Introduction
Gastric cancer, one of the most common cancer types,
is the second leading cause of cancer-related deaths worldwide. In
recent years, the prognosis of gastric cancer patients has improved
owing to the combined application of surgical techniques and
chemotherapies. Nonetheless, the 5-year survival rate remains low
(1,2). Thus, new strategies to overcome
gastric cancer are urgently required.
Compared with normal cells, most cancer cells
preferentially depend on glycolysis to produce adenosine
triphosphate (ATP) for growth and proliferation (3). This phenotype, referred to as 'aerobic
glycolysis', was first observed by Warburg (4). Furthermore, our previous experiments
and other researches have confirmed that gastric cancer utilizes
glycolysis to meet the energy demand (5–7). ATP
reduction by inhibiting glycolysis induces DNA degradation and cell
apoptosis in tumors (8,9). Therefore, glycolysis suppression may
be an appropriate target for inhibiting cancer cell growth and/or
inducing apoptosis. Hexokinase (HK), phosphofructokinase 1 (PFK-1),
and pyruvate kinase (PK) are crucial enzymes that regulate the rate
of glycolysis (10–13). HK binds to the mitochondrial
membrane to catalyze the first rate-regulating step of glycolysis,
and also enhances cell proliferation and suppresses apoptosis
(14). PFK-1 catalyzes the
conversion of fructose-6-phosphate and ATP into fructose
1,6-bisphosphate and ADP, while PK converts phosphoenolpyruvate and
ADP into ATP in the glycolysis pathway. Therefore, if suppression
of glycolysis can induce cancer cell growth inhibition and/or
apoptosis, then HK, PFK-1 and PK may be potential targets for
developing novel anticancer agents (15).
Both glycolysis and apoptosis have been regarded as
independent pathways crucial for tumor cell survival (16,17).
Apoptosis can be activated through the endoplasmic reticulum, death
ligand, and mitochondrial pathways (18,19).
The mitochondrial pathway is regulated by the activity of pro- and
anti-apoptotic members in the Bcl-2 family. Pro-apoptotic proteins,
such as Bax, increase mitochondrial membrane permeability, causing
the secretion of cytochrome c (Cyt-C), which activates the
caspase cascade and initiates cell apoptosis (20–22).
On the other hand, Bcl-2 prevents the accumulation of Cyt-C and the
activation of the caspase cascade by stabilizing mitochondrial
permeability, thereby inhibiting apoptosis (23,24).
Thus, any agent with the ability to regulate Bcl-2 family members
and/or caspases in tumor cells may induce mitochondrial-mediated
apoptosis. Similarly, survivin is a member of the inhibitor of
apoptosis (IAP) family that is overexpressed in a variety of human
cancers (25,26), and may be an important anticancer
target for gastric tumors.
Bromopyruvate (3-BrPA) is an alkylating agent that
inhibits tumor growth and induces cell apoptosis by a variety of
biochemical mechanisms (27–29).
In our previous study, we found that both 3-BrPA and sodium citrate
(SCT) can inhibit cancer cell proliferation in vitro
(6,7). However, their underlying inhibitory
mechanisms require further investigation. In the present study, we
developed an orthotopic transplantation tumor model in nude mice
using human gastric cancer cells. We chose this animal model as the
biological behaviors of the gastric orthotopic transplantation
tumor model are more similar to the processes of growth and
metastasis of human gastric cancer than the conventional xenograft
models (subcutaneous or intraperitoneal injection of cancer cells)
(30,31). We aimed to explore the specific
inhibitory mechanisms of 3-BrPA and SCT and their effects on
apoptosis-related genes in gastric cancer. Moreover, we aimed to
determine whether an intraperitoneal injection is an effective form
of administration of 3-BrPA and SCT.
Materials and methods
Reagents
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA).
3-BrPA, SCT and the chemotherapeutic agent 5-fluorouracil (5-FU)
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and animals
The human gastric cancer cell line SGC-7901 was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI-1640 medium
supplemented with 10% FBS, 100,000 U/l penicillin, and 100 mg/l
streptomycin at 37°C in an incubator with 5% CO2. The
cells were harvested after trypsinization by 0.025% trypsin with
0.02% EDTA and washed twice with phosphate-buffered saline (PBS).
