Introduction
Food, including milk, vegetables, vegetable oil, and
even school meals have been found to contain multiple types of
phthalates in recent years (1–4). These
different types of phthalates are transferred to food during
vegetable cultivation, food packaging and food processing (5–7).
Phthalates have been detected in the serum of infants, children and
adults as it is difficult to avoid daily dietary exposure at
present (8,9). Moreover, metabolites of dibutyl
phthalate (DBP), di-2-ethylhexyl phthalate (DEHP) and benzyl butyl
phthalate (BBP), have been found in urine, serum, breast milk and
saliva. Studies have revealed that phthalate exposure is associated
with many diseases, such as airway obstruction, allergies, asthma,
reproductive disease and breast cancer (10–12).
BBP has been demonstrated to induce neoplastic transformation of
breast epithelial cells and to increase the proliferation and
progression of both estrogen-dependent and -independent breast
cancer stem cells and cancer cells (13–16).
However, the influence of phthalates on cancer therapy has not been
evaluated to date.
Breast cancer is the most common female cancer, and
is one of the major causes of cancer-related mortality among women.
There were nearly 1.7 million new breast cancer cases diagnosed and
521,900 deaths due to breast cancer worldwide in 2012 (17,18).
Although chemotherapy significantly improves the outcome of
patients with breast cancer, chemoresistance is still a major
obstacle to successful treatment and such resistance to
chemotherapeutic drugs frequently results in subsequent tumor
recurrence and tumor metastasis (19,20).
However, most of the mechanisms responsible for resistance to
chemotherapeutic agents are still unknown. A better understanding
of the causes and mechanisms of chemoresistance can be helpful for
identifying and developing novel therapeutic agents that could
decrease metastasis, chemoresistance, and even cancer-related
death.
The tumor microenvironment (TME) not only plays a
critical role during tumorigenesis, cancer progression and
metastasis, but also influences therapeutic efficacy (21). Tumor environment-mediated drug
resistance (EMDR) is mediated by a multitude of reciprocal
interactions between cancer cells and various cell types existing
in the TME. Tumor-associated immune cells are a hallmark of most
solid malignancies, and the presence of various immune cells
significantly influences clinical outcome (22). Tumor-associated macrophages have
been indicated to increase the resistance of cancer against
cytotoxic agents. Therefore, inhibiting the infiltration of
macrophages improves the efficacy of chemotherapy and reduces
cancer metastasis (23,24). Similar to macrophages,
tumor-associated dendritic cells (TADCs) have been postulated as
being involved in cancer progression (25–27).
TADCs produce a number of potent growth factors and cytokines,
which may be mediators that potentiate chemoresistance (28,29).
The present study is the first to assess the effects of BBP on
breast cancer, and to demonstrate that BBP induces a change in the
chemo-sensitivity of breast cancer to doxorubicin/cyclophosphamide
by altering the cancer microenvironment.
Materials and methods
Cell culture and conditioned medium
Human breast adenocarcinoma cell line MDA-MB-231
(ATCC HTB-26™; American Type Culture Collection, ATCC, Manassas,
VA, USA) was cultured in Leibovitz medium (L15) supplemented with
1% antibiotic solution and 10% fetal bovine serum (FBS) (all from
Thermo Fisher Scientific, Waltham, MA, USA) in a
CO2-free incubator. Human umbilical vein endothelial
cells (HUVECs) (BCRC H-UV001; Bioresource Collection and Research
Center, Hsinchu, Taiwan) and 4T1 mouse mammary tumor cell line
(ATCC CRL-2539; ATCC) were cultured in complete EGM2 medium (Lonza,
Walkersville, MD, USA), or RPMI-1640 (Gibco-BRL, Gaithersburg, MD,
USA) medium containing 10% FBS in a 5% CO2 incubator. To
prepare the conditioned media (CM), the MDA-MB-231 cells
(2×106/100 mm dish) were seeded, and the supernatant was
harvested and filtered (0.22 mm), after 48 h of incubation.
Isolation of CD14+ monocytes
and differentiation of dendritic cells
Monocytes were isolated from peripheral blood
mononuclear cells (PBMCs) provided by healthy consenting donors.
PBMCs were isolated from blood by Ficoll-Hypaque gradient (GE
Healthcare Bio-Sciences, Little Chalfont, UK). CD14+
monocytes were purified from PBMCs by using CD14+
monoclonal antibody-conjugated magnetic beads (MACS MicroBeads;
Miltenyi Biotec Ltd., Bergisch Gladbach, Germany), according to the
manufacturer's procedure. Monocyte-derived dendritic cells (mdDCs)
or TADCs were generated by culturing CD14+ monocytes in
RMPI-1640 medium containing control 20% L15 (for mdDCs) or
MDA-MB-231-CM (for TADCs) presenting in 10 ng/ml GM-CSF and IL-4
(R&D Systems, Minneapolis, MN, USA), with or without BBP (1 or
10 µM; Sigma-Aldrich, St. Louis, MO, USA) for 5 days. The
mdDC and TADC supernatants were collected and filtered (0.22 mm) to
be used as CMs. The Institutional Review Board (IRB) of Kaohsiung
Medical university Hospital (Kaohsiung, Taiwan) approved the study
protocol, and all of the participants provided written informed
consent in accordance with the Declaration of Helsinki (IRB no.
KMUH-IRB-990174 and KMUH-IRB-20120362).
Measurement of secreted factors
Supernatants from the mdDCs or TADCs treated with or
without BBP were collected. CXCL1/GROα and VEGF were determined by
Milliplex MAP kit (Millipore, Billerica, MA, USA). S100A8 and
S100A9 were assessed and quantified using the DuoSet ELISA
(enzyme-linked immunosorbent assay; R&D Systems).
Cell viability/apoptosis
MDA-MB-231 cells were treated with control or
doxorubicin/cyclophosphamide (10/100 µM) with or without BBP
(1 µM) presenting in various CMs (20%) for 48 h. Cell
viability was determined by Premixed WST-1 Cell Proliferation
reagent (Clontech Laboratories Inc., Mountain View, CA, USA) in
accordance with the manufacturer's instructions. Quantitative
analysis of apoptosis was determined by the terminal nucleotidyl
transferase-mediated nick end labeling (TUNEL) method using the BD
ApoAlert DNA Fragmentation Assay kit (BD Biosciences Clontech, Palo
Alto, CA, USA).
Tube formation analysis
Tube formation assays were carried out as described
previously after modification (30). Growth factor-reduced Matrigel (200
µl) was loaded in each well of a 24-well plate, which was
incubated at 37°C for 60 min. HUVECs were mixed with the various
CMs (20%) and the CXCL1/GROα antibody. Cell suspension solution
(500 µl) was added on top of the Matrigel. The plate was
then incubated at 37°C, and the formation of capillary-like tubes
was detected and stained using Calcein-AM (Life Technologies,
Carlsbad, CA, USA) after 10 h using a fluorescence microscope.
RNA isolation and quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer's
protocol, and reverse transcribed to cDNA using a SuperScript III
Reverse Transcriptase kit (Invitrogen). PCR mixture was prepared
using the SYBR Green qPCR kit (Invitrogen) and using the following
primers as follows: CXCL1/GROα (forward, 5′-agggaattcaccccaagaac-3′
and reverse, 5′-TAACTATGGGGGATGCAGGA-3′); S100A8 (forward,
5′-atgccgtctacagggatgac-3′ and reverse, 5′-ACGCCCATCTTTATCACCAG-3′)
and GAPDH (forward, 5′-TTCACCACCATGGAGAAGGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGA-3′). All qRT-PCR reactions were performed
using the StepOne Real-Time PCR system (Applied Biosystems, Foster
City, CA, USA). Quantitative analysis normalized to GAPDH was
performed according to the comparative cycle threshold (Ct)
method.
