Introduction
Colon cancer is one of the most common malignancies
in the digestive system. Current treatments include surgical
resection, radiotherapy, chemotherapy and targeted therapy alone or
in combination. However, the prognosis remains unsatisfactory
(1,2). Thus, there is a great clinical need to
explore new agents for the treatment of colon cancer.
Numerous herbs or their components have been
clinically used as potential candidates for anticancer agents, such
as camptothecin, vincristine and taxol (3–5).
Polyphenolic compounds are highly present in red wine, and some
reports and clinical epidemiologic studies have demonstrated that
red wine contributes to a reduction in cardiovascular diseases and
cancer risk (6,7). Resveratrol (3,5,4′-trihydroxystilbene,
Res), a polyphenolic compound from beans and grapes, was discovered
in red wine by Siemann and Creasy in 1992, and can be used as a
platelet aggregation inhibitor, cardiac-protection and anticancer
agent (8,9). It has been reported that Res can
inhibit the proliferation and promote the apoptosis of various
types of cancer cells, such as colon, breast and prostate (10–12).
For colon cancer, although the anticancer effects of Res have been
validated (10), the precise
mechanisms underlying these activities remain unclear.
Bone morphogenetic proteins (BMPs) belong to the
transforming growth factor-β (TGF-β) superfamily (13). Approximately 20 BMPs have been
identified to date, and they exert multifunctions to regulate
physiological processes, such as proliferation, differentiation,
adhesion, migration and apoptosis (14,15).
Thus, the aberrant expression of BMPs and/or downstream signal
cascades have been implicated in the pathogenesis of various types
of cancer, such as colon cancer (16,17).
Therefore, BMPs and/or the downstream signal cascades may be used
as potential targets for colon cancer treatment (15,18).
BMP9 is the most potent BMP member to induce osteogenic
differentiation in mesenchymal stem cells to date (19). Apart from promoting osteogenic
differentiation, BMP9 is also implicated in cancer, although the
outcome is controversial. BMP9 inhibits the proliferation of breast
and prostate cancer cells (20,21),
but promotes the proliferation of osteosarcoma, ovarian and liver
cancer cells (22–24). The response of cancer cells to BMP9
may greatly differ even in the same type of cancer by inhibiting or
promoting proliferation, such as in osteosarcoma (22,25).
BMP9 often onsets its signaling through the canonical BMP/Smad
pathway. namely, BMP9 binds with type II bone morphogenetic protein
receptor (BMPR) or type I BMPR, then phosphorylates Smad1/5/8 and
forms a complex with Smad4, followed by translocation to the
nucleus and regulation of downstream targets (19,26).
In addition, BMP9 can also exert its function through the
non-canonical BMP/Smad pathway, such as p38 MAPK and PI3K/Akt
(26,27). The p38 MAPK signal is involved in
cell differentiation, apoptosis and autophagy (28). Activation of p38 MAPK is critical
for BMP9 to induce osteogenic differentiation in mesenchymal stem
cells, but the relationship between p38 MAPK and BMP9 in cancer is
not yet known.
Although the anticancer effect of Res in colon
cancer cells has been well validated, it remains unknown whether
BMP9 is associated with this activity. In the present study, we
investigated the possible role of BMP9 in the antiproliferative
effect of Res on LoVo cells, and we elucidated how BMP9 exerts this
function. Our findings demonstrated that Res can effectively
inhibit the proliferation and promote the apoptosis of LoVo cells,
which may be partly mediated by upregulation of BMP9 to activate
p38 MAPK. Hence, Res may be used as an effective anticancer agent
for colon cancer treatment alone or in combination with other
agents, and BMP9/p38 MAPK signaling may be a potential therapeutic
target for colon cancer treatment.
Materials and methods
Reagents and cell culture
Res was purchased from Xi'an Hao-xuan Biotechnology
Co. Ltd. (Xi'an, China). For the in vivo experiments, Res
was prepared with 0.5% carboxymethylcellulose sodium (CMC-Na) as a
suspension. The LoVo cell line was obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). All antibodies were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
LDN-193189 and SB203580 were purchased from Selleckchem (Houston,
TX, USA). Cells were maintained in Dulbecco's modified Eagle's
medium (DMEM) with 10% fetal bovine serum (FBS), penicillin (100
U/ml) and streptomycin (100 μg/ml) at 37°C in 5%
CO2.
Crystal violet assay
The crystal violet assay was carried out as
previously described (10).
Experimentally, LoVo cells were treated with different
concentrations of Res. Cells were carefully washed at the scheduled
time points with cold phosphate-buffered saline (PBS; 4°C), and
were stained with 0.5% crystal violet solution for 20–30 min (at
room temperature). Plates were gently washed with tap water, air
dried at room temperature, then scanned and quantified. For
quantification, 20% acetic acid was used to extract the crystal
violet for 20 min, with gentle shaking at room temperature. The
absorbance at 570 nm was measured.
Flow cytometric analysis of cell cycle
distribution and apoptosis
Cells were seeded into 6-well plates. For cell cycle
analysis, the cells were treated with different concentrations of
Res or DMSO. After 48 h, the cells were washed with cold PBS (4°C),
collected and washed with cold (4°C) 70% ethanol followed by
washing with 50 and 30% ethanol, and PBS. Then, the cells were
incubated with 1 ml of 20 mg/ml propidium iodide (PI) containing
RNase (1 mg/ml) for 30 min (in PBS), followed by fluorescence
activated cell sorting (FACS) analysis. For the apoptosis analysis,
the cells were collected after treatment with the different
concentrations of Res or DMSO for 48 h. Then, the cells were washed
with cold PBS, incubated with Annexin V-EGFP and PI according to
the procedure provided in the kit (#KGA104; KeyGen Biotech, China).
Finally, the cells were subjected to FACS assay.
Western blot assay
Cells were seeded into 6-well plates, and were
treated with different concentrations of Res or DMSO. At the
scheduled time points, the cells were lysed and the lysates were
boiled for 10 min. Total protein was separated with SDS-PAGE,
transfered to polyvinylidene difluoride (PVDF) membranes, blocked
with 5% BSA and probed with the antibody tagged with HRP. The
target bands were developed with ECL substrate (#34095; Thermo,
USA).
Total RNA extraction and reverse
transcription polymerase chain reaction (RT-PCR) assay
The cells were seeded in a T25 flask and were
treated with different concentrations of Res or DMSO. Total RNA was
extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA),
followed by RT reaction to obtain cDNA. Finally, the cDNA products
were used as templates to detect the expression of the target genes
with PCR. Primer sequences are available upon request.
