Introduction
Cancer is one of the leading causes of mortality in
humans. However, this problem is exasperated by the complex
molecular nature of cancer and therefore requires a complex
therapeutic strategy. Efforts have focused on the ability of
natural compounds to induce programmed cell death and activate the
relevant apoptotic cascades (1–4).
Violacein is a violet pigment that is produced from
tryptophan by action of a cluster of five genes, designated as
vioABCDE, naturally by the bacterium Chromobacterium
violaceum (5,6). It has significant biological and
pharmacological activities, including antimicrobial,
antiparasitary, anti-diarrhoeal, and antifungal activities
(7–12). Violacein has been shown to possess
anticancer properties (13–21) and has been overexpressed in E.
coli K12 JM109 by cloning of the violacein gene cluster
(22). Ahmetagic et al found
that expression of the violacein gene cluster in the pPSX vector
(JM109 E. coli K12 strain) and mutations in the promoter
region produced hyper-producing strains of violacein (22).
Anticancer studies of violacein have shown efficacy
in a number of cell lines of both neoplastic and haematological
malignant origins. The most effective activity of violacein was
against MOLT-4 leukaemia, NCI-H460 non-small-cell lung cancer and
KM12 colon-cancer cell lines (GI50 ~30–60 nM) (23). Furthermore, Saraiva et al
found that uveal melanoma cell lines, 92.1 and OCM-1, were found to
be sensitive to violacein (GI50 ~1.69–2.21 μM) (21). Additionally, violacein was found to
inhibit the growth and proliferation of colorectal cancer cell
lines (16). The mechanism of
action in HCT116 cells was identified as induction of apoptosis.
Violacein was found to potentiate the effects of 5-fluorouracil in
HCT116 cells. The in vivo efficacy of violacein has been
assessed in intraperitoneal models of Ehrlich ascites tumour, which
showed increased lifespan in tumour-bearing mice (13).
In terms of the mechanism of action of violacein,
its role in the induction of apoptosis has been investigated and
evidence suggests cell type-dependent activity (23). Findings of previous studies have
indicated caspase-dependent apoptosis and production of reactive
oxygen species as the mechanism of action (14,16).
The most comprehensive mechanistic study was undertaken by Ferreira
et al and Kodach et al on leukaemia cells and
colorectal cancer cells, respectively (15,16).
Violacein was found to activate TNF receptor 1-mediated apoptosis
in HL60 leukaemia cells (15).
Furthermore, in HCT116 cells violacein was found to block cell
cycle at G1, upregulate the expression of p53, p27 and p21 levels
and decrease cyclin D1 expression. Most significantly, violacein
was found to inhibit the phosphorylation of Akt, a
serine-threonine-specific protein kinase involved in the
proliferation and survival of many cancer types (16).
This study aimed to compare the cytotoxic effects of
violacein in a number of cancer cells under normaxic and hypoxic
conditions. Furthermore, we investigated the in vivo effect
of violacein in subcutaneous tumour models. The results showed that
hypoxia significantly potentiated the cytotoxic effects of
violacein. Violacein was also found to cause tumour regression and
have an increased lifespan in tumour-bearing mice. The results
suggest additional investigations into the potential antitumour
capacity of violacein.
Materials and methods
Mammalian cell culture conditions
Human cancer cell lines, A549 (lung), PC3
(prostate), HCT116 (colon), HT29 (colon), MCF-7 (breast), A431
(melanoma), HN5 (head and neck), and HeLa (cervix), were obtained
from the American Type Culture Collection. The cell lines were
cultured in Dulbecco’s modified essential medium (Invitrogen-Life
Technologies, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 110 mg/l sodium
pyruvate, 25 mM HEPES, 100 U/ml of penicillin and 100 μg/ml of
streptomycin solution, in an atmosphere of 5% CO2 and
95% air at 37°C. Hypoxic conditions were achieved by placing
culture plates in a GasPak EZ pouch (BD) and incubating at
37°C.
Expression and extraction of
violacein
Violacein was expressed in the recombinant E.
coli strain JM109 containing the violacein expression vector
pBSX-vio-opv. This strain was kindly provided by Professor John
Pemberton, The University of Queensland, Australia. The recombinant
violacein expression vector pBSX-vio-opv was also transformed into
the tumour-targeting Salmonella typhimurium strain, VPN20009
(24). Rosenberg et al,
showed that VPN20009 is able to colonise the tumour
microenvironment 10,000-fold higher than that in liver in mice
(24). The violacein-producing
strains were cultured on PYE agar for 48 h until dark purple
colonies were visible, indicating the production of violacein
(22). The colonies were
subsequently scraped from the culture plates and resuspended in
water and centrifuged at 14,000 × g. After centrifugation, the
cells were resuspended in ethanol to extract the violacein. After
centrifugation at 14,000 × g the supernatant, containing the
violacein, was collected and stored at 4°C until further use. The
concentration of the extracted violacein was determined according
to the methods of Duràn et al (25).
