Introduction
microRNAs (miRNAs) are endogenous 19-25 nucleotide
short RNAs and play critical roles in cancer progression through
either protein translation repression or mRNA degradation to
regulate their target mRNAs (1).
miRNAs have a strong impact on all the main aspects of
tumorigenesis by controlling the major regulators of cell cycle
progression, senescence, apoptosis and autophagy, along with tumor
cell motility, invasion and metastasis (2-4). The
pathogenic, diagnostic and prognostic roles of miRNAs have been
extensively studied in breast cancer (5). miRNAs have emerged as important
biomarkers for breast cancer risk stratification and outcome
prediction (6). In addition, miRNAs
represent promising therapeutic tools against breast cancer, as
shown by the tumor regression achieved by the in vivo
delivery of let-7 or miR-34 family members (7,8).
The cell cycle regulatory protein p27Kip1 is
frequently inactivated in many human cancers. This inactivation is
caused by enhancing its degradation, repressing its transcription,
or by promoting its cytoplasmic sequestration, in different tumor
types and in different subsets of patients within tumor types using
distinct mechanisms (9,10). miRNA-mediated control is one of the
post-transcriptional mechanisms responsible for p27Kip1
downregulation in cancer cells. To date, a set of miRNAs has been
verified to target p27Kip1, such as miR-25 (11), miR-200 (12), miR-802 (13), miR-194 (14), miR-181b (15), miR-196a (16) and miR-221 (17).
In the present study, the role of miR-24-3p and
p27Kip1 in breast cancer tissues and cells was investigated. Our
findings verified that the expression of miR-24-3p was upregulated,
while p27Kip1 was downregulated in breast cancer tissues and
inversely correlated with the expression of miR-24-3p.
Overexpression of miR-24-3p promoted breast cancer cell growth,
colony formation, cell cycle and inhibited cell apoptosis.
Furthermore, we experimentally validated that miR-24-3p directly
targeted the 3′untranslated region (3′UTR) of p27Kip1 and
suppressed its expression. Finally, we determined that knockdown of
p27Kip1 promoted cell proliferation and overexpression of p27Kip1
abrogated the miR-24-3p promoted cell proliferation in breast
cancer cells. Therefore, our results demonstrated that miR-24-3p
can mediate its tumor oncogenic function, at least in part, by
suppressing the expression of p27Kip1. Altogether, our study
characterized a novel microRNA-mediated mechanism of p27Kip1
regulation and may provide a novel therapeutic target for breast
cancer.
Materials and methods
Patient samples
Breast cancer specimens and adjacent normal tissues
were collected at the Third Affiliated Hospital of Harbin Medical
University (Harbin, China) from January 2011 to December 2013. All
the patients recruited into the present study did not receive
radiotherapy, chemotherapy or any other treatment before and after
operation. Surgical specimens of the resected tumor were collected,
and lumps of tumors as well as adjacent normal tissues, which were
at least 2 cm distal to the tumor margins, were snap-frozen in
liquid nitrogen for miR-24-3p and p27Kip1 assays. Written informed
consent was obtained from all the study participants. The use of
tissue samples was approved by the Ethics Committees of the Third
Affiliated Hospital of Harbin Medical university.
Cell culture and transfection
Three widely used cell lines, MCF-10A, MDA-MB-435
and MDA-MB-468 were used in the present study, and were obtained
from the American Type Culture Collection (ATCC) and maintained in
RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1%
antibiotics (both from Invitrogen, Grand Island, NY, USA).
Transfection of the cells with miR-24-3p mimics, miR-control,
anti-miR-24-3p or anti-NC (GenePharma, Shanghai, China) was
performed using Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer’s instructions.
Detection of cell phenotypes
The effect of miR-24-3p on the proliferation of
breast cancer cells was evaluated by the MTT assay. MDA-MB-435 and
MDA-MB-468 cells were plated in 96-well culture plates
(3×103/well). After a 24-h incubation, the cells were
transfected with miR-24-3p mimics (30 pmol) or miR-control (30
pmol) for 12, 24 and 48 h. Then MTT (0.5 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) was added to each well (20 μl/well). After 4
h of additional incubation, the MTT solution was discarded and 200
ml of DMSO (Sigma-Aldrich) was added, and the plates were shaken
gently. The absorbance was measured on a microplate reader
(VersaMax; Molecular Devices, Sunnyvale, CA, USA) at a wavelength
of 570 nm. For the colony formation assay, cells were counted and
seeded in 12-well plates (in triplicate) at 100 cells/well.
Fresh-cultured medium was replaced every 3 days. The number of
viable cell colonies was determined after 14 days and the colonies
were fixed with methanol, stained with crystal violet, photographed
and counted under a microscope (IX71; Olympus, Tokyo, Japan). Each
experiment was performed in triplicate.
Cell cycle and apoptosis assays
Transfected MDA-MB-435 and MDA-MB-468 cells were
seeded into 6-well plates for 24 h in complete medium. Then, the
cells were deprived of serum for 48 h followed by returning the
complete medium for an additional 24 h. After that, the cells were
collected by centrifugation, fixed in 95% ethanol, incubated at
−20°C overnight and washed with phosphate-buffered saline (PBS).
The cells were then resuspended in 1 ml of FACS solution [PBS, 0.1%
Triton X-100, 60 μg/ml propidium iodide (PI), 0.1 mg/ml
DNase-free RNase and 0.1% trisodium citrate] with a final
incubation on ice for 30 min. The cells were analyzed using a
FACSCalibur flow cytometer (Beckman Coulter, Fullerton, CA, USA). A
total of 10,000 events were counted for each sample. For the
Annexin V assay, the MDA-MB-435 and MDA-MB-468 cells were
transfected. After 48 h, the DNA content was determined by PI
staining as described by Yi et al (18), and Annexin V staining was performed
with the Vybrant Apoptosis Assay kit (Invitrogen, Carlsbad, CA,
USA).