CXCL1/GROα and S100A9 knockdown
CD14+ monocytes were transfected with 1
µM non-targeting, CXCL1/GROα or S100A9 Accell™ SMARTpool
siRNA (Thermo Fisher Scientific) containing IL-4 and GM-CSF for 5
day. The medium containing siRNA and IL-4/GM-CSF was replaced on
day 3. The knockdown efficacy of siRNA was measured by qRT-PCR.
Animal experiments and drug
treatment
For the orthotopic metastasis assay, mouse breast
cancer 4T1 cells were transplanted into the mammary fat pads of
8-week-old female BALB/c mice. Mice were injected once a week with
either PBS vehicle, BBP, doxorubicin hydrochloride (2
mg/kg)/cyclophosphamide monohydrate (60 mg/kg) or a combination of
BBP and PBS vehicle, BBP, doxorubicin hydrochloride (2
mg/kg)/cyclophosphamide monohydrate (60 mg/kg) for 3 weeks. For
experiments involving CXCL1/GROα inhibition, the mice were injected
intraperitoneally with either IgG (vehicle) or with CXCL1/GROα once
per week (50 µg/mouse). All immunohistochemical reactions
were performed on 5-µm paraffin sections. In brief, the
sections were deparaffinized in xylene and rehydrated, and then
incubated in target retrieval solution (DAKO) in an autoclave for 8
min to retrieve the antigens. Endogenous peroxidase activity was
blocked by 10 min of incubation with a 3% solution of
H2O2. The expression of CD31 antigen was
assessed using the mouse monoclonal anti-CD31 (dilution 1:100)
antibody. The sections were incubated with the primary antibodies
overnight at 4°C. The antigens were then visualized using
biotinylated antibodies and streptavidin, conjugated with
horseradish peroxidase. Diaminobenzidine (DakoCytomation, Glostrup,
Denmark) served as the substrate, and all of the sections were
counter-stained with hematoxylin.
Isolation of dendritic cells
(CD11c+F4/80−) and myeloid-derived suppressor
cells (MDSCs) from the mice
Mouse mammary tumors (4T1-bearing mice) were
collected and minced. Single-cell suspensions were obtained after
enzymatic digestion (1 mg/ml collagenase A; Roche Diagnostics) and
100 IU/ml type I Dnase (Sigma-Aldrich) for 2 h at 37°C in RPMI-1640
medium. A single-cell suspension was filtered through a
70-µm nylon mesh (BD Biosciences), and cells were washed
twice with PBS. CD11c+ cells were purified using
anti-CD11c monoclonal antibody-conjugated magnetic beads (MACS
MicroBeads). F4/80+ cells were depleted from
CD11c+ cells by using F4/80+ antibody-biotin
beads (MACS MicroBeads). MDSCs were isolated from tumors using the
Myeloid-Derived Suppressor Cell Isolation kit from Miltenyi Biotec.
Cell purity was checked by flow cytometric (BD Biosciences,
Franklin Lakes, NJ, USA) analysis using anti- CD11b and Gr-1
antibodies (>75%), and the viability was assessed by trypan blue
dye exclusion.
Statistical analysis
Data are expressed as means ± SD. Statistical
analyses between the control and experimental groups were analyzed
by an unpaired Student's t-test. Multiple comparisons were
evaluated by one-way ANOVA, and differences in the mean values
among groups were conducted by a Turkey post hoc analysis. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
BBP increases chemoresistance in a 4T1
orthotopic metastasis model
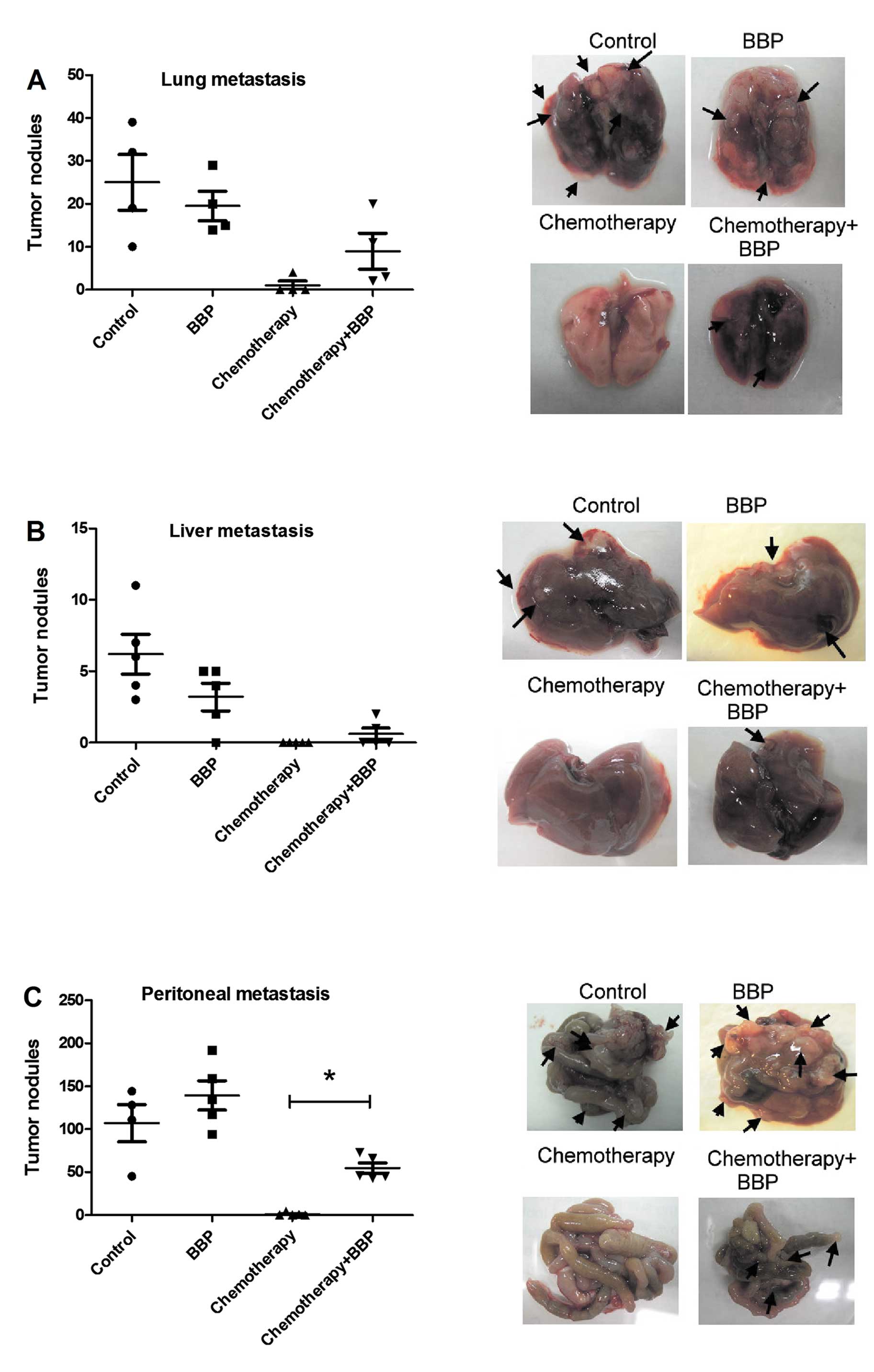
First, we assessed the influence of BBP on
chemotherapeutic efficacy in breast cancer in a mouse model. As
shown in Fig. 1, combination of
doxorubicin hydrochloride (2 mg/kg body weight) and
cyclophosphamide monohydrate (60 mg/kg body weight), a doublet
chemotherapy frequently used in the clinic, exhibited a markedly
inhibitory effect on cancer metastasis (lung, liver and peritoneal
metastasis). Exposure of mice to BBP alone did not affect the
metastasis of 4T1 in the mouse model, but did reduce the inhibitory
effect of chemotherapy on breast cancer peritoneal metastasis in
the mice (Fig. 1).
