Introduction
Lung cancer is a malignant tumor with the highest
morbidity and mortality worldwide, and its incidence is on the
increase. Approximately 80% of the disease may be attributed to
non-small cell lung cancer (NSCLC) (1). Due to the limitations of early
diagnostic techniques and the lack of early specific clinical
manifestations, 70–80% of patients are diagnosed at advanced stages
(2). Therefore, identification of a
safe and effective drug treatment for NSCLC is crucial.
Recent findings have shown that the incidence,
development and transfer of NSCLC are not only influenced by
genetic factors, but that epigenetic change is also important,
including small molecule non-coding RNA (ncRNA) (3). MicroRNA (miRNA), a class of endogenous
small ncRNA with a length of ~21–25 bases, widely existing in
eukaryotes, can identify the mRNA of a specific target gene
(4). Over 50% of the miRNAs genes
are mapped in NSCLC-related fragile sites or genomic regions,
suggesting that miRNA expression may be closely associated with
NSCLC. As reported, the expression of a large number of miRNAs in
NSCLC show disorder and the imbalance of miRNAs promote the
progression of NSCLC cell cycle, anti-apoptotic effect, enhanced
cell invasion and metastasis of non-small cell lung cancer
(5).
The PI3K/Akt signaling pathway plays an important
role in growth factor-mediated cell survival. Previous findings
have shown that the disorder of the PI3K/Akt/mTOR pathway may play
an important role in the formation of NSCLC (6). It has also been reported that the cell
proliferation signal generated by the combination of a number of
transmembrane receptors and ligands can activate the signal
transduction of PI3K/Akt/mTOR, which is closely associated with
NSCLC proliferation and survival status (7). These receptors include c-met,
epidermal growth factor receptor (EGFR), c-kit and insulin-like
growth factor receptor (IGF-IR).
Curcumin is a natural active ingredient extracted
from dry rhizome of the turmeric Curcuma genus plant, turmeric and
Curcuma, with extensive pharmacological effects, low toxicity, and
good tolerance, and due to its economic value, it has become a hot
spot for exploration (8). Findings
of studies focusing on curcumin have identified a wide range of
pharmacological activities such as anti-inflammation,
anti-oxidation, lipid, anti-virus, anti-infection, anticancer,
anticoagulant, anti-liver fibrosis and atherosclerosis, low
toxicity and few side effects (9–13).
However, the effect of curcumin on NSCLC and the underlying
molecular mechanism remains unclear. In the present study, we
examined the anticancer effect of curcumin on cell proliferation
and apoptosis of human NSCLC and identified a possible relationship
between this effect and the miRNA-192-5p-modulated PI3K/Akt
signaling pathway.
Materials and methods
Chemicals
Dulbecco's modified Eagle's medium (DMEM) and fetal
calf serum (FBS) were purchased from Gibco (Carlsbad, CA, USA) and
(South America). 3-(4,5-Dimethyl-thylthiazol-2-yl)-2,5
diphenyltetrazolium bromide (MTT) was purchased from Sangon Biotech
(Shanghai, China). The Apoptosis Detection kit I was purchased from
BD Biosciences (San Jose, CA, USA). The caspase-3 activity assay
kit was purchased from Promega (Madison, WI, USA).
Cell culture
Human normal NCL-H460 and BEAS-2E lung epithelial
cells, and human A549 lung cancer cells were obtained from the Cell
Resource Center of the Second Military Medical University. These
cells were cultured in DMEM supplemented with 10% FBS (both from
Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C and a humidifid incubator with 5% CO2. The
concentrations (5,10,20 and 40 µM) and time periods (12 and
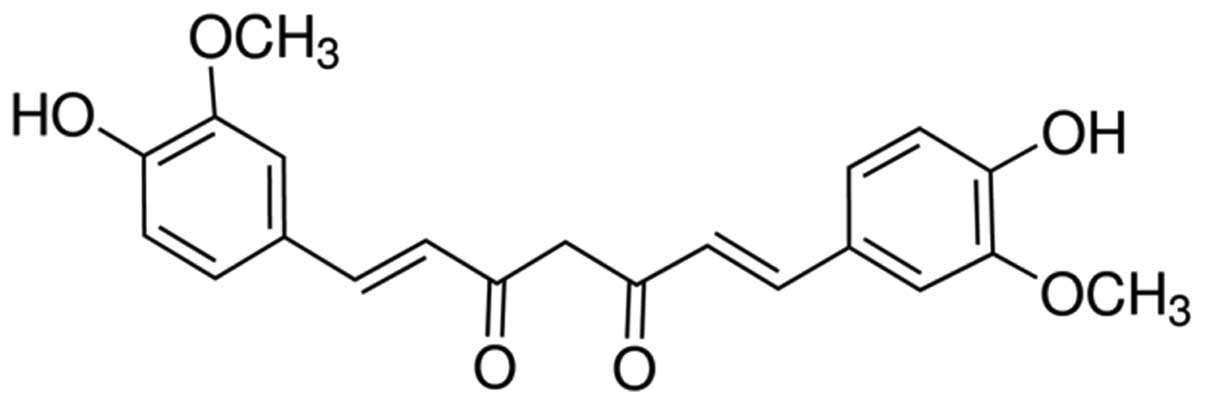
24 h) of curcumin against A549 cells were examined. The chemical
structure of curcumin is shown in Fig.
1.
Cell viability analysis
Cells were seeded at a density of
5×103/well in 96-well plates. Briefly, cell viability
was measured by MTT assay (Sangon Biotech). Ten microliters MTT (5
mg/l) were added into each well and incubated for 4 h at 37°C in a
humidified incubator with 5% CO2. Dimethyl sulfoxide
(150 µl; Invitrogen Life Technologies, Carlsbad, CA, USA)
were added to each well and agitated for 20 min at room
temperature. The absorbance was measured at 450 nM using a
microplate reader.
Annexin V-FITC/propidium iodide (PI)
apoptosis analysis
Cells were seeded at a density of
1×106/well in 6-well plates. Cultured cells were washed
twice with ice-cold PBS and then stained with Annexin V-FITC and PI
using the Apoptosis Detection kit I according to the manufacturer's
instructions (BD Biosciences). Apoptosis was analyzed via flow
cytometric using CellQuest Pro (IVD) software (both from BD
Biosciences).
Caspase-3 activation analysis
Cells were seeded at a density of
5×103/well in 96-well plates. Caspase-3 activity was
measured using a caspase-3 activity assay kit according to the
manufacturer's instructions (Promega). One hundred microliters
reaction buffer with 10 µl substrate Asp-Glu-Val-Asp
(DEVD)-p-nitroaniline (pNA) was added into 10 µl protein
cell lysate/well and incubated at 37°C for 6 h. Caspase-3 activity
was analyzed at an absorbance of 405 nm.
Cell transfection
The miR-192-5p, anti-miR-192-5p and negative control
mimics (miR-NC) were all purchased from Sangon Biotech. The cells
were seeded in 6-well plates and grown to 50–60% confluence prior
to transfection. The mimics were transfected with Lipofectamine
2000 into cells according to the manufacturer's instructions
(Invitrogen Life Technologies). Gene or protein was isolated 24 h
after transfection and used for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) or western blot analysis,
respectively.
RT-qPCR
Cells were seeded at a density of
5×103/well in 96-well plates. Total RNA was extracted
from the cells using TRIzol reagent (Invitrogen Life Technologies).