The cells were split for further culture once they reached ~80%
confluency. Experiments were not conducted until the cells were in
logarithmic growth phase. The 5-to-6-week old female BALB/c nude
mice (weighing between 18 and 20 g) were purchased from the Animal
Experimental Center of Guangxi Medical University (Guangxi, China)
and fed under specific pathogen-free conditions. The experimental
protocol was carried out under the supervision of the Ethics
Committee of Guangxi Medical University, and in accordance with
internationally recognized guidelines on Animal Welfare.
Cell viability assay
Cell viability was assessed with the modified
tetrazolium salt
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT)
method. Briefly, SGC-7901 cells were exposed to different
concentrations of 3-BrPA or SCT (or 5-FU or the PBS control) for 24
or 48 h after being seeded onto 96-well plates (~2,000 cells/well)
for 24 h. The 3-BrPA and SCT solutions were prepared in RPMI-1640
medium, adjusted to pH 7.4 with NaOH, and then sterilized using a
0.22-µm filter unit (Millipore, Billerica, MA, USA). To each
well, 0.01 ml MTT solution (5 mg/ml) was added and incubated at
37°C for 4 h. Formazan crystals were then dissolved with DMSO, and
the optical densities at 490 nm were measured using a microplate
reader (Bio-Rad, Hercules, CA, USA). The IC50 value
(concentration of 50% inhibition) was obtained from three
independent repetitive trials.
Analysis of cell morphology
SGC-7901 cells were cultured with 5-FU (0.5 mmol/l)
and different concentrations of 3-BrPA and SCT for 24 h. The
morphological changes in cells were photographed using inverted
microscopy (Nikon, Tokyo, Japan).
Analysis of cell apoptosis
The apoptosis of SGC-7901 cells was analyzed by flow
cytometry (FCM) with the Annexin V:PE Apoptosis Detection kit I (BD
Biosciences, USA). Briefly, the cells were collected after being
treated for 24 or 48 h with 3-BrPA, SCT, 5-FU or the PBS control,
resuspended in 100 µl of binding buffer with 5 µl
Annexin V-PE and 5 µl 7-amino-actinomycin D (7-AAD), washed
twice with cold PBS, and then incubated in the dark at room
temperature for 15 min. The samples were then analyzed by FCM
(Beckman Coulter, Miami, FL, USA).
Cell cycle assay
SGC-7901 cells were collected after being treated
for 24 h with 3-BrPA, SCT, 5-FU or the PBS control, washed twice
with PBS and fixed in 70% cold ethanol. The cells were then
incubated with RNase A (0.1 mg/ml) at 37°C for 30 min, and then
with propidium iodine (PI, 0.2 mg/ml) at 4°C for 60 min before
analysis. Cell cycle progression was measured using the Cell Cycle
Detection kit (Keygen, Nanjing, China) and analyzed by FCM.
Glycolytic enzyme, ATP and lactate
assays
According to the manufacturer's instructions in the
HK, PFK-1, PK, ATP and lactate assay kits (Jiancheng, Nanjing,
China), we measured the activity or concentration of HK, PFK-1, PK,
ATP and lactate spectrophotometrically (UV-2450; Shimadzu, Japan)
at an absorbance of 340 nm. Samples were obtained from SGC-7901
cells after being treated for 24 or 48 h with 3-BrPA, SCT, 5-FU or
the PBS control. The activities of the enzymes were calibrated with
cellular protein concentration.
Quantitative real-time PCR (RT-qPCR)
assay
Total RNA was prepared by using the total RNA
Extraction kit (Axygen, Union City, CA, USA) and
reverse-transcribed with the PrimeScript® RT reagent kit
(Takara, Tokyo, Japan). The primers for Bax, Bcl-2, Cyt-C,
survivin, and GAPDH synthesized by Sangong Biotech (Shanghai,
China) are shown in Table I. The
reaction was carried out using the SYBR Green PCR Master Mix
(Roche, USA). Real-time PCR assays were performed using Applied
Biosystems® 7500 Real-Time PCR systems (Life
Technologies, MA, USA) according to the manufacturer's
instructions. PCR was carried out for 40 cycles of 95°C for 15 sec
and 60°C for 30 sec. The mRNA expression levels were calculated
relative to GAPDH by using the 2−ΔΔCT method.
