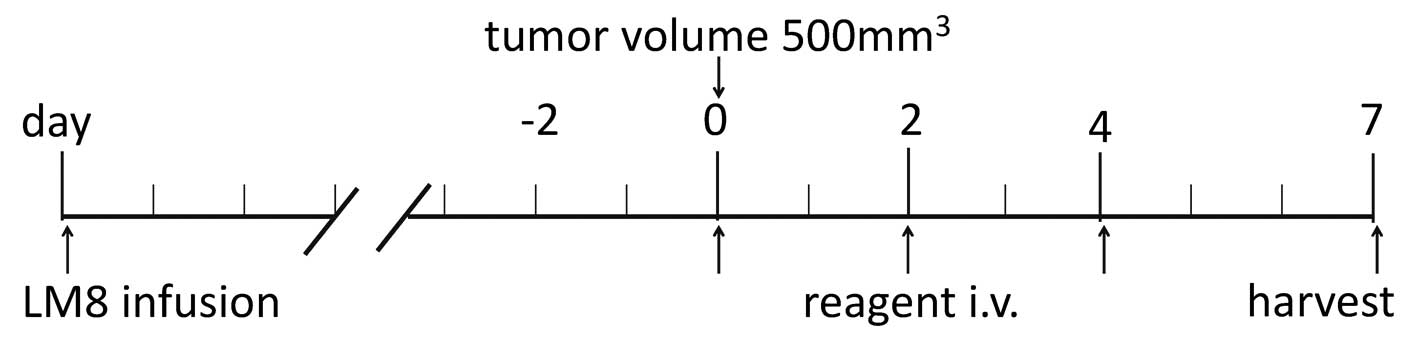
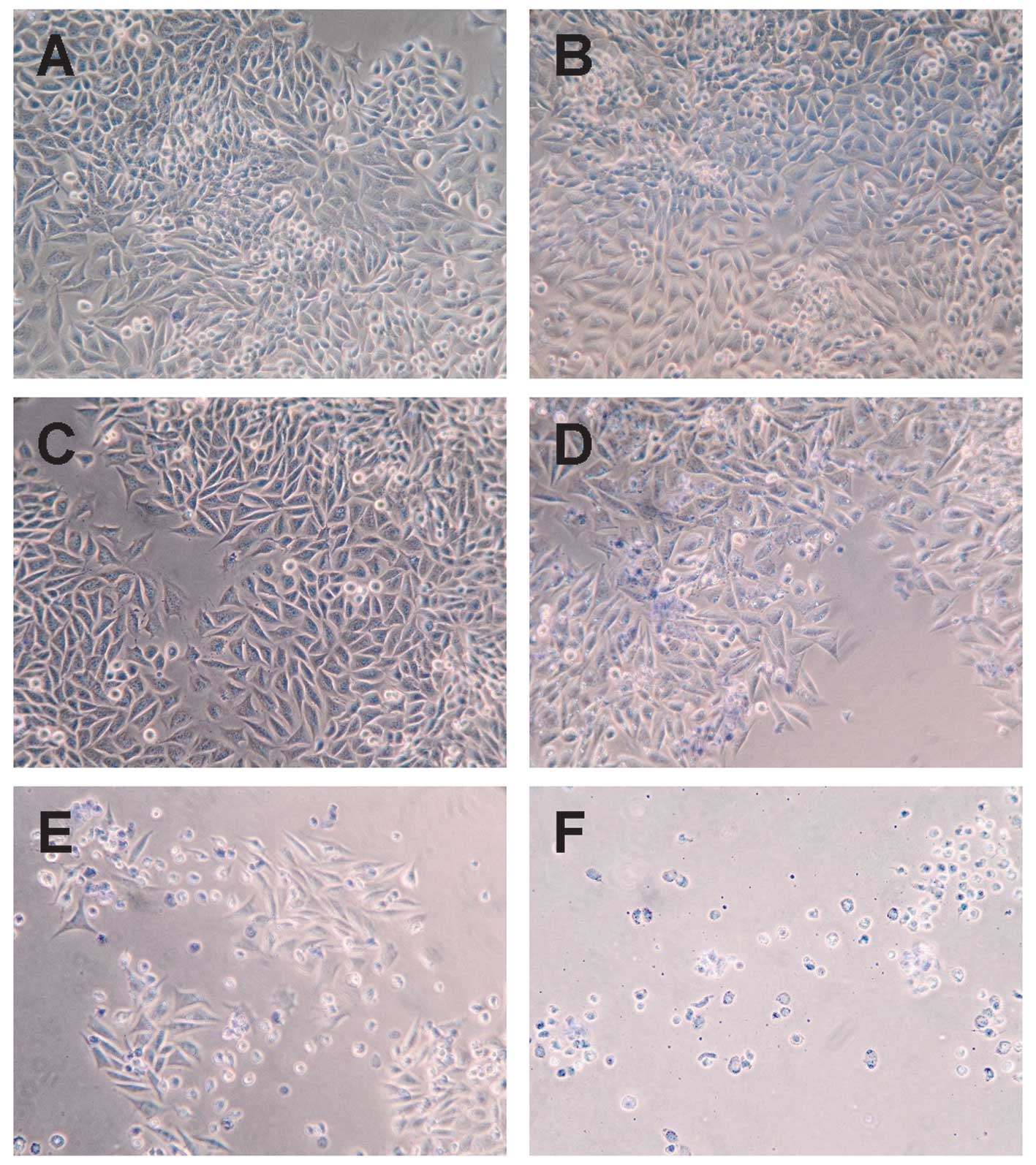
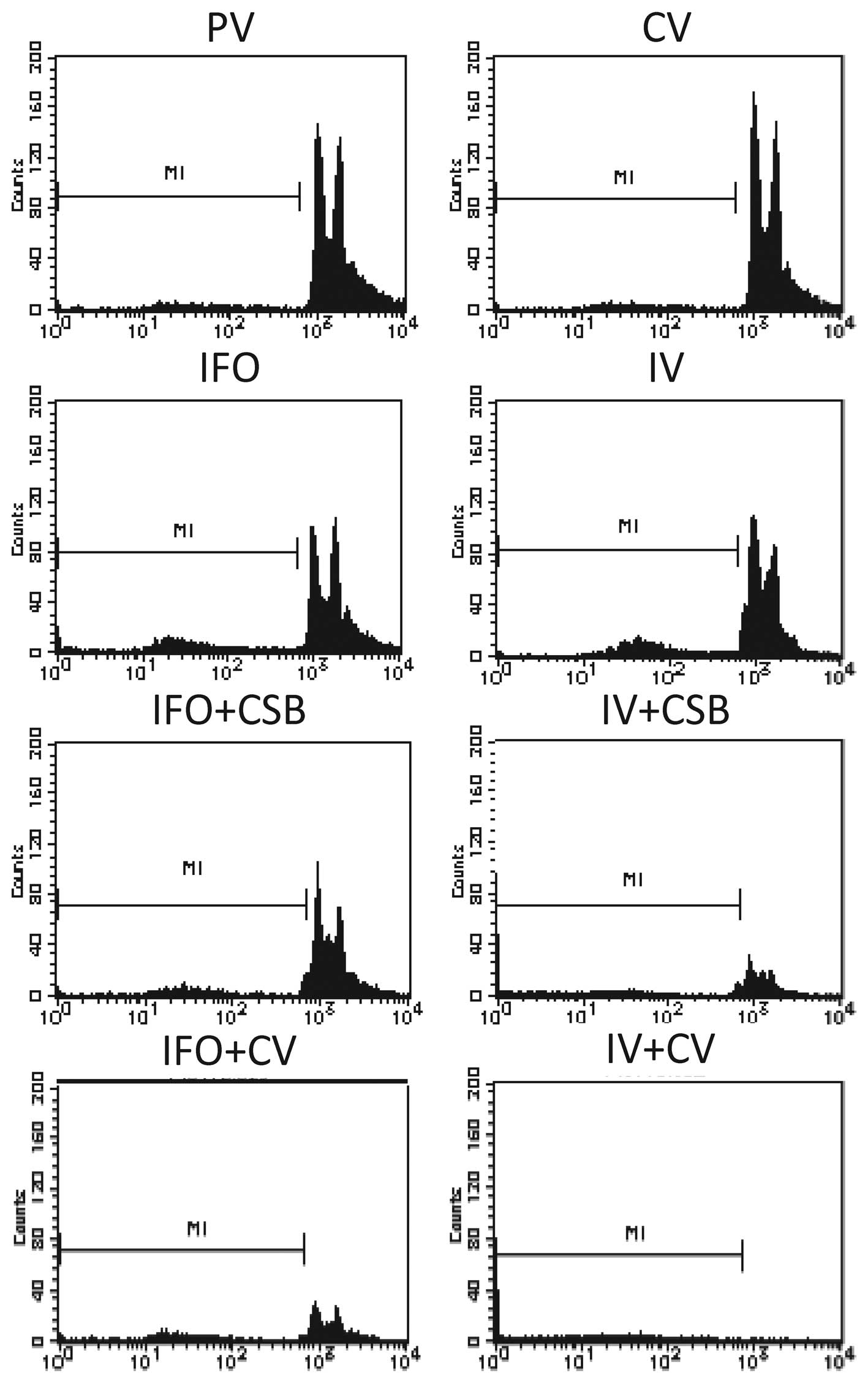
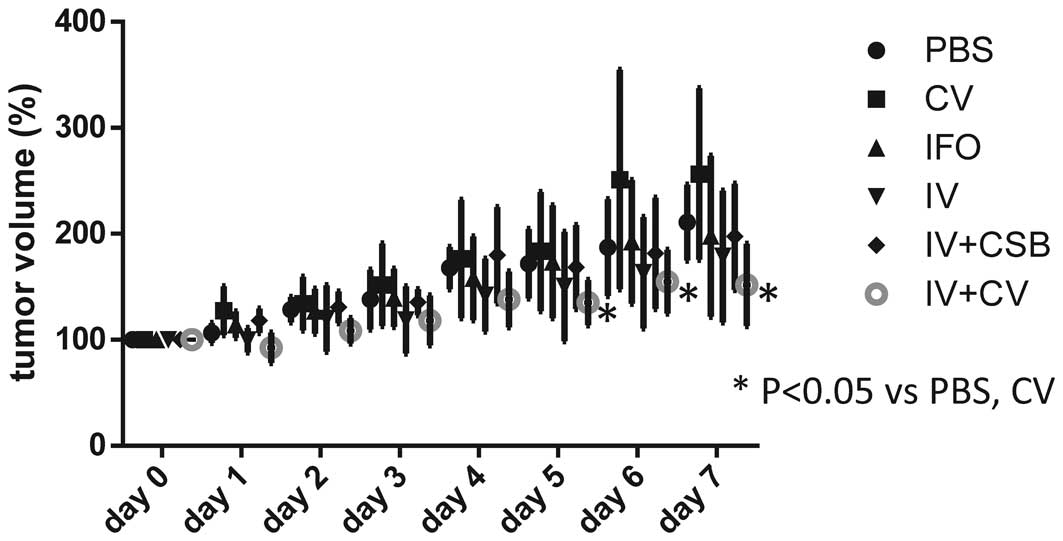
|
1
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar
|
|
3
|
Kimura H, Tsuchiya H, Shirai T, et al:
Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J
Orthop Sci. 14:556–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuchiya H, Tomita K, Mori Y, et al:
Caffeine-assisted chemotherapy and minimized tumor excision for
nonmetastatic osteosarcoma. Anticancer Res. 18:657–666.
1998.PubMed/NCBI
|
|
5
|
Hayashi K, Tsuchiya H, Yamamoto N, et al:
Impact of serum caffeine monitoring on adverse effects and