Western blotting
Cells were plated at a concentration of
106 cells/ml. After transfection with the miR-24-3p
mimics or anti-miR-24-3p for 48 h, the cells were lysed with RIPA
buffer (1X pbS, 1% Np-40, 0.1% SDS, 5 mM EDTA, 0.5% sodium
deoxycholate, 1 mM sodium orthovanadate and 1% PMSF). After
centrifuging at 12,000 rpm for 15 min at 4°C to remove the cell
debris, the supernatant was transferred and the protein
concentration were determined using Bradford protein dye reagent
(Bio-Rad, Hercules, CA, USA). The samples were resolved by 10%
SDS-PAGE and transferred to a nitrocellulose membrane. After
blocking with 5% skimmed milk at room temperature for 1 h, the
membrane was incubated with the p27Kip1 or GAPDH antibody (sc-528
and sc-25778, dilution 1:1,000; Santa Cruz biotechnology, Santa
Cruz, CA, USA) at 4°C overnight. After washing, HRP-conjugated
rabbit secondary monoclonal antibody (#7074, dilution 1:1,000; Cell
Signaling Technology, Beverly, MA, USA) was added and incubated at
room temperature for 1 h. Densitometric analysis of the band
intensity was performed using the software NIH ImageJ (version
1.32j).
Quantitative RT-PCR for miR-24-3p
The small RNA fraction was isolated from the cells
using the mirVana miRNA isolation kit (Ambion, Austin, TX, USA)
according to the manufacturer’s instructions. Quantitative RT-PCR
was performed using the mirVana qRT-pCR miRNA Detection kit and the
miR-24-3p and U6 snRNA primer sets (Ambion) in a Roche LightCycler
(Roche, basel, Switzerland). The LightCycler software package
version 5.3.2 was used to determine the expression of miR-24-3p
relative to that of U6 snRNA. Relative changes in miR-24-3p levels
were calculated using a standard curve constructed from serial
dilutions of control RNA.
Construction of 3′UTR reporter plasmid
and luciferase assay
The full length 3′UTR of p27Kip1 was cloned by
standard procedures into the pMIR-Report vector (Ambion),
immediately downstream of the stop codon of the luciferase gene to
generate the pMIR-p27Kip1-3′UTR luciferase reporter plasmid.
Mutagenesis of the pMIR-p27Kip1-3′UTR was performed using a
QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA,
USA). The two binding sites of miR-24-3p on the p27Kip1 3′UTR were
mutated simultaneously to generate pMIR-p27Kip1-3′UTR-mut and to
analyze their functional role. Cells were co-transfected with 2 mg
wild-type pMIR-p27Kip1-3′UTR or pMIR-p27Kip1-3′UTR-mut, 50 pmol
miRNA mimics and 0.01 mg Renilla in 24-well plates.
