Introduction
Primary and metastatic bone cancers are often
accompanied by severe pain. Bone cancer pain occurs in 75–90% of
patients with metastatic cancer (1)
and is often debilitating and intractable, affecting the quality of
life of patients (2,3). Bone cancer pain is associated with
breakthrough pain, which is defined as 'a transitory exacerbation
of pain experienced by patients who have relatively stable and
adequately controlled baseline pain' (2). Hence, controlling bone cancer pain
would be of great significance in the management of patients with
bone cancer.
Currently, there are many therapeutic options
available for alleviating bone cancer pain including external beam
radiotherapy, local surgery, opioid analgesia, non-steroidal
anti-inflammatory drugs (NSAIDs), bisphosphonates and other
anesthetic techniques (4). However,
these therapies either have poor responses or require high dosages
that are often associated with severe adverse effects (5–8). For
instance, although bisphosphonates are effective in reducing
skeletal morbidity from bone metastases and bone cancer pain, their
efficacy remains unclear (9,10).
Therefore, there is an urgent need for the discovery and
development of new therapeutic reagents to control bone cancer
pain, particularly for breakthrough pain.
We and other researchers have shown that some
Chinese herbal medicines have potent antinociceptive activity, and
could effectively control cancer pain including bone cancer pain in
clinical practice (11–14). Indeed, it is reported that ~41–62%
of cancer patients use herbs as a complementary or alternative
medical therapy for cancer pain management (15). Topical treatment is an attractive
and effective option, particularly for bone cancer pain management;
since oral administration of herbal medicines causes several
side-effects such as nausea, vomiting and diarrhea, affecting the
quality of life of patients (11–13,16).
Xiaozheng Zhitong Paste (XZP) is a herbal analgesic paste that is
prepared from six herbs including Xuejie (Dragon's blood), Yanhusuo
(Corydalis Rhizoma), Ruxiang (Olibanum), Moyao (Myrrha),
Qingdai (Natural Indigo) and Bingpian (Borneolum Syntheticum). Our
previous studies showed that XZP significantly alleviated cancer
pain including bone cancer pain in patients with middle and late
stages of cancer (16,17). However, its underlying mechanism has
not been systemically explored.
Specific cellular and molecular mechanisms
underlying bone cancer pain remain largely obscure (18,19).
While several lines of studies have demonstrated remarkable
sensitization of peripheral nociceptors in bone, resulting from the
tumor-induced acidosis and cytokine synthesis; emerging evidence
indicates that central sensitization exists in the spinal dorsal
horn of a rodent model of bone cancer pain such as the activation
of astrocytes (20–22) and altered glutamatergic
synaptogenesis (23,24).
Proteinase-activated receptors (PARs) are a family
of the G-protein coupled receptors that are activated by proteases,
which liberates a tethered ligand by cleaving the N-terminus of the
receptors initiating several intracellular signal pathways
(25). Among the four PAR subtypes,
PAR1, PAR3 and PAR4 are preferentially activated by thrombin, while
PAR2 is preferentially activated by trypsin and trypsin-like
proteinases (26,27). All four PARs are expressed
throughout the peripheral and central nervous system (26–33).
In the spinal dorsal horn, PAR2 receptors are located in the
microglia, astrocytes, neurons and terminals of afferent fibers
originating from dorsal root ganglions (26). Previous studies have demonstrated
the critical involvement of PAR2 in the pathogenesis of several
types of inflammatory or neuropathic pain (26). Activation of PAR2 signaling
participates in the induction of peripheral nociceptor
sensitization in a rodent model of bone cancer pain (34). We have also demonstrated that the
activation of PAR2 signaling contributes to the central
sensitization in rats with bone cancer pain (26,27).
However, there is no information on the impact of XZP treatment on
peripheral and central sensitization, and on the PAR2 signaling
pathway. Given that XZP effectively alleviates bone cancer pain, we
hypothesized that XZP treatment inhibits the PAR2 signaling
pathway; mitigating peripheral and central sensitization. The
present study was designed to test this hypothesis, utilizing a rat
model of bone cancer nociception to evaluate the therapeutic effect
of XZP on bone cancer pain and explore its potential underlying
mechanism. We believe that our results have an impact on the
development of XZP as an effective topical treatment for bone
cancer pain, and future discoveries of novel PAR2-targeted therapy
for cancer pain in general.
Materials and methods
XZP preparation
XZP is composed of the six aforementioned
traditional Chinese medicines. High performance liquid
chromatography (HPLC) analysis has shown that XZP contained
tetrahydropalmatine (0.0568%), imperatorin (0.0041%),
isoimperatorin (0.0122%), coptisine (0.0358%) and palmatine
chloride (0.112%) (16).
Animals
Animal use and care protocols were reviewed and
approved by the Animal Care and use Committee of the China Academy
of Chinese Medical Sciences, Beijing, China. All animal studies
were carried out in accordance with the guidelines of the
International Association for the Study of Pain (35). Female Wistar rats, weighing 150–180
g, were obtained from the Department of Experimental Animal
Sciences, Peking University Health Science Center (Beijing, China).
Individual rats were housed in specific pathogen-free (SPF)
facilities under strict temperature (24±1°C) and humidity (60%)
control on a 12/12 h light/dark cycle with free access to standard
food and tap water.
Rat bone cancer pain model and XZP
treatment
Wister rat Walker 256 breast sarcocarcinoma cells
were prepared, and a rat bone cancer pain model was established as
previously reported (32). Briefly,
Wistar rats were intraperitoneally (i.p) injected with Walker 256
cells (2×107 in 0.5 ml of Hank's solution). Seven to 14
days later, cells in the produced ascites were collected. After
being anesthetized, 50 rats were injected with 105
Walker 256 cells in 10-µl of Hank's solution into the left
tibia of the hind paw. Then, the injection site was covered by bone
wax and the wound was closed. Sham control rats (n=10) received the
same surgery and volume of vehicle injection. All rats were
subjected to the same post-operative care.
Rats with bone cancer were randomly divided into
five groups (n=10/group): one placebo control group, topically
treated with inert paste; three XZP groups, treated with low (15.75
g/kg), medium (31.5 g/kg) and high (63 g/kg) doses of XZP; one OPG
group, subcutaneously injected with 5 mg/kg of OPG twice per day.
Sham control rats were also topically treated with inert paste. XZP
and placebo pastes were evenly applied on the skin of tumor-bearing
tibias; which were then covered with gauze and a layer of plastic
film, sealed and fixed with desensitized adhesive plaster.
Treatments were performed twice a day at 8:00 am and 20:00 pm for a
total of 21 days, beginning at day one after cancer cells were
implanted into the tibias (17).
Radiological analysis of bone
lesions
Cancer-related osteolytic lesions in rat tibias were
examined by X-ray radiology at day 21 after cell inoculation. Rats
were anaesthetized with sodium pentobarbital (45 mg/kg) by i.p
injection and exposed to X-ray (Emerald 125) at 40 kVp for 1/20
sec. X-ray film (Henry Schein blue sensitive film; Shanghai Han
Ruixiang Trade Co., Ltd.) was developed with a film developer
(Konica SRX-101; Suzhou Zhongyi Electronic Technology Co., Ltd.).
Tibias were scanned and reconstructed with an isotropic voxel size
of 8-µm on a micro-CT system (eXplore Locus SP; GE Medical
Systems, Zhongtong Shanghai Automation & Electrics Co., Ltd.).
Reconstructed 3D images of femurs were analyzed using Microviewer
(GE Medical Systems, Zhongtong Shanghai Automation & Electrics
Co., Ltd.) as previously described (36). Bone mineral density (BMD) of left
tibias in individual rats were measured by dual energy X-ray
absorptiometry using a PIXImus Mouse Densitometer; and bone mineral
contents were calculated using small animal analysis software (both
from GE Lunar Medical System, Zhongtong Shanghai Automation &
Electrics Co., Ltd.) at day 21, after cell inoculation.