 | Table IPrimers of related genes used for
RT-qPCR analysis. |
Table I
Primers of related genes used for
RT-qPCR analysis.
| Genes | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) |
|---|
| Bax |
CCGATTCATCTACCCTGCTG |
TGAGCAATTCCAGAGGCAGT |
| Bcl-2 |
GAGGATTGTGGCCTTCTTTG |
GTGCCGGTTCAGGTACTCA |
| Cyt-C |
TGTCGGCATTAAGAAGAAGGA |
TAAATCAGGACTGCCCAACA |
| Survivin |
TCAAGGACCACCGCATCTCT |
CAGTGGGGCAGTGGATGAA |
| GAPDH |
GTCAGCCGCATCTTCTTT |
CGCCCAATACGACCAAAT |
Western blot analysis
The total protein was extracted from the SGC-7901
cells after being treated for 24 h with 3-BrPA, SCT, 5-FU or the
PBS control, and protein concentration was determined using the BCA
method. A 15% separation gel was used to separate the proteins
using SDS-PAGE at a constant voltage (80 mV) for 90 min. A 5%
stacking gel and water bath method were used to transfer the
membranes at a constant current (180 mA) for 30–75 min. The NC
membrane (Millipore) was blocked with 5% skimmed milk for 2 h at
room temperature. The rabbit antibodies against Bcl-2, Bax, Cyt-C,
survivin, GAPDH and the anti-rabbit IgG DyLight 800 conjugated
antibody were purchased from Cell Signaling Technology (Boston, MA,
USA). The rabbit antibody against cleaved caspase-3 (P17KD
fragment) was purchased from Neomarker (USA). The primary
antibodies were diluted with primary antibody dilution buffer
(Beyotime, Shanghai, China) and incubated at 4°C in the
refrigerator overnight. The secondary antibodies were added to the
slides, and then incubated for 90 min at room temperature. The gray
scale values of the protein bands were determined by Odyssey
software (LI-COR, Lincoln, NE, USA).
Tumor model of gastric orthotopic
transplantation in nude mice
The back of the axillary region of each nude mouse
was subcutaneously injected with 0.2 ml (2×106/ml) of
the SGC-7901 cell suspension. When the subcutaneous tumor became
palpable (i.e., ~5 mm after ~2 weeks), the tumor tissue was excised
from the nude mouse, and then minced. The minced tumor tissue
became the subcutaneous transplant for the next group of nude mice,
and this subcutaneous transplantation was repeated for six
generations. The tumor tissue from the sixth generation nude mice
was minced to 1–2 mm3 in size before being orthotopic
transplanted inside the seromuscular layer of greater curvature. We
chose the seromuscular layer near the antrum of the greater
curvature as the preferable operation site for its rich blood flow.
On account of its secure wound adhesion, we used 1–2 drops of
medical OB glue (32) to bind the
serosal layer incision, before covering it with the greater
omentum. We waited for ~10 sec for the glue to fully congeal to
avoid the tumor tissue masses falling off. The tumor was allowed to
grow for 2 weeks before treatment was initiated.
Mice were then randomized into 8 groups (8
mice/group) as follows: 3-BrPA low-dose group (3-BrPA-L, 1.85
mg/kg/day), 3-BrPA medium-dose group (3-BrPA-M, 2.23 mg/kg/day),
3-BrPA high-dose group (3-BrPA-H, 2.67 mg/kg/day), SCT low-dose
group (SCT-L, 7.5 mg/kg/day), SCT medium-dose group (SCT-M, 15
mg/kg/day), SCT high-dose group (SCT-H, 30 mg/kg/day), 5-FU group
(5-FU, 10 mg/kg/day), and control group (PBS, 10 ml/kg/day).