Forty-eight hours after transfection, the cells were washed and
lysed with passive lysis buffer (Promega, Madison, WI, USA), and
the luciferase activity was measured using a luminometer (Sirius;
Titertek-berthold, pforzheim, germany).
Knockdown of p27Kip1 by siRNA and
contruction of the p27 overexpression plasmid
The transient transfection of p27Kip1-siRNA or
pCDNA-p27Kip1 was performed using Lipofectamine 2000 according to
the manufacturer’s instructions. After 48-72 h, the cells were
analyzed to determine the transfection efficiency by western blot
analysis. The siRNA targeting p27Kip1 was purchased from Santa Cruz
biotechnology (sc-29429). The full-length human p27Kipl cDNA (1.5
kb) was subcloned into the EcoRI and BamHI sites of
the eukaryotic expression vector pCDNA3 in the sense orientation
resulting in the pCDNA-p27 plasmid.
Statistical analysis
A Student’s t-test was performed to analyze the
significance of differences between the sample means obtained from
three independent experiments. Differences were considered
statistically significant at p<0.05.
Results
Relative expression of miR-24-3p in the
breast cancer tissues and cells
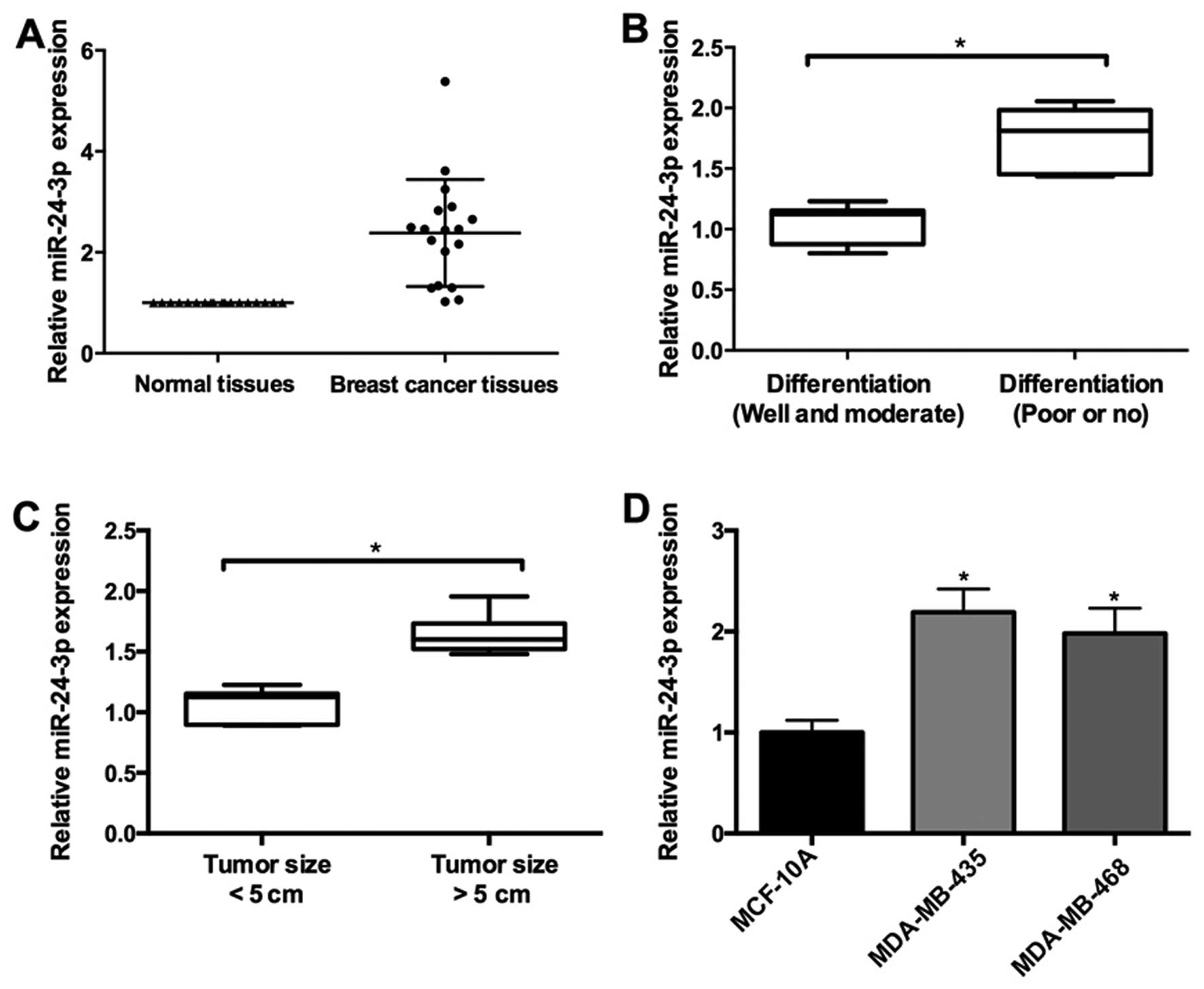
In the present study, the expression level of
miR-24-3p was measured with qRT-pCR in 26 pairs of breast cancer
and adjacent normal tissues. miR-24-3p was upregulated in the
breast cancer tissues (Fig. 1A). In
addition, miR-24-3p expression was associated with pathological
differentiation and tumor size in the breast cancer tissues. As
shown in Fig. 1b, the expression of
miR-24-3p was upregulated in breast cancer tissues with well and
moderate differentiation compared with tissues with poor or no
differentiation. Furthermore, higher expression of miR-24-3p was
observed in breast cancer tissues with tumor size >5 cm compared
to the tissues with tumor size <5 cm (Fig. 1C).
To further confirm the role of miR-24-3p during
breast cancer progression, we determined the expression of
miR-24-3p in two breast cancer cell lines (MDA-MB-468 and
MDA-MB-435) and non-malignant breast epithelial MCF-10A cells.
Compared to the MCF-10A cells, the expression of miR-24-3p was
higher in the MDA-MB-468 and MDA-MB-435 cells (Fig. 1D).
miR-24-3p promotes breast cancer
proliferation and inhibits apoptosis
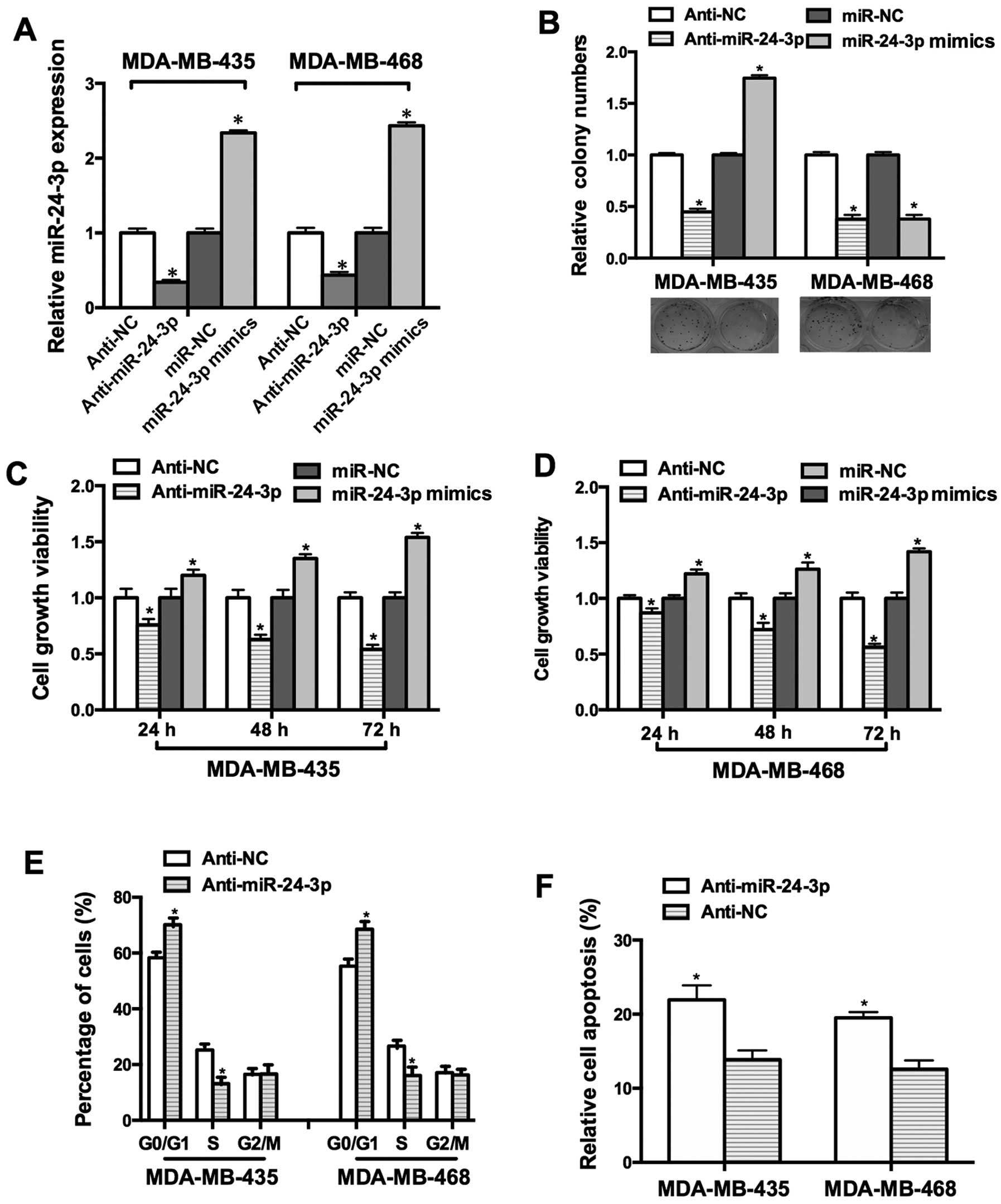
To investigate the function of miR-24-3p in breast
cancer cell lines, MDA-MB-435 and MDA-MB-468 were transfected with
miR-24-3p mimics. As shown in Fig.