Determination of mechanical threshold and
paw withdrawal latency
The effects of XZP treatment on spontaneous
nociceptive behavior were determined using mechanical threshold and
paw withdrawal latency (PWL), as previously described (11,16,27,28,30,32).
In brief, individual rats were placed in an inverted plastic
chamber on the glass surface of a Paw Thermal Stimulator System
(UCSD, San Diego, CA, USA) for 30 min before the test. Mechanical
hyperalgesia was measured using a single rigid filament attached to
a handheld transducer (automatic plantar analgesia tester;
Institute of Biomedical Engineering, Chinese Academy of Medical
Science, Tianjin, China). Animals were acclimated to their
surroundings daily for 10 min for three consecutive days in a
plexiglass box on a metal grid surface prior to testing. On testing
days, rats were allowed to acclimate for 5–10 min. A rigid filament
was pressed perpendicularly against the medial plantar surface of
the hind paw with increasing force. Brisk paw withdrawal or paw
flinching accompanied by head turning, biting and/or licking upon
application of the increasing force was considered as a positive
response. Paw withdrawal threshold (PWT) was defined as the minimal
force (g) required in evoking the cited positive responses. Each
hind paw was tested three times and data were averaged. Interval
between consecutive tests of the same paw was 5 min. The same
procedure was performed on day 3, 6, 9, 12, 15, 18 and 21 after
cell inoculation. Each hind paw was stimulated with a focused beam
of radiant heat using an analgesiometer (37360; Ugo Basile, Italy)
underneath the glass surface. When the paw was withdrawn from the
stimulus, PWL was automatically recorded to the nearest 0.1 sec.
Stimuli intensity was adjusted to generate an average baseline PWL
of ~10.0 sec in naive animals. Maximum stimulation was controlled
to <20 sec to prevent potential tissue damage. Paws were
alternated randomly to preclude 'order' effects. Individual rats
were subjected to four tests with 5-min intervals before surgery;
and 2, 5, 8, 11, 14, 17 and 20 days after cell inoculation. Mean
PWL values were calculated for each time point for individual
rats.
Histological evaluation
Rats were deeply anesthetized by pentobarbital and
transcardially perfused with saline on day 0 and 21 after
inoculation (n=8/group). The left tibia of each rat was dissected,
fixed in 10% formalin overnight, decalcified in 15%
EDTA-phosphate-buffered saline (PBS) for seven days and
paraffin-embedded. Tissue sections (6-µm) were prepared and
stained with hematoxylin and eosin (H&E) for pathological
analysis.
Analysis of trypsin, TNF-α and IL-1β
serum by enzyme-linked immunosorbent assay (ELISA)
Orbital venous blood samples from each rat were
collected on day 0, 7, 14 and 21 after cell implantation.
Inflammatory mediator levels were determined using ELISA kits
(Beijing Jia Mei Nuo Si Biotechnology Co., Ltd.; trypsin, ng/ml,
catalog no. ABIN1117631, detection range 0.16–10 ng/ml, detection
minimum 0.09 ng/ml; interleukin-1β, pg/ml, catalog no. ABIN365189,
detection range 62.5–4,000 pg/ml, detection minimum 62.5 pg/ml;
tumor necrosis factor-α, pg/ml, catalog no. ABIN365380, detection
range 6.25–400 pg/ml, detection minimum 6.25 pg/ml).
Quantitative RT-PCR
To analyze c-Fos, GFAP, IBA1 and CGRP mRNA levels,
total RNA was isolated from the L4-L5 spinal cord using the TRizol
reagent (Invitrogen, Beijing, Mao Jian united Stars Technology Co.,
Ltd., Beijing, China) according to the manufacturer's instructions.
Total RNA (5 µg) was reverse transcribed to cDNA with a
first-strand cDNA synthesis kit (Invitrogen). Quantitative PCR was
performed using a LightCycler system (Roche, Beijing Mao Jian
United Stars Technology Co., Ltd.) with SYBR-Green. Each PCR was
performed in triplicate to a final solution volume of 20-µl:
10 µl of SYBR-Green dye, 1 µl of diluted cDNA
products,0.2 µM of each paired primer and 8.6 µl of
deionized water. Protocols were as follows: initial denaturation
for 5 min at 95°C, followed by 35 cycles denaturation for 15 sec at
95°C, and extension for 30 sec at 56°C. Last cycle for dissociation
of SYBR-Green probe was 15 sec at 95°C, 30 sec at 56°C, and 15 sec
at 95°C. Primer sequences are shown in Table I. Relative mRNA levels of c-Fos,
GFAP, IBA1 and CGRP were normalized to GAPDH.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Target gene | Primers
sequences | Size (bp) |
|---|
| c-Fos | F
5′-GGGCATCGGGGATCTTGC-3′ | 510 |
| R
5′-GGGCTCTGTCAAC-3′ | |
| GFAP | F
5′-GAGAGGAAGGTTGAGTCGCT-3′ | 241 |
| R
5′-CGTCTGTGAGGTCTGCAAAC-3′ | |
| IBA1 | F
5′-ATGCTGGAGAAACTTGGGGT-3′ | 190 |
| R
5′-CAGTTGGCTTCTGGTGTTCT-3′ | |
| CGRP | F
5′-ACTGCATCCTGAATATCAGTCTC-3′ | 223 |
| R
5′-CAGTTGTACAAGGAAGTCACCTT-3′ | |
| PAR2 | F
5′-TGGCAACGACTGGACCTAT-3′ | 497 |
| R
5′-TGGGAGCGAAGCAGATGAAG-3′ | |
| PKC-γ | F
5′-TCTTCCCAGAAACCCCACTC-3′ | 232 |
| R
5′-AGAACATGGAAGGGAGGTGG-3′ | |
| PKA | F
5′-GGCTTCCAACTCCAACGATG-3′ | 240 |
| R
5′-GTGTGCTCGATCTGCTTCAG-3′ | |
| TRPV1 | F
5′-TGCACAATGGGCAGAATGAC-3′ | 151 |
| R
5′-GTCATGTTCCGCCGTTCAAT-3′ | |
| GAPDH | F
5′-CCCCCAATGTATCCGTTGTG-3′ | 118 |
| R
5′-TAGCCCAGGATGCCCTTTAGT-3′ | |
To analyze PAR2, PKC-γ, PKA and TRPV1 mRNA levels
from rat bones with tumor invasion, total RNA was isolated using a
TRIzol reagent and RT-PCRs were performed as above. Primer
sequences are shown in Table I.
Western blot assay
To analyze c-Fos, GFAP, IBA1 and CGRP protein
levels, total proteins from the L4-L5 spinal cords in various
groups were extracted by sonication in RIPA buffer containing
protease inhibitors (Roche). Extracted proteins were dialyzed in
PBS. After measuring protein content with a BCA protein assay kit
(Thermo Scientific, Rockford, IL, USA), protein samples with equal
amounts of total protein were separated on SDS-PAGE gels and
transferred to nitrocellulose (NC) membranes (Millipore). Western
blot assay was performed, as previously reported (16,27,28,31).
Primary antibodies used in the present study were obtained from
Abcam (Cambridge, MA, USA) including anti-c-Fos (phospho T325)
(ab27793), anti-GFAP (ab4674), anti-IBA1 (ab107159) and anti-CGRP
[EPR9670(B)] (ab139264) antibodies. Target protein bands were
detected using horseradish peroxidase-conjugated secondary
antibodies and visualized by an enhanced chemiluminescence
detection system (Beijing Mao Jian United Stars Technology Co.,
Ltd.). Relative protein expression levels were quantified by
ImageQuant™ LAS 4000 (GE Healthcare) and normalized to GAPDH.
To analyze PAR2, PKC-γ, PKA and TRPV1 protein levels
in rat bones with tumor invasion, total proteins were extracted,
quantified and analyzed by western blotting as previously
described. Primary antibodies used in experiments were also
obtained from Abcam including anti-PKC γ (phospho T514) (ab109539),
anti-PAR2 (ab180953), anti-PKA α + β (catalytic subunits) (phospho
T197) (ab5815) and anti-VR1 (ab63083) antibodies.