In vitro efficacy of violacein
Cytotoxicity of violacein in all the cell lines was
studied by using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays (Invitrogen). Cells were seeded at a density of
1×104 cells per well in a 96-well plate. After seeding,
the cells were exposed to violacein treatment for another 24 h, and
then MTT reagent was added following the manufacturer’s
instructions. The absorbance was read at 570 nm and IC50
values were calculated by Prism 5 (GraphPad Software).
Apoptosis assays
Cancer cell lines were seeded at a density of
1×104 cells per well in white μClear 96-well plates
(Greiner) and exposed to violacein for 24 h. Caspase-3 activity was
evaluated using Caspase-Glo 3/7 assay systems (Promega, Madison,
WI, USA) according to the manufacturer’s instructions. Luciferase
intensity was measured by POLARstar Omega at 24 h post-violacein
addition.
Mouse xenograft models and violacein
antitumour evaluation
Experiments involving animals were approved by the
Griffith University animal ethics committee (AEC No. MSC/01/08). To
establish mouse models, 5×106 HN5 cells in PBS (200 μl)
were subcutaneously injected into the right flank of female BALB/c
nude mice. When tumours reached a size of 320 mm3, the
mice (aged 8 weeks and weighing 16–18 g) were randomly divided into
two groups (6 mice per group): the treatment group, which was
administered 0.7 mg/kg body weight violacein, while an equal amount
of injection solution without violacein was administered to the
control group. Treatments were delivered by intraperitoneal
injection. Tumour volume was measured using a calliper and
calculated using the formula: 0.5(l × w2). When the
tumour volume reached 1 cm3, mice were sacrificed by
cervical dislocation.
Statistical analysis
Experiments were repeated at least three times to
obtain statistically significant data. The results are presented as
means with standard error of mean. The Student’s t-test was used to
analyse the data and p<0.05 was considered statistically
significant.
Results
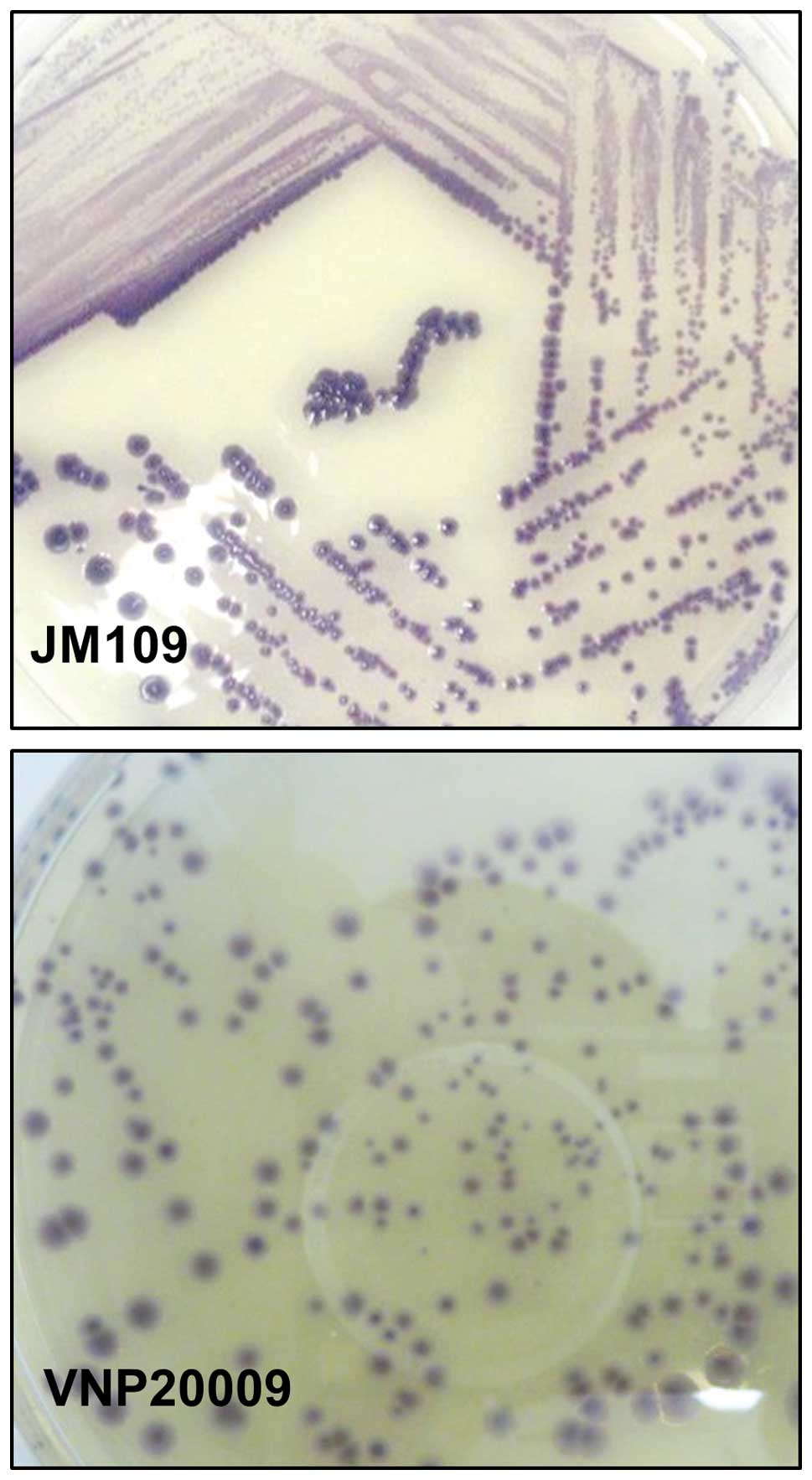
Production of violacein
Recombinant E. coli and Salmonella
typhimurium strain VPN20009 containing the violacein
biosynthetic clusters were cultured on PYE agar. After 48 h intense
purple-coloured colonies were visible in the two strains (Fig. 1). The intense purple colourisation
in the VPN20009 plates showed that it is capable of producing
violacein from the pBSX-vio-opv expression vector. Following
extraction and purification, the violacein content was measured by
spectrophotometry at 570 nm. From a batch of 20 plates, 5 ml of 3
mM violacein was extracted in ethanol. This extract was
subsequently used in the cytotoxic assays and in the xenograft
tumour models.