The concentration RNA was determined using a NanoDrop®
ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). Quantification of the miRNAs was performed by
a TaqMan miRNA assay kit (Applied Biosystems, Foster City, CA,
USA). Total RNA was subjected to first-strand cDNA synthesis and
examined using a PrimeScript RT reagent kit (both from Takara,
Shiga, Japan). qPCR was performed using SYBR-Green PCR Master Mix
(Takara) on the ABI 7500HT System. The primers used in the
reactions are shown in Table I.
 | Table IThe PCR primers used in the
reactions. |
Table I
The PCR primers used in the
reactions.
| Gene | Primer sequences
(forward) | Primer sequences
(reverse) |
|---|
| miR-192-5p |
5′-GGACTTTCTTCATTCACACCG-3′ |
5′-GACCACTGAGGTTAGAGCCA-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACATATACT-3′ |
5′-ACGCTTCACGAATTTGCGTGTC-3′ |
Western blot analysis
Cells were seeded at a density of
5×103/well in 96-well plates. Cultured cells were washed
twice with ice-cold PBS and incubated with ice-cold lysis buffer
for 30 min on ice. Cell liquid was collected and centrifuged at
12,000 × g for 10 min at 4°C. The soluble protein concentration was
determined using a BCA protein assay kit (Sigma, St. Louis, MO,
USA). Total proteins (10 µg) were run on 12% sodium dodecyl
sulfate (SDS)-polyacrylamide gels and transferred to polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked with
Tris-buffered saline (TBS) containing 5% non-fat milk to block
non-specific binding sites. The membranes were incubated with
anti-PI3K (1:1,500) and anti-Akt (1:1000) (both from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and anti-β-actin (1:500;
Sangon Biotech) overnight at 4°C. After being washed with 1%
Tween-20/PBS, the membranes were incubated with the secondary
antibodies (Tiangen, Beijing, China) for 2 h at room temperature.
The proteins were detected using enhanced chemiluminescence
(Vilber, Marne La Vallée, France).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software. The results are presented as mean ± SD. The results were
analyzed using the Student's t-test or one-way analysis of variance
(ANOVA). P<0.05 was considered statistically significant.
Results
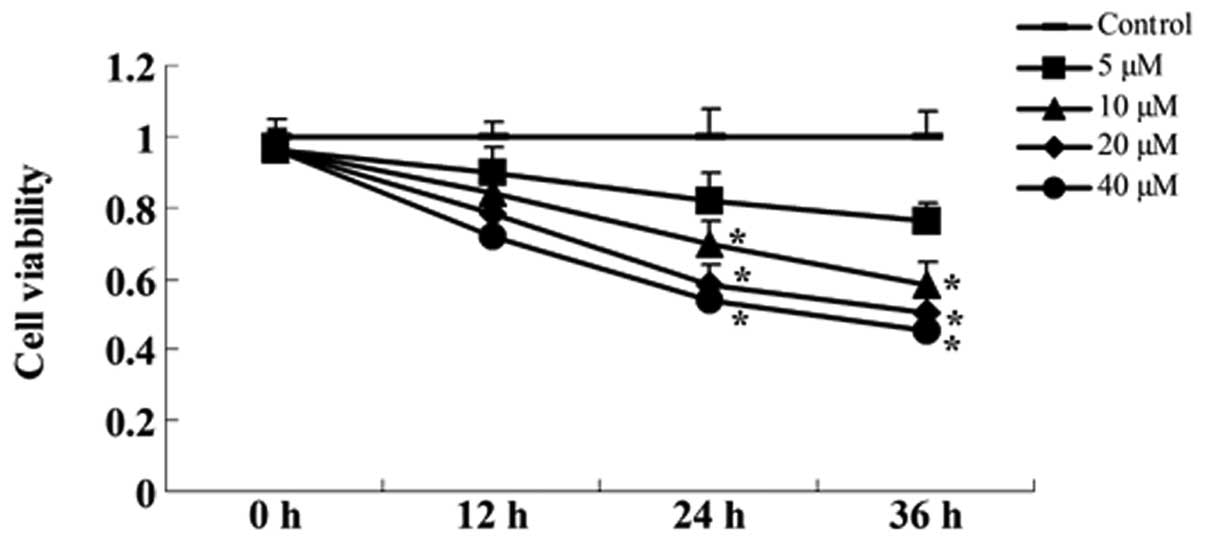
Curcumin inhibits viability of A549
cells
The concentrations (5, 10, 20 and 40 µM) and
time periods (12, 24 or 36 h) of curcumin against A549 cells were
assessed using the MTT assay. Fig.
2 shows that the treatment of A549 cells for 12, 24 or 48 h
with 10, 20 and 40 µM curcumin inhibited cell viability in a
dose- and time-dependent manner. The MTT assay indicated that
curcumin (20 and 40 µM) treatment for 24 or 36 h resulted in
significant cell viability, compared with the control group
(Fig. 2).
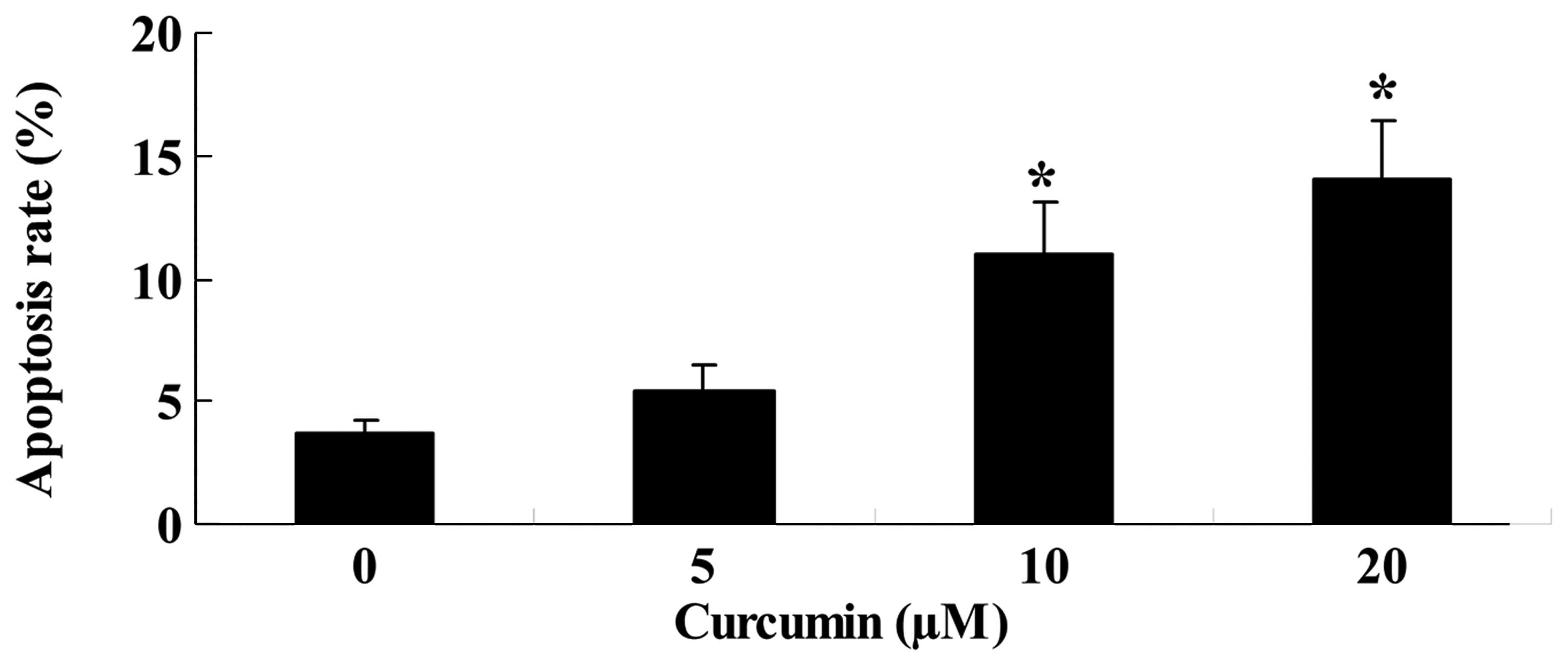
Curcumin induces apoptosis of A549
cells
Prior to the treatment, the apoptotic rate of
curcumin-treated (10, 20 or 40 µM) A549 cells was also
measured. Following treatment with 20 or 40 µM curcumin for
24 h, the apoptotic rate markedly increased in a dose-dependent
manner, compared with the control group (Fig. 3).
