Introduction
Colorectal cancer (CRC) is one of the most common
cancers in the world and the third leading cause of cancer-related
death in both males and females (1). It was estimated that there were more
than 1.4 million CRC cases and 693,900 deaths in 2012 worldwide
(2). Smoking, red/processed meat
consumption, obesity and excessive alcohol consumption are the
modifiable risk factors for CRC (3). With a reduction in smoking and other
modifiable risk factors, along with CRC screening and research into
the molecular pathological epidemiology of CRC, decreased CRC
mortality rates have been observed (4,5).
However, the death rate is still high, and the molecular
pathological epidemiology of CRC is not completely understood, thus
further extensive investigations are required.
MicroRNAs (miRNAs) are 18–23-nucleotide small RNAs
that function as negative regulators of gene expression through
binding the 3′ untranslated region (3′UTR) of mRNAs (6). Since evidence of the involvement of
miR-15 and miR-16 in leukemia was initially reported, researchers
have investigated the important roles of miRNAs in cancer (7,8).
Subsequently, more and more miRNAs have been found to be involved
in the development of human tumors (9). miRNAs can regulate cell proliferation,
apoptosis and carcinogenesis by targeting related mRNAs in cancer
(10). In CRC, eight miRNAs,
including miR-25, miR-345, miR-7 and miR-331-3p, have been found to
be significantly and differentially expressed when compared to the
levels in healthy tissues (11).
miR-331-3p is a member of the miR-331 family,
located on 12q22n, and has been shown to be a tumor-suppressor
miRNA in the prostate (12).
Aberrant expression of miR-331-3p has been observed and found to be
associated with proliferation and migration in lymphocytic leukemia
(13), lung cancer (14), glioblastoma (15), gastric cancer (16) and prostate cancer (17). However, the role of miR-331-3p in
the tumorigenesis of CRC remains unknown (18).
The human epidermal growth factor receptor (EGFR)
family, which consists of EGFR (HER1 or ErbB1), HER2 (HER2/neu or
ErbB2), HER3 (ErbB3) and HER4 (ErbB4), plays an important role in
regulating cell proliferation, survival, and differentiation in
cancers (19,20). HER2 is an important regulator of
EGFR family signaling (21,22). Overexpression of HER2 is found in
~30% of all breast cancers and is frequently associated with poor
prognosis, greater invasiveness and higher apoptotic resistance
potential (23–26). It has been found that HER2 is a key
factor in CRC, and cytoplasmic HER2 is overexpressed in almost 30%
of CRC patients (27). However, the
prognostic value of HER-2 expression remains controversial in CRC.
Thus, the present study was designed to validate the potential
function of miR-331-3p in CRC.
Materials and methods
Human tissue samples
Human colon cancer tissue samples and normal
adjacent colon tissues were obtained from 29 colon cancer patients
before any therapeutic intervention at the First Affiliated
Hospital of Xi'an Jiaotong University. All patients provided
informed written consent. Samples were selected and stored in
liquid nitrogen immediately after surgical resection. The study was
approved by the Human Research Ethics Committee of the
hospital.
Cell culture
Human colon cancer cell lines HCT-116, LoVo, HT-29,
SW480, DLD-1 and Caco2 and the human normal colon epithelial cell
line CRL-1831 (American Type Culture Collection, ATCC, Manassas,
VA, USA) were grown in RPMI-1640 medium containing 5% fetal bovine
serum (FBS) (both from Gibco-BRL, Gaithersburg, MD, USA), 1%
penicillin and streptomycin (Sigma-Aldrich, Castle Hill, NSW,
Australia), and maintained in a humidified incubator at 37°C with
5% CO2.
Real-time quantitative polymerase chain
reaction (RT-qPCR)
MicroRNAs were isolated from the tissue samples and
cells using the miRNeasy Mini kit (Qiagen, Valencia, CA, USA). One
Step PrimeScript miRNA cDNA Synthesis kit (Takara Biotechnology,
Dalian, China) was used to synthesis cDNA. Total RNA was extracted
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
reverse-transcribed into cDNA using M-MLV reverse transcriptase
(Clontech, Palo Alto, CA, USA) according to the standard protocol.
RT-qPCR was performed using SYBR Green qPCR Master Mix (Thermo
Fisher, Shanghai, China). U6 (RiBoBio, Guangzhou, China) or β-actin
was used as the normalizer for miRNA or mRNA, respectively. The
data obtained were assessed using the 2−ΔΔCt method and
evaluated by statistical analysis as described previously (28).
Plasmid vectors and transfection
HCT-116 cells were transfected with the miR-331-3p
precursor (pre-miR-331-3p) or negative control RNA oligonucleotides
(pre-miR-control) (both from Ambion Corporation, Austin, TX, USA);
the miRNA inhibitor of miR-331-3p (AS-miR-331-3p) or the negative
control (AS-miR-control); and HER2-siRNA (29) (sense, 5′-GGUGAAGGUGCUUGGAUCUUU-3′
and antisense, 5′-AGAUCCAAGCACCUUCACCUU-3′) or control siRNA using
Lipofectamine 2000 according to the manufacturer's procedure
(Invitrogen) and cultured for 48 h. The expression levels of
miR-331-3p and HER2 in the HCT-116 cell line were assayed by
RT-qPCR 48 h after transfection.
Caspase-3 activity assay
A caspase-3 assay kit (Abcam, Cambridge, MA, USA)
was used to measure the enzymatic activity of caspase-3. HCT-116
cells transfected with pre-miR-331-3p or AS-miR-331-3p were seeded
in a 24-well plate and cultured for 48 h. The cells were then
harvested and the enzymatic activity of caspase-3 was detected
according to the manufacturer's protocol.