2A, the miR-24-3p mimics increased the expression of miR-24-3p
by 2.3-fold and 2.4-fold in the MDA-MB-435 and MDA-MB-468 cells,
while anti-miR-24-3p reduced the expression of miR-24-3p by
0.3-fold and 0.4-fold, respectively. MTT and colony formation
assays were performed to detect the effects of miR-24-3p on cell
growth. Our data demonstrated that the miR-24-3p mimics facilitated
cell growth and anti-miR-24-3p inhibited cell growth (Fig. 2B–D).
To address the mechanism underlying
miR-24-3p-modulated cell growth, FACS assay was used to detect the
effect of miR-24-3p on the cell cycle. The proportion of cells in
the G0/G1 phase was increased in the MDA-MB-435 and MDA-MB-468
cells transfected with anti-miR-24-3p and the proportion of cells
in the S phase was decreased (Fig.
2E). Moreover, the Annexin V apoptosis assay showed that
anti-miR-24-3p promoted the apoptosis of the MDA-MB-435 and
MDA-MB-468 cells (Fig. 2F).
miR-24-3p directly targets and inhibits
p27Kip1 protein expression
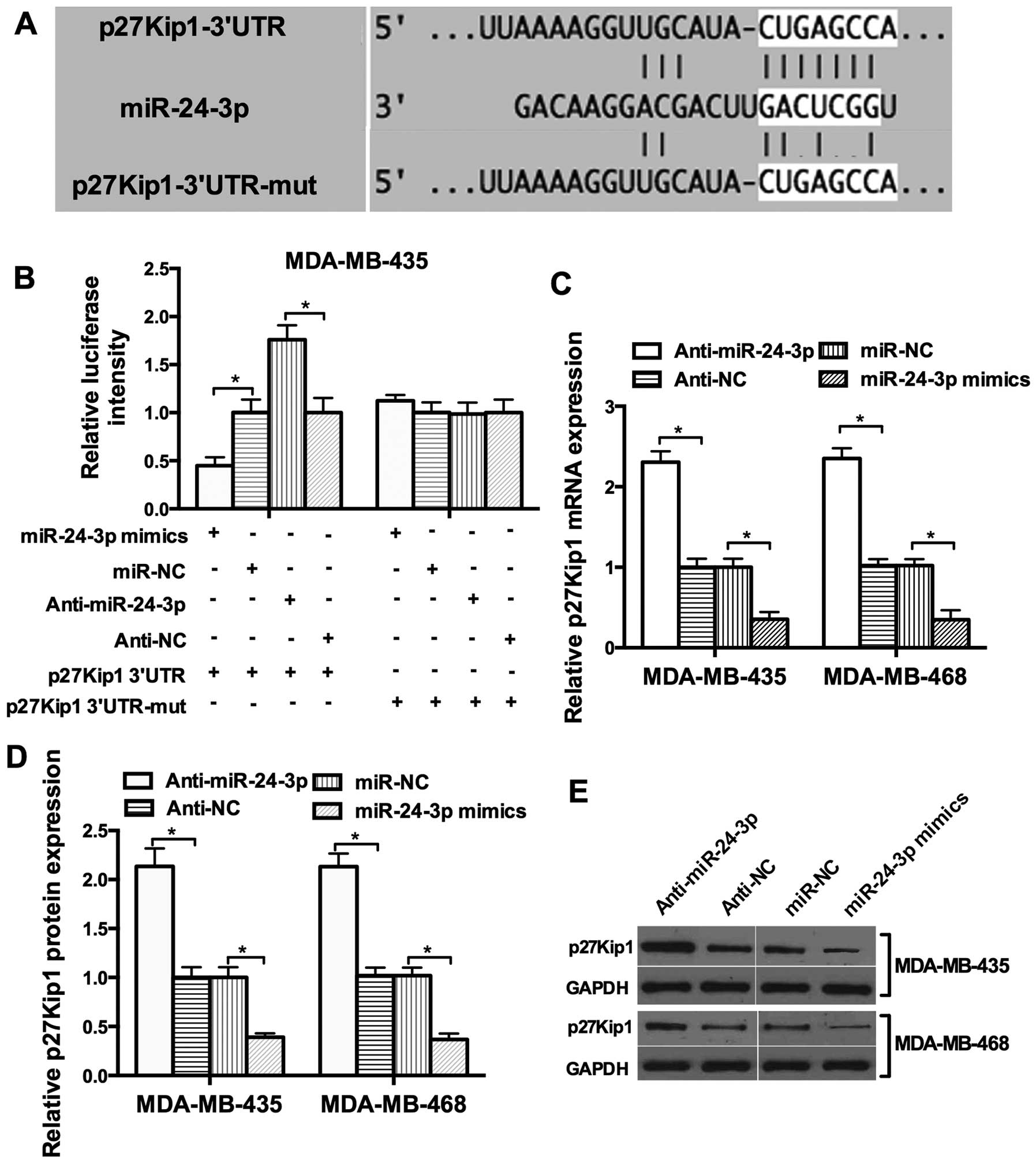
To explore the potential target of miR-24-3p,
bioinformatic analysis with TargetScan and miRanda was used and a
set of different target genes were predicted. Among these candidate
targets, p27Kip1 attracted our attention immediately since it was
predicted by the two algorithms (Fig.
