Introduction
Gastric cancer (GC) remains a severe public health
problem worldwide. In China, GC is often diagnosed at an advanced
clinical stage, characteristic of obvious lymphatic tumor
dissemination (1). Radiotherapy is
the main modality for unresectable GC (http://www.nccn.org/index.asp) (2).
However, radiation is also a double-edged sword; it
not only kills tumor cells, yet also promotes radioresistance and
induces distant metastases, and destroys normal tissues (3). The intricate process of metastasis
includes a series of divergent steps. Epithelial-mesenchymal
transition (EMT) is one of the main programs. The EMT process leads
to acquisition of mesenchymal characteristics, including motility,
invasiveness, chemoresistance and radioresistance (4). Uncovering the relationship between
irradiation and EMT is essential. E-cadherin, which facilitates and
ensures the continuous adhesive epithelium, is a biomarker of the
EMT process (5). The loss of
E-cadherin leads to the lack of stable intercellular junctions;
therefore cell dispersion is accelerated due to the decrease in
cellular adhesive forces. Jung et al reported that the
morphology of cells changes after irradiation and cells appeared
similar to fibroblasts corresponding to a mesenchymal phenotype
(6).
The Notch axis is a key participant in EMT. Notch
signaling is a conserved family of transmembrane receptors that
determines cell fate (7). It
includes 4 Notch family members (Notch-1–4), 5 Notch ligands, 3
δ-like ligands (Dll1/3/4) and 2 serrate-like ligands (Jagged1/2)
(8). Notch is dysregulated in
various types of cancers, accompanied by poor clinical outcomes
(9). Notch signaling can be
activated under radiation and there is accumulating evidence
confirming the use of Notch inhibitors as promising
radiosensitizers in cancer treatment. Notch has also been proven to
mediate radioresistance in nasopharyngeal carcinoma and glioma
cells (10–12).
MicroRNAs (miRNAs) are a class of small non-coding
RNAs of 21–23 nucleotides that control specific mRNA translation or
induce mRNA degradation (13).
Dysregulated miRNA expression is well correlated with various
malignancies. The deficiency in members of the miR-200 family
(miR-200s), miR-124 and let-7, are observed in certain types of
cancers, and are regarded as tumor suppressors (14). In contrast, various overexpressed
miRNAs, such as miR-373 and miR-21, are regarded as oncogenes,
involved in cell proliferation and metastasis (15). In GC tissues, the expression levels
of miR-221, miR-222, miR 21 and miR-103 were found to be
significantly higher than levels in normal samples. However, the
expression of miR-143 and miR-195 in cancer samples was
significantly lower than levels in normal tissues (16). Moreover, miR-200c deficiency
promoted cancer metastasis, EMT and aggressiveness (17).
As a polymethoxylated flavonoid abundant in citrus
fruits, tangeretin (5,6,7,8,4′-pentamethoxyflavone) has been
reported to display apoptotic (18), antimetastatic (19) and antioxidant properties in various
cancer models. It inhibited the proliferation and induced G1 phase
arrest of MCF-7 cells after a 4-day treatment (20). Tangeretin induced the apoptosis of
human leukemia cells (18) and
enhanced the cytotoxic effect of doxorubicin on breast cancer cells
(21). Importantly, tangeretin
possessed little toxicity as there was no significant weight loss
and no obvious change in the gross behavior of rats (22). In our previous studies (unpublished
data), we screened for effective radiosensitizers of GC cells.
Recently, we observed the potential radiosensitizing effects of
tangeretin on GC cells, which led us to explore the mechanism
involved.
Materials and methods
Materials
Tangeretin (98%) was purchased from the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China). Tangeretin was dissolved in dimethylsulfoxide
(DMSO) for all in vitro experiments. DMSO, RPMI-1640 medium,
fetal calf serum (FBS), Tween-20, sodium dodecyl sulfate (SDS),
phenylmethylsulphonyl fluoride (PMSF), trypsin and carbonylcyanide
p-trifluoromethoxyphenylhydrazone (FCCP) were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). Notch-1 siRNA and miR-410 mimics
were obtained from Dharmacon (Chicago, IL, USA). The Notch-1 cDNA
plasmid was purchased from OriBioGene Biological Inc. (Shanghai,
China). Deionized water was purified by a Milli-Q water
purification system (Millipore, Milford, MA, USA). All other
reagents were of analytical grade and obtained from Nanjing
Chemical Reagent Co. (Nanjing, China).
Cell lines and cell viability
GC cell lines (MGC80-3, AGS, SGC7901 and MKN45) and
normal gastric mucosa GES-1 cells were purchased from the American
Type Culture Collection (ATCC; Rockville, MD, USA) and were
cultured in RPMI-1640 supplemented with 10% FBS. Cell viability was
determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Radiosensitivity was examined by colony formation assay.
Cells were exposed to increasing doses of irradiation (2, 4, 6 and
8 Gy) and tangeretin for 24 h. Irradiation was produced with a
4-MeV electron beam accelerator (Elekta, Sweden). After being
washed with fresh medium, cells were allowed to grow for 14 days to
form colonies, and cells were then fixed with 4% formaldehyde
(Sigma). Fixed cells were then stained with 0.5% crystal violet
(Sigma) for 15 min, and the colonies were counted. D0 values were
calculated by a multitarget-single hit model.
In vivo study protocol
Athymic male nude mice weighing ~20 g (SLARC
Laboratory Animal Center, Shanghai, China) were used and maintained
under standard pathogen-free conditions. SGC7901 cells were
harvested and injected subcutaneously into the nude mice. After the
onset of tumor development, tangeretin [30 mg/kg dissolved in 1 ml
of phosphate-buffered saline (PBS) containing 0.1% DMSO/nude mice]
was administered intraperitoneally for 3 weeks. After the mice were
anesthetized, the tumors were exposed to radiation with 2 Gy 5
times a week for 3 weeks. After 3 weeks of administration, all mice
were sacrificed and the tumors were subjected to further analysis.
The numbers of lung metastatic lesions were counted under a
microscope (Zeiss Axio Observer A1; Zeiss, Germany).
Western blot analysis
Cells were extracted in extraction buffer. Equal
amounts of protein extracts were separated on 10% polyacrylamide
gels and electrophoretically transferred onto polyvinylidene
difluoride membranes (Invitrogen, Carlsband, CA, USA) with a
semi-dry blot system. After blocking, the membranes were incubated
with each primary antibody (Nanjing Bioworld Biotech Co., Nanjing,
China) and then with IgG.
Cell invasion assays
After co-treatment with tangeretin (10 and 30
μM) and radiation (8 Gy) for 24 h, the cells were then
placed in the upper chamber of a Transwell coated with BD
Matrigel™. Medium with 10% FBS was placed in the lower chamber.
After 18 h, the cells that had migrated to the lower surface were
photographed and quantified in 5 fields at a magnification of ×100
under a microscope (Zeiss Axio Observer A1).
Wound healing assay
Cells were seeded in 24-well plates and grown until
reaching confluency. In each well, a scratch was made with a 1-ml
pipette tip. The cells were washed thrice with PBS to remove
detached cells. Then the cells were treated with irradiation (8 Gy)
and tangeretin (10 and 30 μM), and images of the wound area
were captured at 0 and 18 h under a microscope (Zeiss Axio Observer
A1).