Cell proliferation assay
HCT-116 cells transfected with pre-miR-331-3p or
AS-miR-331-3p or HER2-siRNA or HER2-siRNA and AS-miR-331-3p were
seeded in a 96-well plate. Twenty-four, 48, 72 and 96 h later, the
medium was replaced by fresh medium, and 20 µl MTT (5 mg/ml)
was added in each well and incubated for 4 h. Then, the medium was
removed, and 200 µl DMSO was added. The OD490 was read after
shaking for 10 min to fully dissolve the crystals.
Cell apoptosis assay
HCT-116 cells transfected with pre-miR-331-3p or
AS-miR-331-3p or HER2-siRNA or HER2-siRNA and AS-miR-331-3p were
seeded in 24-well plates and cultured for 48 h. Cells were
harvested and the apoptotic cells were evaluated using the
FITC-Annexin V apoptosis detection kit (BD Biosciences, Piscataway,
NJ, USA) according to the manufacturer's instructions.
Western blot analysis
HCT-116 cells transfected with pre-miR-331-3p or
AS-miR-331-3p were seeded in a 6-well plate and cultured for 48 h.
Cells were harvested and the total protein was extracted using
mammalian protein extraction reagent (Pierce, Rockford, IL, USA)
supplemented with a protease inhibitor cocktail (Sigma, St. Louis,
MO, USA). After measuring the concentration, the proteins were
separated using 10% SDS-PAGE and then transferred onto a
nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membranes
were blocked in 5% (v/v) dried milk and incubated with anti-HER2
(Abcam), anti-Bax, anti-Bcl-2, anti-Akt, anti-p-Akt, anti-ERK1/2
and anti-p-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
at 4°C overnight. The secondary HRP-conjugated goat anti-mouse IgG
antibody (Santa Cruz Biotechnology) was incubated for 1 h. β-actin
(Cell Signaling Technology, Danvers, MA, USA) was used as the
reference protein.
Luciferase activity assay
The human HER2 wild-type 3′UTR containing the
miR-331-3p binding site and mutated HER2-3′UTR sequence (18) were constructed into the pGL3
luciferase reporter plasmid (Promega, Madison, WI, USA). HCT-116
cells were plated in 24-well plates and co-transfected with 10 nM
of either pre-miR 331-3p or the pre-miR-control, and 500 ng of
pGL3-HER2 or pGL3-mutHER2 according to the manufacturers' protocols
for the use of Lipofectamine (Invitrogen) and the luciferase assay
kit (Promega) (16). Cells were
collected and cell lysates were assayed for luciferase activity
using a dual-luciferase reporter assay kit (Promega) 48 h after
transfection.
Statistical analysis
Results are presented as the mean ± standard
deviation. One-way analysis of variance and the Student's t-test
were used to analyze differences between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-331-3p is downregulated in human
colon cancer tissues and cells
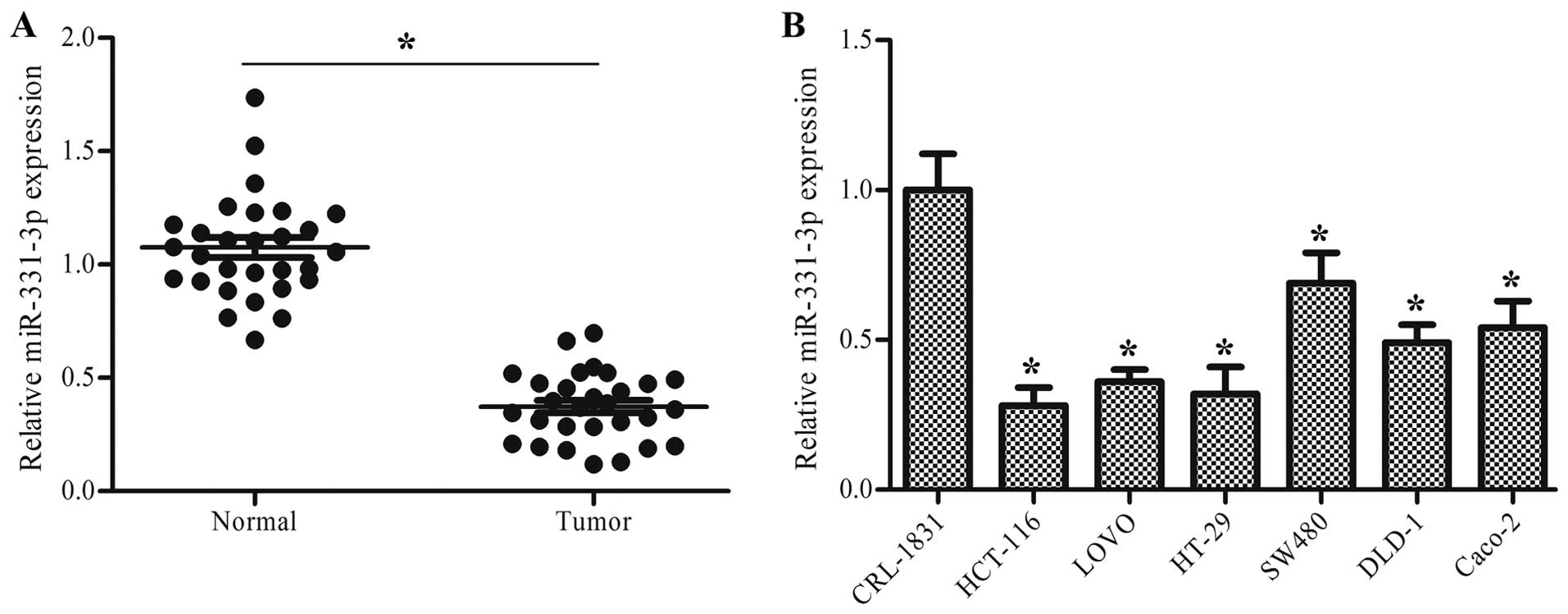
To explore the role of miR-331-3p in human colon
cancer, the expression level of miR-331-3p in human colon cancer
tissues and cells was measured using RT-qPCR. The results showed
that the expression level of miR-331-3p was significantly
downregulated in the colon cancer tissues compared to this level in
the normal tissues (P<0.05, Fig.