3-BrPA, SCT and 5-FU were dissolved in PBS, adjusted to pH 7.4 with
NaOH, and then sterilized with a 0.22-µm filter unit. All
mice were intraperitoneally injected with 0.2 ml of the drug or PBS
once per day for 4 weeks. Any changes observed in the nude mice,
such as behavior, eating and excretion were monitored. The tumors
were harvested after 4 weeks of treatment, and tumor volume was
calculated as 0.5 × length × width × thickness.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptosis in the tumor tissue was investigated by
TUNEL staining using the In Situ Cell Death Detection kit (Roche,
USA). The percentages of positive cells from total cells were
considered as apoptotic indices (AI) after being counted in six
different high power fields. The tumor tissue sections were
observed under an IX73 inverted microscope (Olympus, Tokyo,
Japan).
Transmission electron microscope (TEM)
assay
The ultra-structure of the tumors was observed by
TEM. The tumor tissues were fixed in 2.5% glutaraldehyde and 1%
osmium tetroxide, dehydrated by graded ethanol, and embedded in
Epon. Ultrathin sections were stained with 2% uranyl acetate and
lead citrate, and then examined under a JEM-2000EX transmission
electron microscope (Jeol, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Chicago, IL, USA). The data are presented as
the mean ± standard deviation (SD). Differences between multiple
groups were analyzed with one-way analysis of variance. Pairwise
comparison was performed using the t-test, with significant
differences determined as a value of P<0.05 (P<0.05 vs. the
control group and P<0.05 vs. the 5-FU group).
Results