BBP increases the chemoresistance of
breast cancer via the TADC-mediated response
Since exposure to BBP increases the resistance of
cancer to doxorubicin/cyclophosphamide treatment, we assessed
whether BBP affects the sensitivity of breast cancer to
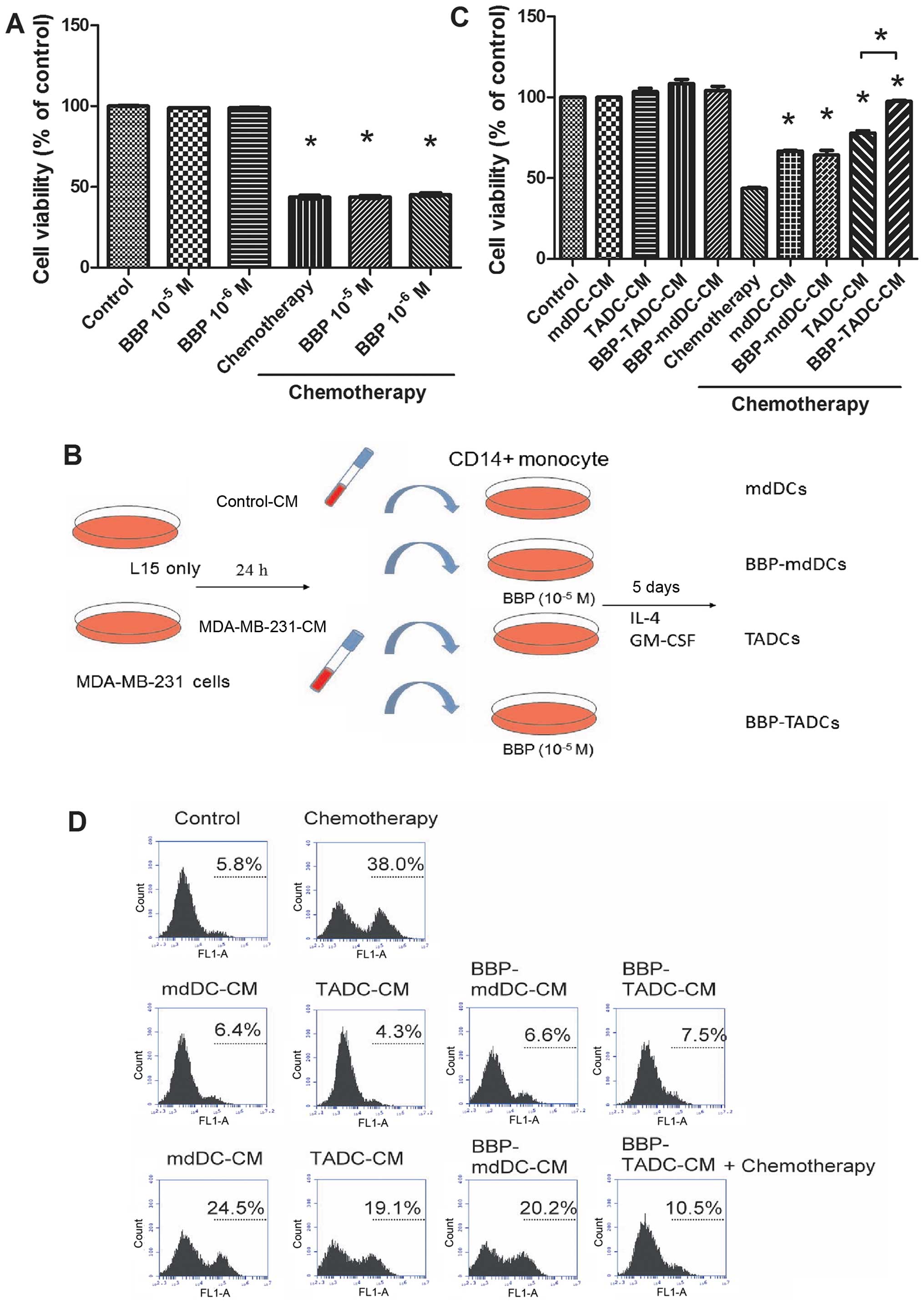
doxorubicin/cyclophosphamide. As shown in Fig. 2A, doxorubicin/cyclophosphamide
(10/100 µM) decreased the cell viability of MDA-MB-231 cells
~60% after a 48-h treatment. However, BBP did not affect the
sensitivity of breast cancer cells to the
doxorubicin/cyclophosphamide combination (Fig. 2A). TME is considered to determine
the efficacy of chemotherapy (31).
To assess whether BBP increases the chemo-resistance of breast
cancer by stimulating TADCs, we generated mdDCs, TADCs and
BBP-stimulated mdDCs and TDACs (BBP-mdDCs and BBP-TADCs) and
collected the condition media (CM) as described in Fig. 2B. MDA-MB-231 cells were treated with
doxorubicin/cyclophosphamide in regular culture medium, mdDC-CM,
TADC-CM, BBP-mdDC-CM or BBP-TADC-CM containing medium. TADC-CM
significantly decreased the sensitivity of the MDA-MB-231 cells to
doxorubicin/cyclophosphamide. BBP further desensitized breast
cancer to doxorubicin/cyclophosphamide (Fig. 2C). Next, we investigated whether the
chemoresistance induced by TADC-CM and BBP-TADC-CM was mediated by
reducing doxorubicin/cyclophosphamide-induced apoptosis. TADC-CM
markedly reduced the percentage of TunEL-positive MDA-MB-231 cells
following treatment with doxorubicin/cyclophosphamide relative to
mdDC-CM. A statistically significant reduction in apoptosis
induction was noted after exposure to BBP-TADC-CM (Fig. 2D). These data suggest that BBP
stimulated soluble factor(s) secreted from TADCs to induce
doxorubicin/cyclophosphamide resistance in breast cancer.