Statistical analysis
Behavioral data are expressed as mean ± standard
deviation (SD) and analyzed with repeated measures ANOVA, while
ELISA data were analyzed by one-way ANOVA followed by Newman-Keuls
test. SPSS 13.0 statistical software was used in the present study.
P<0.05 was considered to indicate a statistically significant
result.
Results
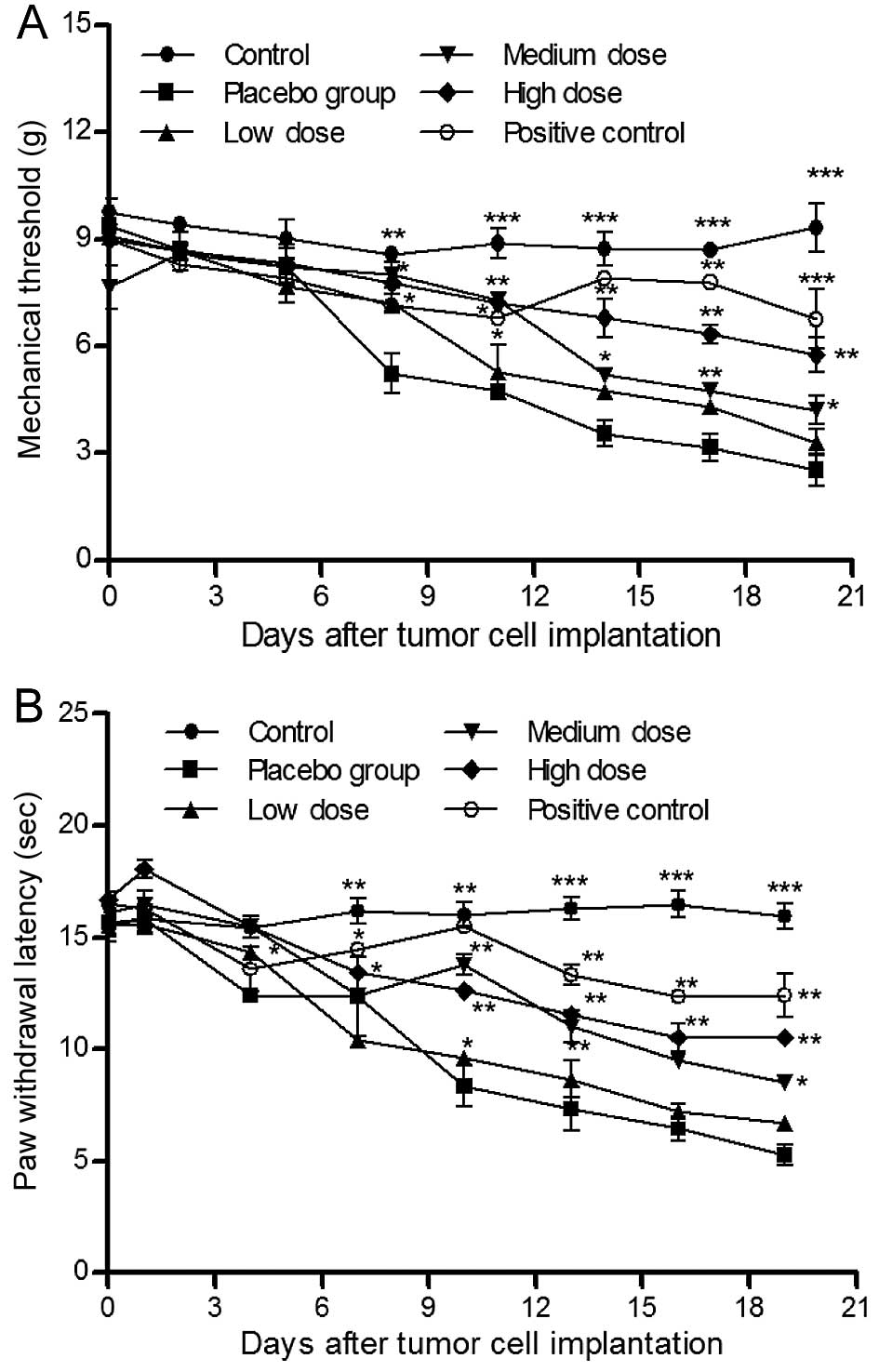
XZP treatment reduce nociceptive
responses in rats with bone cancer
We first determined the effects of XZP treatments on
bone cancer-related nociceptive behavior in bone cancer bearing
Wister rats. While constant levels of mechanical thresholds were
observed in sham control rats, levels of mechanical thresholds were
gradually reduced throughout the observation period in rats in the
placebo group (Fig. 1A). In
contrast, treatment with different doses of XZP significantly
mitigated mechanical allodynia in a dose- and time-dependent
manner; although mechanical threshold values were lower than
OPG-treated positive controls. Similar PWL patterns were observed
in different groups of rats (Fig.
1B). These data indicated that XZP treatment reduced mechanical
and thermal nociceptive behavior in rats with bone cancer.
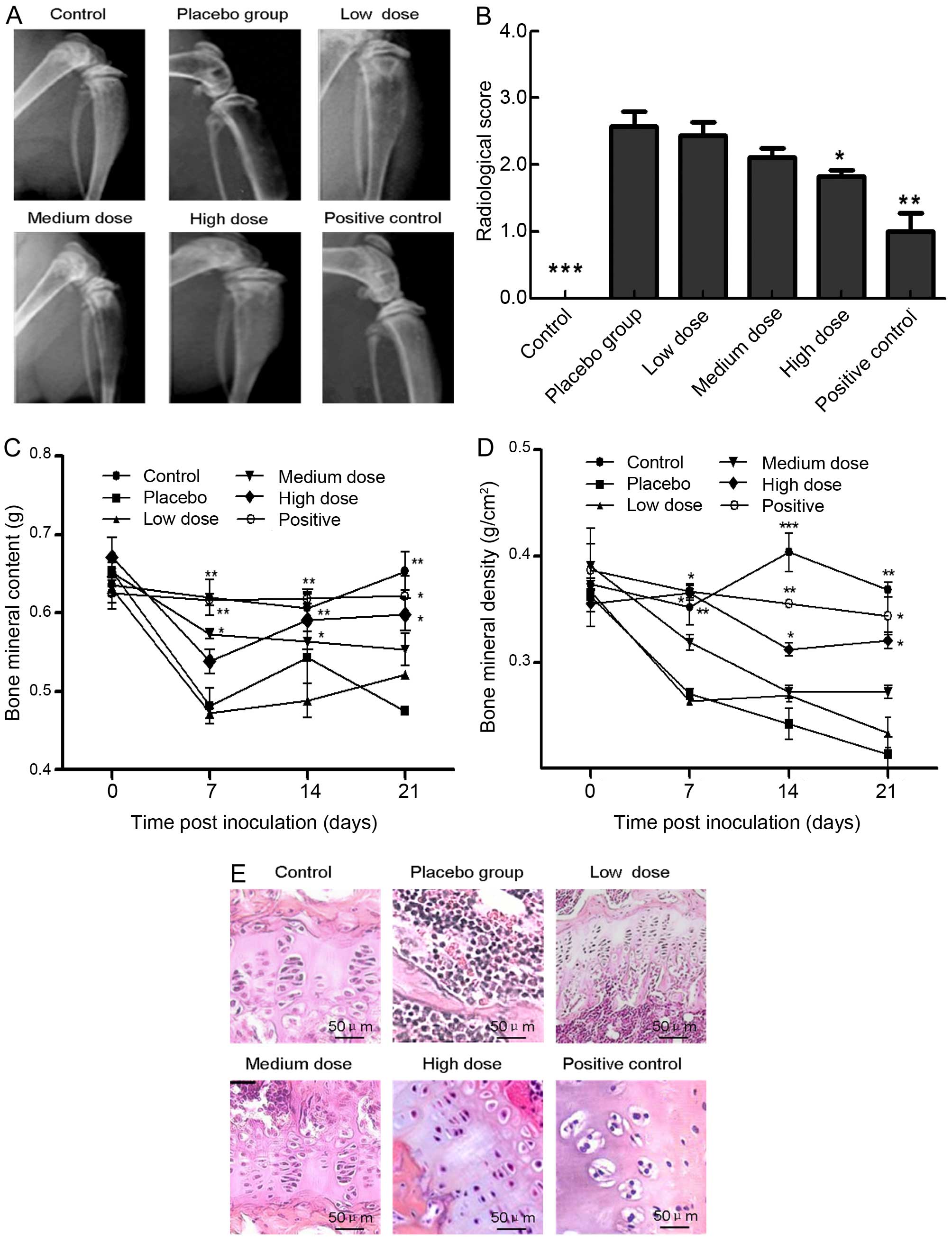
XZP treatment mitigates bone damage in
rats with bone cancer
Next, we examined the effects of XZP treatment on
tibia cancer-induced bone structural damage. X-ray images indicated
less severe loss of medullary bone and cortical bone erosion were
observed in positive control (OPG) rats and XZP-treated rats at
various doses at day 21 of post cell inoculation, while
full-thickness bicortical bone loss was accompanied by displaced
fractures in rats with bone cancer in the placebo group (Fig. 2A). Quantitative analysis revealed
that radiological scores in rats that received different doses of
XZP were significantly less than the placebo group, yet remained
higher than OPG-treated positive controls (Fig. 2B). Quantitative analyses of BMC and
BMD revealed that high-dose XZP treatment, similar to OPG,
significantly preserved BMC at day 7, 14 and 21 post cell
inoculation (P<0.05, P<0.01, Fig.