Morphological changes induced by 3-BrPA
and SCT
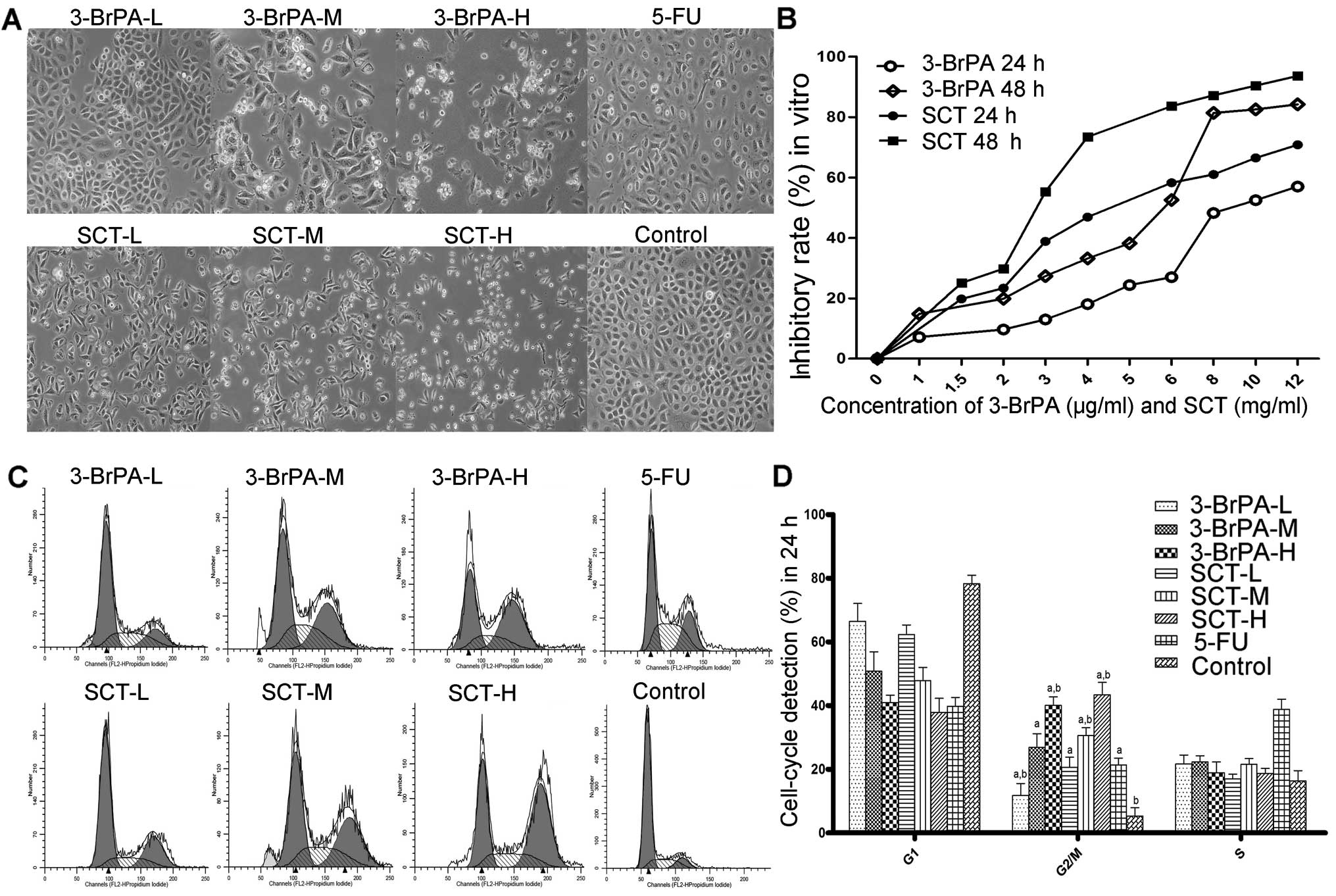
As shown in Fig. 1A,
untreated cells attached closely to one another, and were polygonal
in shape. However, the cells in the 5-FU-, 3-BrPA-, or SCT-treated
groups became round or inflated, with fewer cellular contacts.
Cell viability is reduced by 3-BrPA and
SCT
We measured the proliferative effect of 3-BrPA and
SCT on SGC-7901 cells using the MTT assay. As shown in Fig. 1B, we found a time- and
dose-dependent decrease in cell viability after exposure to 3-BrPA
or SCT. The IC50 values of 3-BrPA against SGC-7901 cells
after being treated for 24 or 48 h were 9.88±1.07 and 5.97±0.95
µg/ml, respectively; however, the IC50 values of
SCT were 6.47±0.87 and 3.84±0.59 mg/ml. Therefore, for our in
vitro study we created the following eight treatment groups:
3-BrPA low-dose group (3-BrPA-L, 4 µg/ml), 3-BrPA
medium-dose group (3-BrPA-M, 6 µg/ml), 3-BrPA high-dose
group (3-BrPA-H, 8 µg/ml), SCT low-dose group (SCT-L, 1.5
mg/ml), SCT medium-dose group (SCT-M, 3 mg/ml), SCT high-dose group
(SCT-H, 6 mg/ml), 5-FU group (5-FU, 0.5 mmol/l), and control group
(untreated).
Cell cycle arrest is induced by 3-BrPA
and SCT
The effect of 3-BrPA and SCT on the cell cycle was
detected by FCM. As shown in Fig. 1C
and D, after exposure to 3-BrPA, SCT or 5-FU for 24 h, the cell
population in the G2/M phases was significantly increased
(P<0.05). The cell population in the S phase of the 5-FU group
was also obviously increased (P<0.05). The results suggest that
one of the inhibitory mechanisms of 3-BrPA or SCT is to induce cell
cycle arrest to increase the rate of apoptosis.