Immunofluorescence microscopy
After exposure to irradiation (8 Gy) for 24 h,
immunofluorescence assay was performed to examine EMT biomarkers in
the SGC7901 cells. After fixation and permeabilization, E-cadherin
and N-cadherin were measured following staining with
anti-E-cadherin and anti-N-cadherin antibodies. Nuclei were
counterstained with 4′,6-diamidine-2′-phenylindole dihydrochloride
(DAPI) (Sigma Chemical Co.). The stained cells were observed with a
fluorescence inverted microscope (Zeiss Axio Observer A1).
miRNA microarray analysis
Total RNA was harvested using miRNeasy Mini kit
(Qiagen, Copenhagen, Denmark) according to the manufacturer’s
instructions (23,24). The profiles of samples were
generated using Agilent Human miRNA Microarray V3 (Agilent
Technologies, Inc., Santa Clara, CA, USA). The scanned images were
imported into GenePix Pro 6.0 software (Axon Instruments, Molecular
Devices Corp., Foster City, CA, USA) for data extraction. Real-time
PCR was carried out to confirm the miRNA expression profiling with
U6 as the internal reference gene.
Statistical analysis
Data are expressed as the mean ± SD from 3
independent experiments. Group results were analyzed using one-way
analysis of variance (ANOVA). Two groups were analyzed by the
Student’s t-test. P-value <0.05 was considered to indicate a
statistically significant result.
Results
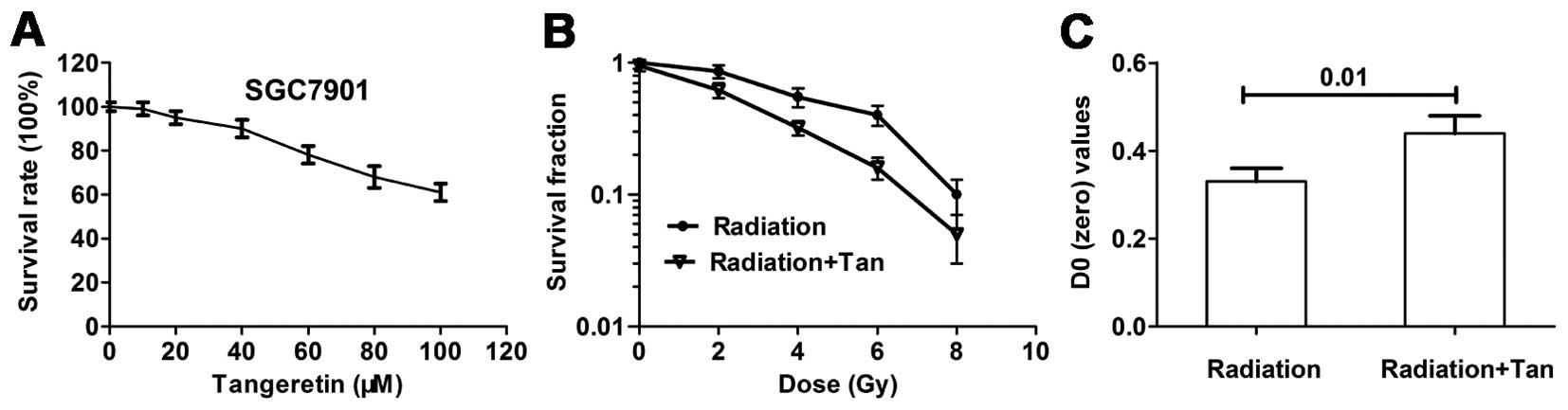
Tangeretin decreases cell viability and
increase radiosensitivity in the SGC7901 cells
Cytotoxicity was detected by MTT assay. Cells were
treated with tangeretin (0–100 μM) for 24 h. Tangeretin
caused a cytotoxicity effect on the SGC7901 cells in a
dose-dependent manner. Tangeretin did not affect cell viability
until reaching concentrations of 30 μM (Fig. 1A). The non-cytotoxic concentrations
used for the present study were identified, to ensure that the
radiosensitizing effect of tangeretin was not caused by a direct
cytotoxic effect.
A colony forming assay was carried out to explore
whether tangeretin increased radiosensitivity in the SGC7901 cells.
Cell survival curves measured by clonogenic survival assay are
illustrated in Fig. 1B. Tangeretin
considerably increased radiation-induced cell clonogenic death. D0
of the tangeretin plus radiation group was significantly increased
(P=0.01), compared with the radiation alone group (Fig. 1C).
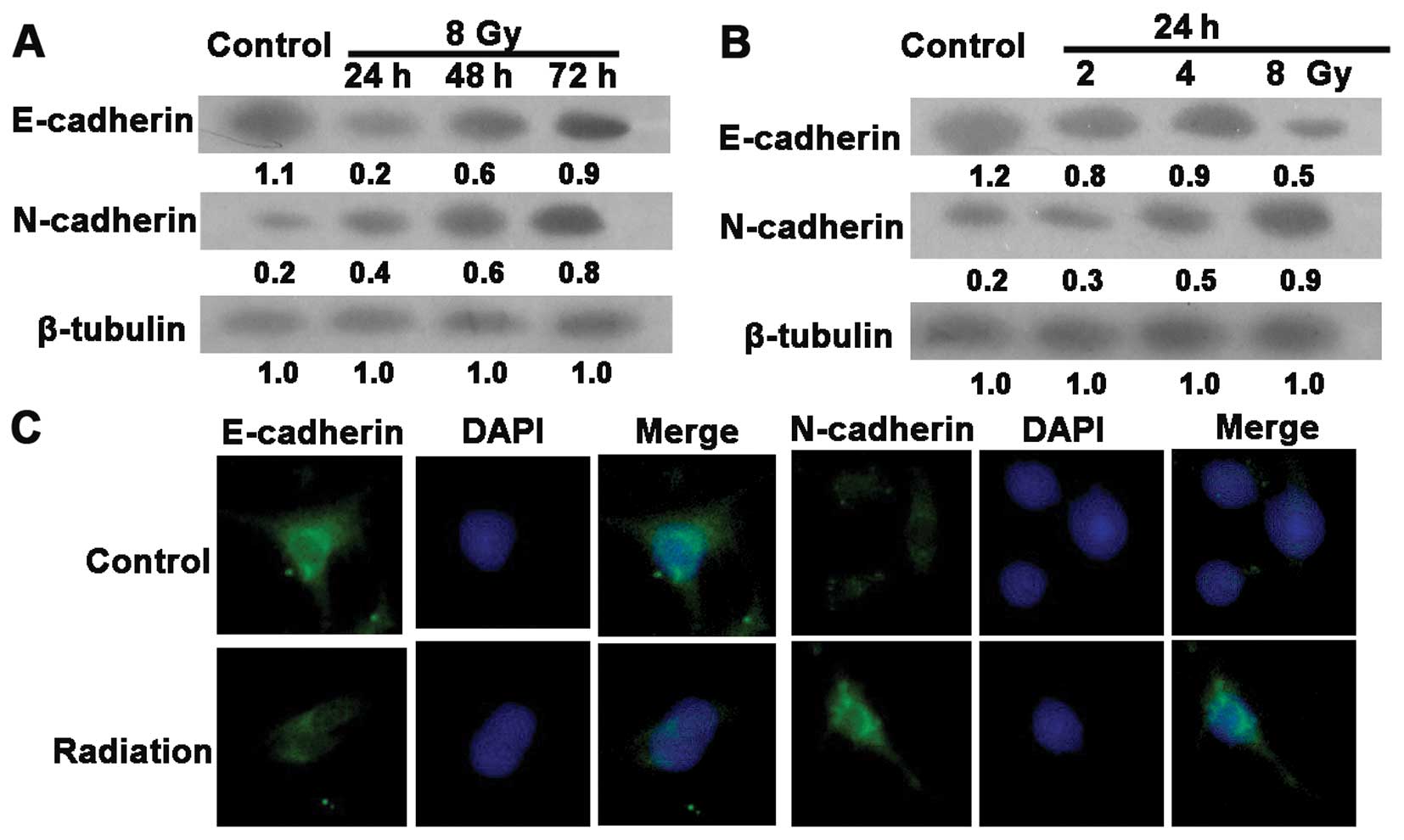
Radiation induces EMT in the gastric
cancer cells
The loss of the epithelial phenotype marker,
E-cadherin, is obvious in the EMT process. Features of the
mesenchymal phenotype, such as augmented formation of pseudopodia
and the appearance of spindle-shaped cells, were observed at 24 h
post-irradiation (data not shown). To determine whether this
alteration correlates with EMT, we examined EMT-specific markers by
immunofluorescence assay and western blotting. As shown in Fig. 2A and B, radiation induced a decrease
in E-cadherin and promoted the expression of the mesenchymal marker
N-cadherin in the SGC7901 cells. Similar results were observed in
the immunofluorescence assay (Fig.