Construction of recombinant adenoviruses
for exogenous expression of BMP9, GFP and small interference RNA
fragments for BMP9
The recombinant adenoviruses for exogenous
expression of BMP9 (AdBMP9) or GFP (AdGFP) were generated following
the AdEasy system as previously reported (29,30),
as well as the recombinant adenoviruses expressing small
interfering RNA (siRNA) fragments for BMP9 (AdsiBMP9). AdGFP was
used as a vehicle control.
Ectopic tumor model of human colon cancer
and histological evaluation
All experiments followed the guideline of the
Institutional Animal Care and Use Committee of Chongqing Medical
University (Chongqing, China). Athymic nude mice (female, 4–6 weeks
old, 5/group) were purchased from the Animal Center of Chongqing
Medical university (Chongqing, China). LoVo cells were cultured and
resuspended in PBS (4°C) for implantation into the flanks of the
athymic nude mice as previously reported (10,31).
The mice were treated with Res (50 or 150 mg/kg) or the same volume
of solvent through intragastric administration 1 week after
implantation, once a day, up to 4 weeks. At the end of the 4 week,
all nude mice were sacrificed. The tumor samples were harvested,
fixed in 10% formalin and embedded in paraffin. Sections of the
samples were subjected to hematoxylin and eosin (H&E)
staining.
Immunohistochemical staining
The deparaffinized slides were subjected to antigen
retrieval and were probed with an anti-BMP9 antibody or isotype IgG
control, followed by incubation with a biotin secondary antibody
and streptavidin-HRP. The proteins of interest were visualized by
3,3′-diaminobenzidine staining.
Statistical analysis
All experiments were performed in triplicates and
the results were repeated in at least three independent
experiments. Statistical analysis of the results was conducted
following a t-test (Microsoft Excel). Data are expressed as mean ±
standard deviation (SD).
Results
Res decreases the proliferative ability
of LoVo cells
It has been reported that Res exhibits
antiproliferative activity in various types of cancer cells,
including colon cancer cells. In this investigation, we first
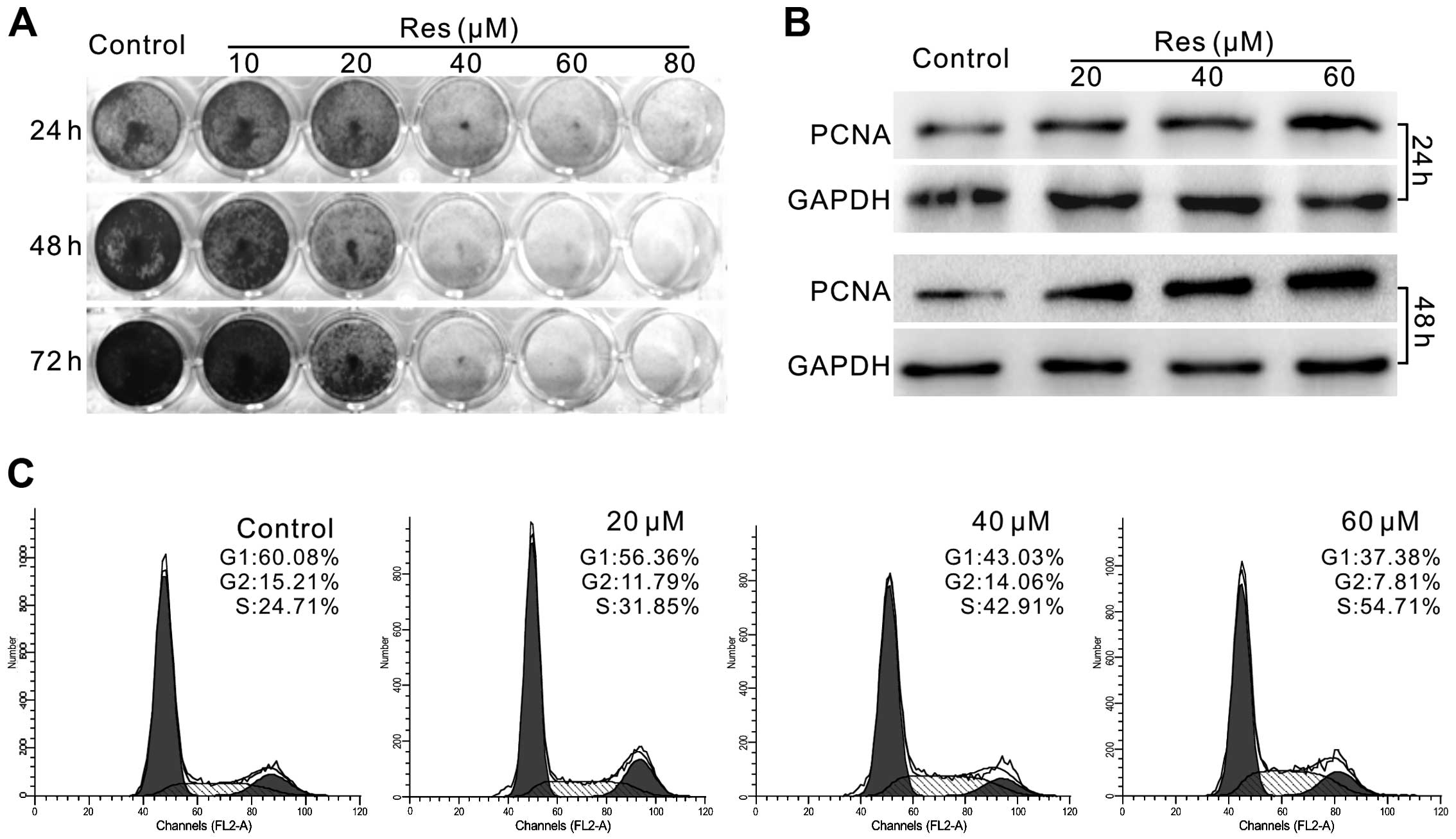
confirmed the effect of Res on LoVo cells. The results showed that
Res inhibited the proliferation of LoVo cells in a
concentration-dependent manner (Fig.
1A). The level of proliferating cell nuclear antigen (PCNA) was
also markedly increased in a concentration-dependent manner
(Fig. 1B). Cell cycle analysis
results showed that Res induced cell cycle arrest at the S phase in
the LoVo cells (Fig. 1C). All these
data suggest that Res inhibits the proliferation of LoVo cells.