2C). Furthermore, medium- or high-dose XZP treatment, similar
to OPG treatment, significantly mitigated BMD loss at day 7, 14 and
21 post cell inoculation (Fig. 2D).
Histological examinations revealed that very few cancer cells
invaded into bone tissues in positive controls and in rats treated
with different doses of XZP, while a significant number of cancer
cells invaded bone tissues and destroyed bone structure in the
placebo group (Fig. 2E).
Collectively, XZP treatment mitigated cancer invasion-mediated bone
damage and structural changes.
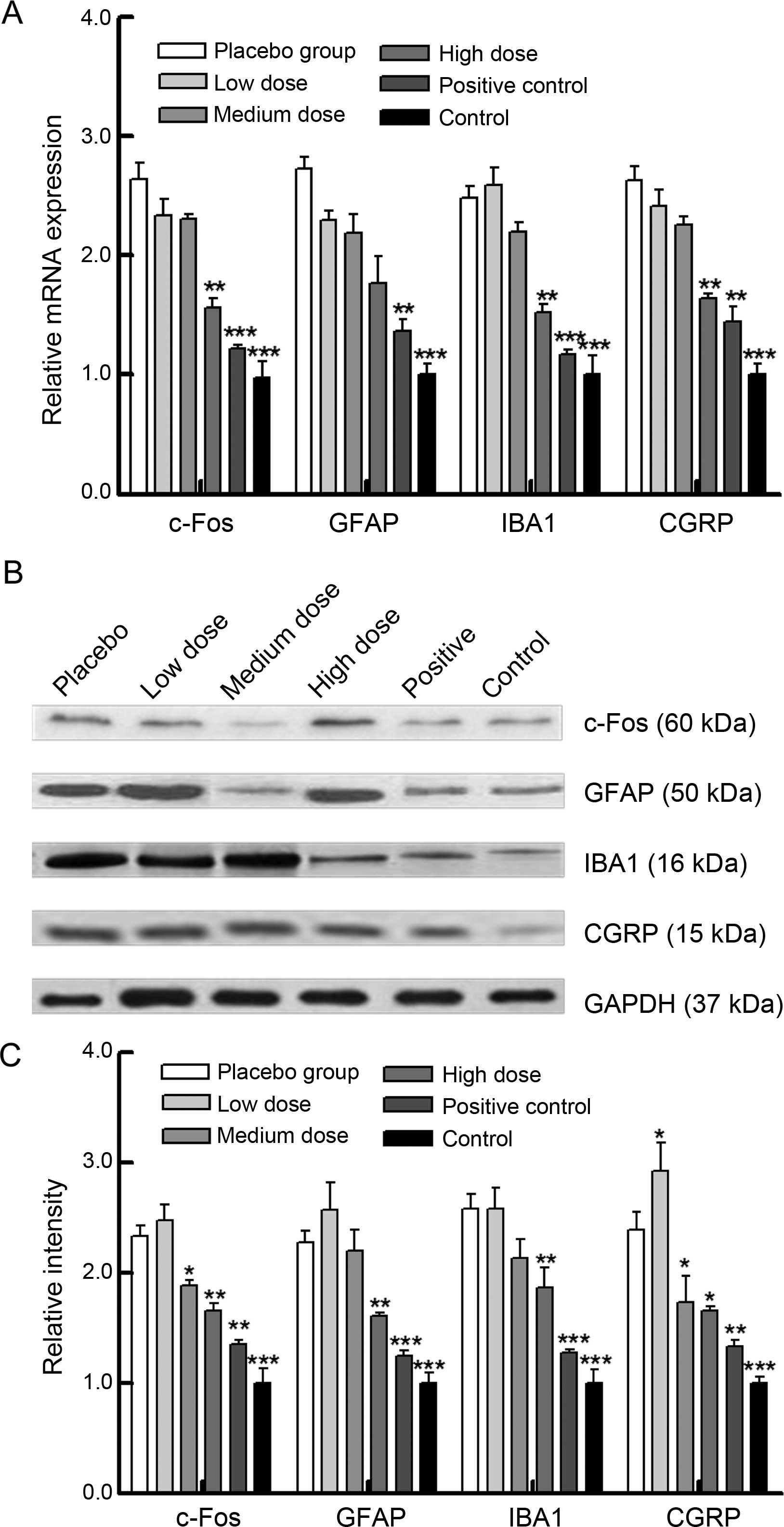
XZP treatment inhibits activation of
astrocytes and microglial cells in rats with bone cancer
To determine potential mechanisms underlying the
action of XZP, we analyzed microglial IBA1 and astrocyte GFAP
markers, and neurotransmitters c-Fos and CGRP, in the spinal cord
of rats (Fig. 3). Significantly
increased levels of c-Fos, GFAP, IBA1 and CGRP mRNA transcripts
were detected in rats with bone cancer, compared with the sham
controls (Fig. 3A); while relative
levels of their gene mRNA transcripts were significantly reduced in
rats treated with XZP, similar to rats that received OPG treatment.
A similar pattern was detected when protein expressions in the
spinal cord were analyzed (Fig. 3B and
C). Thus, XZP treatment inhibited the activation of astrocytes
and microglial cells, contributing to its therapeutic effects in
rats with bone cancer.