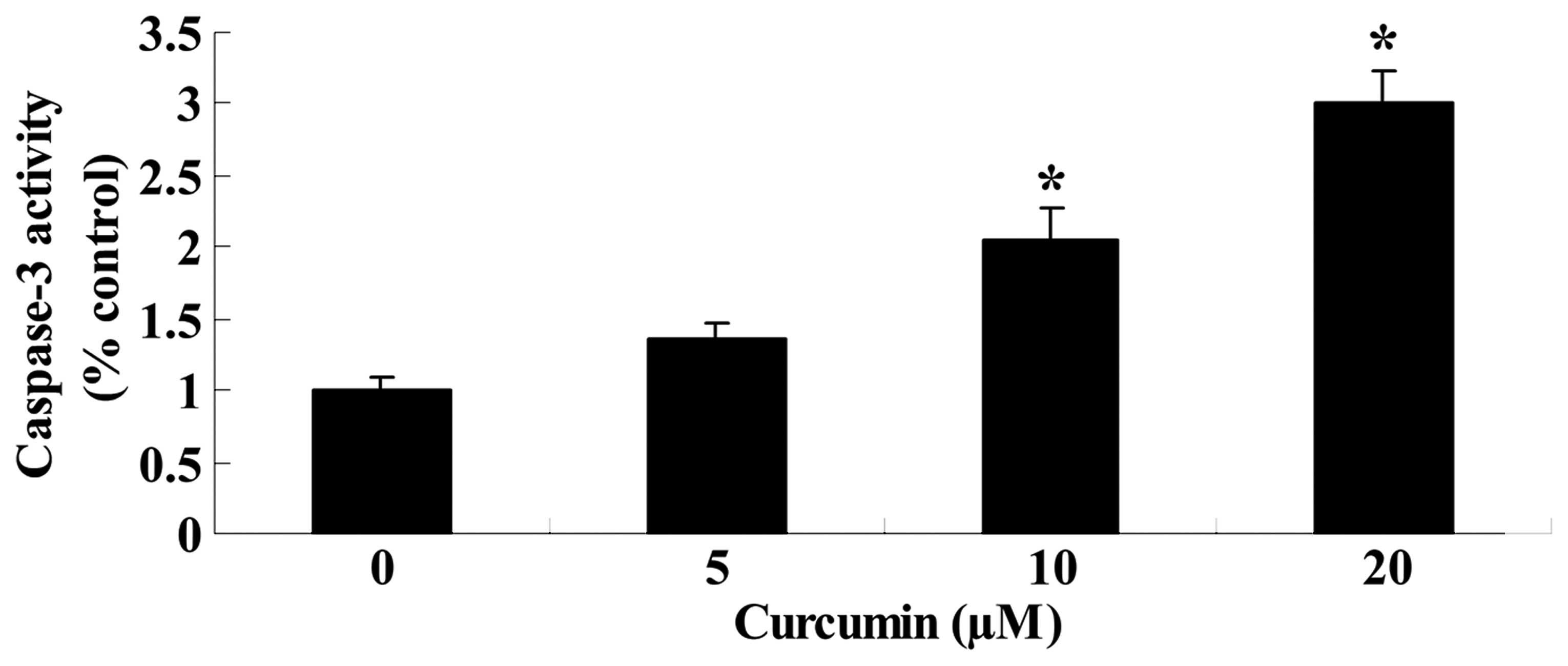
Curcumin induces caspase-3 activity of
A549 cells
To determine whether curcumin induces caspase-3
activity of A549 cells, caspase-3 activity was measured using a
caspase-3 activity assay kit. Caspase-3 activity also increased
significantly in a dose-dependent manner after curcumin (20 or 40
µM) treatment for 24 h, compared with the control group
(Fig. 4). These results indicated
that curcumin induced apoptosis in A549 cells.
miR-192-5p inhibits cell viability and
induces apoptosis of A549 cells
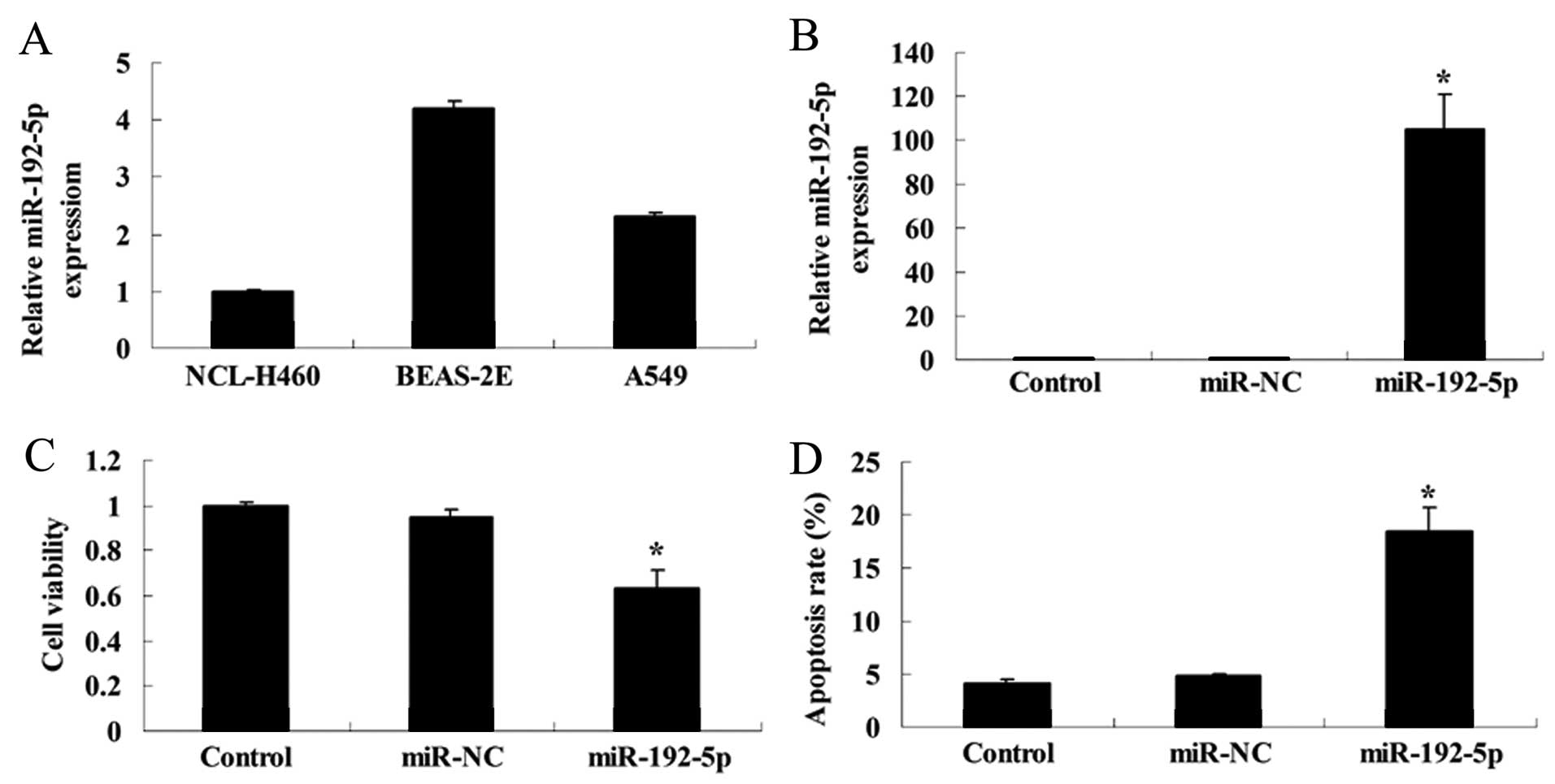
To examine the expression and significance of
miR-192-5p in human normal lung epithelial and lung cancer cells,
the miR-192-5p relative expression was detected using RT-qPCR.