chemotherapeutic responses to caffeine-potentiated chemotherapy for
osteosarcoma. J Orthop Sci. 14:253–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang C and Fu ZX: Liposomal delivery and
polyethylene glycol-liposomal oxaliplatin for the treatment of
colorectal cancer (Review). Biomed Rep. 2:335–339. 2014.PubMed/NCBI
|
|
7
|
Sultana S, Khan MR, Kumar M, Kumar S and
Ali M: Nanoparticles-mediated drug delivery approaches for cancer
targeting: a review. J Drug Target. 21:107–125. 2013. View Article : Google Scholar
|
|
8
|
Brochu H, Polidori A, Pucci B and Vermette
P: Drug delivery systems using immobilized intact liposomes: a
comparative and critical review. Curr Drug Deliv. 1:299–312. 2004.
View Article : Google Scholar
|
|
9
|
Kato K, Walde P, Koine N, et al:
Temperature-sensitive nonionic vesicles prepared from Span 80
(sorbitan monooleate). Langmuir. 24:10762–10770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashi K, Shimanouchi T, Kato K, Miyazaki
T, Nakamura A and Umakoshi H: Span 80 vesicles have a more fluid,
flexible and ‘wet’ surface than phospholipid liposomes. Colloids
Surf B Biointerfaces. 87:28–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashi K, Tatsui T, Shimanouchi T and
Umakoshi H: Membrane interaction between Span 80 vesicle and
phospholipid vesicle (liposome): Span 80 vesicle can perturb and
hemifuse with liposomal membrane. Colloids Surf B Biointerfaces.