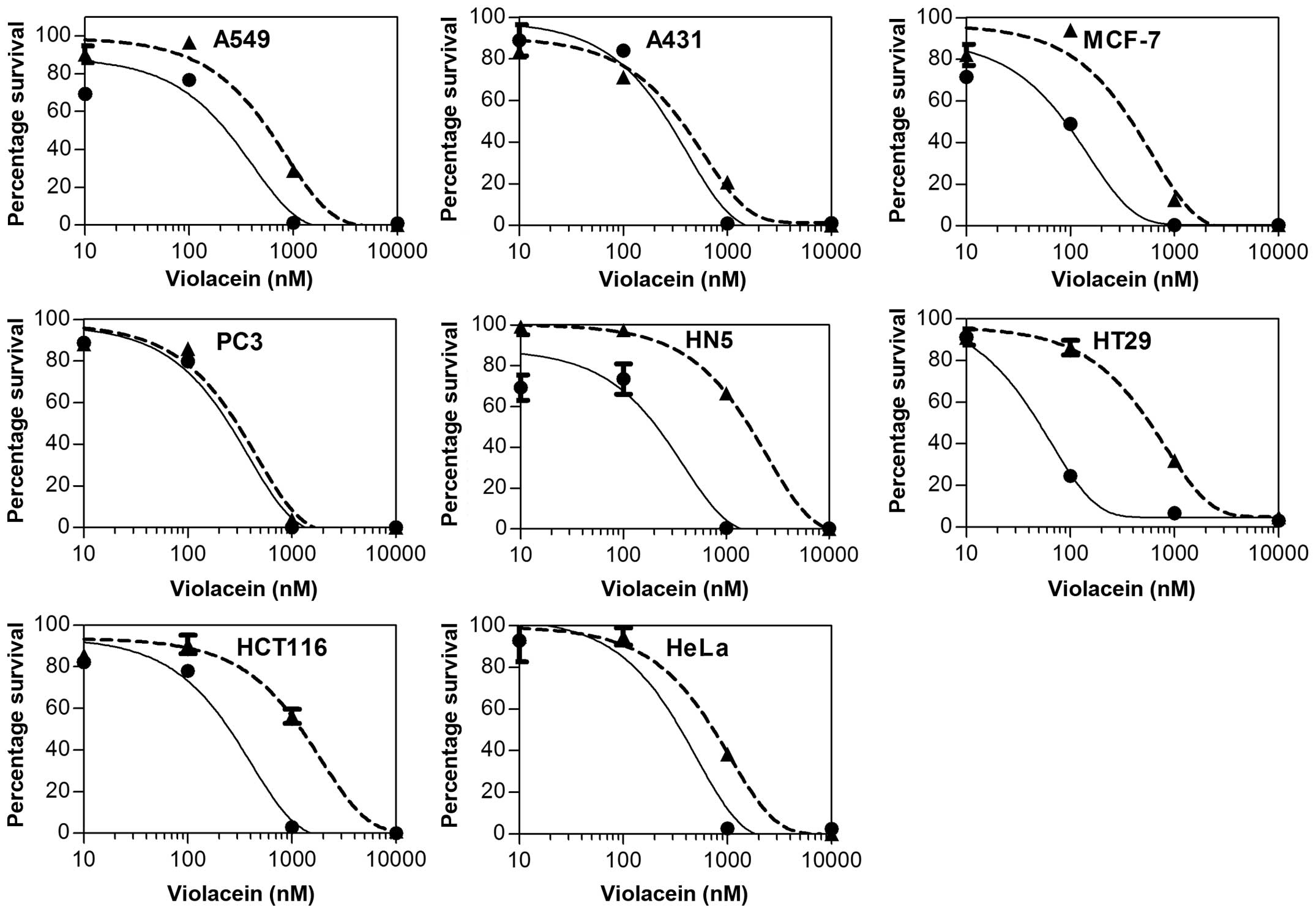
Violacein is a potent inhibitor of cancer
cell proliferation
To examine the effects of violacein on cells MTT
proliferation assays were used. MTT was converted to formazan by
living cells and was detected by spectrophotometric quantification.