Fig. 5A shows that miR-192-5p
relative expression of NCL-H460 cells was relatively lower, that of
A549 cells was higher, with BEAS-2E cells being the most highly
expressed.
To analyze whether miR-192-5p influenced cell
viability and apoptosis of A549 cells, we transfected miR-192-5p
mimics into A549 cells. The cells transfected with miR-192-5p
mimics showed a significant, 120-fold increase in the miR-192-5p
expression after 48 h transfection, compared with the control or
miR-NC group (Fig. 5B).
Overexpression of miR-192-5p decreased the viability of A549 cells,
compared with the control or miR-NC group (Fig. 5C). However, overexpression of
miR-192-5p induced the apoptotic rate of A549 cells, compared with
the control or miR-NC group (Fig.
5D).
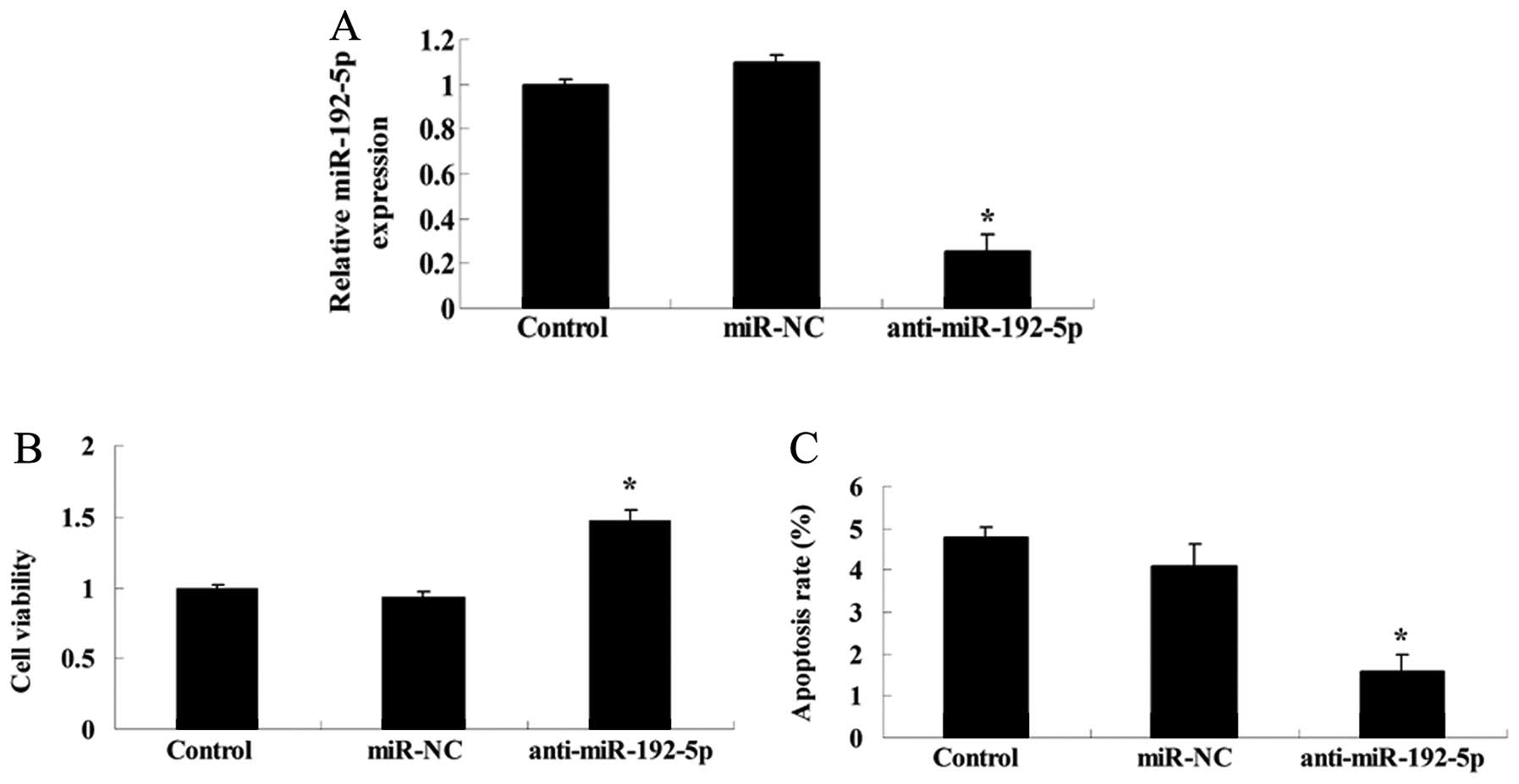
Inhibition of miR-192-5p suppresses cell
viability and induces apoptosis of A549 cells
To determine whether anti-miR-192-5p influenced the
cell viability and apoptosis of A549 cells, we transfected
anti-miR-192-5p mimics into A549 cells. The expression level of
miR-192-5p was detected using RT-qPCR after 48 h transfection.
Anti-miR-192-5p mimics effectively suppressed the miR-192-5p
relative expression of A549 cells (Fig.
6A). Downregulation of miR-192-5p promoted cell viability and
inhibited the apoptotic rate of A549 cells, respectively, compared
with the control or miR-NC group (Fig.
6B and C).
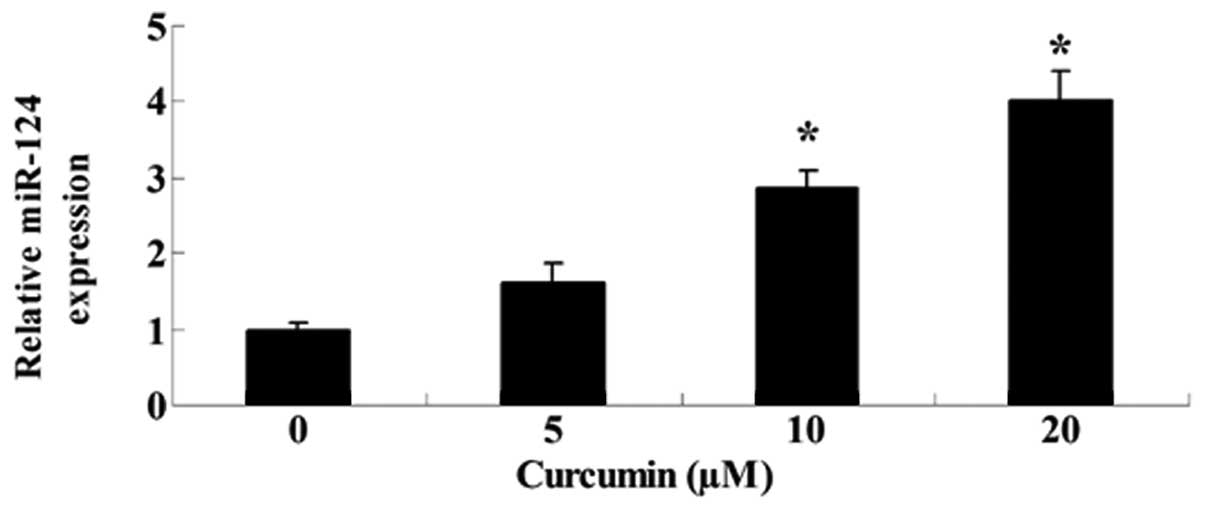
Curcumin inhibits miR-192-5p gene
expression of A549 cells
To determine whether curcumin influenced miR-192-5p
gene expression of A549 cells, the expression level of miR-192-5p
was detected using RT-qPCR following treatment with curcumin for 24
h. Fig. 7 shows that the expression
level of miR-192-5p was markedly enhanced by curcumin (20 or 40
µM).