TADC-mediated S100A8/A9 increases breast
cancer chemoresistance
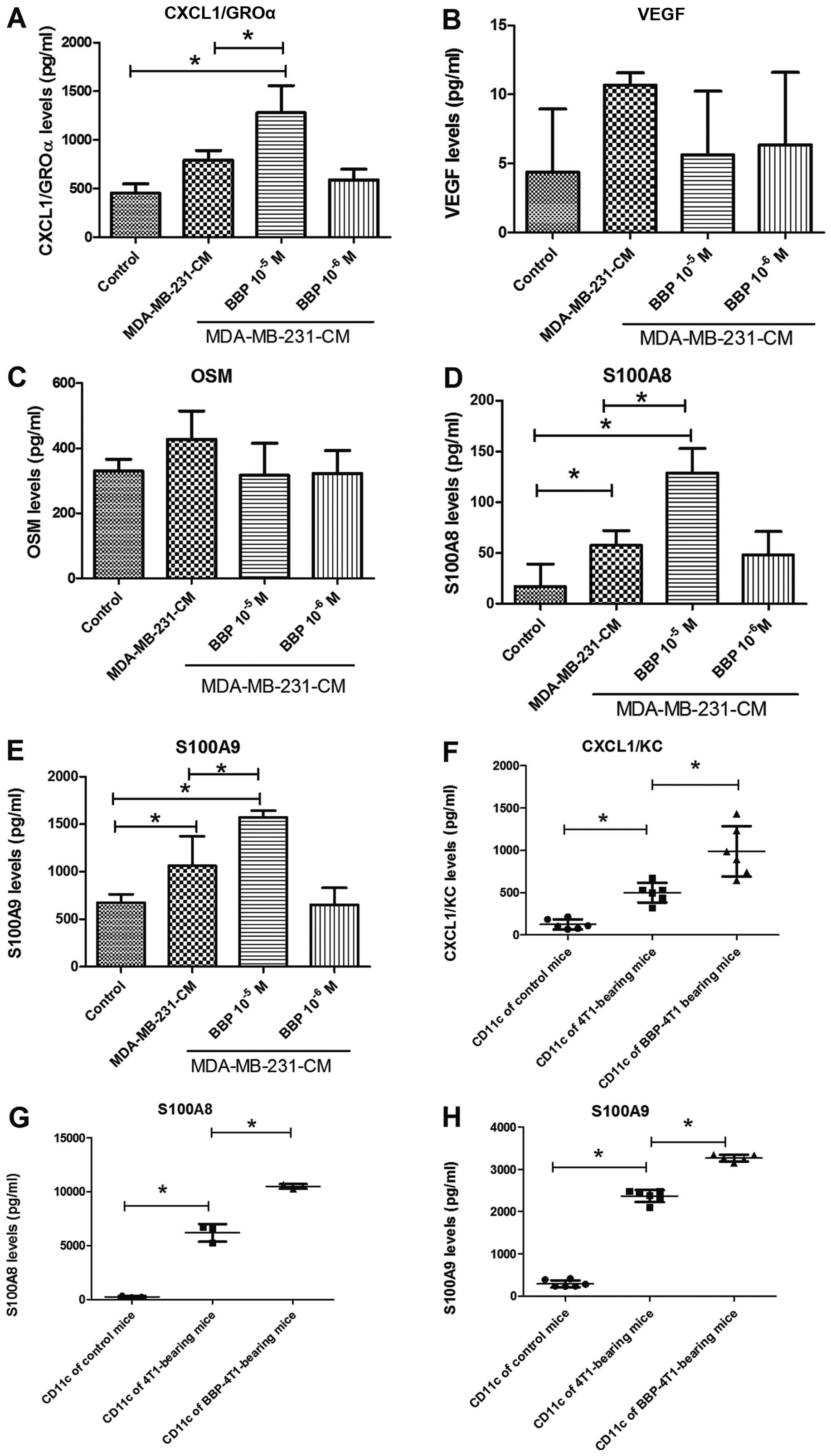
Next, we assessed the influence of BBP on the
expression of secretory cytokines in TADCs, which have been
reported to be involved in the development of chemoresistance. The
expression levels of CXCL1/GROα, S100A8 and S100A9 were increased
in the TADCs, in comparison to the levels in the mdDCs. BBP further
enhanced the stimulatory effect of breast cancer cells in regards
to the secretion of CXCL1/GROα, S100A8 and S100A9, but not VEGF and
OSM (Fig. 3A-E). Transplantation of
the 4T1 cell into mice increased the expression of CXCL1/GROα,
S100A8 and S100A9 in the TADCs
(CD11c+F4/80−), compared to the control mice.
Exposure to BBP further increased expression of CXCL1/GROα, S100A8
and S100A9 in the TADCs in the the 4T1-bearing mice (Fig. 3F and G).
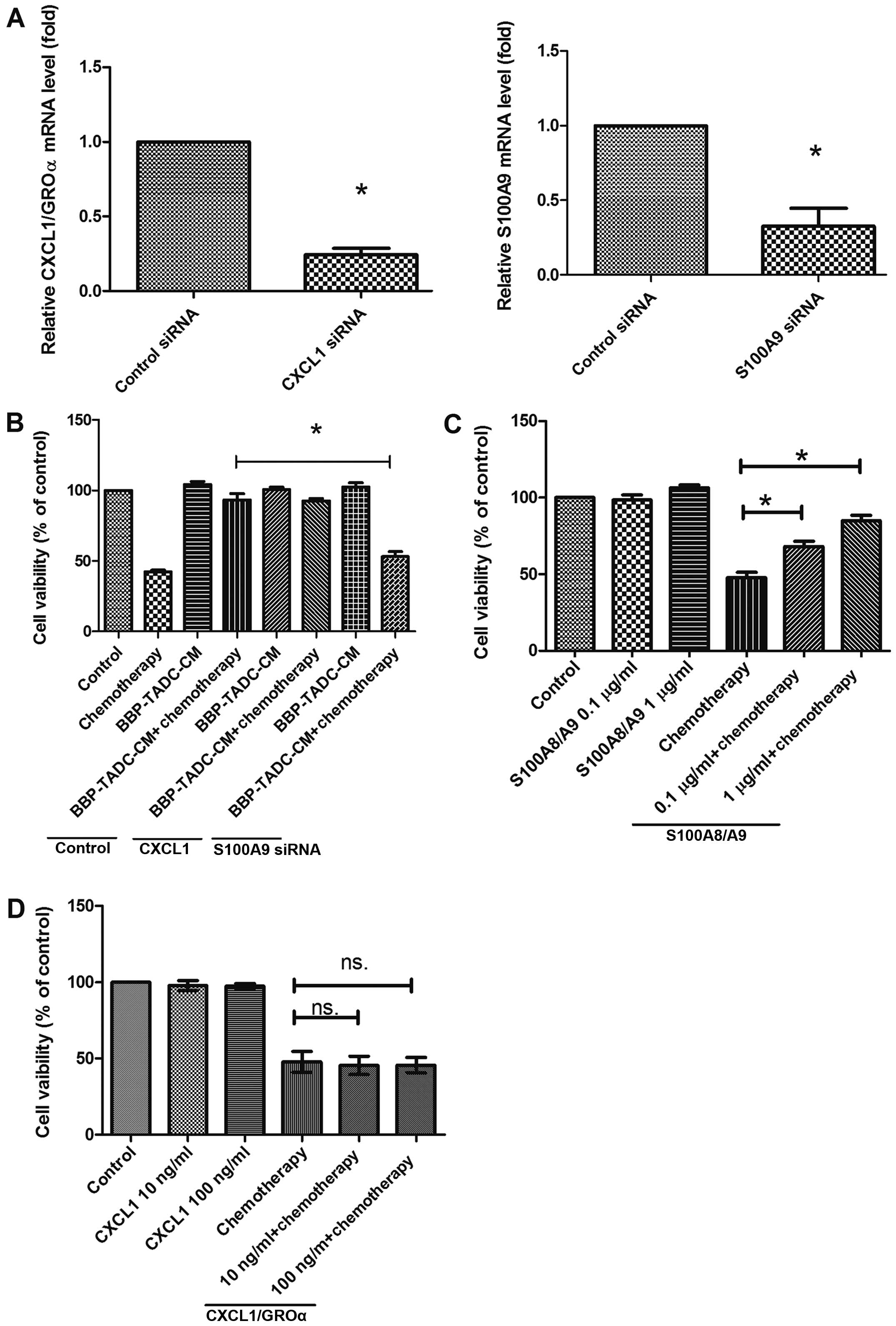
To explore which secretory factors contribute to the
chemoresistance of MDA-MB-231 cells, we inhibited the expression of
CXCL1/GROα or S100A9 using siRNA transfection. Transfection of
CD14+ monocytes decreased the CXCL1/GROα and S100A9
expression by 80 and 90%, respectively (Fig. 4A). Inhibition of S100A9 expression
prevented the effect of CMs of BBP-derived TADCs on the
chemoresistance of the MDA-MD-231 cells (Fig. 4B). However, knockdown of CXCL1/GROα
did not restore the chemosensitivity of MDA-MB-231 cells to
doxorubicin/cyclophosphamide in presenting CMs of BBP-derived TADCs
(Fig. 4B). Similarly, only
recombinant human S100A8/A9 (rhS100A8/A9) reduced the cytotoxicity
of doxorubicin/cyclophosphamide, whereas recombinant human
CXCL1/GRO (rhCXCL1/GRO) did not affect the cytotoxicity of
doxorubicin/cyclophosphamide in the MDA-MB-231 cells (Fig. 4C and D).