Violacein was tested at a concentration range of 0–10 μM to yield a
dose response for subsequent IC50 calculation. The
results showed that violacein was highly potent against A549
non-small cell lung cancer, A431 Melanoma, MCF-7 breast cancer, PC3
prostate cancer, HT29 colon cancer, and HeLa cervical cancer
(Fig. 2). Furthermore, violacein
was effective against HCT116 colon and HN5 head and neck cancer,
although not as potent as against the other cell lines. The dose
response curve was used to calculate IC50 concentrations
for the various cell lines (Table
I). Based on the IC50 values violacein was most
effective against A431, MCF-7 and PC3 cancer cells, while HN5 and
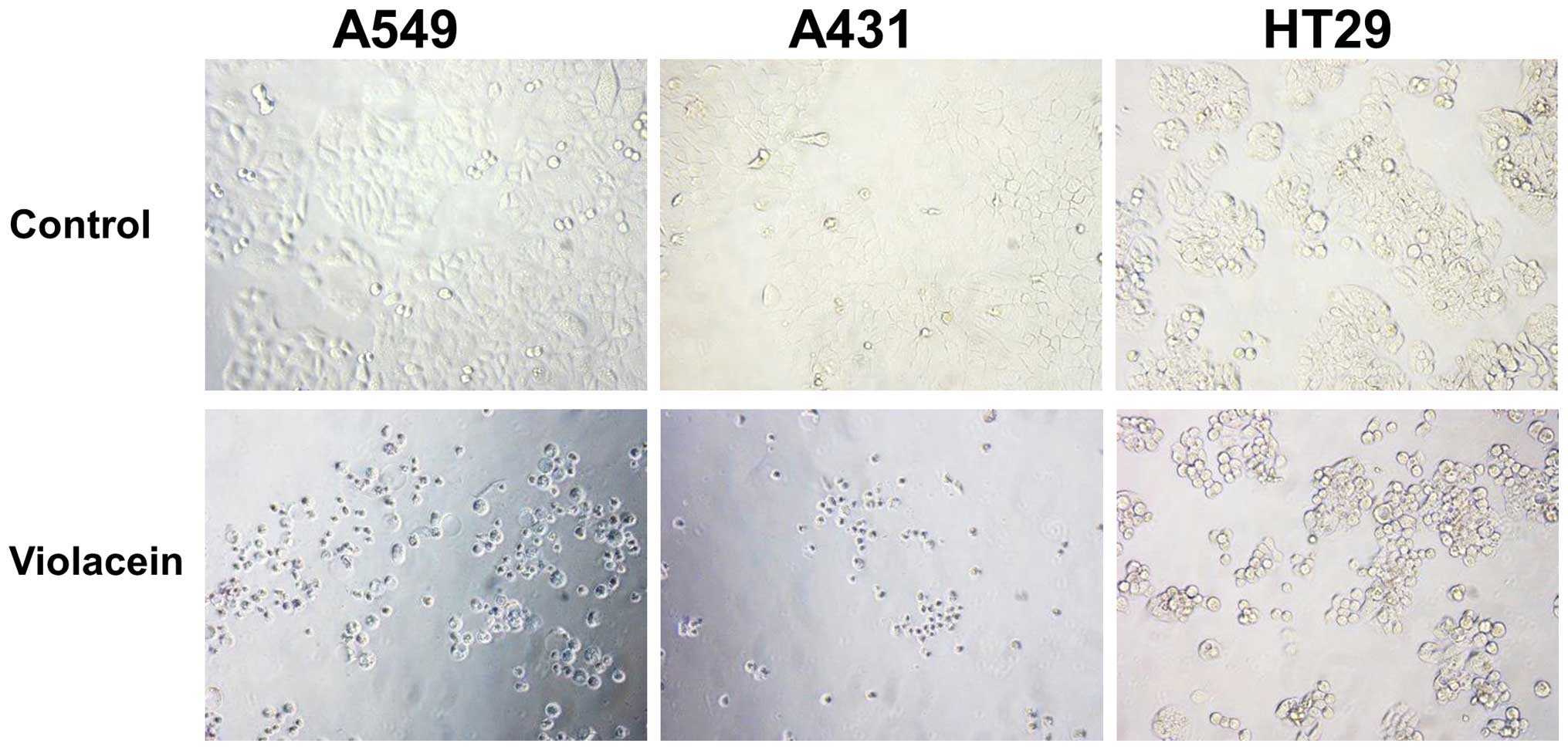
HCT116 were the least affected. A microscopic analysis of the
treated cells revealed fewer and dead cells compared to the
non-treated group (Fig. 3).