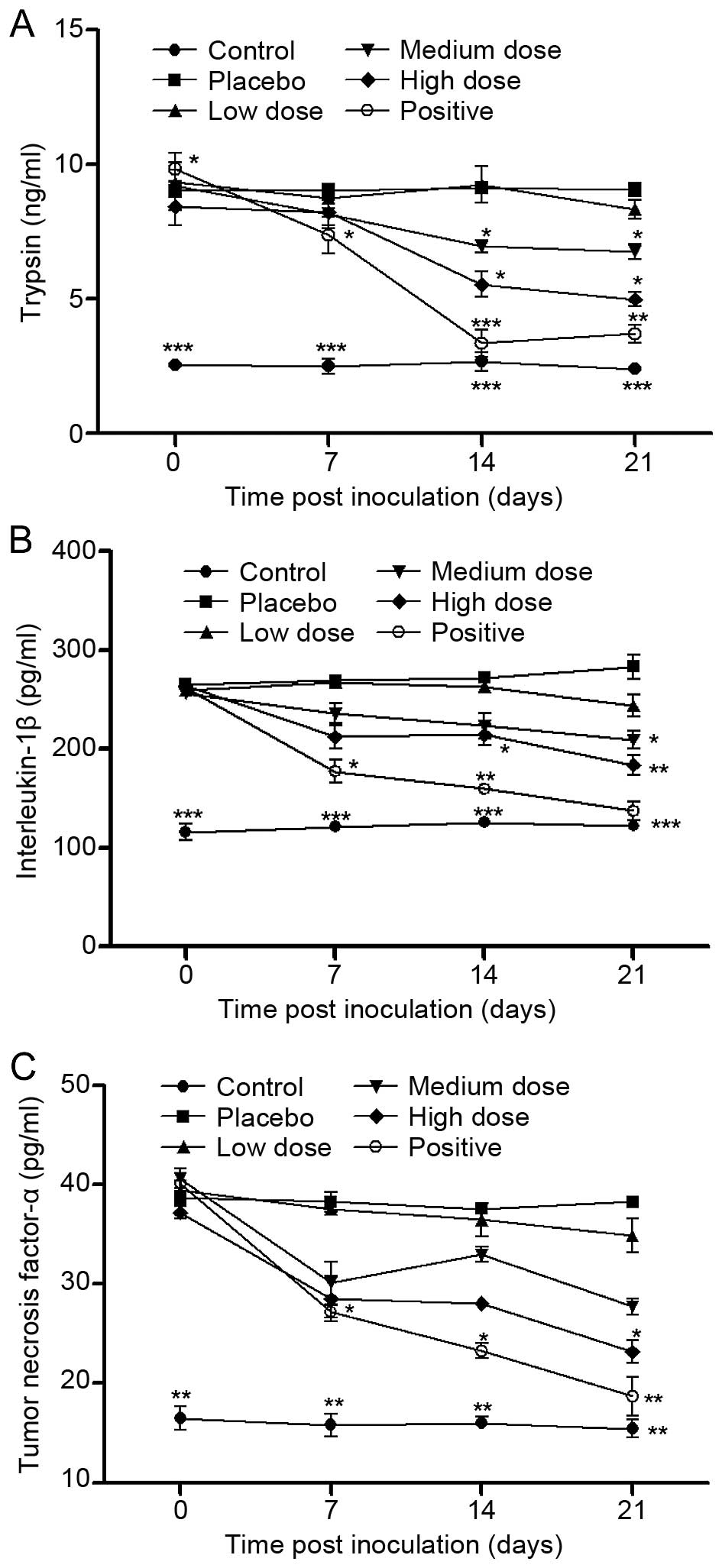
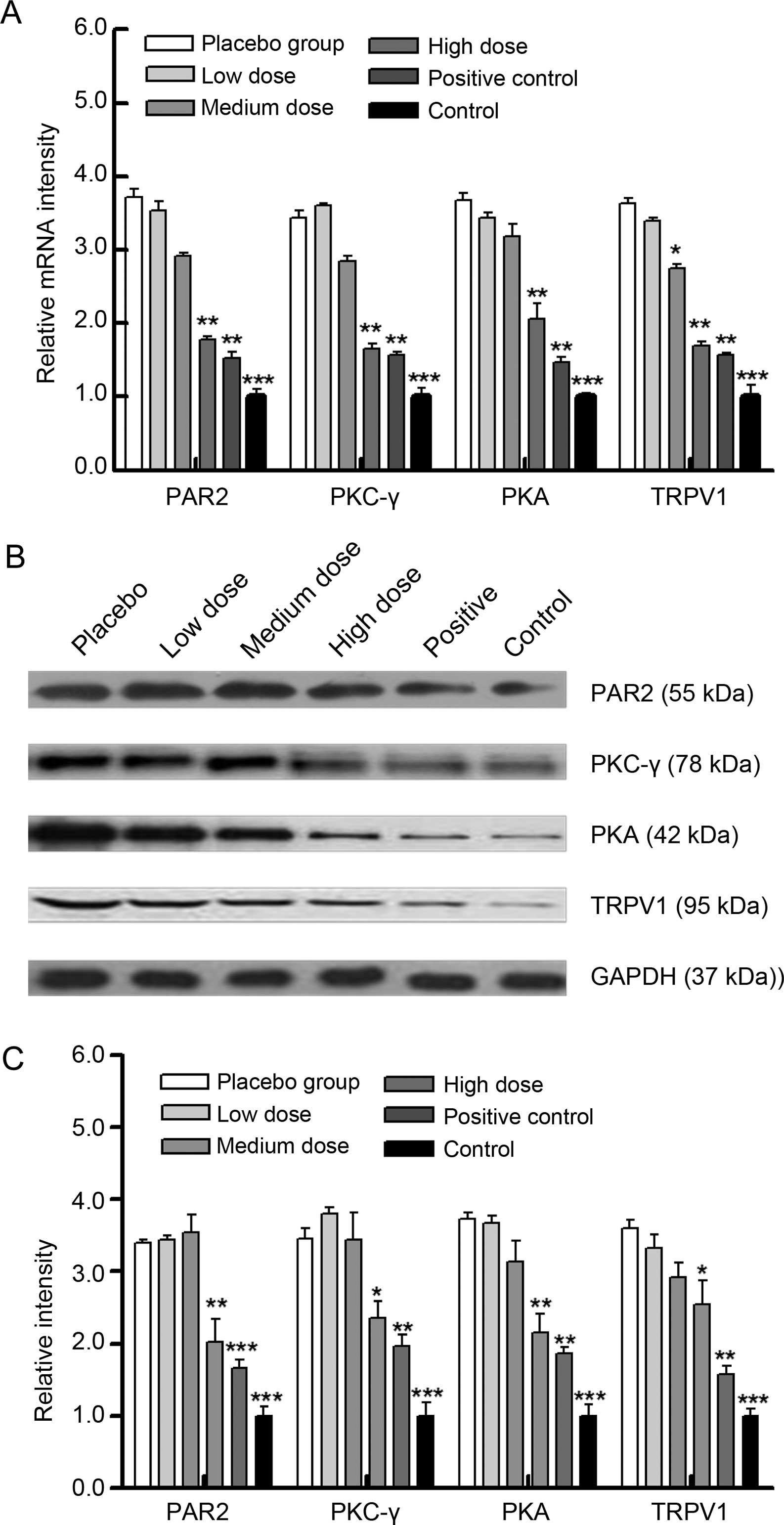
XZP treatment inhibits the PAR2 signaling
pathway in rats with bone cancer
ELISA analysis indicated that upstream levels of the
PAR2 signaling pathway including trypsin, TNF-α and IL-1β serum in
rats treated with XZP were significantly reduced at day 7, 14 or 21
post-inoculation, compared with placebo controls (Fig. 4); and these effects appeared to be
dose-dependent. Furthermore, relative downstream levels of PAR2
signaling pathway mediators including PAR2, PKC-γ, PKA and TRPV1
mRNA transcripts in bone lesions were significantly increased in
the rats with bone cancer, compared with the sham controls
(Fig. 5A); while relative levels of
these gene mRNA transcripts were significantly reduced in the rats
that received XZP treatment, similar to rats with OPG treatment. A
similar pattern was detected when protein expression was analyzed
in bone lesions in rats in the different groups (Fig. 5B and C). Collectively, these data
indicate that XZP treatment inhibited the PAR2 signaling pathway in
rats with bone cancer, which contributes to the observed
antinociceptive effects.
Discussion
The present study aimed to demonstrate the
therapeutic effects of topical XZP treatment in bone cancer pain by
establishing a rat model and exploring its underlying mechanism.
Our major findings from the present study revealed that various
doses of XZP treatment significantly alleviated bone cancer-related
nociception; and inhibition of the PAR2 signaling pathway in a
dose-dependent manner was most likely the underlying mechanism of
action. We anticipate that these results may be beneficial to the
development of this TCM for treating bone cancer pain in clinical
practice.