106:258–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omokawa Y, Miyazaki T, Walde P, et al: In
vitro and in vivo anti-tumor effects of novel Span 80 vesicles
containing immobilized Eucheuma serra agglutinin. Int J Pharm.
389:157–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hotz MA, Gong J, Traganos F and
Darzynkiewicz Z: Flow cytometric detection of apoptosis: comparison
of the assays of in situ DNA degradation and chromatin changes.
Cytometry. 15:237–244. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asai T, Ueda T, Itoh K, et al:
Establishment and characterization of a murine osteosarcoma cell
line (LM8) with high metastatic potential to the lung. Int J
Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elbayoumi TA and Torchilin VP: Enhanced
cytotoxicity of monoclonal anticancer antibody 2C5-modified
doxorubicin-loaded PEGylated liposomes against various tumor cell
lines. Eur J Pharm Sci. 32:159–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patterson WP and Khojasteh A:
Ifosfamide-induced renal tubular defects. Cancer. 63:649–651. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skinner R, Pearson AD, Coulthard MG, et
al: Assessment of chemotherapy-associated nephrotoxicity in
children with cancer. Cancer Chemother Pharmacol. 28:81–92. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skinner R, Sharkey IM, Pearson AD and
Craft AW: Ifosfamide, mesna, and nephrotoxicity in children. J Clin
Oncol. 11:173–190. 1993.PubMed/NCBI
|
|
19
|
McCune JS, Friedman DL, Schuetze S, Blough
D, Magbulos M and Hawkins DS: Influence of age upon
Ifosfamide-induced nephrotoxicity. Pediatr Blood Cancer.
42:427–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oberlin O, Fawaz O, Rey A, et al:
Long-term evaluation of Ifosfamide-related nephrotoxicity in
children. J Clin Oncol. 27:5350–5355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yonemoto T, Ishii T, Takeuchi Y, Hagiwara
Y, Iwata S and Tatezaki S: Recently intensified chemotherapy for
high-grade osteosarcoma may affect fertility in long-term male
survivors. Anticancer Res. 29:763–767. 2009.PubMed/NCBI
|
|
22
|
Ridola V, Fawaz O, Aubier F, et al:
Testicular function of survivors of childhood cancer: a comparative
study between ifosfamide- and cyclophosphamide-based regimens. Eur
J Cancer. 45:814–818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Longhi A, Macchiagodena M, Vitali G and
Bacci G: Fertility in male patients treated with neoadjuvant
chemotherapy for osteosarcoma. J Pediatr Hematol Oncol. 25:292–296.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomson B, Hawkins D, Felgenhauer J and
Radich J: RT-PCR evaluation of peripheral blood, bone marrow and
peripheral blood stem cells in children and adolescents undergoing
VACIME chemotherapy for Ewing’s sarcoma and alveolar
rhabdomyo-sarcoma. Bone Marrow Transplant. 24:527–533. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lotz JP, Andre T, Donsimoni R, et al: High
dose chemotherapy with ifosfamide, carboplatin, and etoposide
combined with autologous bone marrow transplantation for the
treatment of poor-prognosis germ cell tumors and metastatic
trophoblastic disease in adults. Cancer. 75:874–885. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams D, Crofton PM and Levitt G: Does
ifosfamide affect gonadal function? Pediatr Blood Cancer.
50:347–351. 2008. View Article : Google Scholar
|
|
27
|
Hayashi K, Tatsui T, Shimanouchi T and
Umakoshi H: Enhanced cytotoxicity for colon 26 cells using
doxorubicin-loaded sorbitan monooleate (Span 80) vesicles. Int J
Biol Sci. 9:142–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iwamoto T: Clinical application of drug
delivery systems in cancer chemotherapy: review of the efficacy and
side effects of approved drugs. Biol Pharm Bull. 36:715–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eckes J, Schmah O, Siebers JW, et al:
Kinetic targeting of pegylated liposomal doxorubicin: a new
approach to reduce toxicity during chemotherapy (CARL-trial). BMC
Cancer. 11:3372011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maeng JH, Lee DH, Jung KH, et al:
Multifunctional doxorubicin loaded superparamagnetic iron oxide
nanoparticles for chemotherapy and magnetic resonance imaging in
liver cancer. Biomaterials. 31:4995–5006. 2010. View Article : Google Scholar : PubMed/NCBI
|