2C). These data suggest that radiation induced EMT in the human
GC SGC7901 cells.
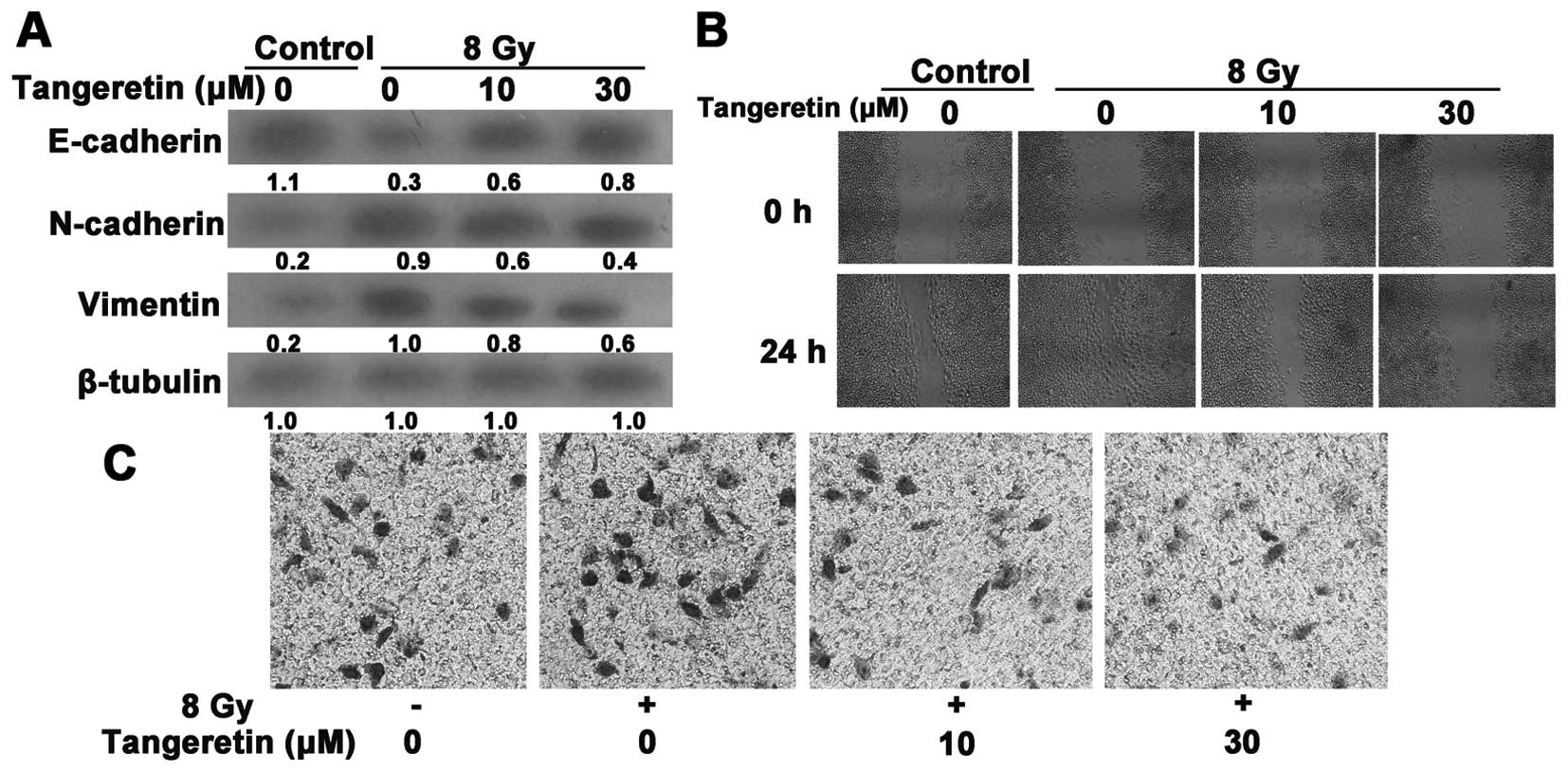
Tangeretin reduces radiation-induced EMT
and invasion and migration in the SGC7901 cells
Tangeretin suppressed radiation-induced EMT, as
revealed by a reduced reduction in expression of vimentin and
N-cadherin and an increase in expression of E-cadherin (Fig. 3A). The EMT process is also
accompanied with enhanced cell invasion and migration. We further
evaluated the invasive and migratory properties of SGC7901 cells
via Matrigel Transwell and wound-healing assays. Radiation
accelerated the rate of wound closure (Fig. 3B). Likewise, the acquisition of
increased invasive ability induced by irradiation was prohibited by
tangeretin treatment (Fig. 3C).
These data indicate that tangeretin diminished the
radiation-induced responses in SGC7901 cells.