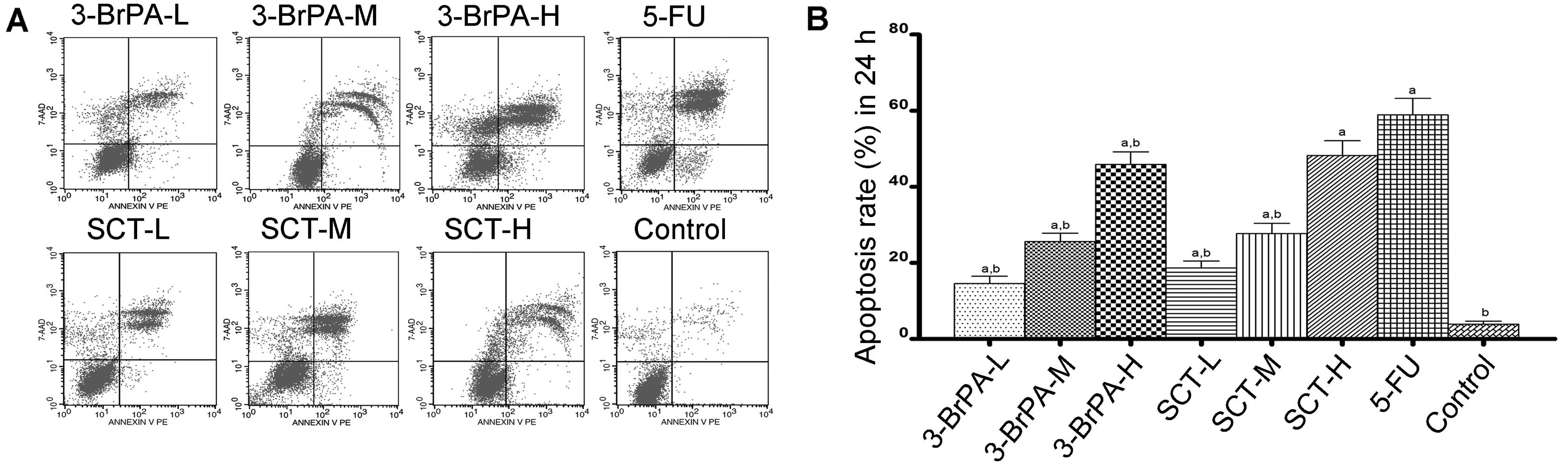
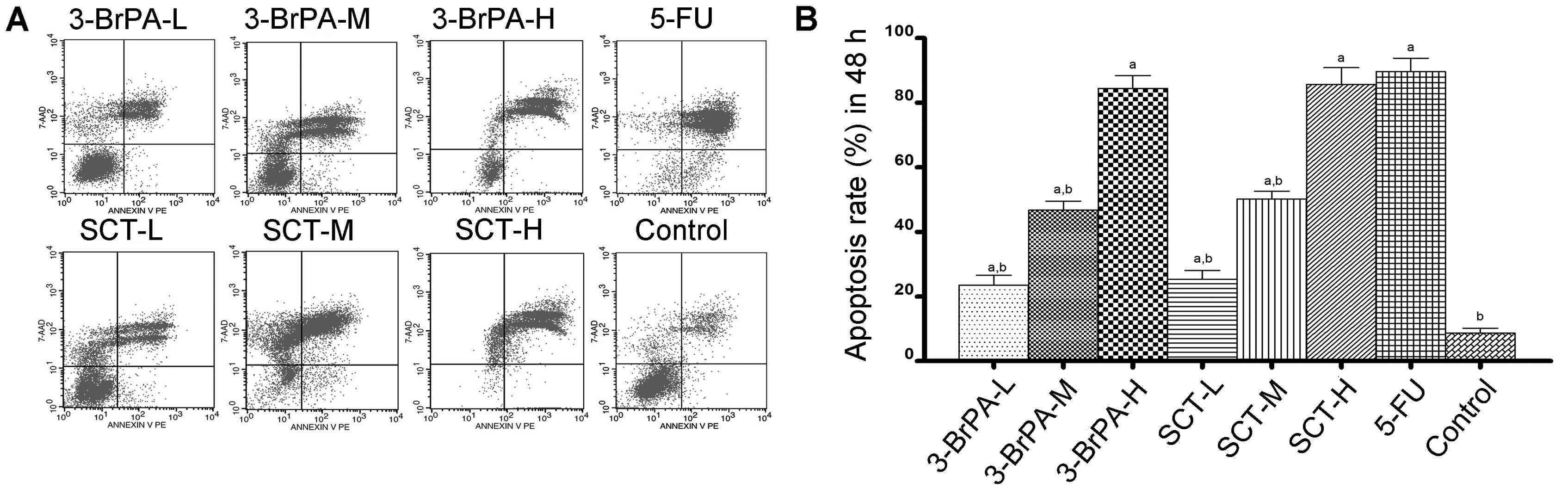
Cell apoptosis is induced by 3-BrPA and
SCT
To determine whether 3-BrPA- and SCT-induced cell
death is related to apoptosis, an Annexin V:PE staining assay was
conducted. As shown in Figs. 2 and
3, the percentage of apoptosis was
increased from 15.60±1.83% after 24 h to 84.45±3.97% after 48 h in
the 3-BrPA-treated groups, and increased from 19.31±1.89 to
88.34±5.22% in the SCT groups. As a positive control, the
percentage was increased from 61.57±4.30 to 92.64±4.15% after
treatment with 5-FU. These increases in apoptosis percentages were
significant compared with the control group (3.24±0.72% after 24 h
and 9.70±1.52% after 48 h) (P<0.05). The result demonstrated
that dose- and time-dependent apoptosis occurs after exposure to
3-BrPA or SCT. Moreover, the reductions in cell viability using the
MTT assay correlated well with the induction of apoptosis by 3-BrPA
or SCT.