Furthermore, the morphology of violacein-treated cells was round
compared to a flat appearance of the control cells.
 | Table IAnticancer activity of violacein. |
Table I
Anticancer activity of violacein.
| Cell line | Tissue origin | Violacein
IC50 (nM) | Fold drop
IC50 |
|---|
|
|---|
| Hypoxia | Normoxia |
|---|
| A549 | Lung | 285.5 | 601.8 | 2.1 |
| A431 | Skin | 288.2 | 412.6 | 1.4 |
| MCF-7 | Breast | 105.6 | 421 | 4 |
| PC3 | Prostate | 268.5 | 318.2 | 1.2 |
| HCT116 | Colon | 283.5 | 1348 | 4.8 |
| HT29 | Colon | 45.2 | 569.4 | 12.6 |
| HN5 | Head and neck | 268.4 | 1738 | 6.5 |
| HeLa | Cervix | 351.9 | 744 | 2.1 |
Hypoxia sensitises cancer cells to
violacein
To determine the effects of violacein on cancer
cells in hypoxic conditions, the cells were cultured in a GasPak EZ
pouch (BD) at 37°C. MTT proliferation assays showed that hypoxia
sensitised all the cancer cells to violacein. The most significant
effect was on MCF-7 breast ductal carcinoma, HCT116 and HT29 colon
cancer, and HN5 head and neck squamous carcinoma cells (Fig. 2). Furthermore, a dose response curve
was used to calculate the IC50 values for violacein
under hypoxia and normoxia. It was found that HT26 was 12.6-fold
more sensitive to violacein under hypoxia when compared to normoxic
conditions (Table I). The
IC50 values for PC3 prostate cancer and A431 melanoma
cells were not significantly affected by hypoxia.
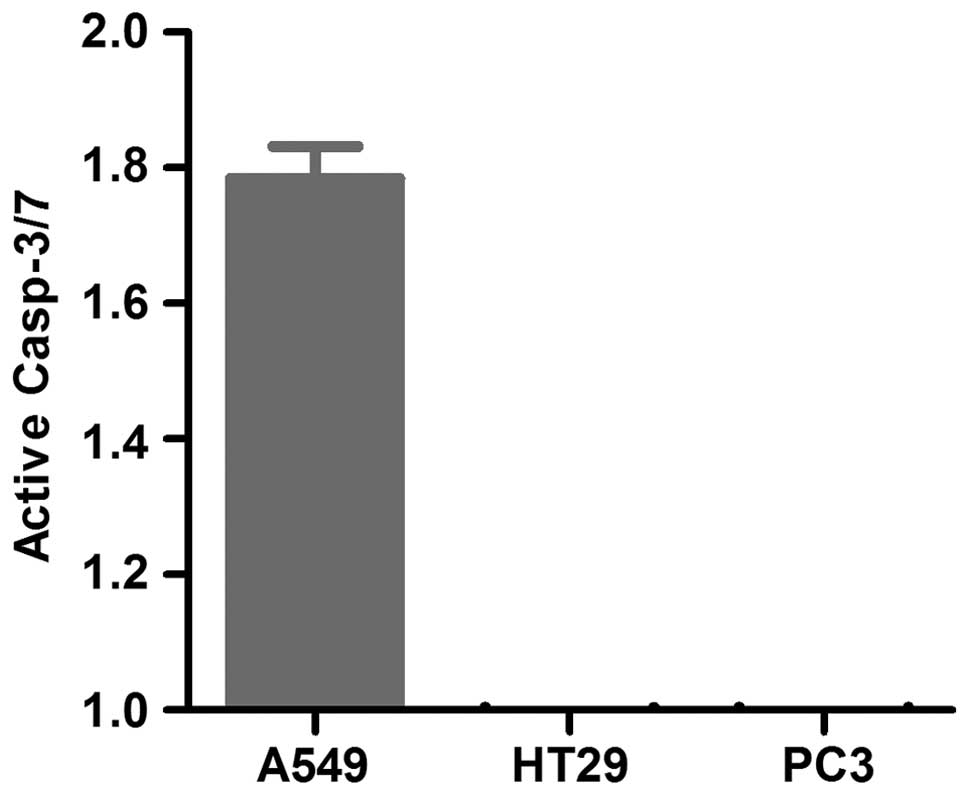
Violacein activates caspase-dependent
apoptosis in A549 cells only
To determine whether violacein activates caspase
dependent apoptosis, the cells were seeded in 96-well white walled
plates, exposed to violacein and the caspase-3/7 activity was
measured. Caspase-3/7 activity was induced in A549 non-small cell
lung cancer cells only when treated with violacein (Fig. 4). The remaining cell lines tested
had similar caspase-3/7 activity in the treated and non-treated
cells.