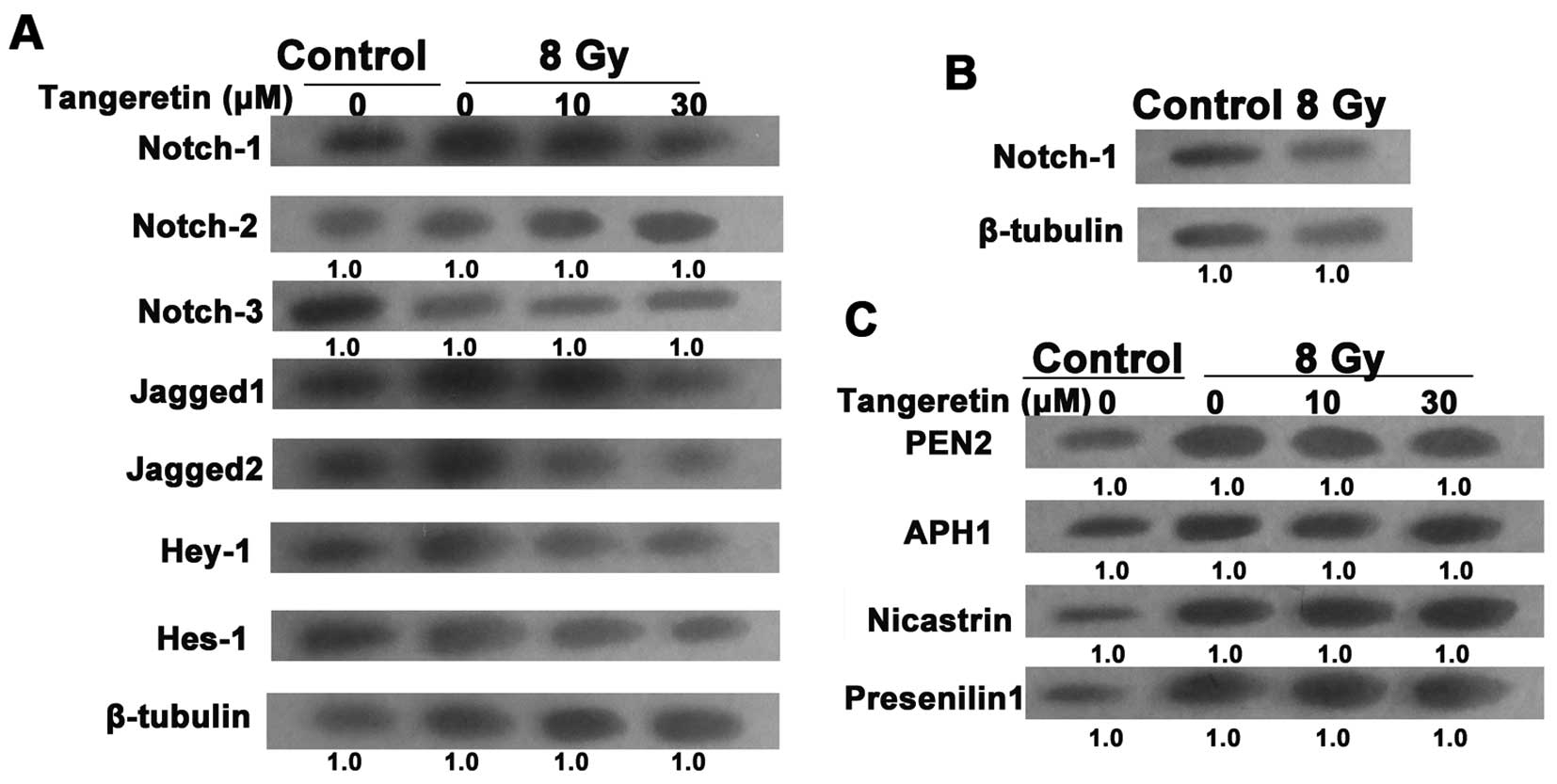
Tangeretin inhibits the Notch-1 pathway
in the irradiated SGC7901 cells
Evidence has proven the essential role of the
Notch-1 pathway in the EMT process (25). To uncover the molecular mechanism
responsible for radiation-induced EMT, the expression of the Notch
pathway in irradiated cells was examined. After treatment with
tangeretin for 24 h, the upregulation of Notch-1, Jagged1/2, Hes-1
and Hey-1 expression levels in the irradiated cells was almost
blocked. However, radiation or/and tangeretin co-incubation failed
to influence the expression of Notch-2/3 (Fig. 4A). Moreover, western blot assays
showed that there was no significant change in Notch-1 expression
in the gastric mucosa GES-1 cells after exposure to irradiation
(Fig. 4B).
After binding to Jagged or δ ligands, the Notch
receptor is activated and goes through a succession of cleavages.
The final cleavage depends on the γ-secretase protease complex,
which contains 4 catalytic subunits such as PEN2, presenilin 1,
APH1 and nicastrin (26), causing
the translocation of Notch into the nucleus (27). We then tested the effect of
tangeretin on the activation of Notch-1 signaling. Tangeretin did
not influence the protein level of the 4 catalytic subunits,
suggesting that tangeretin only suppressed the expression rather
than the activation of Notch-1 (Fig.
4C).
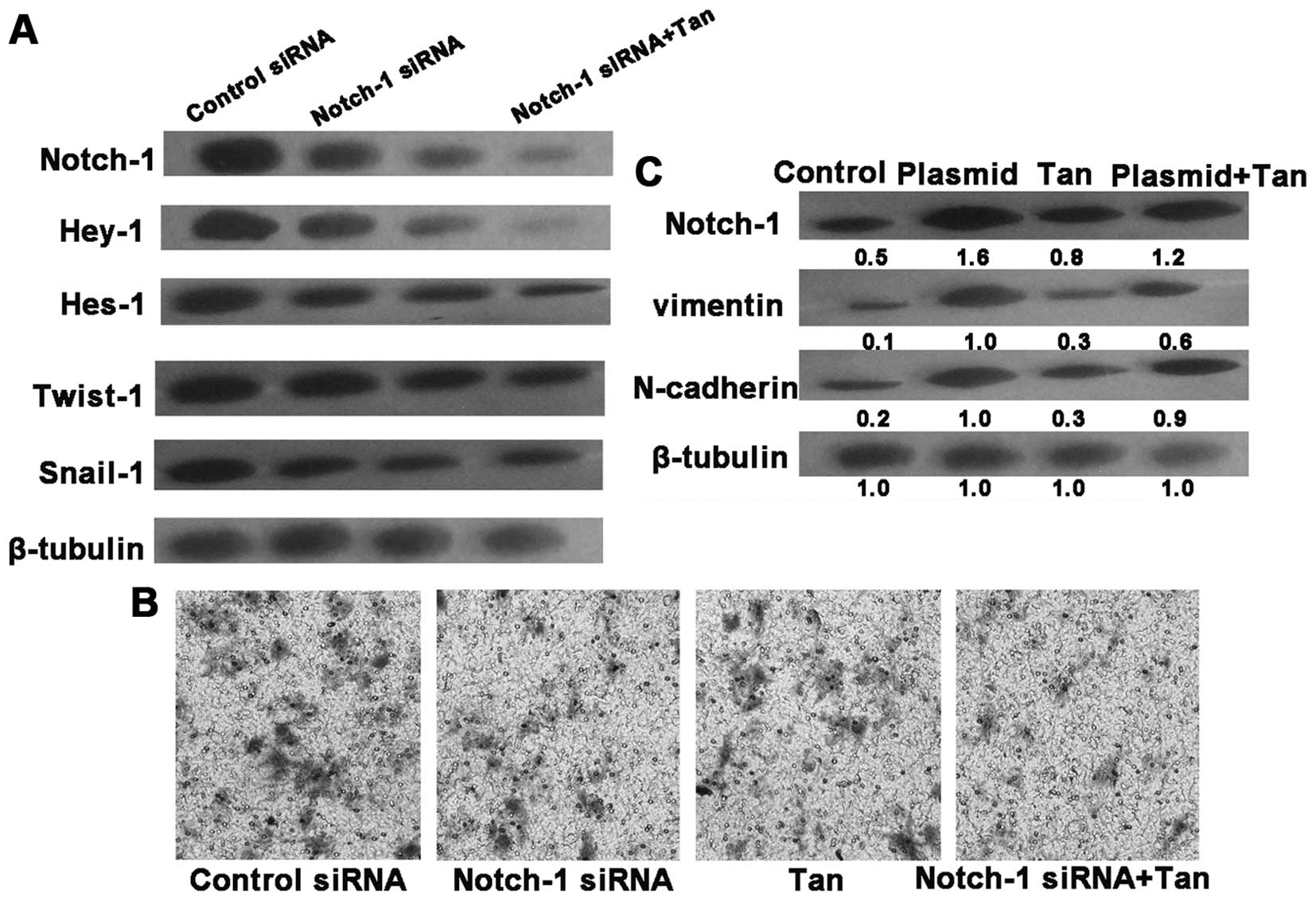
Knockdown of Notch-1 alleviates EMT in
SGC7901 cells
To further demonstrate the essential role of
Notch-1, the Notch-1 gene was knocked down by siRNA. Notch-1 siRNA
transfection downregulated the protein levels of Notch-1, Hey-1,
Hes-1, Snail1 and Twist1 in the irradiated SGC7901 cells,
accompanied with a decline in invasive ability. After 24 h
post-transfection, tangeretin treatment inhibited both Notch-1
activity and cell invasion to a more significant degree in the
irradiated SGC7901 cells than tangeretin treatment alone (Fig. 5A and B). We then explored the
relationship between tangeretin and Notch-1 with the overexpression
plasmid of Notch-1. Overexpression of Notch-1 overrode the
inhibitory effect of tangeretin on EMT (Fig. 5C). Accordingly, we hypothesized that
tangeretin prevented EMT probably via suppressing Notch-1
expression.