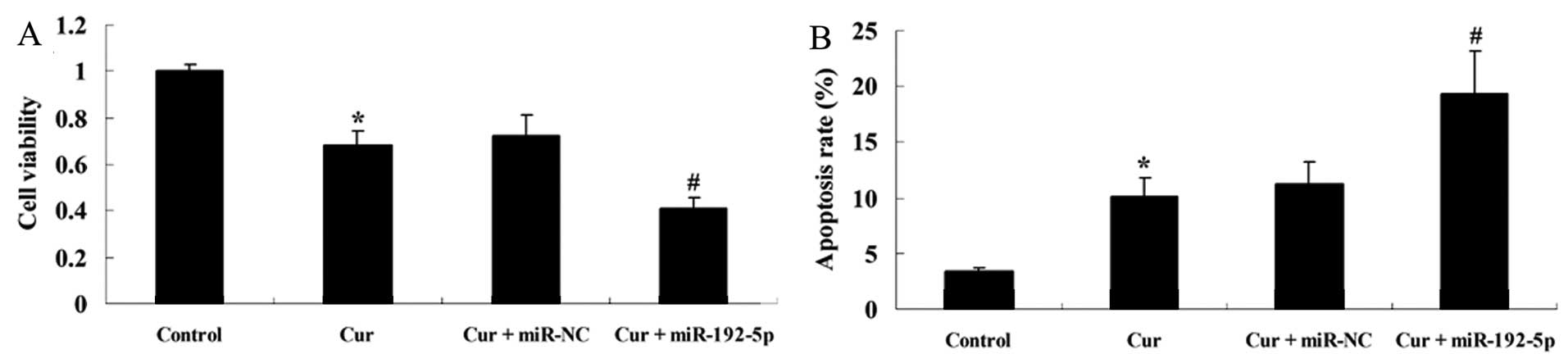
miR-192-5p inhibits the effect of
curcumin on cell viability and induces apoptosis of A549 cells
To examine how the over-expression of miR-192-5p
influenced the effect of curcumin on cell viability and the
apoptotic rate of A549 cells, we transfected miR-192-5p mimics into
A549 cells. Fig. 8 shows that
curcumin suppressed cell viability and increased the apoptotic rate
of A549 cells following treatment with curcumin (20 µM) for
24 h, respectively, compared with the control group. The effect of
curcumin (20 µM) on cell viability and the apoptotic rate of
A549 cells were markedly decreased and increased by miR-192-5p
mimics, respectively, compared with the curcumin-treated group
(Fig. 8A).
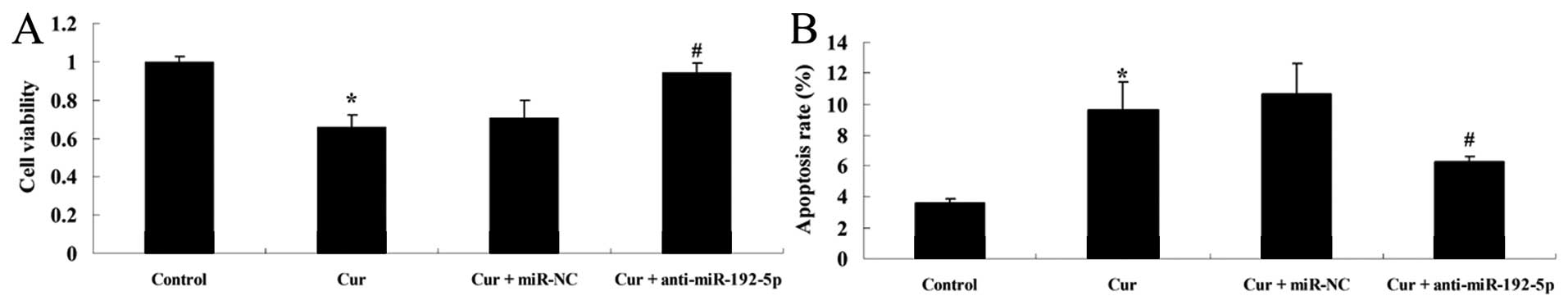
Inhibition of miR-192-5p suppresses the
effect of curcumin on cell viability and induces apoptosis of A549
cells
To examine how the downregulation of miR-192-5p
influenced the effect of curcumin on cell viability and the
apoptotic rate of A549 cells, we transfected anti-miR-192-5p mimics
into A549 cells. Fig. 9 shows that
curcumin suppressed cell viability and increased the apoptotic rate
of A549 cells following treatment with curcumin (20 µM) for
24 h, respectively, compared with the control group. However, the
effect of curcumin (20 µM) on cell viability and the
apoptotic rate of A549 cells were markedly increased and reduced by
anti-miR-192-5p mimics, respectively, compared with the
curcumin-treated group (Fig.
9A).