Res induces the apoptosis of LoVo
cells
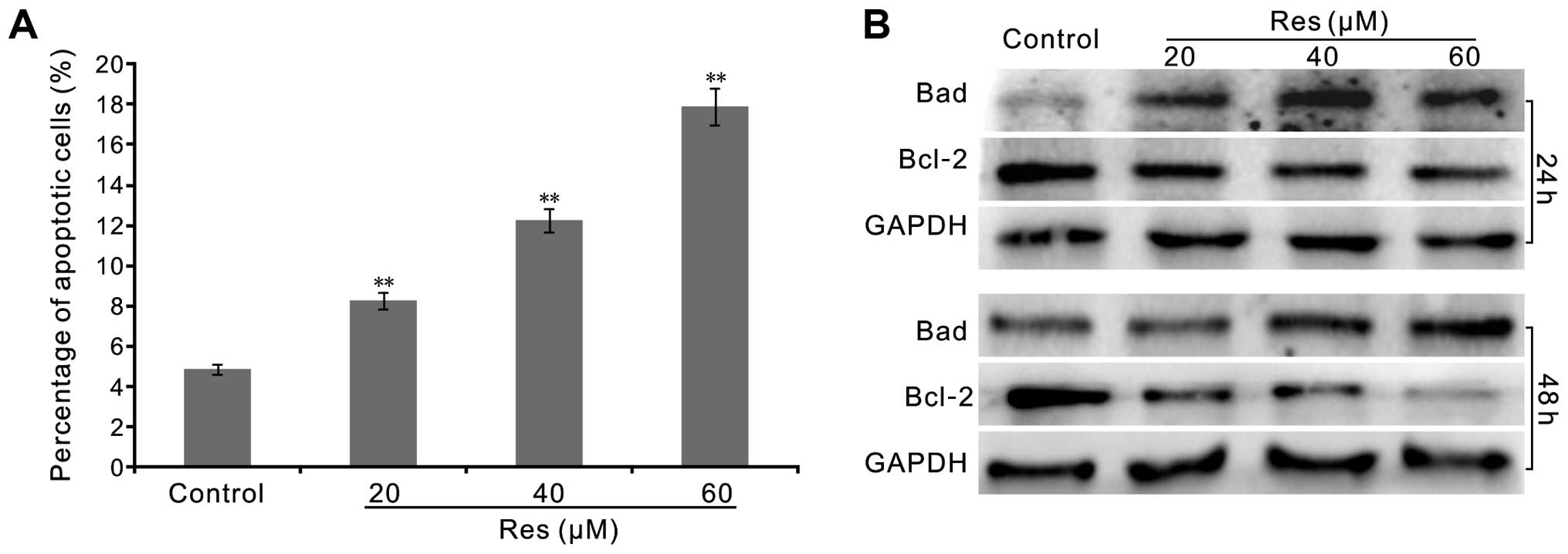
Most anticancer agents induce apoptosis, therefore
we ascertained whether Res induces apoptosis in the LoVo cells. We
employed FACS and western blotting to analyze the effect of Res on
apoptosis in the LoVo cells. FACS assay results showed that Res
notably increased the percentage of apoptotic LoVo cells (Fig. 2A). Western blot assay showed that
Res increased the level of Bad, but markedly decreased the level of
Bcl-2 prominently in a concentration-dependent manner (Fig. 2B). These results confirmed that Res
may be an effective apoptosis inducer in human colon cancer
cells.
Res inhibits tumor growth in an ectopic
tumor model
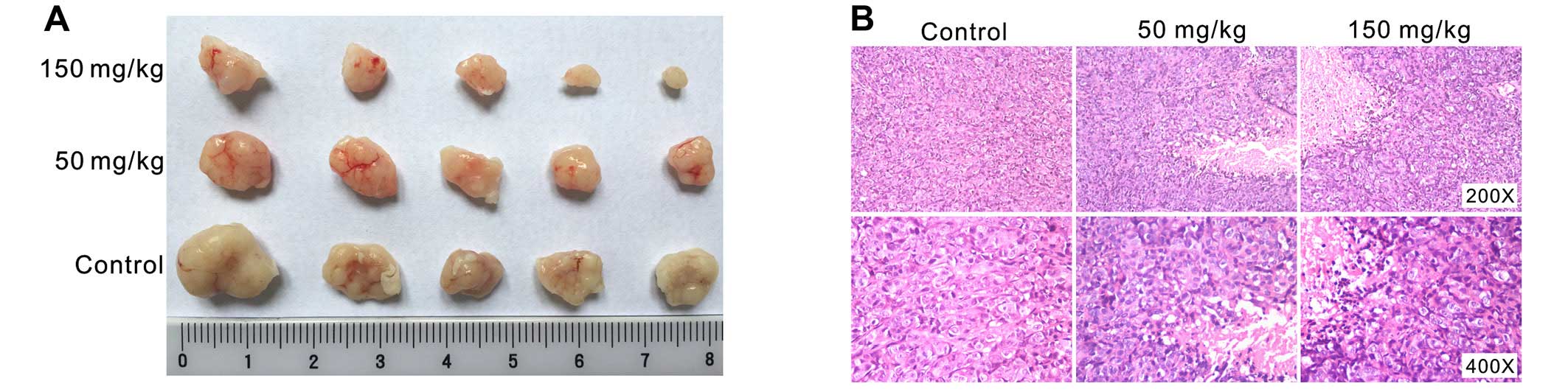
The above findings showed that Res is a potent
proliferation inhibitor in colon cancer cells. We next investigated
the in vivo anticancer activity of Res with a
well-established xenograft tumor model as previously reported
(10,31). We implanted 1×106 LoVo
cells into the flanks of athymic nude mice. One week after
implantation, the mice were treated with Res (50 or 150 mg/kg)
through intragastric administration, once a day, up to 4 weeks. The
results showed that tumor masses from the Res-treated groups were
smaller than those from the control group (Fig. 3A). H&E staining results showed
that cellularity was apparently decreased in the Res-treated groups
(Fig. 3B). These results
demonstrated that Res is capable of effectively inhibiting colon
cancer growth in vivo.