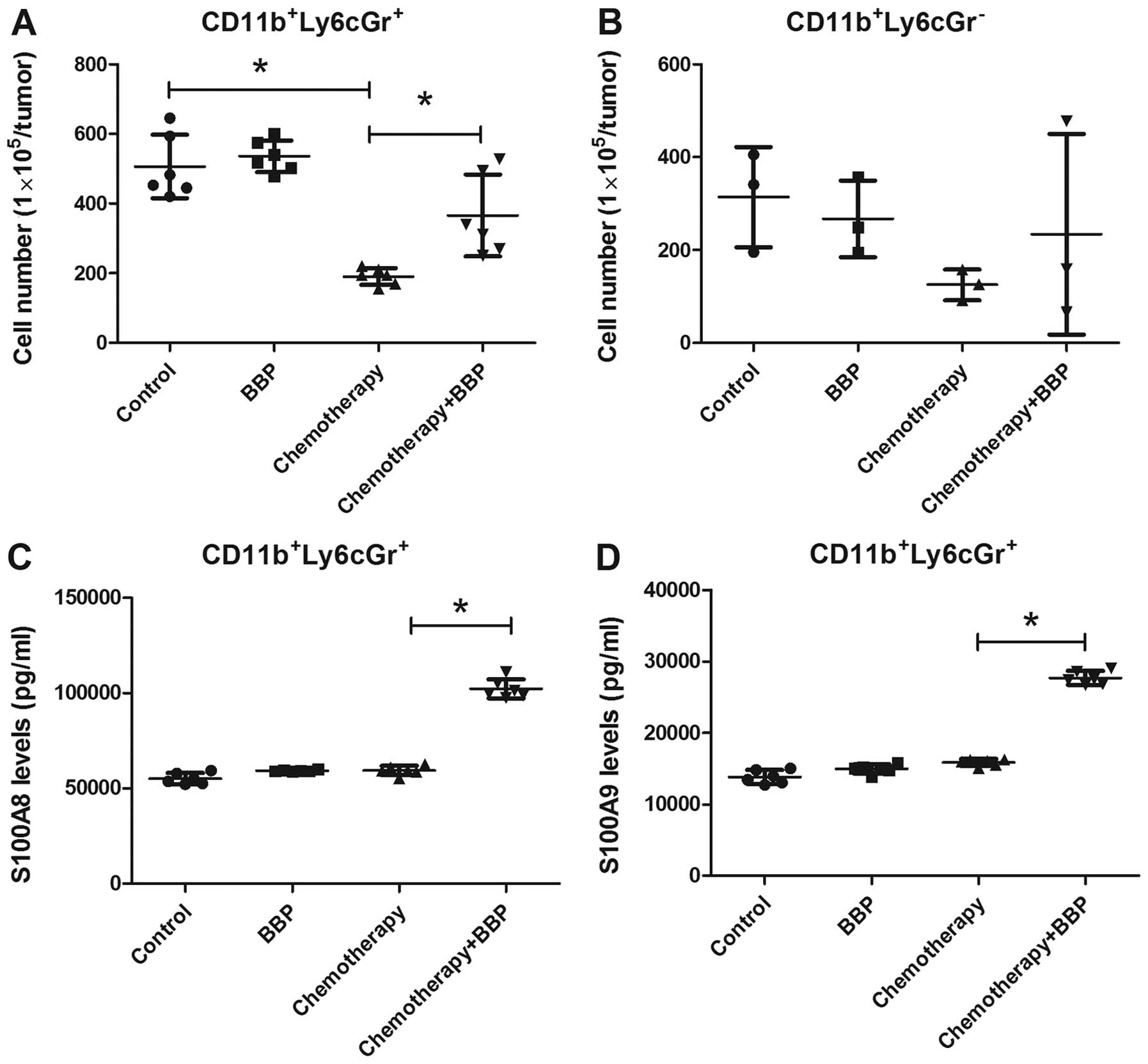
BBP increases the production of S100A8
and S100A9 in MDSCs (CD11b+Ly6CGr-1+) in
breast cancer
Previous research has demonstrated that S100A8/A9
levels are associated with the infiltration of MDSCs in tumors and
enhance chemoresistance (31,32).
Therefore, we assessed whether BBP increases the infiltration of
MDSCs in the 4T1 cell-bearing mice. As shown in Fig. 5A and B, doxorubicin/cyclophosphamide
treatment decreased the infiltration of
CD11b+Ly6CGr-1+ MDSCs in the tumors. BBP
exposure prevented the effect of chemotherapy on the infiltration
of CD11b+Ly6CGr-1+ MDSCs (Fig. 5A). However, BBP exposure did not
further increase the recruitment of
CD11b+Ly6CGr-1− MDSCs, regardless of
doxorubicin/cyclophosphamide treatment in the mice (Fig. 5B). Next, we assessed the expression
of S100A8 and S100A9 in the tumor-infiltrating
CD11b+Ly6CGr-1+ MDSCs. ELISA results showed
that BBP or doxorubicin/cyclophosphamide treatment alone slight
enhanced the expression of S100A8 and S100A9 in the
CD11b+Ly6CGr-1+ MDSCs (Fig. 5C and D). However, exposure of mice
to BBP markedly enhanced the production of S100A8 and S100A9 in the
CD11b+Ly6CGr-1+ MDSCs in the
doxorubicin/cyclophosphamide-treated mice (Fig. 5C and D).