1A). Compared with the normal colon epithelial cell line
CRL-1831, the expression level of miR-331-3p was significantly
lower in all of the colon cancer cell lines, including HCT-116,
LoVo, HT-29, SW480, DLD-1 and Caco2 (P<0.05, Fig. 1B).
miR-331-3p inhibits colon cancer cell
proliferation
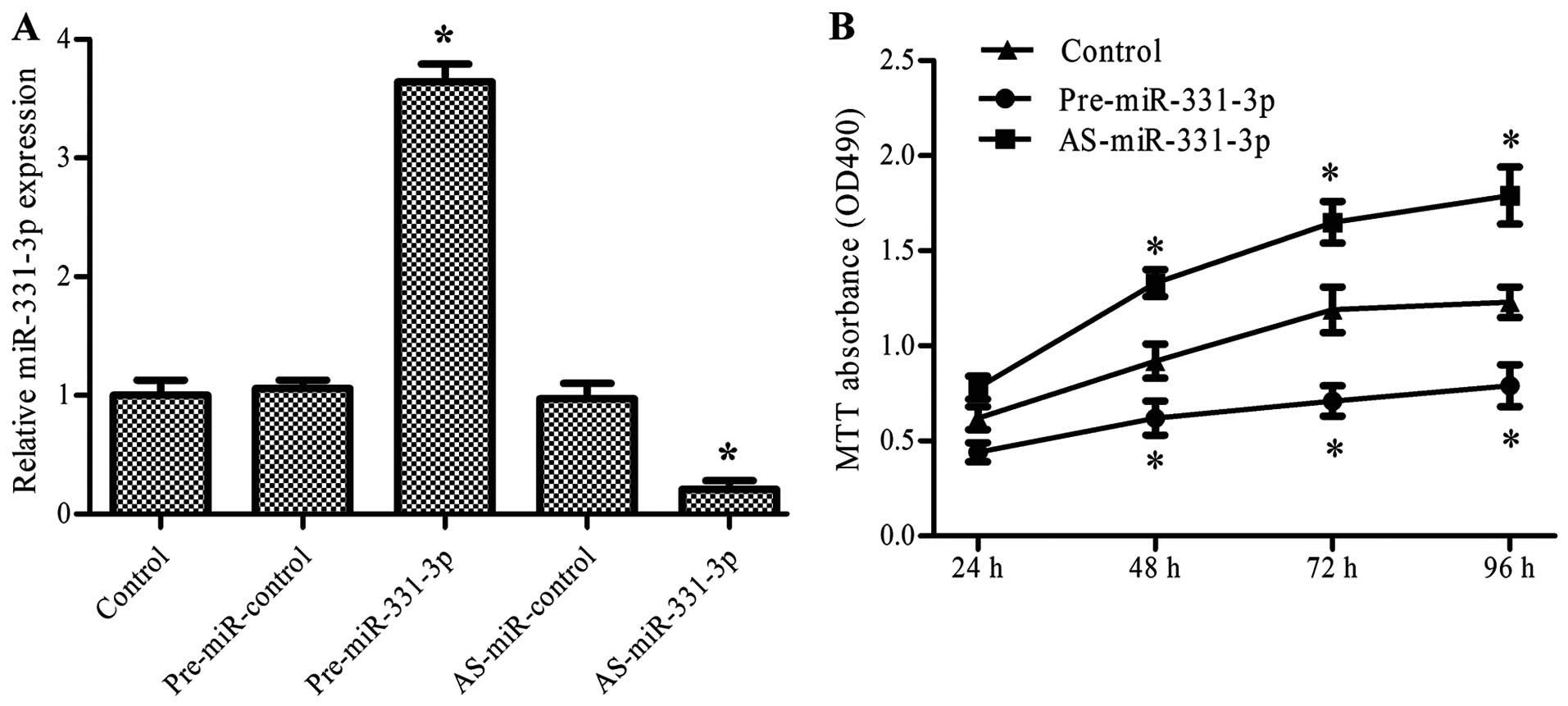
To verify the effect of miR-331-3p on the growth of
colon cancer, we examined the impact of miR-331-3p overexpression
and suppression on the proliferation of colon cancer cells. HCT-116
cells were transfected with pre-miR-331-3p or AS-miR-331-3p and
cultured for various time periods. The MTT assay was then used to
evaluate the proliferation of the colon cancer cells. The results
revealed that pre-miR-331-3p significantly upregulated and
AS-miR-331-3p downregulated the expression of miR-331-3p
(P<0.05, Fig. 2A). The MTT assay
showed that pre-miR-331-3p inhibited and AS-miR-331-3p promoted the
proliferation of the HCT-116 cells in a time-dependent manner
(P<0.05, Fig. 2B).