3A). To investigate whether miR-24-3p directly targets p27Kip1
in breast cancer cells, the 3′UTR luciferase reporter assay was
performed. miR-24-3p had an obvious inhibitory effect on the
luciferase intensity of the wild-type 3′UTR luciferase reporter.
However, the inhibitory effect of miR-24-3p was reduced in the
presence of the mutant 3′UTR luciferase reporter (Fig. 3B). We then tested whether miR-24-3p
affects the expression of endogenous p27Kip1. The restoration of
miR-24-3p expression resulted in a reduction in endogenous p27Kip1
mRNA and protein expression in the MDA-MB-435 and MDA-MB-468 cells
(Fig. 3C–E). These results provide
evidence that miR-24-3p directly targets the 3′UTR of p27Kip1 and
suppresses its expression.
miR-24-3p-promoted cell proliferation is
mediated by p27
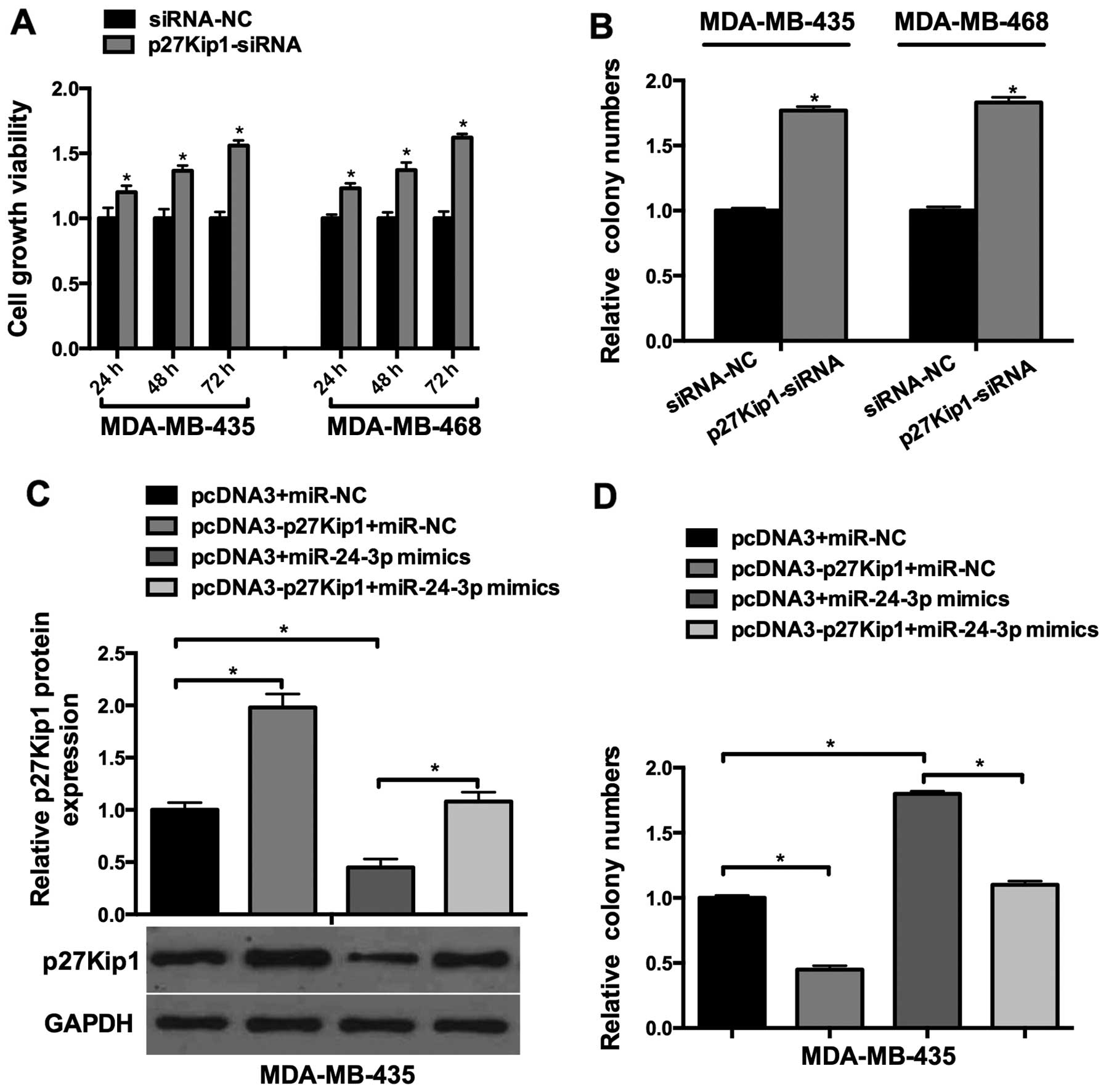
To investigate the function of p27Kip1 in breast
cancer cell lines, p27Kip1-siRNA was transfected into the
MDA-Mb-435 and MDA-Mb-468 cells. As shown in Fig. 4A, p27Kip1-siRNA increased the cell
viability of the MDA-MB-435 and MDA-MB-468 cells in a
time-dependent manner. Colony formation assays showed that relative
cell growth was significantly promoted in the
p27Kip1-siRNA-transfected cells (Fig.