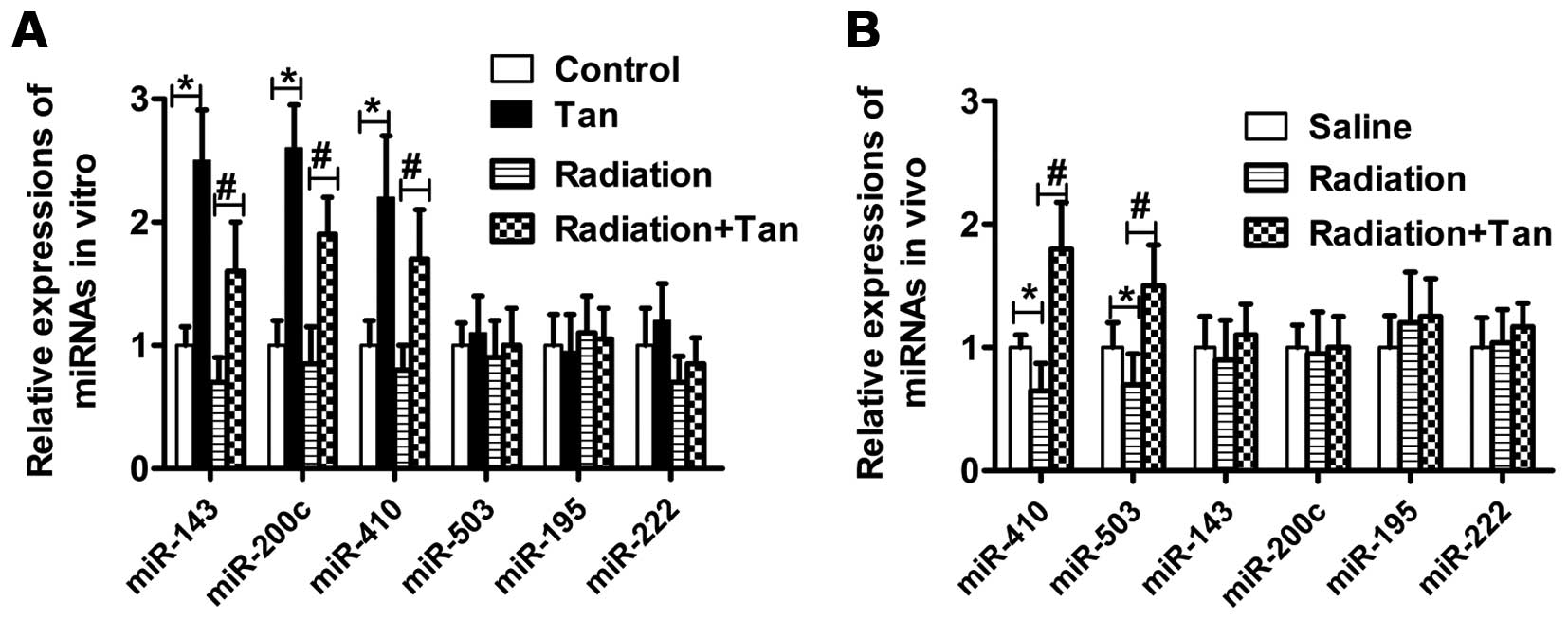
Tangeretin elevates miR-410 expression
both in vitro and in vivo
Radiation regulates miRNA expression, and in turn
affects tumor progression. As demonstrated here, in SGC7901 cells
exposed to tangeretin, enhanced expression of miR-143, miR-200c and
miR-410 was noted when compared to the control cells. Moreover, the
relative change in miR-410 expression was greater in the tangeretin
plus irradiation group, than the radiation alone group (Fig. 6A). In addition, tangeretin promoted
the expression of miR-410 and miR-503 in tumor tissue lysates from
nude mice (Fig. 6B). These results
demonstrated that tangeretin elevated miR-410 expression both in
vitro and in vivo.