miR-331-3p promotes the apoptosis of the
colon cancer cells
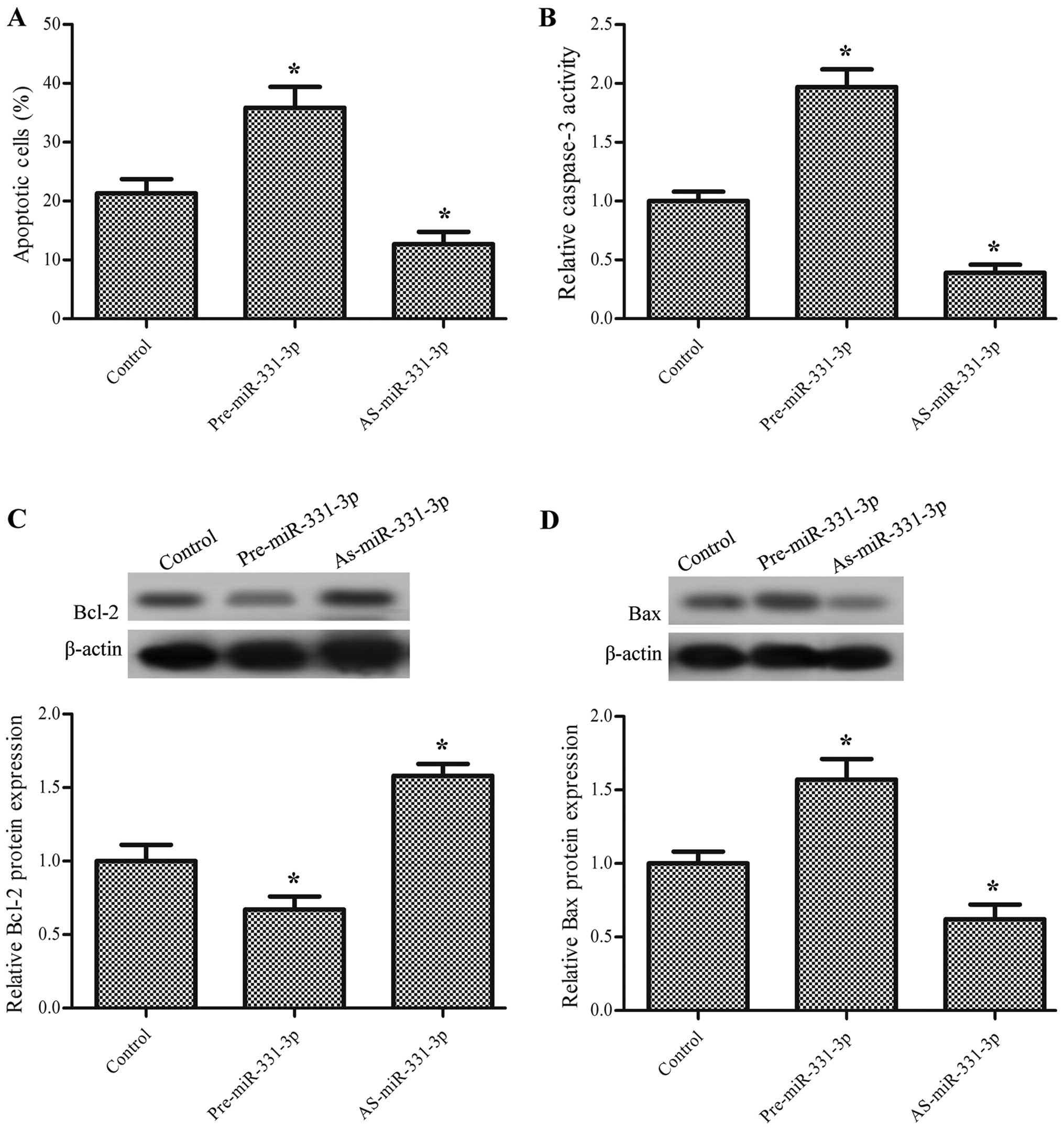
To further confirm the effect of miR-331-3p on the
tumorigenesis of colon cancer, the apoptotic ratio of the HCT-116
cells transfected with pre-miR-331-3p or AS-miR-331-3p was examined
by FITC-Annexin V staining. The results show that overexpression of
miR-331-3p significantly promoted apoptosis while suppression of
miR-331-3p inhibited apoptosis (P<0.05, Fig. 3A). To further study the effect of
miR-331-3p on colon cancer cell apoptosis, the activity of
caspase-3 was also determined. As expected, pre-miR-331-3p
significantly increased the activity of caspase-3 and AS-miR-331-3p
had the opposite effect (P<0.05, Fig. 3B). Additionally, the protein
expression levels of the apoptosis-related proteins Bcl-2 and Bax
were also measured; the results demonstrated that pre-miR-331-3p
decreased the protein expression level of Bcl-2 and increased the
protein expression level of Bax, while AS-miR-331-3p increased the
protein expression level of Bcl-2 and decreased the protein
expression level of Bax (P<0.05, Fig. 3C and D).