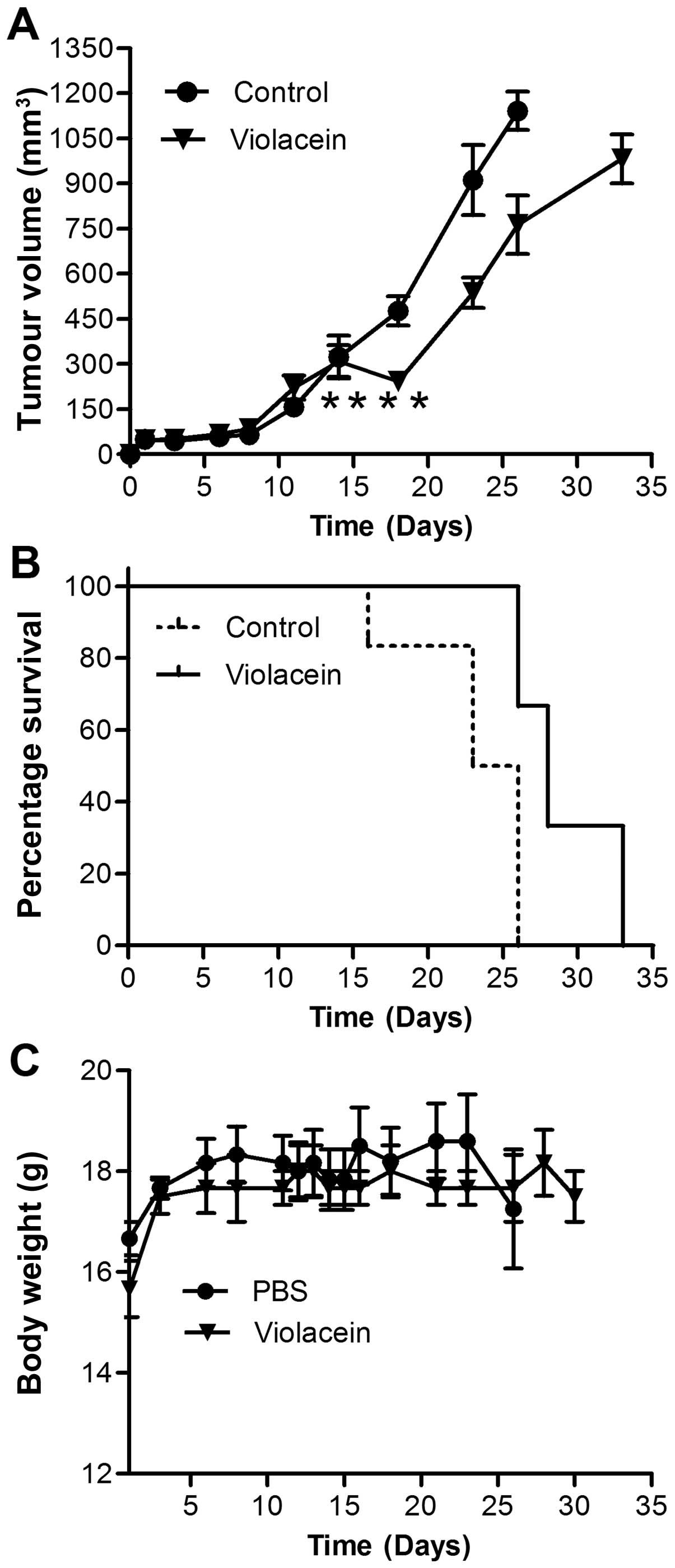
In vivo activity of violacein
Having established the efficacy of violacein in the
in vitro culture system we elucidated its activity in
xenograft tumour models. Head and neck squamous carcinoma
subcutaneous xenograft models were established in BALB/c nude mice
and treated with daily doses of 0.7 mg/kg of body weight for 4
days. It was found that tumours treated with violacein regressed
during the treatment period while the non-treated control tumours
continued to grow (Fig. 5).
Furthermore, violacein-treated tumours had a significantly slower
growth rate compared to the control groups. It was also found that
violacein extended the life span of animals during the treatment
period, however, the mortality rate reached similar levels as that
of the controls when treatment was terminated. Violacein
administration did not affect the weight of the animals and treated
animals were not any different to controls in terms of behaviour or
phenotypic features.
Discussion
Cancer remains a devastating disease inflicting
significant mortality rates to humans worldwide despite significant
breakthroughs in its diagnosis and treatment. Natural compounds
have been extensively studied for their biological activities and
in particular their ability to induce programmed cell death or
apoptosis in cancer cells (1–4).
Understanding the mechanisms by which these drugs kill cancer cells
provides the possibility of engineering novel drugs with improved
anticancer activity.
Violacein produced by the bacterium
Chromobacterium violaceum, a broad spectrum bioactive
compound, has been shown to have anticancer activity (23). Our study focused on understanding
the mechanisms of action of violacein in cancer cells.