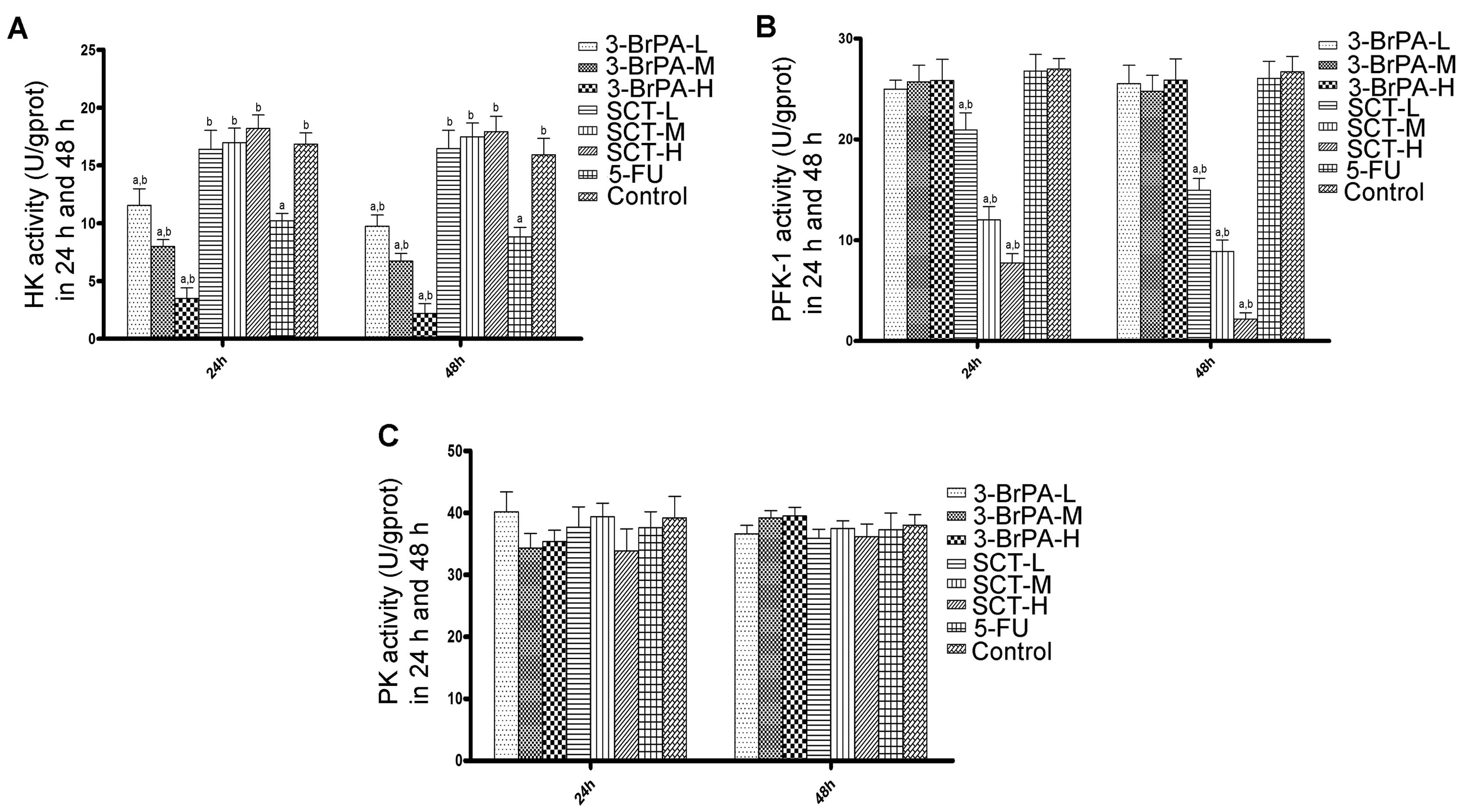
Regulation of glycolytic enzyme activity
by 3-BrPA and SCT
We evaluated the activities of HK, PFK-1 and PK to
investigate whether 3-BrPA and SCT kill cancer cells through
regulation of glycolytic enzymes. As shown in Fig. 4A, 3-BrPA significantly reduced HK activity in
a time- and dose-dependent manner (P<0.05). The HK activity
observed in the 5-FU group was less than that in the 3-BrPA-L group
but more than that in the 3-BrPA-M group (P<0.05). HK activity
in the SCT groups had no difference when compared with the activity
in the control group (P>0.05). As shown in Fig. 4B, PFK-1 activity decreased in a
time- and dose-dependent manner in the SCT groups (P<0.05).