4B). Next, we explored whether miR-24-3p-induced breast cancer
cell growth was mediated by p27Kip1. miR-24-3p mimics and
pcDNA3-p27Kip1 were co-transfected into the breast cancer cells,
which showed that miR-24-3p-induced p27Kip1 downregulation was
rescued by the overexpression of p27Kip1 (Fig. 4C); Furthermore, colony formation
assay showed that miR-24-3p-induced cell proliferation was aborted
by the overexpression of p27Kip1 (Fig.
4C). These results suggested that miR-24-3p-induced breast
cancer cell growth was mediated by p27Kip1.
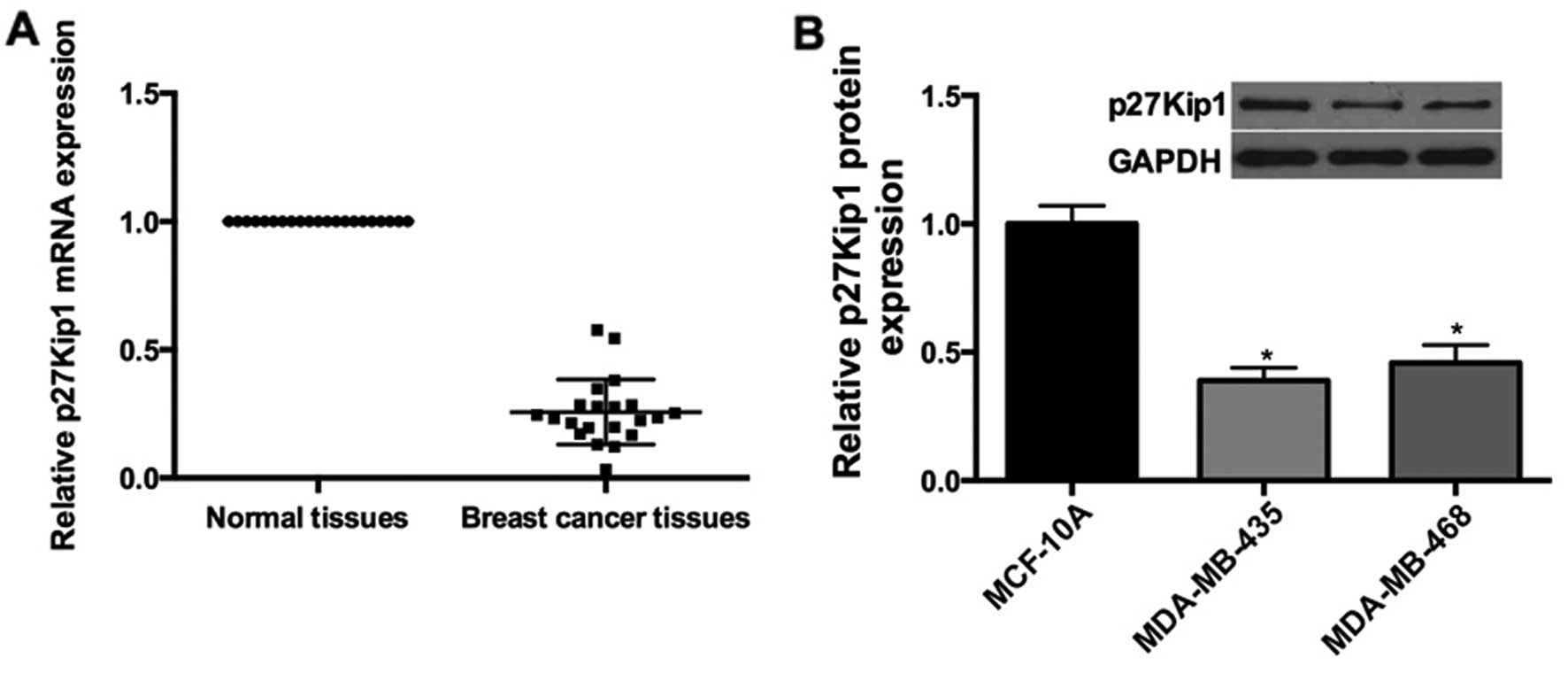
p27 expression is downregulated in breast
cancer tissues and cells
Since we demonstrated the expression levels of
miR-24-3p in breast cancer cell lines and tissues, qRT-pCR was
performed. The results showed that p27Kip1 mRNA was downregulated
in the breast cancer tissues compared with the adjacent normal
tissues (Fig. 5A); Furthermore, the
expression of p27Kip1 was obviously decreased in the MDA-Mb-435
(0.4-fold) and MDA-MB-468 (0.46-fold) cells compared to the level
in the MCF-10A cells (Fig. 5B).
Discussion
Breast cancer is the most common cancer in women
around the world. Breast cancer is influenced by a number of
environmental factors and is characterized by molecular
heterogeneity. Despite the various existing screening programs and
new therapeutic strategies implemented to treat breast cancer which
have significantly reduced mortality rates, the molecular
mechanisms underlying breast cancer pathogenesis are only partially
understood.
Numerous studies have suggested the potential role
of microRNAs as oncogenes or tumor-suppressor genes affecting the
cell cycle and apoptosis via the regulation of different target
genes in cancer (19). A set of
miRNAs with differential expression levels and critical tumor
suppressive roles has been described in breast cancer cells,
including miR-145, miR-122a, miR-125a-5p, miR-126, miR-200c,
miR-10b and miR-96 (20).
Furthermore, multivariate analysis in breast cancer identified that
downregulation of miR-155 and let-7 expression was significantly
correlated with a shorter patient survival rate (21,22).
miR-24-3p is mainly produced from two miRNA clusters,
miR-23a-27a-24-2 and miR-23b-27b-24-1, which is a master regulator
in a variety of tumors (23,24).