Cell proliferation assays were used to determine the
in vitro effects of violacein on different cancer cell
lines. Our results indicated a broad range and cell-specific
violacein-mediated cytotoxicity. Furthermore, active caspase-3/7
measurements showed that apoptosis was induced only in A549 cells,
indicating a differential effect of violacein on inducing
apoptosis. A number of other investigators have shown that
violacein induces apoptosis in colorectal cancer cells (16), Ehrlich ascites tumour cells
(13), and HL60 leukemia cells
(15). Notably, Ferreira et
al found that violacein only induced apoptosis in HL60 leukemia
cells and was ineffective in other types of leukemia cells
indicating a differential apoptosis-inducing activity (15). Furthermore, we found that the
anti-proliferative activity of violacein increased when the cells
were exposed to hypoxic conditions. In addition, the most
significant effect was observed in HT29 (colon cancer) and HN5
(head and neck cancer) cells showing a 12.6- and 6.5-fold decrease,
respectively, in IC50 from normoxic conditions. Of note,
chemotherapeutic drugs that target proliferating cells are less
effective in hypoxic conditions when cancer cells are quiescent
(26). Furthermore, it has been
well established that the quiescent cells, especially cancer stem
cells, are involved in tumour progression and metastasis (27). This has limited the use of many
chemotherapeutic, making them mostly palliative. The apparent
increased in vitro activity of violacein in hypoxic
conditions suggests a capacity in killing non-proliferative cells,
a property with potential promise in combating chemo-resistant
cells.
Since violacein can be produced in E. coli
carrying the recombinant plasmid pBSX-vio-opv, we determined the
ability of the tumour-colonising Salmonella typhimurium
strain VPN20009 to produce violacein from pBSX-vio-opv. Our results
showed that this strain was capable of producing violacein when
transformed with the pBSX-vio-opv plasmid. Furthermore,
cytotoxicity assays showed that the violacein produced was
effective in killing cancer cells. Combining the tumour-colonising
property of Salmonella typhimurium strain VPN20009 with
violacein expression offers a potential target delivery system for
violacein to the tumour core (28).
Future studies are needed to ascertain the efficacy of this
genetically modified strain.
The efficacy of violacein was also assessed in
vivo on xenograft tumour models of HN5 (head and neck cancer)
cells in mice. Our results showed that violacein regressed tumour
volume and increased survival. However, tumour volumes reached the
same levels as those of the controls when treatment was stopped.
Additional studies with a prolonged dose regime are likely to yield
an improved response to violacein. The in vivo results are
encouraging for future studies to employ the tumour-targeting
ability of VNV20009 (24,29) for the local production of violacein
in the tumour microenvironment.
In conclusion, our findings confirm the
anti-proliferative properties of violacein in a number of cancer
cell lines and that this activity is increased in hypoxia-induced
cells. Furthermore, violacein can be expressed in the antitumour
Salmonella typhimurium strain VPN20009, a potentially
promising strategy to deliver violacein locally to tumours. Our
study further showed that violacein apoptotic activity is
differential and in vivo violacein showed anti-proliferative
activity against subcutaneous head and neck tumours. The findings
of this study require further studies to ascertain the anticancer
properties of violacein and its local delivery by genetically
modified tumour-colonising bacteria harbouring the violacein
biosynthetic cluster.
Acknowledgements
This study was supported by the Dr Jian Zhou Smart
State fellowship from the Queensland government, and grants from
the National Health and Medical Research Council and Cancer
Council, Queensland to M.Q.W. The National Natural Science
Foundation of China (grant no. 81160297) supported Tiefeng Xu. We
would like to thank other members of the Wei Laboratory for their
support and helpful comments. Special thanks is extended to
Professor John Pemberton for providing the violacein producing
E. coli strain (pBSX-vio-opv).
Abbreviations:
|
IC50
|
50% inhibitory concentration
|
|
MTT
|
3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Tabata K, Yamazaki Y, Okada M, et al:
Diarylheptanoids derived from Alpinia officinarum induce apoptosis,
S-phase arrest and differentiation in human neuroblastoma cells.
Anticancer Res. 29:4981–4988. 2009.
|
|
2
|
Uddin S, Khan AS and Al-Kuraya KS:
Developing curcumin into a viable therapeutic for lymphoma. Expert
Opin Investig Drugs. 18:57–67. 2009. View Article : Google Scholar
|
|
3
|
Wang TT, Schoene NW, Kim YS, Mizuno CS and
Rimando AM: Differential effects of resveratrol and its naturally
occurring methylether analogs on cell cycle and apoptosis in human
androgen-responsive LNCaP cancer cells. Mol Nutr Food Res.
54:335–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li C, Hashimi SM, Cao S, et al: The
mechanisms of chansu in inducing efficient apoptosis in colon
cancer cells. Evid Based Complement Alternat Med.
2013:8490542013.PubMed/NCBI
|
|
5
|
August PR, Grossman TH, Minor C, et al:
Sequence analysis and functional characterization of the violacein
biosynthetic pathway from Chromobacterium violaceum. J Mol
Microbiol Biotechnol. 2:513–519. 2000.PubMed/NCBI
|
|
6
|
Pemberton JM, Vincent KM and Penfold RJ:
Cloning and heterologous expression of the violacein biosynthesis
gene cluster from Chromobacterium violaceum. Curr Microbiol.