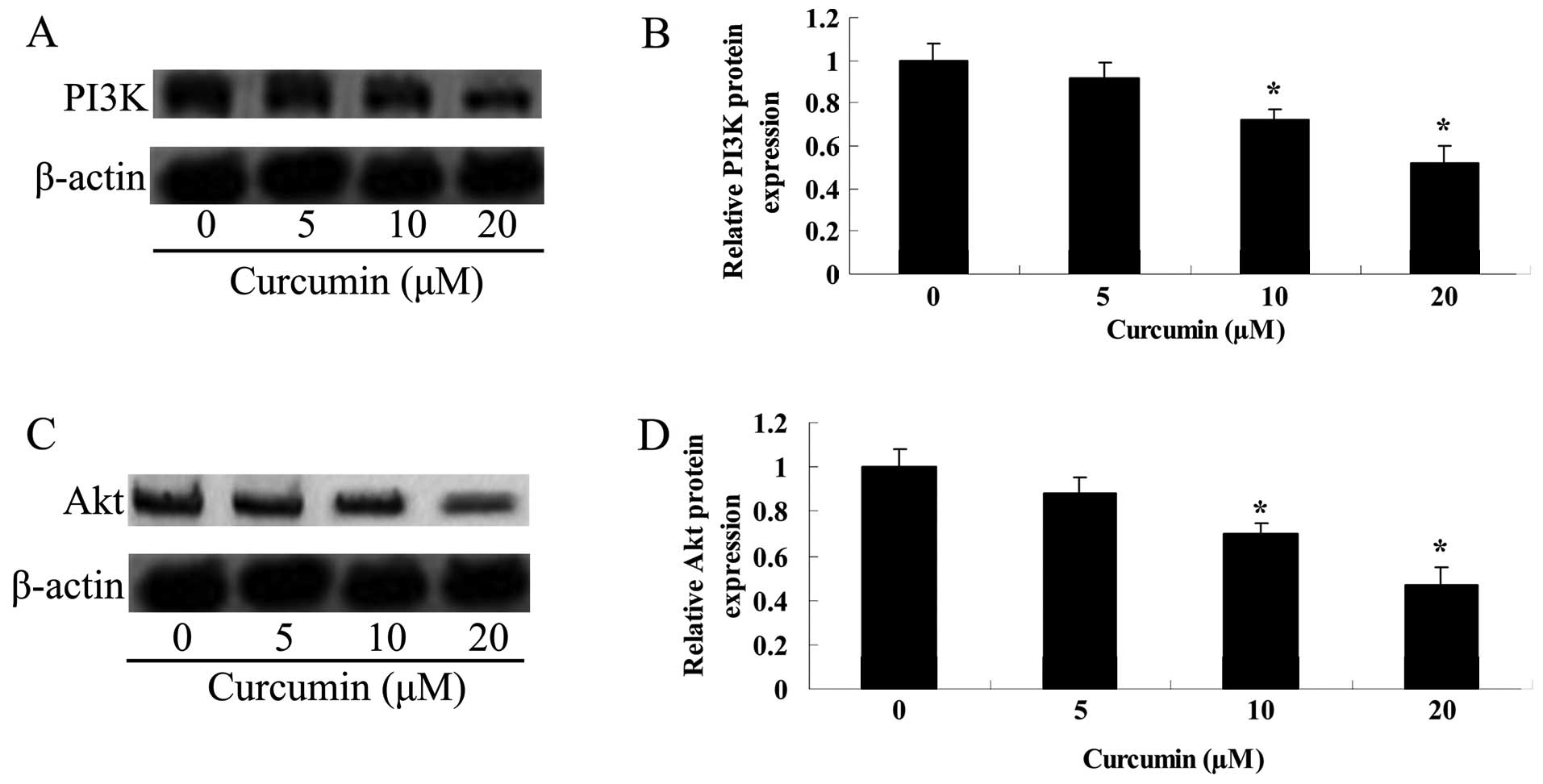
Curcumin inhibits the PI3K/Akt protein
expression of A549 cells
To detemine whether curcumin affected the PI3K/Akt
protein expression of A549 cells, the PI3K/Akt protein expression
was detected using western blot analysis following treatment with
curcumin for 24 h. Fig. 10 shows
that the PI3K/Akt protein expression was markedly enhanced by
curcumin (20 or 40 µM).
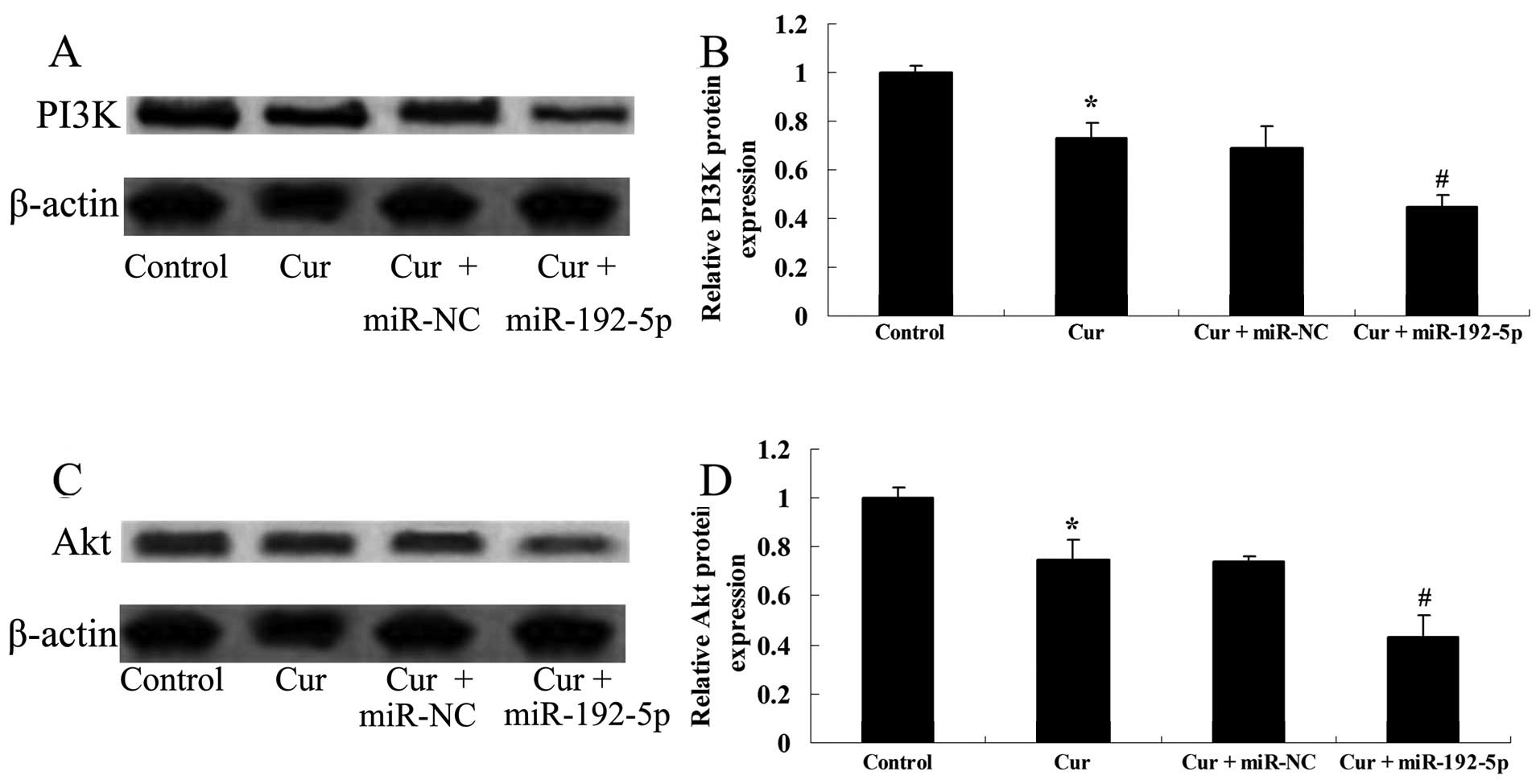
miR-192-5p directly targets PI3K/Akt of
A549 cells
To examine how overexpression of miR-192-5p
influenced the PI3K/Akt protein expression of A549 cells, we
transfected miR-192-5p mimics into A549 cells. Fig. 11A and B shows that curcumin
suppressed the PI3K/Akt protein expressions of A549 cells following
treatment with curcumin (20 µM) for 24 h, compared with the
control group. The effect of curcumin (20 µM) on the
PI3K/Akt protein expression of A549 cells was evidently reduced by
miR-192-5p mimics, compared with the curcumin-treated group
(Fig. 11C and D).