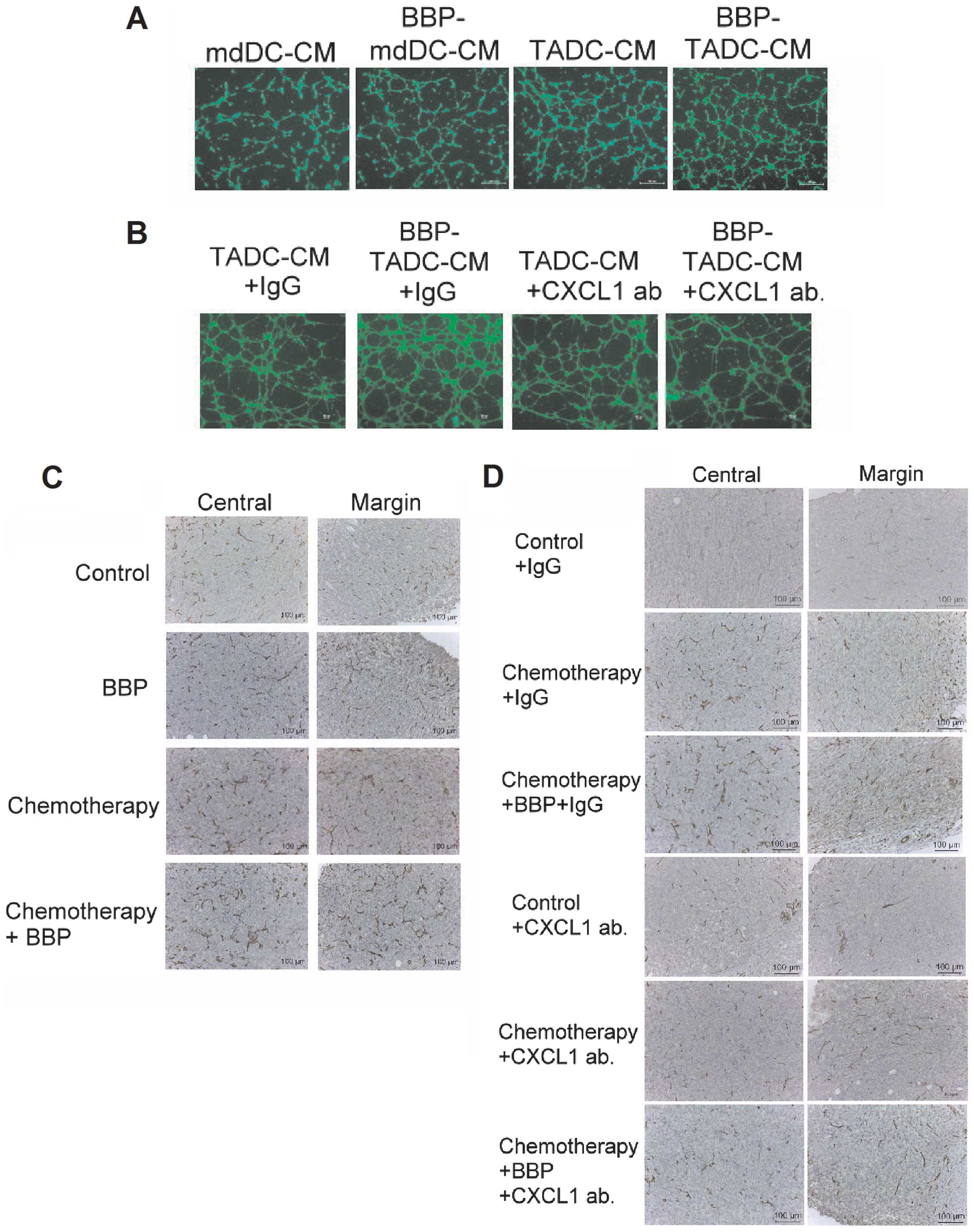
BBP increases angiogenesis by
TADC-derived CXCL1/GROα
Since CXCL1/GROα has been indicated to be an
angiogenic factors in cancer (33),
we assessed the effect of BBP on angiogenesis induced by TADCs.
Compared to mdDC-CM, TADCs increased the tube formation of HUVECs.
In addition, BBP increased the stimulatory effect of TADCs on tube
formation (Fig. 6A). The
synergistic effect of BBP on angiogenesis was prevented by the
neutralizing CXCL1/GROα antibody (Fig.
6B), suggesting that CXCL1/GROα is the major angiogenic factor
in TADC-mediated angiogenesis. Furthermore, exposure of mice to BBP
increased the angiogenesis in primary breast cancers in vivo
(Fig. 6C). To investigate whether
targeting CXCL1/GROα may be a strategy to prevent BBP-induced
angiogenesis, we administered the mice with neutralizing CXCL1/GROα
antibody. Exposure of mice to BBP or chemotherapy increased the
angiogenesis in the breast cancer. The enhancement of angiogenesis
induced by BBP was prevented by the administration of the
CXCL1/GROα antibody in the chemotherapy and BBP +
chemotherapy-treated mice (Fig.
6D).
Discussion
Exposure to phthalates causes various health and
reproductive problems in human. Since phthalate esters are
ubiquitous in the environment and the potential for adverse effects
on human health is great, an understanding of how these factors
influence human health and the underlying mechanisms are urgently
required. This study is the first to investigate the influence of
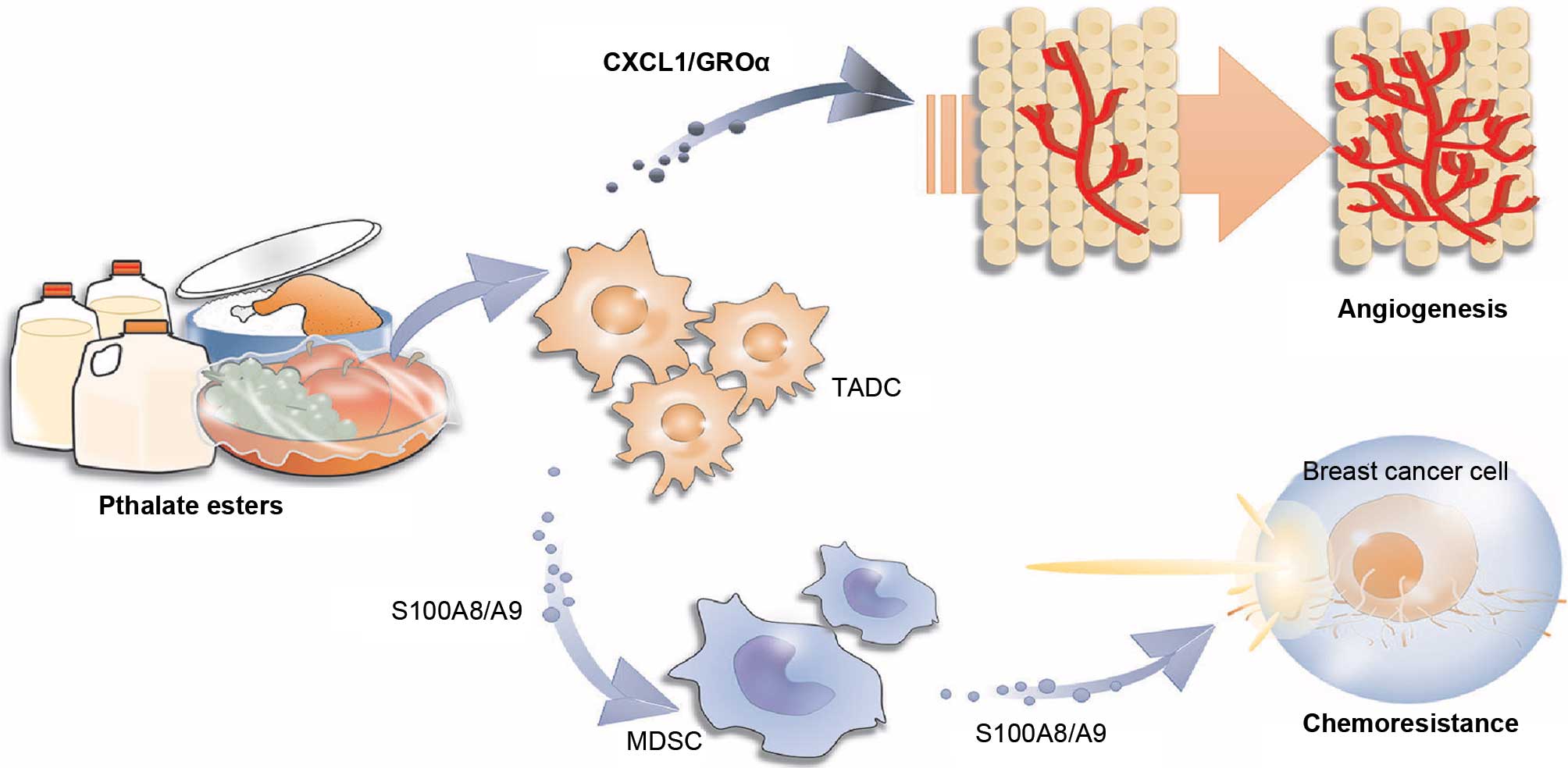
BBP on the chemoresistance of cancer. BBP caused TADCs to produce
S100A8/A9, which directly decreased the sensitivity of breast
cancer cells to doxorubicin/cyclophosphamide treatment. In
addition, BBP also stimulated TADCs to secrete CXCL1/GROα, which
increased the angiogenesis in the tumors, resulting in increased
metastasis of breast cancer. This study raises the possible impact
of BBP on the chemotherapy of breast cancer.