Cancer bone metastasis is common and has a
devastating impact on the quality of life of patients (3,37,38).
Cancer-related bone metastasis cause bone damage and pain, which is
particularly difficult to treat (3,37,38).
Our previous study has shown that XZP treatment alleviates cancer
pain in clinical practice (17).
Our results from the present study indicate that XZP treatment
alleviated cancer-related bone pain through different mechanisms
(39).
Central nervous system (CNS) glia act as immune
effector cells in both normal and pathological conditions, playing
a vital role in initiating processes of persistent pain states
(40–42). Previous studies have shown that
early CNS glial response to peripheral nerve injury is
predominantly due to the activation of spinal microglia, and that
astrocytes subsequently undergo activation and proliferation
(43,44). The activation of astrocytes and
microglial cells leads to the robust release of proinflammatory
cytokines such as IL-1β and TNF-α (45,46).
Cytokines are important factors that contribute to the
establishment of central sensitization (47). For instance, IL-1β increases NMDAR
phosphorylation and NMDAR-mediated intracellular calcium release in
sensory neurons (48). TNF-α
increased neuronal excitability by stimulating neuronal ion
channels (49,50). These proinflammatory cytokines may
also activate glial cells, resulting in the amplification of
glia-mediated pain-related cascades. The activation of astrocytes
and microglial cells, as well as increased activity of
proinflammatory cytokines in the spinal cord, are common mechanisms
underlying pathological pain in a number of pain syndromes with
widely different etiologies, such as peripheral nerve injury,
spinal inflammation and bone cancer neuropathy (40,41,45,51).
In the present study, tumor cell implantation caused
an increase in microglial IBA1 and astrocyte GFAP markers, and
neurotransmitters c-Fos and CGRP; indicating a central
sensitization effect. XZP treatment significantly mitigated IBA1,
GFAP, c-Fos and CGRP expressions in the spinal cord; and treatment
effects appear to be dose-dependent. Our data further supported
previous findings; wherein, targeting the PAR2 signaling pathway
inhibits cancer-induced bone pain (34,52,53).
Proteolytic activity is critical to carcinogenesis
and cancer microenvironment is associated with various proteases.
Cancer-associated serine proteases, such as trypsin, are released
during the early stages of tissue invasion and may directly
activate PAR2 on nociceptive afferents; resulting in acute pain
that is spontaneous and exacerbated during function. PAR2
activation may also sensitize other nociceptive receptors such as
PKA, PKC-γ and TRPV1 (26–33,54,55).
With continued cancer cell proliferation and mediator release,
nociceptive afferents may be persistently activated or sensitized
and consequently maintain a persistent pain state. It has been well
demonstrated that PAR2 is upregulated following various
inflammatory and ischemic insults (56,57).
Mediators, such as trypsin, TNF-α and IL-1β upregulated PAR2
(58). PAR2 upregulation in dorsal
root ganglia has been associated with thermal hyperalgesia
following chronic pancreatitis (59) and cAMP-dependent neuronal
hyperexcitability following chronic nerve compression (60). Additionally, cancer-induced PAR2
upregulation in trigeminal ganglions similarly alters pain
processing and mediate the progression to chronic cancer pain
(34). The complete absence of
cancer-induced functional allodynia in mice lacking PAR2 clearly
demonstrates the critical involvement of PAR2 in acute and chronic
cancer pain (34).
In the present study, we found that XZP treatment
significantly mitigated trypsin, TNF-α and IL-1β serum levels in a
dose-dependent manner. Furthermore, relative downstream levels of
PAR2 signaling pathway mediators including PAR2, PKC-γ, PKA and
TRPV1 mRNA transcripts and protein expression in bone lesions were
significantly reduced in rats that received XZP treatment, similar
to rats with OPG treatment. These findings further supported the
notion that targeting the PAR2 signaling pathway inhibits
cancer-induced bone pain (34,52).
In summary, our data demonstrated that XZP treatment
mitigated bone cancer-related nociceptive behavior, and inhibited
peripheral and central sensitization by inhibiting the PAR2
signaling pathway (Fig. 6). Our
data provides new insights into the molecular mechanisms underlying
the pathogenesis of bone cancer pain and mechanism of action of XZP
in alleviating bone cancer pain in clinical practice. Our findings
may be beneficial in designing new therapeutic agents for
alleviating bone cancer pain.
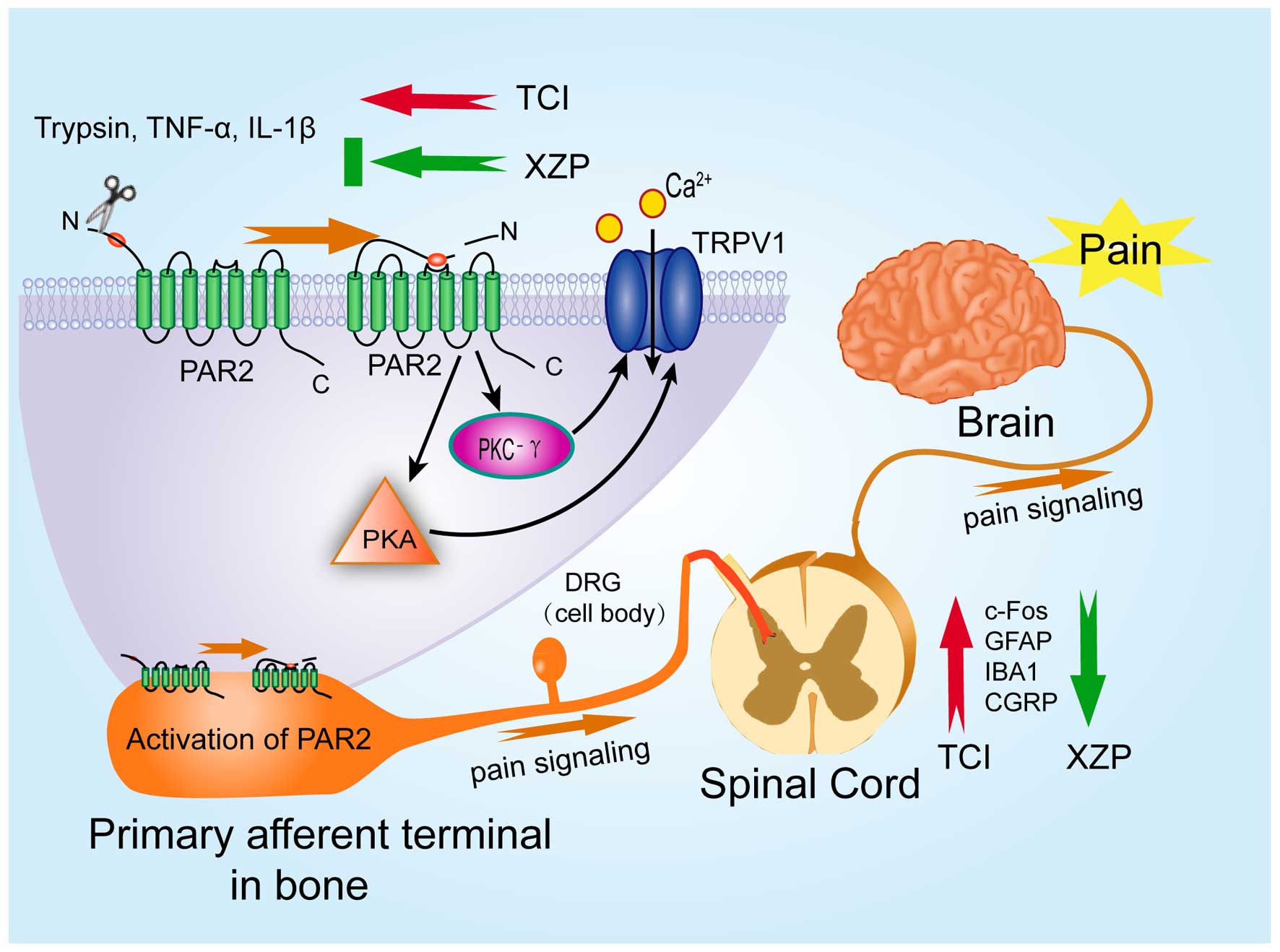 | Figure 6Proposed model of mechanism of action
for Xiaozheng Zhitong Paste (XZP) treatment on bone cancer pain.