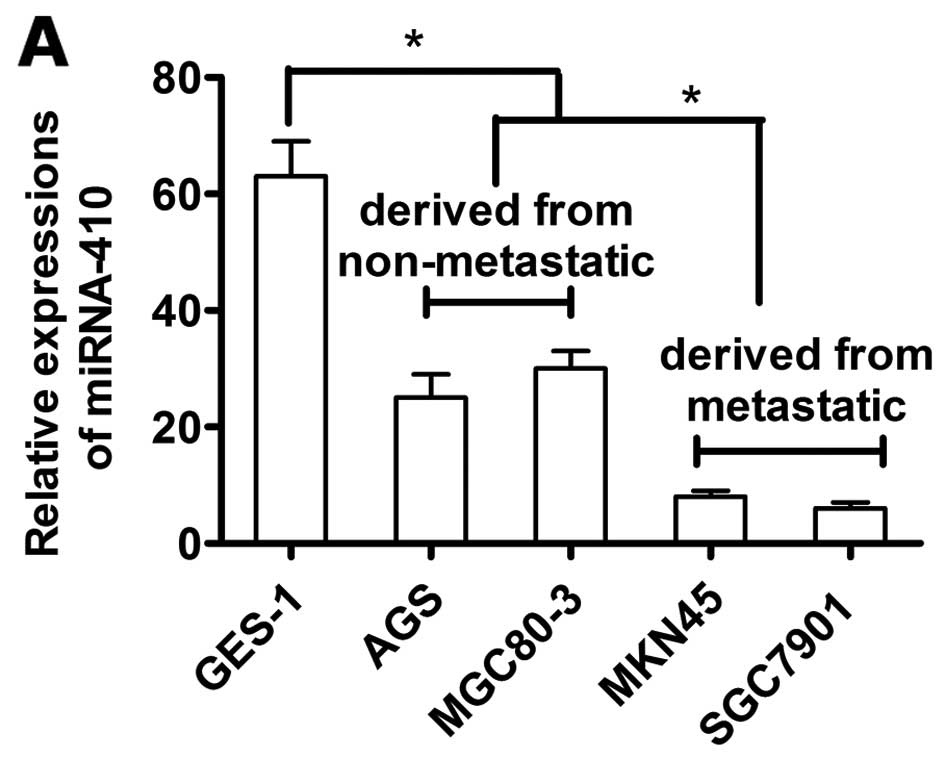
miR-410 expression is reduced in gastric
camcer cells
We then compared miR-410 expression between GC cell
lines (MGC80-3, AGS, SGC7901 and MKN45) and the normal gastric
mucosa GES-1 cells. miR-410 expression was reduced in the GC cell
lines compared to the GES-1 cells. MGC80-3 and AGS cell lines were
derived from non-metastatic tissues, and SGC7901 and MKN45 cell
lines were from metastatic tissues. Expression of miR-410 was
higher in the MGC80-3 and AGS cells than those derived from
metastatic tissues (P<0.05, Fig.
7A).
miR-410 plays a role in the biologic
function of tangeretin in EMT
Since miRNAs are crucial modulators of EMT and
Notch-1 signaling, we further investigated the effect of miR-410 on
the EMT of GC cells subjected to radiation. Enhanced miR-410
expression and weakened Notch-1 expression were observed in the
irradiated-SGC7901 cells after miR-410 mimics were transfected into
the cells (Fig. 7B). Overexpression
of miR-410 also reduced EMT-specific marker expression and invasion
in the irradiated cells, indicating that miR-410 is closely
associated with EMT (Fig. 7C). In
addition, miR-410 expression was upregulated after the knockdown of
Notch-1 by siRNA in the irradiated SGC7901 cells, and these
functions were similar to the effect by tangeretin (Fig. 7D). In summary, miR-410 plays an
essential role in the biologic function of tangeretin in EMT.
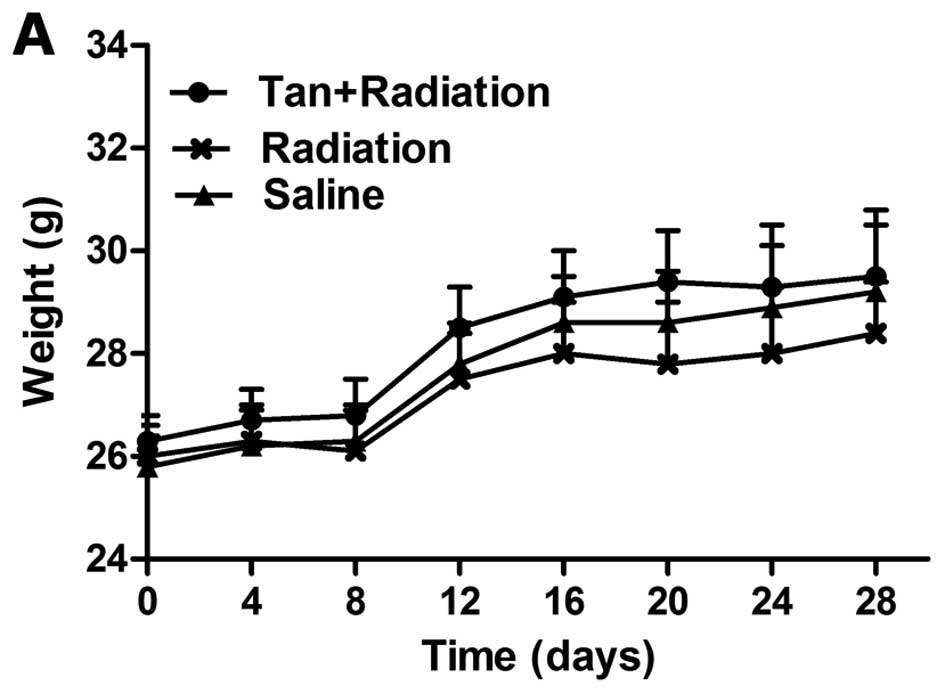
Effects of tangeretin in vivo
Body weight partly reflects a healthy condition. A
decrease in body weight was observed in the group exposed to
radiation for 3 weeks. Tangeretin considerably alleviated
radiation-induced weight loss in nude mice after 3 weeks of
administration. During the period of administration, mice in the
tangeretin + radiation group were in better physical condition and
exhibited higher weight gain, compared with the radiation group
(Fig. 8A).
Following treatment with tangeretin + radiation for
3 weeks, the tumor sizes were smallest among all the groups at the
observation endpoint (Fig. 8B). The
incidence of pulmonary metastasis in the radiotherapy and control
groups was 100 and 50% (6/6 vs. 3/6, P<0.05; Fig. 8C). Compared with the radiation
group, the tangeretin + radiation group had considerably attenuated
lung metastases, with metastatic rates of 16.67% (1/6 vs. 6/6,
P<0.05).
Discussion
The National Comprehensive Cancer Network guidelines
recommend radiotherapy as a standard treatment for gastric cancer
(GC) patients with a high-risk of recurrence (28). However, radiotherapy has shown the
tendency to induce metastasis. Moreover, increased irradiation has
toxic effects on the skin and other normal tissues (29). Identification of radiosensitizers
that maximize radiotherapeutic efficacy and minimize toxicity has
attracted worldwide attention. In the present study, we showed that
tangeretin enhanced the radiosensitivity of SGC7901 cells and
suppressed irradiation-induced epithelial-mesenchymal transition
(EMT) and metastasis both in vitro and in vivo,
probably via inhibition of Notch-1 signaling transduction and the
‘switching on’ of miR-410. This opens perspectives for the
development of novel radiosensitizers in GC therapy. Moreover, the
absorption and pharmacokinetics of tangeretin have been studied
both in vivo and in vitro. In hamsters administered
1% tangeretin flavone for 35 days, total tangeretin and tangeretin
metabolites reached levels equivalent to 21 μM intact
tangeretin in serum and 16–67 μM in liver (30). In rats intraperitoneally
administered tangeretin (50 mg/kg), the concentration of tangeretin
in serum reached a peak of 12.1 μM at 0.5 h (31). In the present study, the
concentrations of tangeretin (10 and 30 μM) used in
vitro were comparable to the levels achievable in vivo.
Nevertheless, the detailed metabolic features of tangeretin in nude
mice bearing tumor xenografts warrant more profound study.
Mounting evidence indicates that radiation is one of
the inducers of EMT, and EMT directly induces radioresistance
(32). Irregular EMT activation in
the stomach is closely related with gastric carcinogenesis
(33). EMT activation endows
gastric epithelial cells with augmented characteristics of
mesenchymal cells and decreases their epithelial features.