miR-331-3p directly downregulates the
expression of HER2 in colon cancer cells
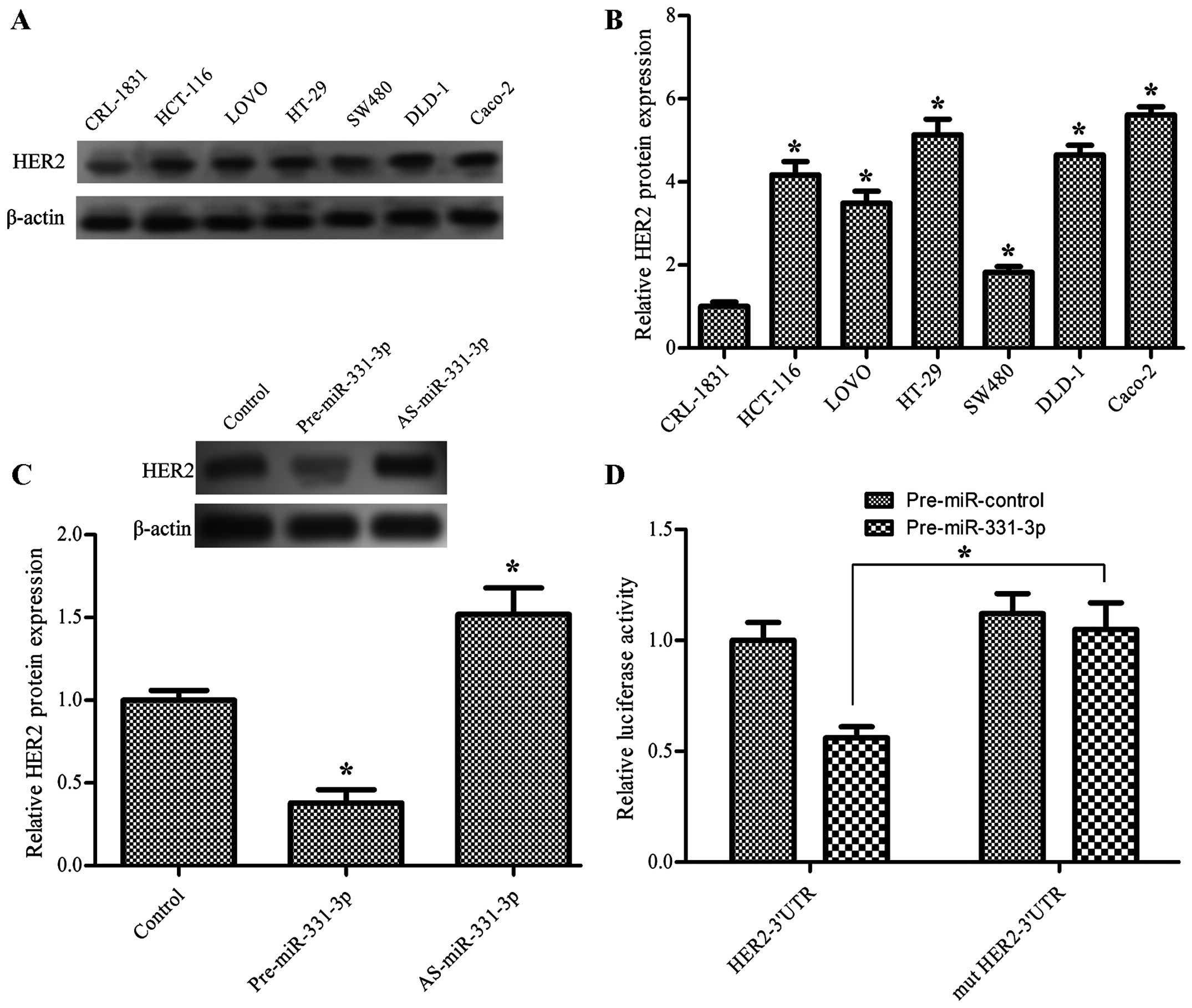
The expression level of HER2 in the human colon
cancer cells was significantly higher than that in the normal
cells, which was inversely related to the expression of miR-331-3p
(P<0.05, Fig. 4A and B). Next,
we explored the relationship between miR-331-3p and HER2 by
transfecting cells with pre-miR-331-3p and AS-miR-331-3p. The
western blot analysis shows that pre-miR-331-3p significantly
downregulated and AS-miR-331-3p significantly upregulated the
protein expression level of HER2 in the HCT-116 cells (P<0.05,
Fig. 4C), suggesting that
miR-331-3p downregulates the expression of HER2 in colon cancer
cells. To examine whether HER2 is a direct target of miR-331-3p in
colon cancer cells, the human HER2 wild-type 3′UTR containing the
miR-331-3p binding site and mutated HER2 3′UTR sequence were cloned
into modified pGL-3 luciferase reporter vectors, which were
co-transfected into the HCT-116 cells with pre-miR-331-3p and
AS-miR-331-3p. The results showed that miR-331-3p overexpression
significantly reduced the luciferase reporter activity in the
pGL3-HER2-3′UTR transfected cells, compared to pGL3-mut HER-3′UTR,
whereas the luciferase activity was not affected by the
pre-miR-control (P<0.05, Fig.
4D).