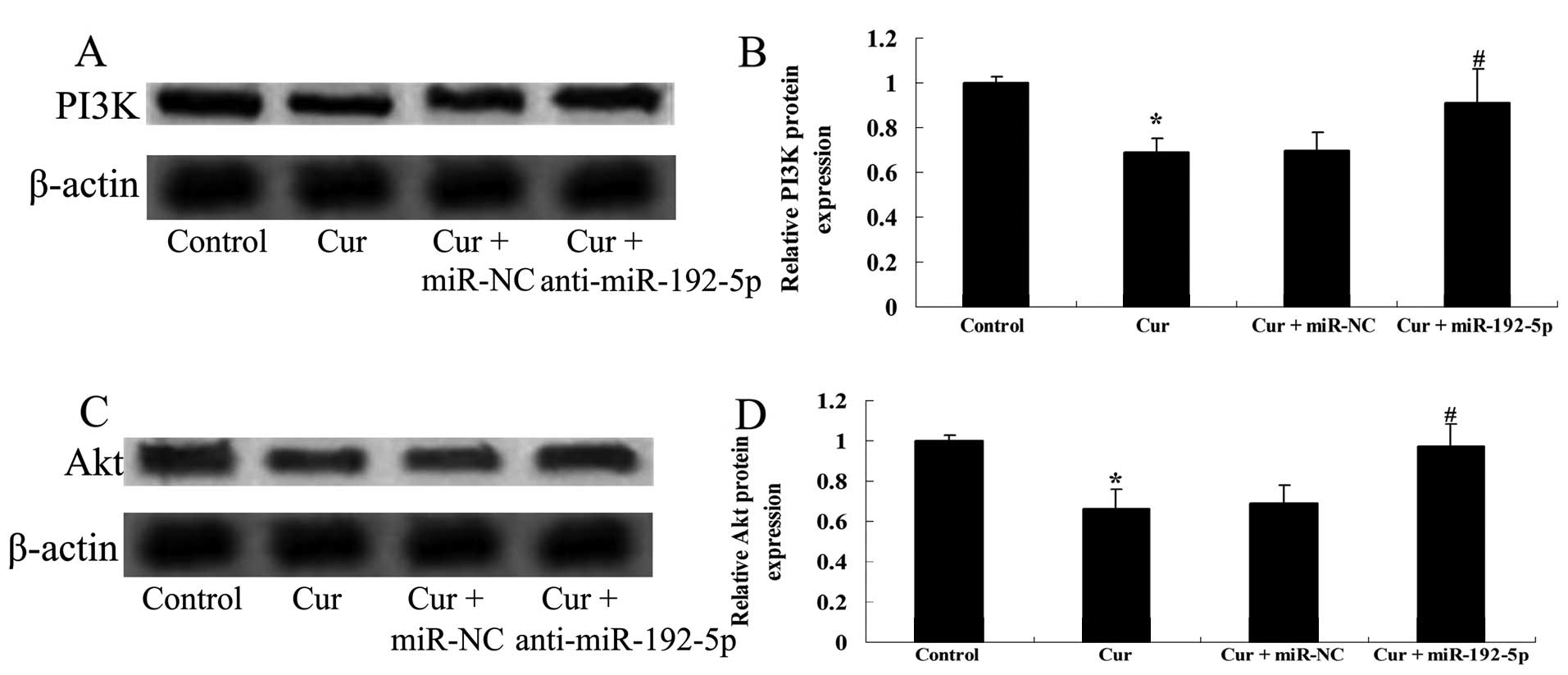
Inhibition of miR-192-5p increases the
PI3K/Akt expression of A549 cells
To determine whether anti-miR-192-5p influenced the
effect of curcumin on PI3K/Akt protein expression of A549 cells, we
transfected anti-miR-192-5p mimics into A549 cells. Fig. 12A and B shows that curcumin
suppressed the PI3K/Akt protein expression of A549 cells following
treatment with curcumin (20 µM) for 24 h, respectively,
compared with the control group. However, the effect of curcumin
(20 µM) on the PI3K/Akt protein expression of A549 cells was
markedly enhanced by anti-miR-192-5p mimics, respectively, compared
with the curcumin-treated group (Fig.
12C and D).
Discussion
One million patients succumb to NSCLC annually
worldwide, and the incidence of NSCLC is gradually on the increase.
Although a number of studies in recent years have focused on NSCLC,
no great progress has been made with regard to treatment. Surgery
remains the most effective treatment method, but in terms of
prevention as well as for the pathogenesis of NSCLC further studies
need to be conducted (14,15). Recently, Liu et al identified
that curcumin inhibits proliferation and significantly induces the
cell apoptotic rate of gastric cancer cells (16). Ma et al demonstrated that
curcumin inhibits cell growth and invasion of pancreatic cancer
cells (17). Collectively, our
findings indicated that curcumin suppressed cell viability, induced
cell apoptosis and increased the caspase-3 activity of A549 cells.
Therefore, curcumin is a potential drug for oncotherapy.
miRNA is a class of small-molecule RNA which has a
very important role in gene expression regulation as recently
identified (18). miRNA genes are
usually located in intron segments of gene. However, miRNAs are
also distributed outside of non-coding exons of the gene and the
intergenic region, which have been found to participate in a
variety of known onc ogenic pathways, such as p53, Bcl-2 and K-Ras
(19). It has been shown that
>50% of defined human miRNAs are located in the fragile sites of
the genome and these fragile sites are often associated with the
occurrence of NSCLC (20). The
expression profiles of miRNAs are associated with the development
of NSCLC. miR-34c, miR-145 and miR-142 can inhibit NSCLC (5). The results of the present study
indicate that miR-192-5p relative expression of NCL-H460 cells was
relatively low, that of A549 cells was higher, with BEAS-2E cells
being the most highly expressed. Overexpression of miR-192-5p
decreased the cell viability and increased the apoptotic rate of
A549 cells. Downregulation of miR-192-5p increased the cell
viability and reduced the apoptotic rate of A549 cells.
Additionally, curcumin inhibited miR-192-5p gene expression of A549
cells. However, the upregulation of miR-192-5p expression enhanced
the effect of curcumin on cell viability and the apoptotic rate of
A549 cells, while the downregulation of miR-192-5p expression
reversed the effect of curcumin on A549 cells. Our results were
consistent with those of other studies. For example, Ye et
al reported that curcumin promotes cell apoptosis by activating
miR-192-5p (21). However, the
probable mechanisms on how curcumin influences miR-192-5p
expression are unclear and future studies to determine this effect
should be performed.
In recent years, the effect of the PI3K/Akt
signaling pathway on human NSCLC has gained much attention
(22). Activation of the PI3K/Akt
signaling pathway is very common in human NSCLC, which can promote
the occurrence of cancer through a variety of mechanisms, including
gene mutations, reduction of cancer suppressor gene PTEN
expression, PI3K mutation or amplification, Akt mutation or
amplification and oncogene receptor activation (7). Activation of each component of this
pathway is the main reason for the unfavourable prognosis of NSCLC,
which can lead to drug resistance of the treatment, thus inhibition
of this pathway can reverse drug resistance and improve
chemotherapy and radiation effects in vivo (23). The data obtained in the present
study revealed that curcumin suppressed the PI3K/Akt protein
expression of A549 cells. Upregulation of miR-192-5p expression
refrained the PI3K/Akt protein expression of A549 cells.
Downregulation of miR-192-5p expression may increase the PI3K/Akt
protein expression of A549 cells. Xu et al have demonstrated
that curcumin inhibits the invasion and migration of FTC133 cell
via downregulation of the PI3K/Akt signaling pathway in thyroid
cancer cells (24). Xu et al
suggested that curcumin inhibits tumor proliferation of lung cancer
through the PI3K/Akt pathway (25).
Schee et al revealed that miR-22-3p, miR-143-3p and
miR-192-5p regulated and were involved in APC, TGFβ and PI3K
pathway in colorectal cancer cells (26).