Tumor cell implantation (TCI) induces release of trypsin, IL-1β and
TNF-α in the serum. Trypsin activates PAR2 on primary afferent
terminals in tumor-bearing tibias. Activation of PAR2 results in
the increase of PKA, PKC-γ and TRPV1 levels, which induce enhanced
influx of Ca2+ ions, and elevate the release of IBA1,
GFAP, c-Fos and CGRP expressions in the spinal cord; resulting in
an enhanced transmission of pain signaling and nociceptive
behaviors. XZP treatment significantly mitigated the expression of
IBA1, GFAP, c-Fos and CGRP in the spinal cord and significantly
inhibit trypsin, TNF-α and IL-1β serum levels. Furthermore,
relative downstream levels of PAR2 signaling pathway mediators
including PAR2, PKC-γ, PKA and TRPV1 in bone lesions were
significantly reduced after XZP treatment. PAR2,
proteinase-activated receptor 2. |
Acknowledgments
This study was partially supported by the National
Natural Science Foundation of China (grant nos. 81302961, 81273718
and 81202931).
References
|
1
|
Sabino MA and Mantyh PW: Pathophysiology
of bone cancer pain. J Support Oncol. 3:15–24. 2005.PubMed/NCBI
|
|
2
|
Mercadante S: Malignant bone pain:
Pathophysiology and treatment. Pain. 69:1–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chow E, Zeng L, Salvo N, Dennis K, Tsao M
and Lutz S: Update on the systematic review of palliative
radiotherapy trials for bone metastases. Clin Oncol. 24:112–124.
2012. View Article : Google Scholar
|
|
5
|
Portenoy RK, Payne D and Jacobsen P:
Breakthrough pain: Characteristics and impact in patients with
cancer pain. Pain. 81:129–134. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan FK: Primer: Managing NSAID-induced
ulcer complications-balancing gastrointestinal and cardiovascular
risks. Nat Clin Pract Gastroenterol Hepatol. 3:563–573. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lapeyre-Mestre M, de Castro AM, Bareille
MP, Del Pozo JG, Requejo AA, Arias LM, Montastruc JL and Carvajal
A: Non-steroidal anti-inflammatory drug-related hepatic damage in
France and Spain: Analysis from national spontaneous reporting
systems. Fundam Clin Pharmacol. 20:391–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneider V, Lévesque LE, Zhang B,
Hutchinson T and Brophy JM: Association of selective and
conventional nonsteroidal antiinflammatory drugs with acute renal
failure: A population-based, nested case-control analysis. Am J
Epidemiol. 164:881–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michaelson MD and Smith MR:
Bisphosphonates for treatment and prevention of bone metastases. J
Clin Oncol. 23:8219–8224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong R and Wiffen PJ: Bisphosphonates for
the relief of pain secondary to bone metastases. Cochrane Database
Syst Rev. (2): CD0020682002.PubMed/NCBI
|
|
11
|
Wang J, Zhang R, Dong C, Jiao L, Xu L, Liu
J, Wang Z, Mao Ying QL, Fong H and Lao L: Topical treatment with
Tong-Luo-San-Jie gel alleviates bone cancer pain in rats. J
Ethnopharmacol. 143:905–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao Y, Kong X, Yang L, Liu R, Shi Z, Li W,
Hua B and Hou W: Complementary and alternative medicine for cancer
pain: An overview of systematic reviews. Evid Based Complement
Alternat Med. 2014:1703962014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanju B, Yang L, Hua B, Hou W, Shi Z, Li
W, Li C, Chen C, Liu R, Qin Y, et al: A systematic review and
meta-analysis on the use of traditional Chinese medicine compound
kushen injection for bone cancer pain. Support Care Cancer.
22:825–836. 2014. View Article : Google Scholar
|
|
14
|
Xu L, Lao LX, Ge A, Yu S, Li J and Mansky
PJ: Chinese herbal medicine for cancer pain. Integr Cancer Ther.
6:208–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gözüm S, Tezel A and Koc M: Complementary
alternative treatments used by patients with cancer in eastern
Turkey. Cancer Nurs. 26:230–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bao Y, Gao Y, Du M, Hou W, Yang L, Kong X,
Zheng H, Li W and Hua B: Topical treatment with Xiaozheng Zhitong
Paste (XZP) alleviates bone destruction and bone cancer pain in a
rat model of prostate cancer-induced bone pain by modulating the
RANKL/RANK/OPG signaling. Evid Based Complement Alternat Med.
2015:2158922015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao YJ, Hua BJ, Hou W, Lin HS, Zhang XB
and Yang GX: Alleviation of cancerous pain by external compress
with Xiaozheng Zhitong Paste. Chin J Integr Med. 16:309–314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van den Beuken-van Everdingen MH, de Rijke
JM, Kessels AG, Schouten HC, van Kleef M and Patijn J: Prevalence
of pain in patients with cancer: A systematic review of the past 40
years. Ann Oncol. 18:1437–1449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mantyh P: Bone cancer pain: Causes,
consequences, and therapeutic opportunities. Pain. 154(Suppl 1):
S54–S62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mao-Ying QL, Wang XW, Yang CJ, Li X, Mi
WL, Wu GC and Wang YQ: Robust spinal neuroinflammation mediates
mechanical allodynia in Walker 256 induced bone cancer rats. Mol
Brain. 5:162012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang XW, Hu S, Mao-Ying QL, Li Q, Yang CJ,
Zhang H, Mi WL, Wu GC and Wang YQ: Activation of c-jun N-terminal
kinase in spinal cord contributes to breast cancer induced bone
pain in rats. Mol Brain. 5:212012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang XW, Li TT, Zhao J, Mao-Ying QL, Zhang
H, Hu S, Li Q, Mi WL, Wu GC, Zhang YQ, et al: Extracellular
signal-regulated kinase activation in spinal astrocytes and
microglia contributes to cancer-induced bone pain in rats.
Neuroscience. 217:172–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke C, Li C, Huang X, Cao F, Shi D, He W,
Bu H, Gao F, Cai T, Hinton AO Jr, et al: Protocadherin20 promotes
excitatory synaptogenesis in dorsal horn and contributes to bone
cancer pain. Neuropharmacology. 75:181–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yanagisawa Y, Furue H, Kawamata T, Uta D,
Yamamoto J, Furuse S, Katafuchi T, Imoto K, Iwamoto Y and Yoshimura
M: Bone cancer induces a unique central sensitization through
synaptic changes in a wide area of the spinal cord. Mol Pain.
6:382010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rothmeier AS and Ruf W: Protease-activated
receptor 2 signaling in inflammation. Semin Immunopathol.
34:133–149. 2012. View Article : Google Scholar
|
|
26
|
Bao Y, Hou W and Hua B: Protease-activated
receptor 2 signalling pathways: A role in pain processing. Expert
Opin Ther Targets. 18:15–27. 2014. View Article : Google Scholar
|
|
27
|
Bao Y, Hou W, Liu R, Gao Y, Kong X, Yang
L, Shi Z, Li W, Zheng H, Jiang S, et al: PAR2-mediated upregulation
of BDNF contributes to central sensitization in bone cancer pain.