Res increases the expression of BMP9 in
LoVo cells
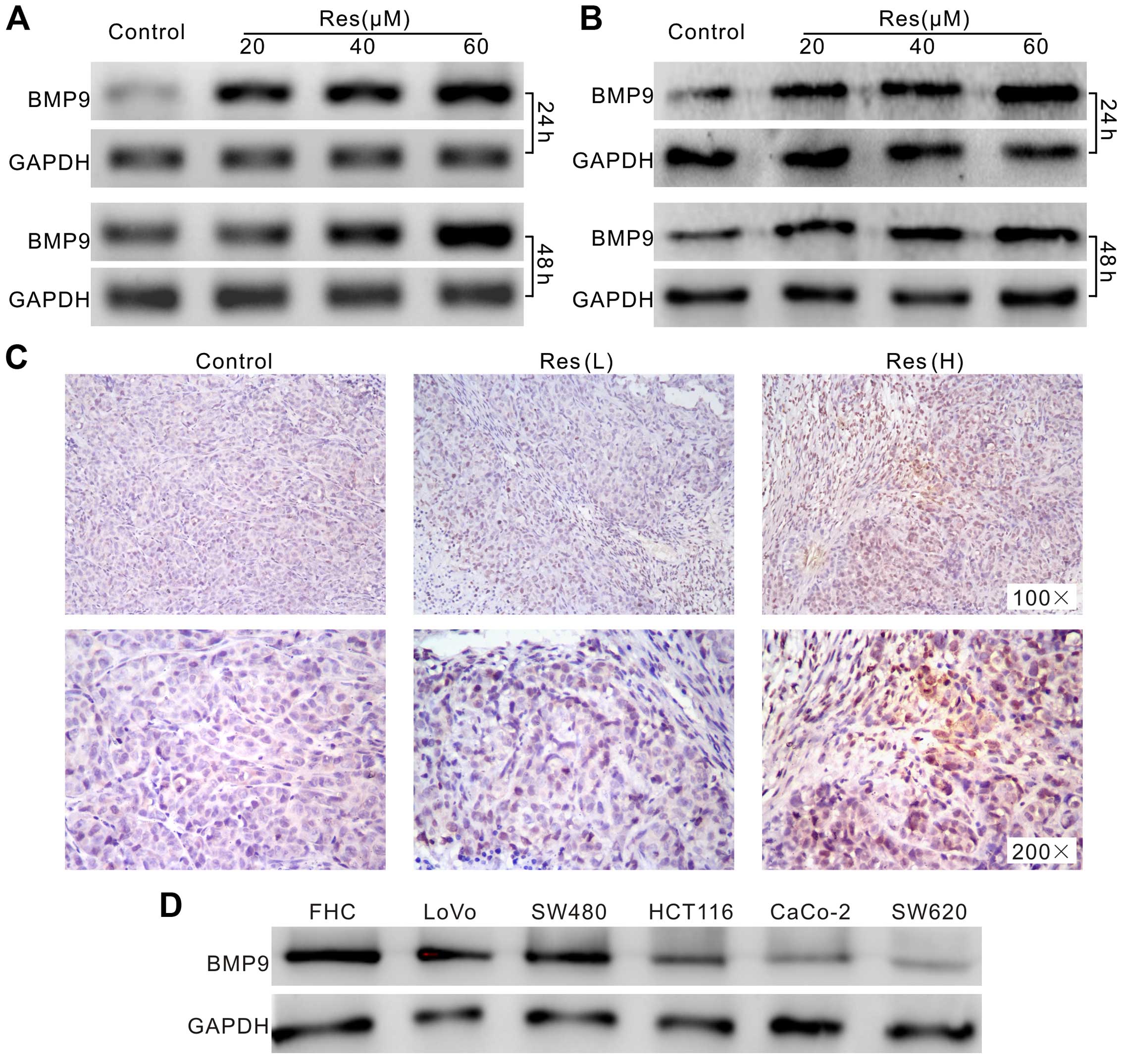
Next, we aimed to ascertain the possible mechanism
underlying the anticancer activity of Res in the colon cancer
cells. Using PCR and western blot assay we found that BMP9 was
highly upregulated by Res in the LoVo cells (Fig. 4A and B). The immunohistochemical
staining of the tumor masses showed similar results (Fig. 4C). Furthermore, western blot assay
found that BMP9 was detectable in all the colon cancer cell lines,
as well as FHC cells. However, the level of BMP9 in the FHC cells
was relative higher than that in the cancer cell lines (Fig. 4D). These data imply that the
upregulation of BMP9 may be related with the anticancer activity of
Res in colon cancer.
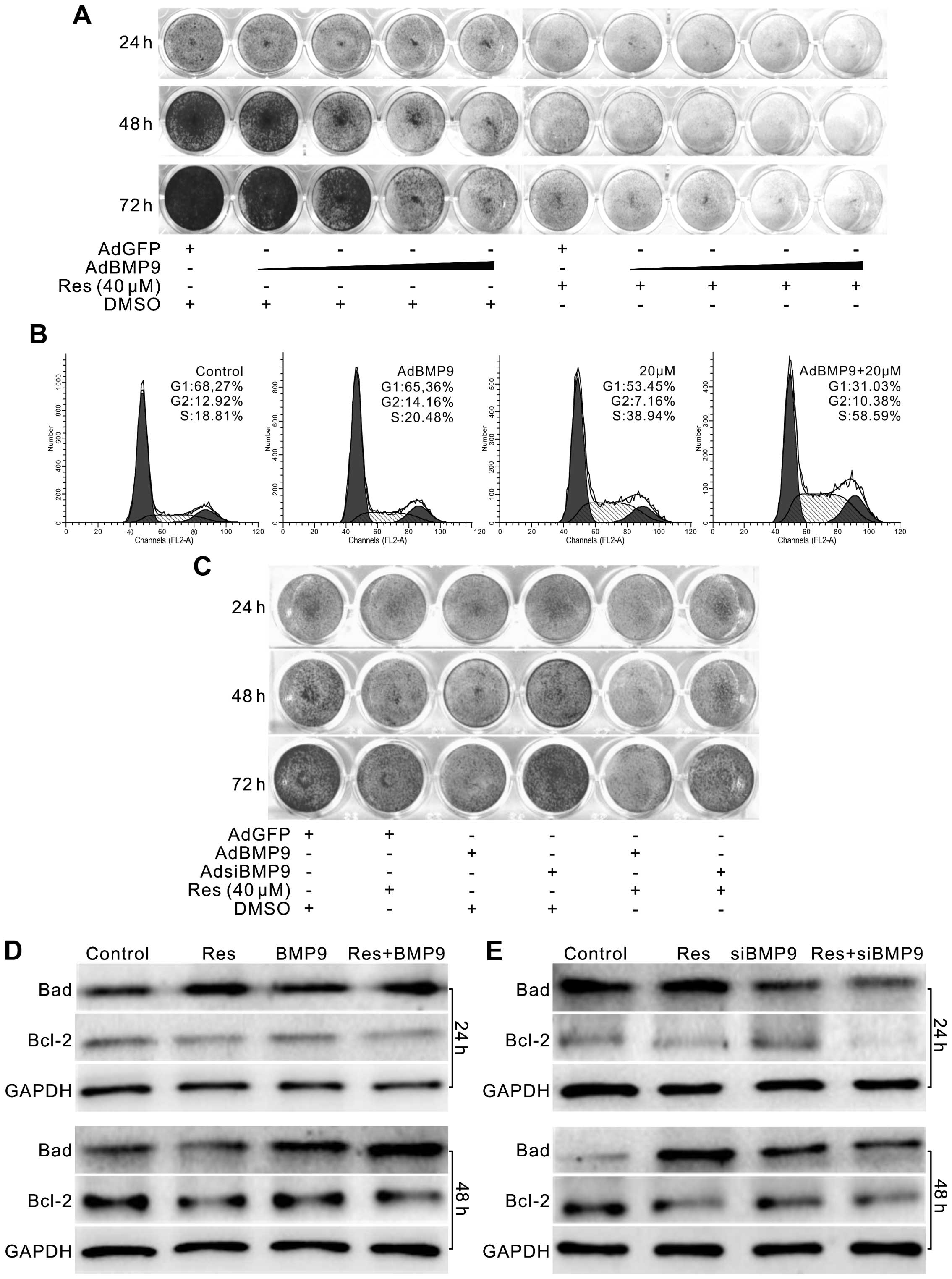
BMP9 enhances the antiproliferative
activity of Res in LoVo cells
As Res upregulates the expression of BMP9 in LoVo
cells and the level of BMP9 is higher in FHC cells, we aimed to
ascertain whether BMP9 affects the anticancer activity of Res in
LoVo cells. Using the AdEasy system, we constructed recombinant
adenoviruses to express BMP9 or BMP9 siRNA fragments in the LoVo
cells. Using a crystal violet staining assay, we found that
exogenous expression of BMP9 inhibited the proliferation of LoVo
cells, and the antiproliferative effect of Res on LoVo cells was
enhanced when combined with BMP9 (Fig.
5A). Cell cycle analysis showed that BMP9 apparently enhanced
the S phase arrest effect of Res in LoVo cells (Fig. 5B). BMP9 knockdown partly reversed