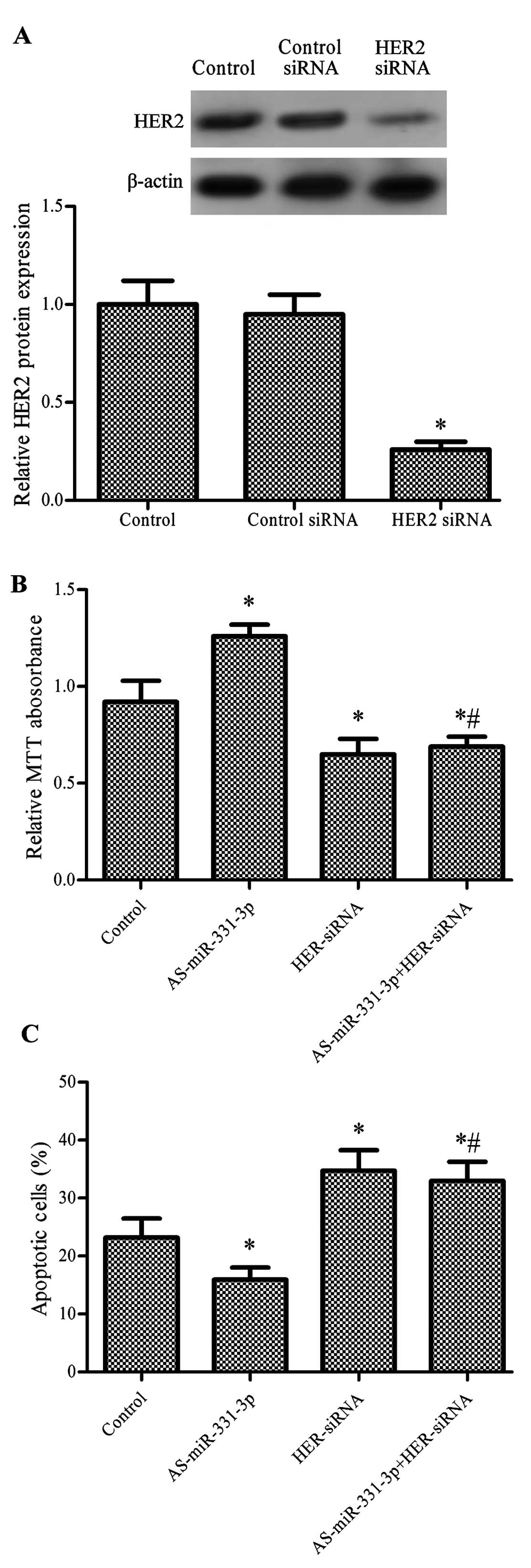
miR-331-3p exerts its function by
targeting HER2
HER2 plays an important role in cell proliferation
and cell survival during the development of cancer (30). The results of RT-qPCR in our study
showed that HER2-siRNA significantly downregulated the protein
expression level of HER2 in the HCT-116 cells (P<0.05, Fig. 5A). HCT-116 cells transfected with
AS-miR-331-3p exhibited significantly increased cell proliferation,
while cells transfected with HER2-siRNA and co-transfected with
AS-miR-331-3p showed significantly decreased proliferation compared
with the control. However, there was no statistical difference
between cells transfected with both AS-miR-331-3p and HER2-siRNA
and treatment with HER2-siRNA alone (P<0.05, Fig. 5B). The results of the FITC-Annexin V
assay showed that pre-miR-331-3p reduced apoptosis, while
HER2-siRNA and the combination of HER2-siRNA and pre-miR-331-3p
promoted apoptosis (P<0.05, Fig.
5C).
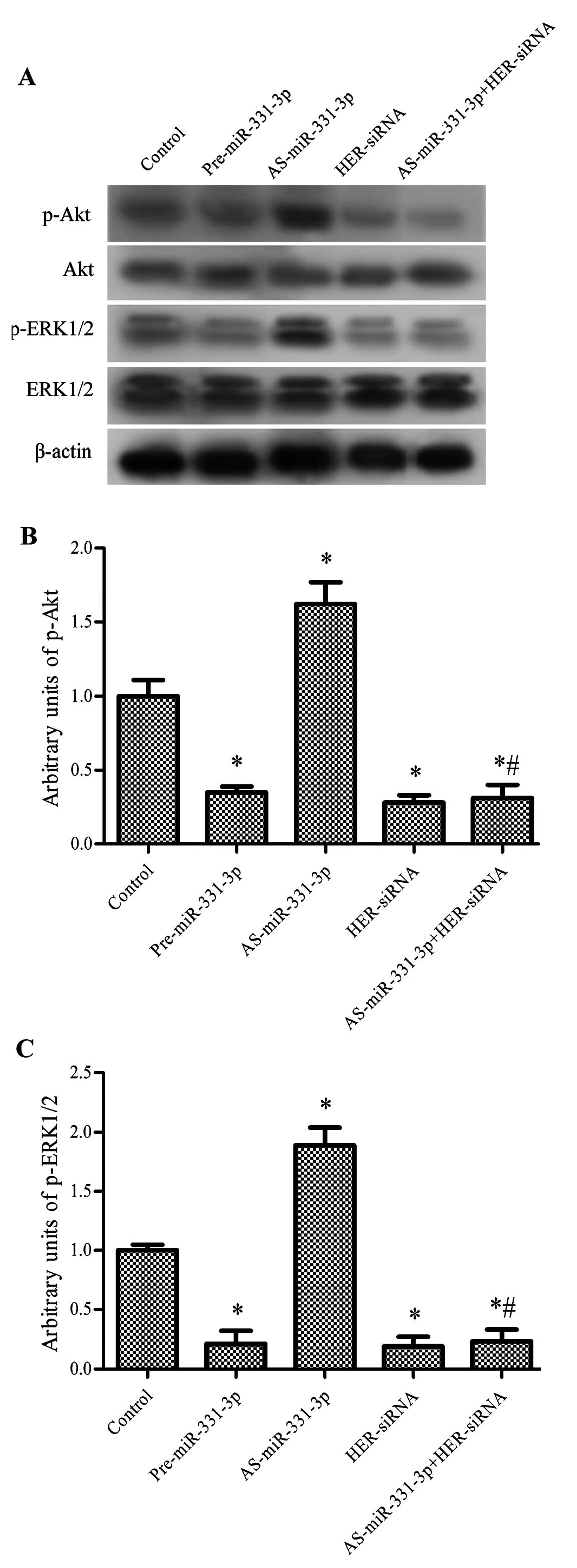
miR-331-3p suppresses the PI3K/Akt and
ERK1/2 signaling pathways
Since HER2 plays important roles in mediating
activation of the PI3K/Akt and ERK1/2 pathways (31,32),
we aimed to ascertain whether miR-331-3p triggers suppression of
HER2, caused by the regulation of PI3K/Akt and ERK1/2 signaling in
human CRC cells. As expected, transfection of pre-miR-331-3p
significantly inhibited the ratios of the levels of phosphorylated
to total protein (arbitrary units) of Akt and ERK1/2, which were
both increased by AS-miR-331-3p. HER2-siRNA significantly decreased
the arbitrary units of p-Akt and p-ERK1/2. After treatment with
HER2-siRNA, the activating effect of AS-miR-331-3p on the arbitrary
units of p-Akt and p-ERK1/2 was abolished and the arbitrary units
of p-Akt and p-ERK1/2 were significantly lower than these values in
the control (P<0.05, Fig.