Radiation-induced metastasis is also closely related to a
mesenchymal phenotype. E-cadherin, an epithelial biomarker, is a
member of a large superfamily of cell-cell adhesion molecules
(34). During EMT, cadherin changes
from E-cadherin to N-cadherin which is expressed in mesenchymal
cells. Thus, decreased E-cadherin and increased N-cadherin are
noted in the EMT process, and both can be identified as EMT
biomarkers. Tangeretin has been found to inhibit invasion of cancer
cells in an E-cadherin-dependent manner (34). Tangeretin also reduced the number of
metastatic nodules in mice bearing B16F10 cell xenografts (35). In the present study, a decrease in
E-cadherin as well as a simultaneous increase in N-cadherin and
vimentin were observed after exposure to radiation, indicating that
the epithelial cells acquired a mesenchymal-like morphology.
Tangeretin successfully inhibited E-cadherin expression, invasion
and migration induced by irradiation.
The Notch-1 pathway plays an important role in EMT
progression (25). Blocking Notch-1
signaling by Hey-1 or Jagged1 knockdown mitigated EMT (36). As an oncogene in various solid
malignancies, Notch-1 is expressed in most GC cell lines and normal
gastric mucosa. The γ-secretase inhibitor DAPT (one of the GSIs)
successfully inhibited the EMT and metastasis of GC cells. There is
crosstalk between the EMT transcription factors and Notch such as
Slug and Snail (37). Notably, Du
et al showed that increased expression of Notch-1 is
associated with non-cardia location, positive lymphovascular
invasion, diffuse type, tumor size >5 cm and distal metastasis
in GC cancer patients (38);
Notch-1 may be regarded as a poor prognostic predictor in GC.
Consistently, we found that Notch-1 was expressed in both SGC7901
cells and normal mucosa GES-1 cells, but a higher level was
observed in SGC7901 cells than in GES-1 cells after exposure to
irradiation, suggesting that Notch-1 is activated under
irradiation. Tangeretin exhibited potent Notch-1 inhibiting
capacity. However, tangeretin only suppressed the expression of
Notch-1, not the activation of Notch-1. Furthermore, the
overexpression of Notch-1 overrode the inhibitory effect of
tangeretin on EMT. Consequently, Notch-1 may be a target of
tangeretin in the inhibitory effect on EMT.
Recently, a mount of evidence strongly supports the
multi-factorial role of miRNAs in critical cellular processes. In
particular, miRNAs may function as tumor-suppressor genes or
oncogenes. In the present study, we compared the expression of 6
miRNAs (miR-410, miR-143, miR-195, miR-222, miR-200c and miR-503)
between GC cell lines/tissues and normal gastric cells/tissue.
Studies indicate that miR-143 and 200c play an important role in
blocking cancer progression (13,39).
Our data revealed that tangeretin promoted miR-143 and miR-200c
expression only in vitro, without a significant effect in
vivo. The miR-503 level has been reported to be considerably
reduced in GC tissues compared to normal mucosa tissues in 76
patients who experienced gastric surgery between 2012 and 2013
(16). miR-503 expression levels
also negatively correlated with metastases in patients. The present
study demonstrated that tangeretin only promoted miR-503 in tumor
tissue from nude mice. Zhang el al reported that
upregulation of miR-222 induced the malignant phenotype of SGC7901
cells, whereas knockdown of miR-222 reversed this phenotype
(40). In the present study,
tangeretin displayed no effect on miR-222 expression in the SGC7901
cells. In the present study, in SGC7901 cells, as well as in tumor
tissues, combined treatment of tangeretin and irradiation
upregulated the expression of miR-410. Gattolliat et al
showed that the miR-410 level was considerably related with
disease-free survival of the non-amplified neuroblastoma (41). Shen et al suggested that
miR-410 acts as a tumor suppressor by targeting the MDM2 gene and
inhibiting GC cell proliferation, migration and invasion (42). Similarly, we found that miR-410
expression was lower in the SGC7901 cells than that in the normal
gastric mucosa cells. Moreover, the miR-410 level was higher in
cells derived from non-metastatic tissues than those derived from
metastatic tissues.
We further investigated the correlation between
miR-410 and Notch-1. Overexpression of miR-410 significantly
weakened Notch-1 expression and inhibited the EMT process in
irradiated GC cells. In addition, Notch-1 siRNA led to the
enhancement of miR-410 expression in the irradiated SGC7901 cells.
Tangeretin treatment also promoted miR-410 expression in the
irradiated SGC7901 cells.
Taken together, we conclude that both Notch-1 and
miR-410 are key effectors in the biologic function of tangeretin.
Inactivation of Notch-1 signaling leads to the reversal of EMT,
along with less invasive characteristics. Tangeretin plus radiation
has noteworthy potential as an effective anti-GC strategy.
References
|
1
|
Bandres E, Bitarte N, Arias F, Agorreta J,
Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ,
et al: microRNA-451 regulates macrophage migration inhibitory
factor production and proliferation of gastrointestinal cancer
cells. Clin Cancer Res. 15:2281–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu W, Huang YJ, Liu C, Yang YY, Liu H,
Cui JG, Cheng Y, Gao F, Cai JM and Li BL: Inhibition of TBK1
attenuates radiation-induced epithelial-mesenchymal transition of
A549 human lung cancer cells via activation of GSK-3β and
repression of ZEB1. Lab Invest. 94:362–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui FB, Liu Q, Li RT, Shen J, Wu PY, Yu
LX, Hu WJ, Wu FL, Jiang CP, Yue GF, et al: Enhancement of
radiotherapy efficacy by miR-200c-loaded gelatinase-stimuli
PEG-Pep-PCL nanoparticles in gastric cancer cells. Int J Nanomed.
9:2345–2358. 2014.
|
|
5
|
Moncharmont C, Levy A, Guy JB, Falk AT,
Guilbert M, Trone JC, Alphonse G, Gilormini M, Ardail D, Toillon
RA, et al: Radiation-enhanced cell migration/invasion process: A
review. Crit Rev Oncol Hematol. 92:133–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung JW, Hwang SY, Hwang JS, Oh ES, Park S
and Han IO: Ionising radiation induces changes associated with
epithelial-mesenchymal transdifferentiation and increased cell
motility of A549 lung epithelial cells. Eur J Cancer. 43:1214–1224.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D’Souza B, Miyamoto A and Weinmaster G:
The many facets of Notch ligands. Oncogene. 27:5148–5167. 2008.
View Article : Google Scholar
|
|
9
|
Espinoza I and Miele L: Notch inhibitors
for cancer treatment. Pharmacol Ther. 139:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Wakeman TP, Lathia JD, Hjelmeland
AB, Wang XF, White RR, Rich JN and Sullenger BA: Notch promotes
radioresistance of glioma stem cells. Stem Cells. 28:17–28.
2010.
|
|
11
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24−/low/CD44+ breast
cancer-initiating cells to radiation. J Natl Cancer Inst.
98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu S, Zhang R, Liu F, Hu H, Yu S and Wang
H: Down-regulation of Notch signaling by a γ-secretase inhibitor
enhances the radio-sensitivity of nasopharyngeal carcinoma cells.