the antiproliferative effect of Res (Fig. 5C). Western blot assay showed that
BMP9 increased the level of Bad but decreased the level of Bcl-2
induced by Res in the LoVo cells (Fig.
5D). However, knockdown of BMP9 decreased the level of Bad
induced by Res in the LoVo cells, although no apparent effect was
noted for Bcl-2 (Fig. 5E). These
data indicate that the anticancer effect of Res in colon cancer may
be mediated by upregulating BMP9.
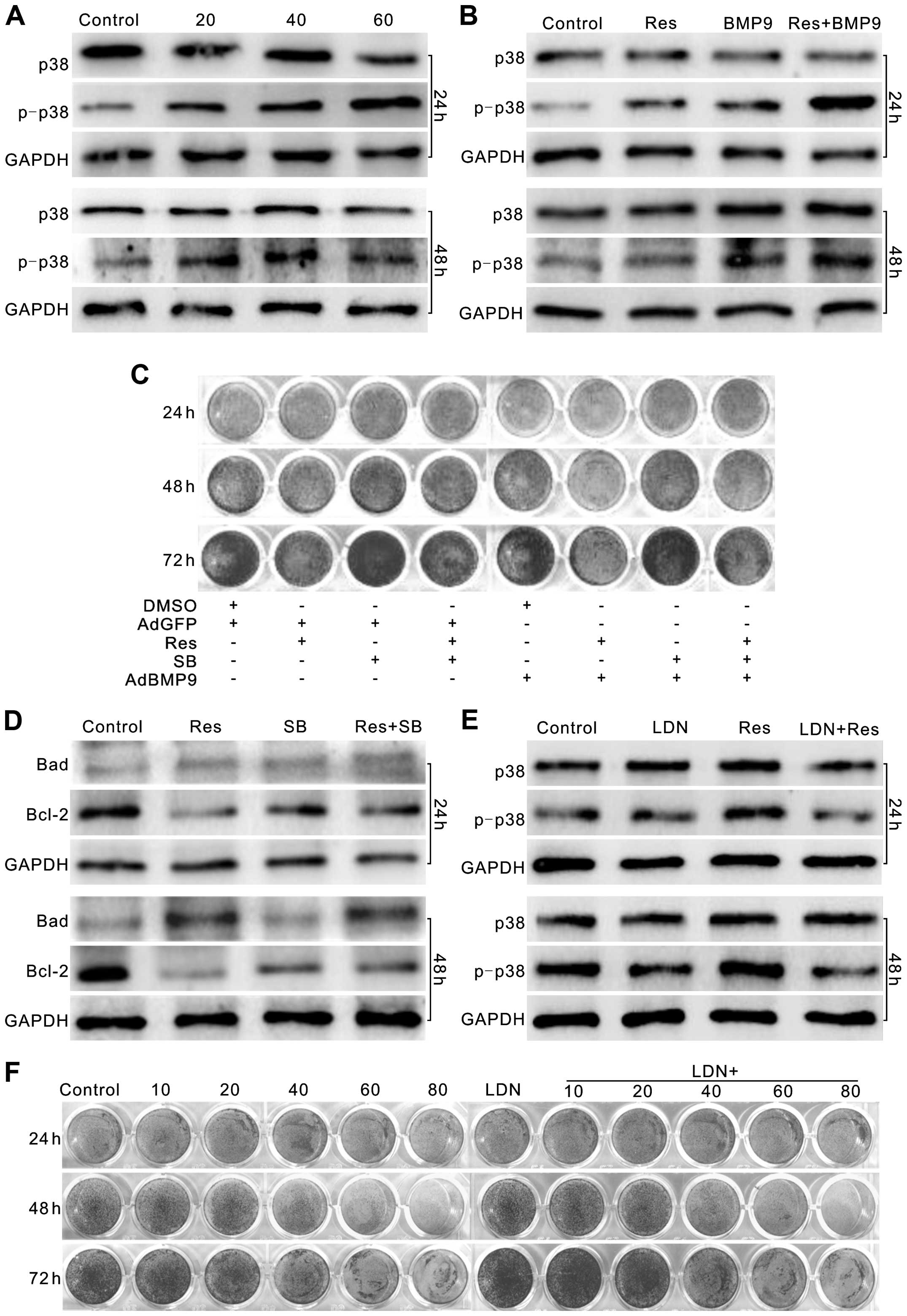
p38 MAPK mediates the effect of BMP9 on
the antiproliferative activity of Res in LoVo cells
BMP9 usually exerts its function through the
canonical BMP/Smad pathway, but our findings showed that Res
exhibited no apparent effect on the phosphorylation of Smad1/5/8
(data not shown). This implied that the effect of BMP9 on the
antiproliferative effect of Res noted in the LoVo cells may not be
mediated through the canonical BMP/Smad pathway, but the
non-canonical BMP/Smad pathway. p38 MAPK is involved in this
signaling pathway, and its activation is essential for BMP9-induced
osteogenesis in mesenchymal stem cells (27). Therefore, we speculated that
Res-induced BMP9 may affect the activation of p38 MAPK in LoVo
cells. Western blot results showed that Res increased the level of
phosphorylated p38 MAPK (p-p38) substantially in the LoVo cells
(Fig. 6A), and exogenous expression
of BMP9 enhanced this effect of Res on p-p38 (Fig. 6B). The p38 MAPK inhibitor, SB203580
(32), increased the proliferation
of LoVo cells, while reducing the antiproliferative effect of Res,
BMP9 and Res combined with BMP9 (Fig.