It is reported that miR-24-3p may function differently regarding
cell proliferation in different cell types (24). For example, miR-24-3p can repress
HeLa cell proliferation, while it facilitates cell proliferation of
TGF-β-treated hepatocellular carcinoma cells (huh7), as well as
lung carcinoma cells (A549) and glioblastoma cell lines (25–27).
Therefore, miRNA expression patterns vary under different
biological conditions (23).
Here, we determined that the miR-24-3p expression
level in breast cancer and normal tissues displayed marked
upregulation by real-time PCR analysis. Furthermore, upregulation
of miR-24-3p was also observed in poorly or undifferentiated
tissues, and tissues with tumor size >5 cm. Thus, these results
suggest the potential tumor oncogenic role of miR-24-3p. This was
further confirmed in the breast cancer cell lines. Using breast
cancer cell lines, MDA-MB-435 and MDA-MB-468, we confirmed that
overexpression of miR-24-3p increased cell proliferation as
determined by MTT and colony formation assays. Recently, an
increasing number of studies have demonstrated that the
dysregulation of miRNAs affects cell cycle progression and
apoptosis in many types of human cancers. Researchers have
identified that miR-15a can inhibit the cell cycle by targeting
CCNE1 in breast cancer cells (28).
Functional assays revealed that MCF-7 cell growth can be suppressed
by miR-497 through increasing the percentage of early apoptotic
cells (29). In the present study,
we identified that overexpression of miR-24-3p significantly
suppressed the apoptosis of MDA-MB-435 and MDA-MB-468 cells.
Furthermore, we confirmed that miR-24-3p can promote cell cycle
transition.
miRNAs exert their function through partially
binding the 3′UTR of mRNAs (30).
Here, we defined a specific function for miR-24-3p in breast
cancer, showing that they repress expression of p27Kip1. Analysis
of the 3′UTR of p27Kip1 suggested that repression of p27Kip1 is a
consequence of the direct binding of miR-24-3p to sites in the
3′UTR. Based on the luciferase reporter data, miR-24-3p was able to
cause at least a 2-fold reduction in the levels of p27Kip1 protein
via direct binding to the 3′UTR of its mRNA. Recent studies have
shown that p27Kip1 is overexpressed in several tumors compared with
normal tissues (31). Furthermore,
research demonstrated that p27Kip1 can exhibit anti-apoptotic
effects or sustain cell growth in several cancer types (32). In the present study, our findings
demonstrated that p27Kip1 was expressed at higher levels in breast
cancer tissues compared with that in the normal tissues. To further
confirm that miR-24-3p can directly target p27Kip1, a knockdown
plasmid of p27Kip1 (p27Kip1-siRNA) was used. p27Kip1-siRNA promoted
MDA-Mb-435 and MDA-MB-468 cell proliferation. Furthermore, the
transfection of the p27Kip1 overexpression plasmid (pcDNA3-p27Kip1)
rescued miR-24-3p-induced p27Kip1 downregulation and abrograted the
miR-24-3p-promoted cell proliferation.
These data that connect miR-24-3p to p27Kip1
suggested that miR-24-3p-promoted breast cancer cell proliferation
was, at least in part, mediated by p27Kip1. Gonzalez et al
previously showed that inhibition of cdk4 activity enhanced the
translation of p27Kip1, providing a link between these two cell
cycle regulators. This effect was shown to be mediated by the 3′UTR
of p27Kip1 (33). In the present
study, we demonstrated that miR-24-3p can suppress the expression
of p27Kip1 through interaction with its 3′UTR, therefore, miR-24-3p
may have a crosstalk with the cdk4/p27Kip1 regulatory axis.
Finally, the data here suggest that miR-24-3p may be
a potential therapeutic target for the treatment of breast cancer.
To date, miR-24-3p inhibition may be a potential way to reduce the
aggressive growth of breast cancer by restoring normal levels of
p27Kip1. This could potentially decrease breast cancer cell
proliferation. The effectiveness of this would in part depend on
what additional targets are regulated by miR-24-3p in both cancer
and normal tissues.
In conclusion, the present study provides new
insights into the specific function of miR-24-3p and its mechanism
in breast cancer proliferation, and suggests that targeting of
miR-24-3p may provide a potential therapeutic strategy for blocking
proliferation in breast cancer.
Acknowledgments
The present study was supported by the Key Fund
project of education (no. 12511z019), the Fund for excellent
Academic Leaders in harbin (no. 2011RFXYS060) and the Chunhui Fund
of the Ministry of Education (no. Z2010006).
References
|
1
|
Iorio MV, Casalini P, Piovan C, Braccioli
L and Tagliabue E: Breast cancer and microRNAs: Therapeutic impact.
Breast. 20(Suppl 3): S63–S70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piovan C, Palmieri D, Di Leva G, Braccioli
L, Casalini P, Nuovo G, Tortoreto M, Sasso M, Plantamura I, Triulzi
T, et al: Oncosuppressive role of p53-induced miR-205 in triple
negative breast cancer. Mol Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang D, Liu D, Gao J, Liu M, Liu S, Jiang
M, Liu Y and Zheng D: TRAIL-induced miR-146a expression suppresses
CxCR4-mediated human breast cancer migration. FEBS J.
280:3340–3353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang S, Li Y, Gao J, Zhang T, Li S, Luo A,
Chen H, Ding F, Wang X and Liu Z: MicroRNA-34 suppresses breast
cancer invasion and metastasis by directly targeting Fra-1.