6).
Discussion
In the present study, we provide evidence that
miR-331-3p is downregulated in CRC tissues and cells.
Overexpression of miR-331-3p plays an important role in inhibiting
proliferation and promoting apoptosis by inhibiting the expression
of HER2 and deactivation of the PI3K/Akt and ERK1/2 signaling
pathways in CRC cells. These results suggest an important role of
miR-331-3p in regulating tumor progression of CRC.
miRNAs function as oncogenes or tumor suppressors in
carcinogenesis, and dysregulation of these miRNAs is believed to be
a common feature of human cancers (33). miRNAs are generally overexpressed or
suppressed in tumor tissues, compared with corresponding healthy
tissues (34,35). Increasing evidence has demonstrated
that miRNAs are crucial regulators of the progression of cancer,
with potential use in cancer diagnosis, prognosis, prediction, and
therapy (9,36). miR-331-3p is regarded as a
cancer-associated miRNA as studies have reported its correlation
with prostate cancer, hepatocellular carcinoma, and gastric cancer
(15,16,37).
In a study by Chang et al (38) on hepatocellular carcinoma,
miR-331-3p was overexpressed in human hepatocellular carcinoma
tissues and was found to be correlated with poor long-term
survival. These authors also demonstrated that miR-331-3p can
promote the proliferation and metastasis of hepatocellular
carcinoma. Based on these results, they suggested that miR-331-3p
may be a potential prognostic biomarker and a novel therapeutic
target. Our study found that miR-331-3p was suppressed both in CRC
tissues and cells compared with healthy colorectal tissues and
cells, which is consistent with the research of Guo et al
(16) who found that miR-331-3p was
downregulated in gastric cancer cell lines. To further understand
the role of miR-331-3p in CRC, the miR-331-3p precursor or miRNA
inhibitor of miR-331-3p was used to upregulate or downregulate the
expression of miR-331-3p, and then cell proliferation and apoptosis
were assessed. The results showed that overexpression of miR-331-3p
significantly inhibited cell proliferation and promoted apoptosis,
suggested a crucial role of miR-331-3p in the development of CRC.
Together with these results, we confer that the role of miR-331-3p
is inconsistent in different types of cancers.
HER2 is overexpressed in a high percentage of CRC
cell lines and has been shown to play an oncogenic role in human
tumors (39). HER2 can activate
signaling pathways including ERK1/2, STAT3, mTOR and Akt, which
play important roles in cell proliferation and survival (31,40,41),
and therefore is crucial to the development of cancer. Ross and
McKenna (42) showed that
overexpression of HER2 is associated with approximately one fourth
of all gastrointestinal tract malignancies. Li et al
(29) found that the expression
level of HER2 was elevated in seven out of eight CRC cell lines.
Our study measured the expression level of HER2 in six frequently
used CRC cell lines, and all showed upregulated expression of HER2,
which suggests that HER2 may be an important factor in the
development of CRC.
miRNAs regulate a variety of cellular pathways
through targeting the expression of multiple target genes (6). Several lines of evidence indicate that
E2F1 and neuropilin-2 are miR-331-3p targets (15,16).
In the present study, we showed that miR-331-3p regulated cell
proliferation by inhibiting the expression of HER2 in CRC, which is
consistent with a study by Epis et al (18), who also concluded that miR-331-3p
has the capacity to regulate the development and progression of
prostate cancer cells by targeting HER2. PI3K/Akt and ERK1/2 are
two important downstream pathways of HER2, and link HER2 to its
biological functions (43). While
in the study by Epis et al, only the PI3K/Akt signaling
pathway was discussed, our study focused on both the PI3K/Akt and
ERK1/2 signaling pathways. The results showed that Akt and ERK1/2
were both deactivated by HER2-siRNA and pre-miR-331-3p, and were
activated by AS-miR-331-3p. Further research found that the
activity of Akt and ERK1/2 were downregulated by the combined
action of HER2 and miR-331-3p. PI3K/Akt and ERK1/2 are crucial
pathways in regulating cell survival in cancer. These results,
taken together, showed that miR-331-3p inhibits proliferation and
promotes apoptosis by suppressing HER2 and deactivating the
PI3K/Akt and ERK1/2 signaling pathways.
In conclusion, our study demonstrated that
miR-331-3p is suppressed in CRC and overexpression of miR-331-3p
inhibits cell proliferation and induces apoptosis by targeting HER2
via activation of the PI3K/Akt and ERK1/2 signaling pathways. Our
findings suggest that miR-331-3p plays an important role in the
development and progression of CRC.
Acknowledgments
The present study was supported by grants from the
Key Science and Technology Program of Shaanxi Province (no.
2013k12-03-14) and Science and Technology Plan Projects of Xian
[SF1203(2)].
Abbreviations:
|
miRNAs
|
microRNAs
|
|
CRC
|
colorectal cancer
|
|
3′UTR
|
3′ untranslated region
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrari P, Jenab M, Norat T, Moskal A,
Slimani N, Olsen A, Tjønneland A, Overvad K, Jensen MK,
Boutron-Ruault MC, et al: Lifetime and baseline alcohol intake and
risk of colon and rectal cancers in the European prospective
investigation into cancer and nutrition (EPIC). Int J Cancer.