6C). The p38 MAPK inhibitor also reversed the Res-induced
decrease in Bcl-2, although no apparent effect on the level of Bad
was noted (Fig. 6D). The BMPR
inhibitor (LDN-193189) abolished the Res-induced activation of p38
MAPK in the LoVo cells (Fig. 6E).
Similar results were also found when BMP9 was knocked down (data
not shown). Further analysis showed that the BMPR inhibitor
promoted the proliferation of LoVo cells, and partly reversed the
antiproliferative effect of Res on LoVo cells (Fig. 6F). These data suggest that BMP9 may
mediate the antiproliferative effect of Res on LoVo cells by
activating p38 MAPK, which may be partly triggered through a
BMPR-dependent manner.
Discussion
Colon cancer, is the most common prevalent
malignancy in the digestive system and accounts for a major
proportion of cancer-induced mortality (33). Hence, there is a great clinical need
to explore new agents or adjuvant therapies for colon cancer
treatment. In the present study, we demonstrated the efficacious
anticancer activity of Res in human colon cancer cells.
Mechanistically, we found that the anticancer effect of Res on
colon cancer cells may be mediated by activating p38 MAPK partly
through upregulation of BMP9.
Challenges for colon cancer treatment include the
toxicity of drugs, drug resistance and cancer cell metastasis. The
serious side-effects associated with traditional chemotherapy drugs
greatly decrease the life quality of patients, while reducing the
effectiveness of the treatment for colon cancer (34,35).
Although targeted therapy agents, such as the VEGF antibody
bevacizumab, and EGFR antibodies cetuximab and panitumumab
(2,36), have been clinically used, natural
products and/or their derivates are still an essential source of
anticancer agents (37).
Resveratrol (Res), a natural polyphenolic compound, is found in the
skin of red grapes or other fruits (8). Increasing evidence suggests that Res
shows antiproliferation-and apoptosis-inducing activities in
breast, prostate and colon cancer cells (10–12).
Since the bioavailability of Res is low, even a high dose of Res
may not provide the effective concentration required for systemic
treatment (38). However, Res may
benefit gastrointestinal cancer treatment. Reports and our studies
have validated the inhibitory effect of Res on the proliferation of
colon cancer cells. Vanamala et al reported that Res induces
apoptosis through suppressing Wnt/β-catenin and activating the p53
signaling pathways in human colon cancer cells (39). Sheth et al found that
microRNA-21 participates in the inhibition of prostate cancer cell
growth and metastasis initialized by Res (40). Recently, it was reported that the
p38 MAPK and PI3K signaling pathways are also involved in the
anticancer activity of Res (41,42).
However, to date, the detail molecular mechanism underlying this
process remains unclear.
BMP9, also termed growth and differentiation factor
2 (GDF2), was first identified in the developing mouse liver as
playing an important role in regulating iron metabolism and the
development of cholinergic neurons (43). Although BMP9 has been reported as
the most potent BMP member to induce osteogenic differentiation in
mesenchymal stem cells, it has also been implicated with
tumorigenesis (20–25). BMP9 exerts its physiological
function through the canonical BMP/Smad pathway or the
non-canonical BMP/Smad pathway. Regarding the canonical BMP/Smad
pathway, BMPs bind with BMP receptor (type I or type II) and
phosphorylate BMP-related Smads (Smad1/5/8), form a complex with
Smad4 and then translocate to the nucleus regulating downstream
targets (19,26). Regarding the non-canonical BMP/Smad
pathway, BMPs activate a serial signaling pathway, such as PI3K/Akt
and p38 MAPK (27,44). However, how BMP9 activates these
signaling pathways remains unknown. The response of cancer cells to
BMP9 may depend on the cell type and/or the microenvironment of the
cells. A few reports have indicated that BMP9 is important for
hepatocellular carcinoma cell proliferation and survival, and
promotes the proliferation of osteosarcoma and ovarian cancer cells
(22,23). On the contrary, it has also been
reported that BMP9 can inhibit the proliferation or metastasis in
various types of cancer cells, such as gastric and breast cancer
cells (15,20). Our results demonstrated that Res can
effectively inhibit the proliferation of LoVo cells, and BMP9 was
significantly increased during this process. This evidence implies
that BMP9 may be critical for the antiproliferative effect of Res
on LoVo cells.
To date, the role of BMP9 in colon cancer remains
unknown. Further analysis found that BMP9 was detectable in FHC
cells and colon cancer cell lines. However, the level of BMP9 in
the FHC cells was distinctly higher than that in the other colon
cancer cell lines. These data may also highlight the importance of
BMP9 in the regulation of proliferation in colon cancer cells. Our
subsequent investigation showed that exogenous expression of BMP9
enhanced the antiproliferation- and apoptosis-inducing effects of
Res in LoVo cells, while BMP9 knockdown reduced these effects.
Thus, the anticancer activity of Res in LoVo cells may be partly
mediated by upregulation of the expression of BMP9. However, how
BMP9 mediates this effect remains unknown.