Mol Pain. 10:282014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bao Y, Gao Y, Hou W, Yang L, Kong X, Zheng
H, Li C and Hua B: Engagement of signaling pathways of
protease-activated receptor 2 and μ-opioid receptor in bone cancer
pain and morphine tolerance. Int J Cancer. Feb 24–2015.Epub ahead
of print. View Article : Google Scholar
|
|
29
|
Bao Y, Gao Y, Yang L, Kong X, Zheng H, Hou
W and Hua B: New insights into protease-activated receptor 4
signaling pathways in the pathogenesis of inflammation and
neuropathic pain: A literature review. Channels. 9:5–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bao Y, Hou W, Yang L, Kong X, Du M, Zheng
H, Gao Y and Hua B: Protease-activated receptor 2 antagonist
potentiates analgesic effects of systemic morphine in a rat model
of bone cancer pain. Reg Anesth Pain Med. 40:158–165. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao Y, Hou W, Yang L, Liu R, Gao Y, Kong
X, Shi Z, Li W, Zheng H, Jiang S, et al: Increased expression of
protease-activated receptor 2 and 4 within dorsal root ganglia in a
rat model of bone cancer pain. J Mol Neurosci. 55:706–714. 2015.
View Article : Google Scholar
|
|
32
|
Bao Y, Hua B, Hou W, Shi Z, Li W, Li C,
Chen C, Liu R and Qin Y: Involvement of protease-activated receptor
2 in nocicep-tive behavior in a rat model of bone cancer. J Mol
Neurosci. 52:566–576. 2014. View Article : Google Scholar
|
|
33
|
Hua B, Gao Y, Kong X, Yang L, Hou W and
Bao Y: New insights of nociceptor sensitization in bone cancer
pain. Expert Opin Ther Targets. 19:227–243. 2015. View Article : Google Scholar
|
|
34
|
Liu S, Liu YP, Yue DM and Liu GJ:
Protease-activated receptor 2 in dorsal root ganglion contributes
to peripheral sensitization of bone cancer pain. Eur J Pain.
18:326–337. 2014. View Article : Google Scholar
|
|
35
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamoureux F, Baud'huin M, Rodriguez
Calleja L, Jacques C, Berreur M, Rédini F, Lecanda F, Bradner JE,
Heymann D and Ory B: Selective inhibition of BET bromodomain
epigenetic signalling interferes with the bone-associated tumour
vicious cycle. Nat Commun. 5:35112014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
DePuy V, Anstrom KJ, Castel LD, Schulman
KA, Weinfurt KP and Saad F: Effects of skeletal morbidities on
longitudinal patient-reported outcomes and survival in patients
with metastatic prostate cancer. Support Care Cancer. 15:869–876.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paqueron X, Conklin D and Eisenach JC:
Plasticity in action of intrathecal clonidine to mechanical but not
thermal nociception after peripheral nerve injury. Anesthesiology.
99:199–204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gosselin RD, Suter MR, Ji RR and Decosterd
I: Glial cells and chronic pain. Neuroscientist. 16:519–531. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakagawa T and Kaneko S: Spinal astrocytes
as therapeutic targets for pathological pain. J Pharmacol Sci.
114:347–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schomberg D and Olson JK: Immune responses
of microglia in the spinal cord: Contribution to pain states. Exp
Neurol. 234:262–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Romero-Sandoval A, Chai N, Nutile-McMenemy
N and Deleo JA: A comparison of spinal Iba1 and GFAP expression in
rodent models of acute and chronic pain. Brain Res. 1219:116–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tanga FY, Raghavendra V and DeLeo JA:
Quantitative real-time RT-PCR assessment of spinal microglial and
astrocytic activation markers in a rat model of neuropathic pain.
Neurochem Int. 45:397–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hald A, Nedergaard S, Hansen RR, Ding M
and Heegaard AM: Differential activation of spinal cord glial cells
in murine models of neuropathic and cancer pain. Eur J Pain.
13:138–145. 2009. View Article : Google Scholar
|
|
46
|
Milligan ED and Watkins LR: Pathological
and protective roles of glia in chronic pain. Nat Rev Neurosci.
10:23–36. 2009. View Article : Google Scholar :
|
|
47
|
Kawasaki Y, Zhang L, Cheng JK and Ji RR:
Cytokine mechanisms of central sensitization: Distinct and
overlapping role of interleukin-1beta, interleukin-6, and tumor
necrosis factor-alpha in regulating synaptic and neuronal activity
in the superficial spinal cord. J Neurosci. 28:5189–5194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Viviani B, Bartesaghi S, Gardoni F,
Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M,
Galli CL, et al: Interleukin-1beta enhances NMDA receptor-mediated
intracellular calcium increase through activation of the Src family
of kinases. J Neurosci. 23:8692–8700. 2003.PubMed/NCBI
|
|
49
|
Dolga AM, Granic I, Blank T, Knaus HG,
Spiess J, Luiten PG, Eisel UL and Nijholt IM: TNF-alpha-mediates
neuroprotection against glutamate-induced excitotoxicity via
NF-kappaB-dependent up-regulation of K 2.2 channels. J Neurochem.
107:1158–1167. 2008.PubMed/NCBI
|
|
50
|
Riazi K, Galic MA, Kuzmiski JB, Ho W,
Sharkey KA and Pittman QJ: Microglial activation and TNFalpha
production mediate altered CNS excitability following peripheral
inflammation. Proc Natl Acad Sci USA. 105:17151–17156. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ren BX, Gu XP, Zheng YG, Liu CL, Wang D,
Sun YE and Ma ZL: Intrathecal injection of metabotropic glutamate
receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer
pain by inhibition of spinal astrocyte activation in a mouse model.
Anesthesiology. 116:122–132. 2012. View Article : Google Scholar
|
|
52
|
Lam DK, Dang D, Zhang J, Dolan JC and
Schmidt BL: Novel animal models of acute and chronic cancer pain: A
pivotal role for PAR2. J Neurosci. 32:14178–14183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu S, Liu YP, Song WB and Song XJ:
EphrinB-EphB receptor signaling contributes to bone cancer pain via
Toll-like receptor and proinflammatory cytokines in rat spinal
cord. Pain. 154:2823–2835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dai Y, Moriyama T, Higashi T, Togashi K,
Kobayashi K, Yamanaka H, Tominaga M and Noguchi K:
Proteinase-activated receptor 2-mediated potentiation of transient
receptor potential vanilloid subfamily 1 activity reveals a
mechanism for proteinase-induced inflammatory pain. J Neurosci.
24:4293–4299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grant AD, Cottrell GS, Amadesi S,
Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi
GW, Bautista-Cruz F, et al: Protease-activated receptor 2
sensitizes the transient receptor potential vanilloid 4 ion channel
to cause mechanical hyperalgesia in mice. J Physiol. 578:715–733.
2007. View Article : Google Scholar
|
|
56
|
Nystedt S, Ramakrishnan V and Sundelin J:
The proteinase-activated receptor 2 is induced by inflammatory
mediators in human endothelial cells. Comparison with the thrombin
receptor. J Biol Chem. 271:14910–14915. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Striggow F, Riek-Burchardt M, Kiesel A,
Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG and Reiser
G: Four different types of protease-activated receptors are widely
expressed in the brain and are up-regulated in hippocampus by
severe ischemia. Eur J Neurosci. 14:595–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ritchie E, Saka M, Mackenzie C, Drummond
R, Wheeler-Jones C, Kanke T and Plevin R: Cytokine upregulation of
proteinase-activated-receptors 2 and 4 expression mediated by p38
MAP kinase and inhibitory kappa B kinase beta in human endothelial
cells. Br J Pharmacol. 150:1044–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang W, Gao J, Zhao T, Wei L, Wu W, Bai
Y, Zou D and Li Z: Proteinase-activated receptor 2 mediates thermal
hyperalgesia and is upregulated in a rat model of chronic
pancreatitis. Pancreas. 40:300–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang ZJ, Li HC, Cowan AA, Liu S, Zhang YK
and Song XJ: Chronic compression or acute dissociation of dorsal
root ganglion induces cAMP-dependent neuronal hyperexcitability
through activation of PAR2. Pain. 153:1426–1437. 2012. View Article : Google Scholar : PubMed/NCBI
|