121:2065–2072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar
|
|
5
|
Bosetti C, Levi F, Rosato V, Bertuccio P,
Lucchini F, Negri E and La Vecchia C: Recent trends in colorectal
cancer mortality in Europe. Int J Cancer. 129:180–191. 2011.
View Article : Google Scholar
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and downregulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
8
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh SR and Rameshwar P: MicroRNA in
Development and in the Progression of Cancer. Springer; New York,
NY: 2014, View Article : Google Scholar
|
|
11
|
Wang S, Xiang J, Li Z, Lu S, Hu J, Gao X,
Yu L, Wang L, Wang J, Wu Y, et al: A plasma microRNA panel for
early detection of colorectal cancer. Int J Cancer. 136:152–161.
2015. View Article : Google Scholar
|
|
12
|
Wang L, Tang H, Thayanithy V, Subramanian
S, Oberg AL, Cunningham JM, Cerhan JR, Steer CJ and Thibodeau SN:
Gene networks and microRNAs implicated in aggressive prostate
cancer. Cancer Res. 69:9490–9497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zanette DL, Rivadavia F, Molfetta GA,
Barbuzano FG, Proto-Siqueira R, Silva-Jr WA, Falcão RP and Zago MA:
miRNA expression profiles in chronic lymphocytic and acute
lymphocytic leukemia. Braz J Med Biol Res. 40:1435–1440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosomes. Cancer. 50:585–597. 2011.
|
|
15
|
Epis MR, Giles KM, Candy PA, Webster RJ
and Leedman PJ: miR-331-3p regulates expression of neuropilin-2 in
glioblastoma. J Neurooncol. 116:67–75. 2014. View Article : Google Scholar :
|
|
16
|
Guo X, Guo L, Ji J, Zhang J, Zhang J, Chen
X, Cai Q, Li J, Gu Q, Liu B, et al: miRNA-331-3p directly targets
E2F1 and induces growth arrest in human gastric cancer. Biochem
Biophys Res Commun. 398:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Epis MR, Giles KM, Kalinowski FC, Barker
A, Cohen RJ and Leedman PJ: Regulation of expression of
deoxyhypusine hydroxylase (DOHH), the enzyme that catalyzes the
activation of eIF5A, by miR-331-3p and miR-642-5p in prostate
cancer cells. J Biol Chem. 287:35251–35259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Epis MR, Giles KM, Barker A, Kendrick TS
and Leedman PJ: miR-331-3p regulates ERBB-2 expression and androgen
receptor signaling in prostate cancer. J Biol Chem.
284:24696–24704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kruser TJ and Wheeler DL: Mechanisms of
resistance to HER family targeting antibodies. Exp Cell Res.
316:1083–1100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mass RD: The HER receptor family: A rich
target for therapeutic development. Int J Radiat Oncol Biol Phys.
58:932–940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holbro T and Hynes NE: ErbB receptors:
Directing key signaling networks throughout life. Annu Rev
Pharmacol Toxicol. 44:195–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marmor MD, Skaria KB and Yarden Y: Signal
transduction and oncogenesis by ErbB/HER receptors. Int J Radiat
Oncol Biol Phys. 58:903–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar
|
|
25
|
Tan M, Yao J and Yu D: Overexpression of
the c-erbB-2 gene enhanced intrinsic metastasis potential in human
breast cancer cells without increasing their transformation
abilities. Cancer Res. 57:1199–1205. 1997.PubMed/NCBI
|
|
26
|
Yu D, Jing T, Liu B, Yao J, Tan M,
McDonnell TJ and Hung MC: Overexpression of ErbB2 blocks
Taxol-induced apoptosis by upregulation of p21Cip1,
which inhibits p34Cdc2 kinase. Mol Cell. 2:581–591.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blok EJ, Kuppen PJ, van Leeuwen JE and
Sier CF: Cytoplasmic overexpression of HER2: A key factor in
colorectal cancer. Clin Med Insights Oncol. 7:41–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li SS, Buchbinder E, Wu L, Bjorge JD,
Fujita DJ and Zhu S: EGFR and HER2 levels are frequently elevated
in colon cancer cells. Discoveries Rep. 1:e12014. View Article : Google Scholar
|
|
30
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nahta R and O'Regan RM: Evolving
strategies for overcoming resistance to HER2-directed therapy:
Targeting the PI3K/Akt/mTOR pathway. Clin Breast Cancer. 10(Suppl
3): S72–S78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Citri A and Yarden Y: EGF-ERBB signalling:
Towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
35
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Epis MR, Barker A, Giles KM, Beveridge DJ
and Leedman PJ: The RNA-binding protein HuR opposes the repression
of ERBB-2 gene expression by microRNA miR-331-3p in prostate cancer
cells. J Biol Chem. 286:41442–41454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang RM, Yang H, Fang F, Xu JF and Yang
LY: MicroRNA-331-3p promotes proliferation and metastasis of
hepatocellular carcinoma by targeting PH domain and leucine-rich
repeat protein phosphatase. Hepatology. 60:1251–1263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kafi SG, Lari S and Nassiri G: HER2/neu
expression in colon adenocarcinoma and its correlation with
clinicopathologic variables. IJBMS. 9:64–69. 2006.
|
|
40
|
Kim SY, Kim HP, Kim YJ, Oh Y, Im SA, Lee
D, Jong HS, Kim TY and Bang YJ: Trastuzumab inhibits the growth of
human gastric cancer cell lines with HER2 amplification
synergistically with cisplatin. Int J Oncol. 32:89–95. 2008.
|
|
41
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ross JS and McKenna BJ: The HER-2/neu
oncogene in tumors of the gastrointestinal tract. Cancer Invest.
19:554–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|