Regarding the canonical BMP/Smad pathway, BMP9 binds
to type I receptor (activin receptor-like kinase, including ALK1
and ALK2), followed by Smad1/5/8 phosphorylation (45). However, our data indicated that Res
exhibited no substantial effect on the phosphorylation of
Smad1/5/8, which implies that the effect of BMP9 on the anticancer
activity of Res may not be mediated through the canonical BMP/Smad
pathway. Therefore, the non-canonical BMP/Smad pathway, such as p38
MAPK and PI3K/Akt, may be implicated in this effect.
It was previously reported that p38 MAPK is involved
in the anticancer effect of Res, and activation of p38 MAPK is also
essential for BMP9-induced osteogenesis (27,41,42).
Hence, we speculated that the effect of BMP9 on the anticancer
activity of Res may be associated with p38 MAPK. Our results showed
that Res can apparently increase the phosphorylation of p38 MAPK in
a concentration-dependent manner, which is consistent with reports
that p38 is involved in the anticancer effect of Res in colon
cancer cells (27,41,42).
However, how p38 MAPK is activated by Res in colon cancer cells
remains unknown. Further assay results showed that exogenous
expression of BMP9 enhanced the phosphorylation of p38 MAPK induced
by Res, and the p38 MAPK inhibitor (SB203580) decreased the
antiproliferative effect of Res, as well as the combination of Res
and BMP9. The p38 MAPK inhibitor also reduced the apoptosis induced
by Res in the LoVo cells. These data suggest that BMP9 may mediate
the anticancer effect of Res partly through activation of p38 MAPK
signaling. In fact, p38 MAPK is important not only in regulating
the proliferation of cancer cells, but also in mediating the
osteogenic differentiation induced by BMP9. The blockage of p38
MAPK greatly reduces BMP9-induced osteogenic differentiation
(27). Thus, p38 MAPK should be
downstream of BMP9. However, how BMP9 activates p38 MAPK remains
unknown. Since Res promotes the activation of p38 MAPK, upregulates
BMP9, and exhibits no substantial effect on Smad1/5/8
phosphorylation, the activation of p38 MAPK by BMP9 may not be
mediated through the canonical BMP/Smad pathway. Following
treatment with the BMP receptor (BMPR) inhibitor (LDN-193189)
(46), the Res-induced activation
of p38 MAPK was almost abolished, so did the antiproliferative
effect of Res in the LoVo cells. Therefore, the activation of BMPR
may be necessary for BMP9 to activate p38 MAPK, although Smad1/5/8
is not involved in. These data demonstrated that p38 MAPK may
mediate the effect of BMP9 on the anticancer activity of Res in
LoVo cells, and BMP9 may activate p38 MAPK through a BMPR-dependent
manner.
Taken together, our findings strongly demonstrated
that Res is a potent anticancer agent and inhibits the
proliferation and promotes the apoptosis of colon cancer cells. The
anticancer activity of Res may be mediated by upregulation of BMP9
in colon cancer, by which to activate p38 MAPK in a BMPR-dependent
manner. Our findings also indicate that TGF-β signaling should be a
potential target for colon cancer treatment. However, the exact
molecular mechanism of how Res regulates the expression of BMP9
requires further investigation.
Acknowledgments
We thank Professor Tong-Chuan He of the University
of Chicago Medical Center (Chicago, IL, USA) for providing the
recombinant adenoviruses. The present study was supported by
research grants from the Natural Science Foundation of China (grant
nos. NSFC 81372120 and 81572226 to B.-C. H.).
References
|
1
|
Binefa G, Rodríguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng QY, Wei Y, Chen JW, Chang WJ, Ye LC,
Zhu DX and Xu JM: Anti-EGFR and anti-VEGF agents: Important
targeted therapies of colorectal liver metastases. World J
Gastroenterol. 20:4263–4275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banjerdpongchai R, Chanwikruy Y,
Rattanapanone V and Sripanidkulchai B: Induction of apoptosis in
the human leukemic U937 cell line by Kaempferia parviflora
Wall.ex.Baker extract and effects of paclitaxel and camptothecin.
Asian Pac J Cancer Prev. 10:1137–1140. 2009.PubMed/NCBI
|
|
4
|
Yan YX, Li WZ, Huang YQ and Liao WX: The
COX-2 inhibitor Celecoxib enhances the sensitivity of KB/VCR oral
cancer cell lines to Vincristine by down-regulating P-glycoprotein
expression and function. Prostaglandins Other Lipid Mediat.
97:29–35. 2012. View Article : Google Scholar
|
|
5
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das S, Santani DD and Dhalla NS:
Experimental evidence for the cardioprotective effects of red wine.
Exp Clin Cardiol. 12:5–10. 2007.
|
|
7
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic phytochemicals –
promising cancer chemopreventive agents in humans? A review of
their clinical properties. Int J Cancer. 120:451–458. 2007.
View Article : Google Scholar
|
|
8
|
Gehm BD, McAndrews JM, Chien PY and
Jameson JL: Resveratrol, a polyphenolic compound found in grapes
and wine, is an agonist for the estrogen receptor. Proc Natl Acad
Sci USA. 94:14138–14143. 1997. View Article : Google Scholar
|
|
9
|
Cottart CH, Nivet-Antoine V and Beaudeux
JL: Review of recent data on the metabolism, biological effects,
and toxicity of resveratrol in humans. Mol Nutr Food Res. 58:7–21.
2014. View Article : Google Scholar
|
|
10
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014.PubMed/NCBI
|
|
11
|
Chin YT, Hsieh MT, Yang SH, Tsai PW, Wang
SH, Wang CC, Lee YS, Cheng GY, HuangFu WC, London D, et al:
Antiproliferative and gene expression actions of resveratrol in
breast cancer cells in vitro. Oncotarget. 5:12891–12907. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kai L, Samuel SK and Levenson AS:
Resveratrol enhances p53 acetylation and apoptosis in prostate
cancer by inhibiting MTA1/NuRD complex. Int J Cancer.