22:355–358. 1991. View Article : Google Scholar
|
|
7
|
Andrighetti-Fröhner CR, Antonio RV,
Creczynski-Pasa TB, Barardi CR and Simões CM: Cytotoxicity and
potential antiviral evaluation of violacein produced by
Chromobacterium violaceum. Mem Inst Oswaldo Cruz. 98:843–848. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonisamy P and Ignacimuthu S:
Immunomodulatory, analgesic and antipyretic effects of violacein
isolated from Chromobacterium violaceum. Phytomedicine. 17:300–304.
2010. View Article : Google Scholar
|
|
9
|
Antonisamy P, Kannan P and Ignacimuthu S:
Anti-diarrhoeal and ulcer-protective effects of violacein isolated
from Chromobacterium violaceum in Wistar rats. Fundam Clin
Pharmacol. 23:483–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cazoto LL, Martins D, Ribeiro MG, Durán N
and Nakazato G: Antibacterial activity of violacein against
Staphylococcus aureus isolated from bovine mastitis. J Antibiot.
64:395–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leon LL, Miranda CC, DeSouza AO and Durán
N: Antileishmanial activity of the violacein extracted from
Chromobacterium violaceum. J Antimicrob Chemother. 48:449–450.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lichstein HC and Van De Sand VF: The
antibiotic activity of violacein, prodigiosin, and phthiocol. J
Bacteriol. 52:145–146. 1946.PubMed/NCBI
|
|
13
|
Bromberg N, Dreyfuss JL, Regatieri CV, et
al: Growth inhibition and pro-apoptotic activity of violacein in
Ehrlich ascites tumor. Chem Biol Interact. 186:43–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Carvalho DD, Costa FT, Durán N and Haun
M: Cytotoxic activity of violacein in human colon cancer cells.
Toxicol In Vitro. 20:1514–1521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferreira CV, Bos CL, Versteeg HH, Justo
GZ, Durán N and Peppelenbosch MP: Molecular mechanism of
violacein-mediated human leukemia cell death. Blood. 104:1459–1464.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar
|
|
17
|
Martins D, Frungillo L, Anazzetti MC, Melo
PS and Durán N: Antitumoral activity of L-ascorbic acid-poly-
D,L-(lactide-co-glycolide) nanoparticles containing violacein. Int
J Nanomed. 5:77–85. 2010. View Article : Google Scholar
|
|
18
|
Melo PS, Justo GZ, de Azevedo MB, Durán N
and Haun M: Violacein and its beta-cyclodextrin complexes induce
apoptosis and differentiation in HL60 cells. Toxicology.
186:217–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melo PS, Maria SS, Vidal BC, Haun M and
Durán N: Violacein cytotoxicity and induction of apoptosis in V79
cells. In Vitro Cell Dev Biol Anim. 36:539–543. 2000. View Article : Google Scholar
|
|
20
|
Queiroz KC, Milani R, Ruela-de-Sousa RR,
et al: Violacein induces death of resistant leukaemia cells via
kinome reprogramming, endoplasmic reticulum stress and Golgi
apparatus collapse. PLoS One. 7:e453622012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saraiva VS, Marshall JC, Cools-Lartigue J
and Burnier MN Jr: Cytotoxic effects of violacein in human uveal
melanoma cell lines. Melanoma Res. 14:421–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmetagic A and Pemberton JM: Stable high
level expression of the violacein indolocarbazole anti-tumour gene
cluster and the Streptomyces lividans amyA gene in E. coli K12.
Plasmid. 63:79–85. 2010. View Article : Google Scholar
|
|
23
|
Durán N, Justo GZ, Ferreira CV, Melo PS,
Cordi L and Martins D: Violacein: properties and biological
activities. Biotechnol Appl Biochem. 48:127–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosenberg SA, Spiess PJ and Kleiner DE:
Antitumor effects in mice of the intravenous injection of
attenuated Salmonella typhimurium. J Immunother. 25:218–225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durán N, Antonio RV, Haun M and Pilli RA:
Biosynthesis of a trypanocide by Chromobacterium violaceum. World J
Microbiol Biotechnol. 10:686–690. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tirino V, Desiderio V, Paino F, et al:
Cancer stem cells in solid tumors: an overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar
|
|
28
|
Hashimi SM, Yu S, Alqurashi N, Ipe DS and
Wei MQ: Immunotoxin-mediated targeting of claudin-4 inhibits the
proliferation of cancer cells. Int J Oncol. 42:1911–1918.
2013.PubMed/NCBI
|
|
29
|
Cunningham C and Nemunaitis J: A phase I
trial of genetically modified Salmonella typhimurium expressing
cytosine deaminase (TAPET-CD, VNP20029) administered by
intra-tumoral injection in combination with 5-fluorocytosine for
patients with advanced or metastatic cancer. Protocol no: CL-017
Version: April 9, 2001. Hum Gene Ther. 12:1594–1596.
2001.PubMed/NCBI
|