Oncol Rep. 26:1323–1328. 2011.PubMed/NCBI
|
|
13
|
Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q,
Dan ZL, Tian DA and Zhang P: MicroRNA-143 suppresses gastric cancer
cell growth and induces apoptosis by targeting COX-2. World J
Gastroenterol. 19:7758–7765. 2013. View Article : Google Scholar
|
|
14
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: MiR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013. View Article : Google Scholar :
|
|
15
|
Ye X, Jiang F, Li Y, Mu J, Si L, Wang X,
Ning S and Li Z: Glabridin attenuates the migratory and invasive
capacity of breast cancer cells by activating microRNA-200c. Cancer
Sci. 105:875–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo B, Li J, Liu L, Hou N, Chang D, Zhao
L, Li Z, Song T and Huang C: Dysregulation of miRNAs and their
potential as biomarkers for the diagnosis of gastric cancer. Biomed
Rep. 1:907–912. 2013.
|
|
17
|
Cortez MA, Valdecanas D, Zhang X, Zhan Y,
Bhardwaj V, Calin GA, Komaki R, Giri DK, Quini CC, Wolfe T, et al:
Therapeutic delivery of miR-200c enhances radiosensitivity in lung
cancer. Mol Ther. 22:1494–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirano T, Abe K, Gotoh M and Oka K: Citrus
flavone tangeretin inhibits leukaemic HL-60 cell growth partially
through induction of apoptosis with less cytotoxicity on normal
lymphocytes. Br J Cancer. 72:1380–1388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo J, Lee HS, Ryoo S, Seo JH, Min BS and
Lee JH: Tangeretin, a citrus flavonoid, inhibits PGDF-BB-induced
proliferation and migration of aortic smooth muscle cells by
blocking AKT activation. Eur J Pharmacol. 673:56–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morley KL, Ferguson PJ and Koropatnick J:
Tangeretin and nobiletin induce G1 cell cycle arrest but not
apoptosis in human breast and colon cancer cells. Cancer Lett.
251:168–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meiyanto E, Hermawan A and Anindyajati:
Natural products for cancer-targeted therapy: Citrus flavonoids as
potent chemopreventive agents. Asian Pac J Cancer Prev. 13:427–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanhoecke BW, Delporte F, Van Braeckel E,
Heyerick A, Depypere HT, Nuytinck M, De Keukeleire D and Bracke ME:
A safety study of oral tangeretin and xanthohumol administration to
laboratory mice. In Vivo. 19:103–107. 2005.PubMed/NCBI
|
|
23
|
Jiang LH, Yang NY, Yuan XL, Zou YJ, Jiang
ZQ, Zhao FM, Chen JP, Wang MY and Lu DX: Microarray analysis of
mRNA and microRNA expression profile reveals the role of
β-sitosterol-D-glucoside in the proliferation of neural stem cell.
Evid Based Complement Alternat Med. 2013:3603022013. View Article : Google Scholar
|
|
24
|
Chen QL, Lu YY, Zhang GB, Song YN, Zhou
QM, Zhang H, Zhang W, Tang XS and Su SB: Characteristic analysis
from excessive to deficient syndromes in hepatocarcinoma underlying
miRNA array data. Evid Based Complement Alternat Med.
2013:3246362013. View Article : Google Scholar
|
|
25
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eliasz S, Liang S, Chen Y, De Marco MA,
Machek O, Skucha S, Miele L and Bocchetta M: Notch-1 stimulates
survival of lung adenocarcinoma cells during hypoxia by activating
the IGF-1R pathway. Oncogene. 29:2488–2498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Capaccione KM and Pine SR: The Notch
signaling pathway as a mediator of tumor survival. Carcinogenesis.
34:1420–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui FB, Li RT, Liu Q, Wu PY, Hu WJ, Yue
GF, Ding H, Yu LX, Qian XP and Liu BR: Enhancement of radiotherapy
efficacy by docetaxel-loaded gelatinase-stimuli PEG-Pep-PCL
nanoparticles in gastric cancer. Cancer Lett. 346:53–62. 2014.
View Article : Google Scholar
|
|
29
|
Lin J, Liu C, Gao F, Mitchel RE, Zhao L,
Yang Y, Lei J and Cai J: miR-200c enhances radiosensitivity of
human breast cancer cells. J Cell Biochem. 114:606–615. 2013.
View Article : Google Scholar
|
|
30
|
Kurowska EM and Manthey JA: Hypolipidemic
effects and absorption of citrus polymethoxylated flavones in
hamsters with diet-induced hypercholesterolemia. J Agric Food Chem.
52:2879–2886. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manthey JA, Cesar TB, Jackson E and
Mertens-Talcott S: Pharmacokinetic study of nobiletin and
tangeretin in rat serum by high-performance liquid
chromatography-electrospray ionization-mass spectrometry. J Agric
Food Chem. 59:145–151. 2011. View Article : Google Scholar
|
|
32
|
Theys J, Jutten B, Habets R, Paesmans K,
Groot AJ, Lambin P, Wouters BG, Lammering G and Vooijs M:
E-Cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vermeulen S, Van Marck V, Van Hoorde L,
Van Roy F, Bracke M and Mareel M: Regulation of the invasion
suppressor function of the cadherin/catenin complex. Pathol Res
Pract. 192:694–707. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martínez Conesa C, Vicente Ortega V, Yáñez
Gascón MJ, Alcaraz Baños M, Canteras Jordana M, Benavente-García O
and Castillo J: Treatment of metastatic melanoma B16F10 by the
flavonoids tangeretin, rutin, and diosmin. J Agric Food Chem.
53:6791–6797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Timmerman LA, Grego-Bessa J, Raya A,
Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick
F, Izpisúa-Belmonte JC, et al: Notch promotes
epithelial-mesenchymal transition during cardiac development and
oncogenic transformation. Genes Dev. 18:99–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du X, Cheng Z, Wang YH, Guo ZH, Zhang SQ,
Hu JK and Zhou ZG: Role of Notch signaling pathway in gastric
cancer: A meta-analysis of the literature. World J Gastroenterol.
20:9191–9199. 2014.PubMed/NCBI
|
|
39
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar :
|
|
40
|
Zhang CZ, Han L, Zhang AL, Fu YC, Yue X,
Wang GX, Jia ZF, Pu PY, Zhang QY and Kang CS: MicroRNA-221 and
microRNA-222 regulate gastric carcinoma cell proliferation and
radioresistance by targeting PTEN. BMC Cancer. 10:3672010.
View Article : Google Scholar
|
|
41
|
Gattolliat CH, Thomas L, Ciafrè SA,
Meurice G, Le Teuff G, Job B, Richon C, Combaret V, Dessen P,
Valteau-Couanet D, et al: Expression of miR-487b and miR-410
encoded by 14q32.31 locus is a prognostic marker in neuroblastoma.
Br J Cancer. 105:1352–1361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen J, Niu W, Zhou M and Zhang H, Ma J,
Wang L and Zhang H: MicroRNA-410 suppresses migration and invasion
by targeting MDM2 in gastric cancer. PLoS One. 9:e1045102014.
View Article : Google Scholar : PubMed/NCBI
|