In conclusion, this study focused on the effect of
curcumin against A549 cells, inhibited cell proliferation and
induced apoptosis of human NSCLC through the upregulation of
miR-192-5p and suppression of the PI3K/Akt signaling pathway. The
conclusions of the present study indicate that curcumin is a
potential target in the treatment of NSCLC. However, the present
study revealed some limitations as we did not examine the detailed
relationship between miR-192-5p and the PI3K/Akt pathway in lung
cancer cells. Additional studies in vivo, clinical and
larger-scale statistical analyses of the present study are to be
performed to verify the results.
References
|
1
|
Kwon SB, Kim MJ, Ham SY, Park GW, Choi KD,
Jung SH and Yoon DY: H9 induces apoptosis via the intrinsic pathway
in non-small-cell lung cancer A549 cells. J Microbiol Biotechnol.
25:343–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kogita A, Togashi Y, Hayashi H, Sogabe S,
Terashima M, De Velasco MA, Sakai K, Fujita Y, Tomida S, Takeyama
Y, et al: Hypoxia induces resistance to ALK inhibitors in the H3122
non-small cell lung cancer cell line with an ALK rearrangement via
epithelial-mesenchymal transition. Int J Oncol. 45:1430–1436.
2014.PubMed/NCBI
|
|
3
|
Lønvik K, Sørbye SW, Nilsen MN and
Paulssen RH: Prognostic value of the MicroRNA regulators Dicer and
Drosha in non-small-cell lung cancer: Co-expression of Drosha and
miR-126 predicts poor survival. BMC Clin Pathol. 14:452014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nana-Sinkam SP and Geraci MW: MicroRNA in
lung cancer. J Thorac Oncol. 1:929–931. 2006. View Article : Google Scholar
|
|
5
|
Garofalo M, Quintavalle C, Di Leva G,
Zanca C, Romano G, Taccioli C, Liu CG, Croce CM and Condorelli G:
MicroRNA signatures of TRAIL resistance in human non-small cell
lung cancer. Oncogene. 27:3845–3855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou L, Luan H, Liu Q, Jiang T, Liang H,
Dong X and Shang H: Activation of PI3K/Akt and ERK signaling
pathways antagonized sinomenine-induced lung cancer cell apoptosis.
Mol Med Rep. 5:1256–1260. 2012.PubMed/NCBI
|
|
7
|
Zito CR, Jilaveanu LB, Anagnostou V, Rimm
D, Bepler G, Maira SM, Hackl W, Camp R, Kluger HM and Chao HH:
Multi-level targeting of the phosphatidylinositol-3-kinase pathway
in non-small cell lung cancer cells. PLoS One. 7:e313312012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen QY, Jiao DM, Wang LF, Wang L, Hu HZ,
Song J, Yan J, Wu LJ and Shi JG: Curcumin inhibits
proliferation-migration of NSCLC by steering crosstalk between a
Wnt signaling pathway and an adherens junction via EGR-1. Mol
Biosyst. 11:859–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu W, Jiang JP, Hu J, Wang J and Zheng MZ:
Curcumin protects against lipopolysaccharide-induced
vasoconstriction dysfunction via inhibition of thrombospondin-1 and
transforming growth factor-β1. Exp Ther Med. 9:377–383.
2015.PubMed/NCBI
|
|
10
|
Tizabi Y, Hurley LL, Qualls Z and
Akinfiresoye L: Relevance of the anti-inflammatory properties of
curcumin in neurodegenerative diseases and depression. Molecules.
19:20864–20879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borra SK, Mahendra J, Gurumurthy P,
Jayamathi, Iqbal SS and Mahendra L: Effect of curcumin against
oxidation of biomolecules by hydroxyl radicals. J Clin Diagn Res.
8:CC01–CC05. 2014.PubMed/NCBI
|
|
12
|
Nahar PP, Slitt AL and Seeram NP:
Anti-inflammatory effects of novel standardized solid lipid
curcumin formulations. J Med Food. 18:786–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ali MS, Pandit V, Jain M and Dhar KL:
Mucoadhesive micropar-ticulate drug delivery system of curcumin
against Helicobacter pylori infection: Design, development and
optimization. J Adv Pharm Technol Res. 5:48–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T, Cui GB, Zhang J, Zhang F, Zhou
YA, Jiang T and Li XF: Inhibition of PI3 kinases enhances the
sensitivity of non-small cell lung cancer cells to ionizing
radiation. Oncol Rep. 24:1683–1689. 2010.PubMed/NCBI
|
|
15
|
Li H, Xie L and Lai RS: Association of
EGFR mutations with low BRCA1 gene expression in non-small cell
lung cancer. Mol Clin Oncol. 1:195–199. 2013.PubMed/NCBI
|
|
16
|
Liu X, Sun K, Song A, Zhang X, Zhang X and
He X: Curcumin inhibits proliferation of gastric cancer cells by
impairing ATP-sensitive potassium channel opening. World J Surg
Oncol. 12:3892014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma J, Fang B, Zeng F, Pang H, Zhang J, Shi
Y, Wu X, Cheng L, Ma C, Xia J, et al: Curcumin inhibits cell growth
and invasion through up-regulation of miR-7 in pancreatic cancer
cells. Toxicol Lett. 231:82–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Zhang Q, Wu L, Jia C, Shi F, Li S,
Peng A, Zhang G, Song X and Wang C: Serum miR-499 as a novel
diagnostic and prognostic biomarker in non-small cell lung cancer.
Oncol Rep. 31:1961–1967. 2014.PubMed/NCBI
|
|
19
|
Pacurari M, Addison JB, Bondalapati N, Wan
YW, Luo D, Qian Y, Castranova V, Ivanov AV and Guo NL: The
microRNA-200 family targets multiple non-small cell lung cancer
prognostic markers in H1299 cells and BEAS-2B cells. Int J Oncol.
43:548–560. 2013.PubMed/NCBI
|
|
20
|
Bandi N, Zbinden S, Gugger M, Arnold M,
Kocher V, Hasan L, Kappeler A, Brunner T and Vassella E: miR-15a
and miR-16 are implicated in cell cycle regulation in a
Rb-dependent manner and are frequently deleted or down-regulated in
non-small cell lung cancer. Cancer Res. 69:5553–5559. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye M and Zhang J and Zhang J, Miao Q, Yao
L and Zhang J: Curcumin promotes apoptosis by activating the
p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer.
Cancer Lett. 357:196–205. 2015. View Article : Google Scholar
|
|
22
|
Yue W, Wang X and Wang Y: The relationship
between the PI3K/Akt/mTOR signal transduction pathway and non-small
cell lung cancer. Zhongguo Fei Ai Za Zhi. 12:312–315. 2009.In
Chinese. PubMed/NCBI
|
|
23
|
Baykara O, Tansarikaya M, Demirkaya A,
Kaynak K, Tanju S, Toker A and Buyru N: Association of epidermal
growth factor receptor and K-Ras mutations with smoking history in
non-small cell lung cancer patients. Exp Ther Med. 5:495–498.
2013.PubMed/NCBI
|
|
24
|
Xu X, Qin J and Liu W: Curcumin inhibits
the invasion of thyroid cancer cells via down-regulation of
PI3K/Akt signaling pathway. Gene. 546:226–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Zhang J, Han J, Pan X, Cao Y, Guo H,
Pan Y, An Y and Li X: Curcumin inhibits tumor proliferation induced
by neutrophil elastase through the upregulation of α1-antitrypsin
in lung cancer. Mol Oncol. 6:405–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schee K, Lorenz S, Worren MM, Günther CC,
Holden M, Hovig E, Fodstad O, Meza-Zepeda LA and Flatmark K: Deep
Sequencing the MicroRNA Transcriptome in Colorectal Cancer. PLoS
One. 8:e661652013. View Article : Google Scholar : PubMed/NCBI
|