Chronic inflammation is strongly associated with
tumor initiation, progression, angiogenesis and drug resistance
(34,35). Elevated inflammatory factors within
the TME have been reported to mediate chemotherapeutic resistance
in cancers (31,36,37).
Infiltrating immune cells are an abundant component of solid
tumors, and have been implicated as the major source of
inflammatory cytokines/chemokines (35,38).
Our data demonstrated that TADCs decreased the sensitivity of
breast cancer to doxorubicin/cyclophosphamide treatment, and this
effect was further exacerbated by BBP exposure. The chemoresistance
of breast cancer occurred by decreasing
doxorubicin/cyclophosphamide-induced apoptotic cell death, while
cancer cells were nourished by BBP-stimulated TADCs. This study
suggests that paracrine signals potentiated by BBP in the TME
maintain cancer survival despite cytotoxic insults.
S100A8 and S100A9, EF-hand calcium-binding proteins,
are recognized as pro-inflammatory factors, which contribute to
various human diseases, including cancer. S100A8 and S100A9 are
constitutively expressed by myeloid cells, including granulocytes,
monocytes, dendritic cells and osteoclasts, but not by lymphocytes
(39). Increased levels of
S100A8/A9 produced by cancer and stroma cells within the TME are
found in many types of cancers, including gastric, esophageal,
colon, pancreatic, bladder, ovarian, thyroid and breast cancer
(39,40). S100A8 and S100A9 proteins are
considered to contribute to the overall pathogenesis of
malignancies, including tumorigenesis, progression, metastasis and
chemoresistance. S100A8 enhances drug resistance by increasing
autophagy in leukemia cells (41).
S100A8/A9 protect breast cancer cells from doxorubicin by
increasing the activation of ERK1/2 and p70S6K (41). We found that BBP may increase the
concentration of S100A8/A9 by two routes. First, BBP potentiates
the stimulatory effect of breast cancer cells on the expression of
S100A8/A9 in TADCs. Inhibition of S100A8 by siRNA prevents
TADC-CM-mediated chemoresistance, supporting the specificity of the
relationship of S100A8/9 with doxorubicin/cyclophosphamide
resistance in breast cancer. The second source of S100A8/A9 is
tumor-infiltrating MDSCs. BBP not only directly enhanced S100A8/A9
secretion, but also potentiated the hyperactivation of chemotherapy
on the enhancement of S100A8 and S100A9 in tumor-infiltrating
CD11b+Ly6CGr-1+ MDSCs. Elevated S100A9 levels
are found in breast cancer patients after chemotherapy treatment,
and this is considered to be a critical factor contributing to
chemoresistance (41). The multiple
effects of BBP on the expression of S100A8/A9 could provide
survival signaling to breast cancer cells, enabling them to resist
chemotherapy.
CXCL1/GROα is an inflammatory chemokine and potent
angiogenic and lymphangiogenic growth factors, which are mediators
that potentiate cancer progression and chemo-resistance (31,42,43).
Phthalate esters have been indicated to induce macrophages to
express inflammatory cytokine CXCL1/GROα, which is implicated as a
mediator of tumor angiogenesis (43,44).
The present study found that BBP increased the expression of
CXCL1/GROα in TDACs in vitro and in vivo. Knockdown
of CXCL1/GROα by siRNA did not affect either TADC-CM or
BBP-TADC-CM-mediated chemo-resistance, suggesting they are not
directly involved in the protection of cancer from anticancer
drugs. However, blockade of CXCL/GROα by a neutralizing antibody
decreased angiogenesis after doxorubicin/cyclophosphamide treatment
in vivo, suggesting that CXCL1/GROα enhanced the development
of BBP-mediated cancer relapse after chemotherapy by altering tumor
angiogenesis.
This is the first study to explore the influence of
BBP on chemotherapy in breast cancer. BBP stimulated TADCs and
MDSCs to express S100A8/A9, which provided a direct protective
effect against chemotherapy. BBP also increased TADCs to produce
CXCL1/GROα, which enhanced the angiogenesis in breast cancer,
resulting in increased cancer metastasis after chemotherapy
(Fig. 7). This study highlights the
potential interference of phthalate esters on the treatment of
breast cancer with specific therapeutic regimens.
Acknowledgments
This study was supported by grants from the national
Science Council of Taiwan (NSC 10.628-B-037-001-MY3; NSC
101-2320-B- 037- 043-MY3; NSC 102-2628-B-037-002-MY3; NSC
102-2632-B-037-001-MY3; and NSC 102-2314-B-037-035-MY3), the
Ministry of Science and Technology (MOST 103-2320-B-037-006-MY3 and
MOST 103-2314-B-037-052), the Excellence for Cancer Research Center
Grant, the Ministry of Health and Welfare, Executive Yuan, Taipei,
Taiwan (MOHW 103-TD-B-111-05), the Kaohsiung Medical university
'Aim for the Top 500 universities Grant, grant no. KMU-DT103008',
and the Kaohsiung Medical University 'Aim for the Top universities
Grant, grant nos. KMU-TP103A19 and KMU-TP103A20'.
References
|
1
|
Cirillo T, Fasano E, Castaldi E, Montuori
P and Amodio Cocchieri R: Children's exposure to Di(2-ethylhexyl)
phthalate and dibutylphthalate plasticizers from school meals. J
Agric Food Chem. 59:10532–10538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casajuana N and Lacorte S: New methodology
for the determination of phthalate esters, bisphenol A, bisphenol A
diglycidyl ether, and nonylphenol in commercial whole milk samples.
J Agric Food Chem. 52:3702–3707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Z, Zhang X, Wu X, Shen G, Du Q and Mo
C: Uptake of di(2-ethylhexyl) phthalate (DEHP) by the plant
Benincasa hispida and its use for lowering DEHP content of
intercropped vegetables. J Agric Food Chem. 61:5220–5225. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Wang S and Wang L: Development of
rapid determination of 18 phthalate esters in edible vegetable oils
by gas chromatography tandem mass spectrometry. J Agric Food Chem.
61:1160–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan H, Cheng X and Yang G: Dummy
molecularly imprinted solid-phase extraction for selective
determination of five phthalate esters in plastic bottled
functional beverages. J Agric Food Chem. 60:5524–5531. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun H, Yang Y, Li H, Zhang J and Sun N:
Development of multi-residue analysis for twenty phthalate esters
in edible vegetable oils by microwave-assisted extraction-gel
permeation chromatography-solid phase extraction-gas
chromatography-tandem mass spectrometry. J Agric Food Chem.
60:5532–5539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu X and Du Q: Uptake of di-(2-ethylhexyl)
phthalate of vegetables from plastic film greenhouses. J Agric Food
Chem. 59:11585–11588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Y, Zhang Z, Liu L, Li Y, Ren N and
Kannan K: Occurrence and profiles of phthalates in foodstuffs from
China and their implications for human exposure. J Agric Food Chem.
60:6913–6919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cirillo T, Latini G, Castaldi MA, Dipaola
L, Fasano E, Esposito F, Scognamiglio G, Francesco FD and Cobellis
L: Exposure to di-2-ethylhexyl phthalate, di-n-butyl phthalate and
bisphenol A through infant formulas. J Agric Food Chem.