126:1538–1548. 2010.
|
|
13
|
Sánchez-Duffhues G, Hiepen C, Knaus P and
Ten Dijke P: Bone morphogenetic protein signaling in bone
homeostasis. Bone. 80:43–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen D, Zhao M, Harris SE and Mi Z: Signal
transduction and biological functions of bone morphogenetic
proteins. Front Biosci. 9:349–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan L, Ye L, Wu R, Wang H, Li X, Li H,
Yuan S, Zha H, Sun H, Zhang Y, et al: Inactivation of the
phosphatidylinositol 3-kinase/Akt pathway is involved in
BMP9-mediated tumor-suppressive effects in gastric cancer cells. J
Cell Biochem. 116:1080–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Epstein NE: Basic science and spine
literature document bone morphogenetic protein increases cancer
risk. Surg Neurol Int. 5(Suppl 15): S552–S560. 2014. View Article : Google Scholar
|
|
17
|
Toofan P, Irvine D, Hopcroft L, Copland M
and Wheadon H: The role of the bone morphogenetic proteins in
leukaemic stem cell persistence. Biochem Soc Trans. 42:809–815.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: The role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JH, Liu YZ, Yin LJ, Chen L, Huang J,
Liu Y, Zhang RX, Zhou LY, Yang QJ, Luo JY, et al: BMP9 and COX-2
form an important regulatory loop in BMP9-induced osteogenic
differentiation of mesenchymal stem cells. Bone. 57:311–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang K, Feng H, Ren W, Sun X, Luo J, Tang
M, Zhou L, Weng Y, He TC and Zhang Y: BMP9 inhibits the
proliferation and invasiveness of breast cancer cells MDA-MB-231. J
Cancer Res Clin Oncol. 137:1687–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye L, Kynaston H and Jiang WG: Bone
morphogenetic protein-9 induces apoptosis in prostate cancer cells,
the role of prostate apoptosis response-4. Mol Cancer Res.
6:1594–1606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo X, Chen J, Song WX, Tang N, Luo J,
Deng ZL, Sharff KA, He G, Bi Y, He BC, et al: Osteogenic BMPs
promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrera B, van Dinther M, Ten Dijke P and
Inman GJ: Autocrine bone morphogenetic protein-9 signals through
activin receptorlike kinase-2/Smad1/Smad4 to promote ovarian cancer
cell proliferation. Cancer Res. 69:9254–9262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrera B, García-Álvaro M, Cruz S, Walsh
P, Fernández M, Roncero C, Fabregat I, Sánchez A and Inman GJ: BMP9
is a proliferative and survival factor for human hepatocellular
carcinoma cells. PLoS One. 8:e695352013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv Z, Wang C, Yuan T, Liu Y, Song T, Liu
Y, Chen C, Yang M, Tang Z, Shi Q, et al: Bone morphogenetic protein
9 regulates tumor growth of osteosarcoma cells through the
Wnt/β-catenin pathway. Oncol Rep. 31:989–994. 2014.
|
|
26
|
Huang J, Yuan SX, Wang DX, Wu QX, Wang X,
Pi CJ, Zou X, Chen L, Ying LJ, Wu K, et al: The role of COX-2 in
mediating the effect of PTEN on BMP9 induced osteogenic
differentiation in mouse embryonic fibroblasts. Biomaterials.
35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Song T, Wang W, Wang J, He J, Wu
N, Tang M, He B and Luo J: P38 and ERK1/2 MAPKs act in opposition
to regulate BMP9-induced osteogenic differentiation of mesenchymal
progenitor cells. PLoS One. 7:e433832012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
32
|
Chen CS, Ho DR, Chen FY, Chen CR, Ke YD
and Su JG: AKT mediates actinomycin D-induced p53 expression.
Oncotarget. 5:693–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tárraga López PJ, Albero JS and
Rodríguez-Montes JA: Primary and secondary prevention of colorectal
cancer. Clin Med Insights Gastroenterol. 7:33–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aggarwal S and Chu E: Current therapies
for advanced colorectal cancer. Oncology. 19:589–595.
2005.PubMed/NCBI
|
|
35
|
Wolpin BM and Bass AJ: Managing advanced
colorectal cancer: Have we reached the PEAK with current therapies?
J Clin Oncol. 32:2200–2202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tol J and Punt CJ: Monoclonal antibodies
in the treatment of metastatic colorectal cancer: A review. Clin
Ther. 32:437–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khazir J, Riley DL, Pilcher LA, De-Maayer
P and Mir BA: Anticancer agents from diverse natural sources. Nat
Prod Commun. 9:1655–1669. 2014.PubMed/NCBI
|
|
38
|
Boocock DJ, Faust GE, Patel KR, Schinas
AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher
AJ, et al: Phase I dose escalation pharmacokinetic study in healthy
volunteers of resveratrol, a potential cancer chemopreventive
agent. Cancer Epidemiol Biomarkers Prev. 16:1246–1252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheth S, Jajoo S, Kaur T, Mukherjea D,
Sheehan K, Rybak LP and Ramkumar V: Resveratrol reduces prostate
cancer growth and metastasis by inhibiting the Akt/MicroRNA-21
pathway. PLoS One. 7:e516552012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Parekh P, Motiwale L, Naik N and Rao KV:
Downregulation of cyclin D1 is associated with decreased levels of
p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of
resveratrol in liver cancer cells. Exp Toxicol Pathol. 63:167–173.
2011. View Article : Google Scholar
|
|
42
|
Gweon EJ and Kim SJ: Resveratrol induces
MMP-9 and cell migration via the p38 kinase and PI-3K pathways in
HT1080 human fibrosarcoma cells. Oncol Rep. 29:826–834. 2013.
|
|
43
|
Herrera B, Dooley S and Breitkopf-Heinlein
K: Potential roles of bone morphogenetic protein (BMP)-9 in human
liver diseases. Int J Mol Sci. 15:5199–5220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Liu Y, Zhang R, Wang X, Huang F,
Yan Z, Nie M, Huang J, Wang Y, Wang Y, et al: All-trans retinoic
acid modulates bone morphogenic protein 9-induced osteogenesis and
adipogenesis of preadipocytes through BMP/Smad and Wnt/β-catenin
signaling pathways. Int J Biochem Cell Biol. 47:47–56. 2014.
View Article : Google Scholar
|
|
45
|
Luo J, Tang M, Huang J, He BC, Gao JL,
Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, et al: TGFbeta/BMP type I
receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic
signaling in mesenchymal stem cells. J Biol Chem. 285:29588–29598.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sanvitale CE, Kerr G, Chaikuad A, Ramel
MC, Mohedas AH, Reichert S, Wang Y, Triffitt JT, Cuny GD, Yu PB, et
al: A new class of small molecule inhibitor of BMP signaling. PLoS
One. 8:e627212013. View Article : Google Scholar : PubMed/NCBI
|