Oncogene. 32:4294–4303. 2013. View Article : Google Scholar
|
|
5
|
Sun EH, Zhou Q, Liu KS, Wei W, Wang CM,
Liu XF, Lu C and Ma DY: Screening miRNAs related to different
subtypes of breast cancer with miRNAs microarray. Eur Rev Med
Pharmacol Sci. 18:2783–2788. 2014.PubMed/NCBI
|
|
6
|
Mulrane L, Klinger R, Mcgee SF, Gallagher
WM and O’Connor DP: microRNAs: A new class of breast cancer
biomarkers. Expert Rev Mol Diagn. 14:347–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barh D, Malhotra R, Ravi B and Sindhurani
P: MicroRNA let-7: An emerging next-generation cancer therapeutic.
Curr Oncol. 17:70–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
9
|
Lane HA, Beuvink I, Motoyama AB, Daly JM,
Neve RM and Hynes NE: ErbB2 potentiates breast tumor proliferation
through modulation of p27(Kip1)-Cdk2 complex formation: Receptor
overexpression does not determine growth dependency. Mol Cell Biol.
20:3210–3223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motti ML, Califano D, Troncone G, De Marco
C, Migliaccio I, Palmieri E, Pezzullo L, Palombini L, Fusco A and
Viglietto G: Complex regulation of the cyclin-dependent kinase
inhibitor p27kip1 in thyroid cancer cells by the
pI3K/AKT pathway: Regulation of p27kip1 expression and
localization. Am J pathol. 166:737–749. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XH, Cai P, Wang MH and Wang Z:
microRNA-25 promotes osteosarcoma cell proliferation by targeting
the cell cycle inhibitor p27. Mol Med Rep. 10:855–859.
2014.PubMed/NCBI
|
|
12
|
Tong J, Fu Y, Xu X, Fan S, Sun H, Liang Y,
Xu K, Yuan Z and Ge Y: TGF-β1 stimulates human Tenon’s capsule
fibroblast proliferation by miR-200b and its targeting of p27/kip1
and RND3. Invest Ophthalmol Vis Sci. 55:2747–2756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao ZQ, Shen Z and Huang WY: MicroRNA-802
promotes osteosarcoma cell proliferation by targeting p27. Asian
Pac J Cancer Prev. 14:7081–7084. 2013. View Article : Google Scholar
|
|
14
|
Wu X, Liu T, Fang O, Leach LJ, Hu X and
Luo Z: miR-194 suppresses metastasis of non-small cell lung cancer
through regulating expression of BMP1 and p27(kip1). Oncogene.
33:1506–1514. 2014. View Article : Google Scholar
|
|
15
|
Wang B, Li W, Guo K, Xiao Y, Wang Y and
Fan J: miR-181b promotes hepatic stellate cells proliferation by
targeting p27 and is elevated in the serum of cirrhosis patients.
Biochem Biophys Res Commun. 421:4–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun M, Liu XH, Li JH, Yang JS, Zhang EB,
Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ, et al: miR-196a is
upregulated in gastric cancer and promotes cell proliferation by
downregulating p27(kip1). Mol Cancer Ther. 11:842–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Wang S, Zhao P, Wang X, Wang J,
Wang Y, Song L, Zou Y and Hui R: miR-221 promotes cardiac
hypertrophy in vitro through the modulation of p27 expression. J
Cell biochem. 113:2040–2046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi M, Parthiban P, Hwang J, Zhang X, Jeong
H, Park DH and Kim DK: Effect of a bispidinone analog on
mitochondria-mediated apoptosis in HeLa cells. Int J Oncol.
44:327–335. 2014.
|
|
19
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Liu L, Xu Q, Wu P, Zuo X and Ji A:
MicroRNA as a novel drug target for cancer therapy. Expert Opin
Biol Ther. 12:573–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Wang BC and Tang JH: Clinical
significance of microRNA-155 expression in human breast cancer. J
Surg Oncol. 106:260–266. 2012. View Article : Google Scholar
|
|
22
|
Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J,
Zhao JJ, Mao SS, Zhang GH, Xu XC and Zhang N: miR-340 inhibition of
breast cancer cell migration and invasion through targeting of
onco-protein c-Met. Cancer. 117:2842–2852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chhabra R, Dubey R and Saini N:
Cooperative and individualistic functions of the microRNAs in the
miR-23a~27a~24–2 cluster and its implication in human diseases. Mol
Cancer. 9:2322010. View Article : Google Scholar
|
|
24
|
Lal A, Navarro F, Maher CA, Maliszewski
LE, Yan N, O’Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O,
et al: miR-24 inhibits cell proliferation by targeting E2F2, MYC,
and other cell-cycle genes via binding to ̔seedless’ 3′UTR microRNA
recognition elements. Mol Cell. 35:610–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ciafrè SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Q, Li X, Li J, Kong X, Zhang J, Chen
L, Huang Y and Fang L: miR-15a is underexpressed and inhibits the
cell cycle by targeting CCNE1 in breast cancer. Int J Oncol.
43:1212–1218. 2013.PubMed/NCBI
|
|
29
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012.PubMed/NCBI
|
|
30
|
Zhou P, Xu W, Peng X, Luo Z, Xing Q, Chen
X, Hou C, Liang W, Zhou J, Wu X, et al: Large-scale screens of
miRNA-mRNA interactions unveiled that the 3′UTR of a gene is
targeted by multiple miRNAs. PLoS One. 8:e682042013. View Article : Google Scholar
|
|
31
|
Bellan C, De Falco G, Lazzi S, Micheli P,
Vicidomini S, Schürfeld K, Amato T, Palumbo A, Bagella L, Sabattini
E, et al: CDK9/CYCLIN T1 expression during normal lymphoid
differentiation and malignant transformation. J Pathol.
203:946–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Falco G and Giordano A: CDK9: From
basal transcription to cancer and AIDS. Cancer biol Ther.
1:342–347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
González T, Seoane M, Caamaño P, Viñuela
J, Domínguez F and Zalvide J: Inhibition of Cdk4 activity enhances
translation of p27kip1 in quiescent Rb-negative cells. J
biol Chem. 278:12688–12695. 2003. View Article : Google Scholar
|