63:3303–3310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaspar FW, Castorina R, Maddalena RL,
Nishioka MG, McKone TE and Bradman A: Phthalate exposure and risk
assessment in California child care facilities. Environ Sci
Technol. 48:7593–7601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Lee Jn,
Chai CY, Wang SC and Tsai EM: Phthalates induce proliferation and
invasiveness of estrogen receptor-negative breast cancer through
the AhR/HDAC6/c-Myc signaling pathway. FASEB J. 26:778–787. 2012.
View Article : Google Scholar
|
|
12
|
Carran M and Shaw IC: New Zealand Malayan
war veterans' exposure to dibutylphthalate is associated with an
increased incidence of cryptorchidism, hypospadias and breast
cancer in their children. NZ Med J. 125:52–63. 2012.
|
|
13
|
Fernandez SV and Russo J: Estrogen and
xenoestrogens in breast cancer. Toxicol Pathol. 38:110–122. 2010.
View Article : Google Scholar :
|
|
14
|
Chen FP and Chien MH: Lower concentrations
of phthalates induce proliferation in human breast cancer cells.
Climacteric. 17:377–384. 2014. View Article : Google Scholar
|
|
15
|
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Hsi E,
Suen JL, Hung CH, Lee JN, Chai CY, Wang SC, et al: n-Butyl benzyl
phthalate promotes breast cancer progression by inducing expression
of lymphoid enhancer factor 1. PLoS One. 7:e427502012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Lee JN,
Chai CY, Hou MF, Chang CC, Long CY, Ko YC, et al: Phthalates
stimulate the epithelial to mesenchymal transition through an
HDAC6-dependent mechanism in human breast epithelial stem cells.
Toxicol Sci. 128:365–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
19
|
Roché H and Vahdat LT: Treatment of
metastatic breast cancer: Second line and beyond. Ann Oncol.
22:1000–1010. 2011. View Article : Google Scholar
|
|
20
|
Hu G, Chong RA, Yang Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poor-prognosis breast cancer. Cancer Cell. 15:9–20. 2009.
View Article : Google Scholar :
|
|
21
|
Klemm F and Joyce JA: Microenvironmental
regulation of therapeutic response in cancer. Trends Cell Biol.
25:198–213. 2015. View Article : Google Scholar
|
|
22
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weizman N, Krelin Y, Shabtay-Orbach A,
Amit M, Binenbaum Y, Wong RJ and Gil Z: Macrophages mediate
gemcitabine resistance of pancreatic adenocarcinoma by upregulating
cytidine deaminase. Oncogene. 33:3812–3819. 2014. View Article : Google Scholar
|
|
24
|
Mitchem JB, Brennan DJ, Knolhoff BL, Belt
BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L,
Piwnica-Worms D, et al: Targeting tumor-infiltrating macro phages
decreases tumor-initiating cells, relieves immunosuppression, and
improves chemotherapeutic responses. Cancer Res. 73:1128–1141.
2013. View Article : Google Scholar :
|
|
25
|
Hsu YL, Hung JY, Tsai YM, Tsai EM, Huang
MS, Hou MF and Kuo PL: 6-shogaol, an active constituent of dietary
ginger, impairs cancer development and lung metastasis by
inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in
tumor-associated dendritic cells. J Agric Food Chem. 63:1730–1738.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kan JY, Wu DC, Yu FJ, Wu CY, Ho YW, Chiu
YJ, Jian SF, Hung JY, Wang JY and Kuo PL: Chemokine (C-C motif)
ligand 5 is involved in tumor-associated dendritic cell-mediated
colon cancer progression through non-coding RNA MALAT-1. J Cell
Physiol. 230:1883–1894. 2015. View Article : Google Scholar
|
|
27
|
Kuo PL, Huang MS, Cheng DE, Hung JY, Yang
CJ and Chou SH: Lung cancer-derived galectin-1 enhances tumorigenic
potentiation of tumor-associated dendritic cells by expressing
heparin-binding EGF-like growth factor. J Biol Chem. 287:9753–9764.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang XH, Deng S, Li M and Lu MS: The
anti-tumor effect of cross-reacting material 197, an inhibitor of
heparin-binding EGF-like growth factor, in human resistant ovarian
cancer. Biochem Biophys Res Commun. 422:676–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian DZ, Rademacher BL, Pittsenbarger J,
Huang CY, Myrthue A, Higano CS, Garzotto M, Nelson PS and Beer TM:
CCL2 is induced by chemotherapy and protects prostate cancer cells
from docetaxel-induced cytotoxicity. Prostate. 70:433–442.
2010.
|
|
30
|
Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey
NB and Popel AS: Inhibition of breast cancer growth and metastasis
by a biomimetic peptide. Sci Rep. 4:71392014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Acharyya S, Oskarsson T, Vanharanta S,
Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N,
Seshan VE, et al: A CXCL1 paracrine network links cancer
chemoresistance and metastasis. Cell. 150:165–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Chang EWY, Wong SC, Ong SM, Chong
DQY and Ling KL: Increased myeloid-derived suppressor cells in
gastric cancer correlate with cancer stage and plasma S100A8/A9
proinflammatory proteins. J Immunol. 190:794–804. 2013. View Article : Google Scholar
|
|
33
|
Miyake M, Goodison S, Urquidi V, Gomes
Giacoia E and Rosser CJ: Expression of CXCL1 in human endothelial
cells induces angiogenesis through the CXCR2 receptor and the
ERK1/2 and EGF pathways. Lab Invest. 93:768–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Palucka K, Coussens LM and O'Shaughnessy
J: Dendritic cells, inflammation, and breast cancer. Cancer J.
19:511–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coussens LM, Zitvogel L and Palucka AK:
Neutralizing tumor-promoting chronic inflammation: A magic bullet?
Science. 339:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Angst E, Reber HA, Hines OJ and Eibl G:
Mononuclear cell-derived interleukin-1 beta confers chemoresistance
in pancreatic cancer cells by upregulation of cyclooxygenase-2.
Surgery. 144:57–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen R, Alvero AB, Silasi DA and Mor G:
Inflammation, cancer and chemoresistance: Taking advantage of the
toll-like receptor signaling pathway. Am J Reprod Immunol.
57:93–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Srikrishna G: S100A8 and S100A9: New
insights into their roles in malignancy. J Innate Immun. 4:31–40.
2012. View Article : Google Scholar :
|
|
40
|
Ichikawa M, Williams R, Wang L, Vogl T and
Srikrishna G: S100A8/A9 activate key genes and pathways in colon
tumor progression. Mol Cancer Res. 9:133–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang M, Zeng P, Kang R, Yu Y, Yang L, Tang
D and Cao L: S100A8 contributes to drug resistance by promoting
autophagy in leukemia cells. PLoS One. 9:e972422014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zou A, Lambert D, Yeh H, Yasukawa K,
Behbod F, Fan F and Cheng N: Elevated CXCL1 expression in breast
cancer stroma predicts poor prognosis and is inversely associated
with expression of TGF-β signaling proteins. BMC Cancer.
14:7812014. View Article : Google Scholar
|
|
43
|
Pecot CV, Rupaimoole R, Yang D, Akbani R,
Ivan C, Lu C, Wu S, Han HD, Shah MY, Rodriguez-Aguayo C, et al:
Tumour angiogenesis regulation by the miR-200 family. Nat Commun.
4:24272013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishioka J, Iwahara C, Kawasaki M,
Yoshizaki F, Nakayama H, Takamori K, Ogawa H and Iwabuchi K:
Di-(2-ethylhexyl) phthalate induces production of inflammatory
molecules in human macrophages. Inflamm Res. 61:69–78. 2012.
View Article : Google Scholar
|