However, PFK-1 activity was not inhibited by 3-BrPA or 5-FU
(P>0.05). As shown in Fig. 4C,
there were no significant difference noted in PK activity in the
5-FU, 3-BrPA, and SCT groups compared with the control group
(P>0.05).
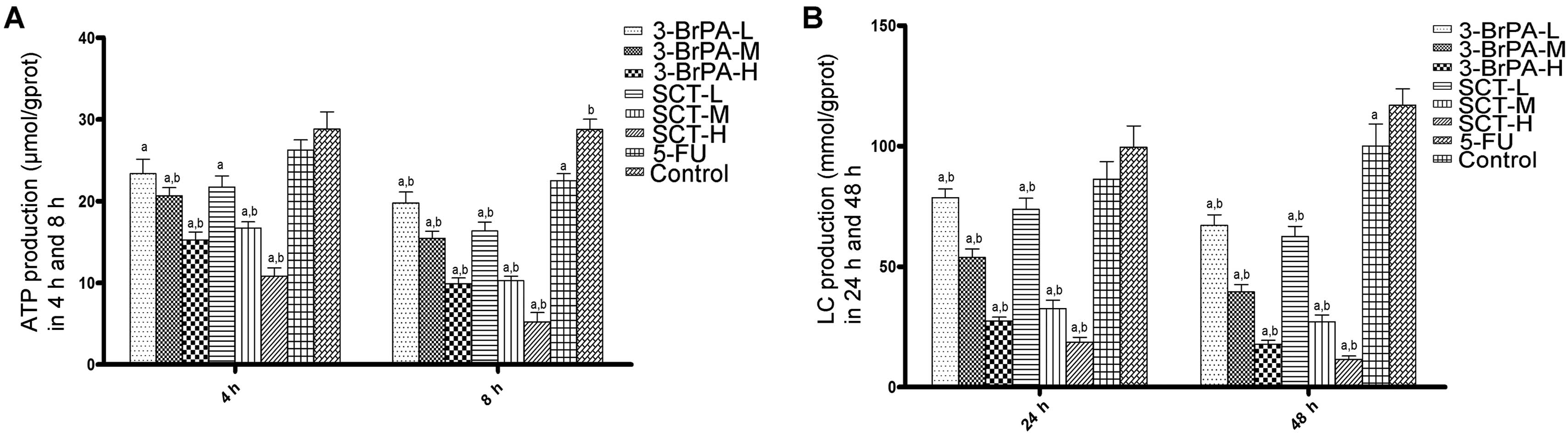
ATP and lactate production is reduced by
3-BrPA and SCT
ATP and lactate are the main end products of
glycolysis, and are regarded as efficient indicators of the
glycolysis rate. As shown in Fig.
5, both the 3-BrPA and SCT groups exhibited a significant time-
and dose-dependent decrease in cellular ATP levels and lactate
production (P<0.05). Moreover, the cellular ATP and lactate
levels in the 5-FU group after treatment for 8 h were obviously
reduced compared with the control group (P<0.05). These results
indicate that 3-BrPA and SCT may cause apoptosis by blocking
glycolysis, which is required for energy metabolism.
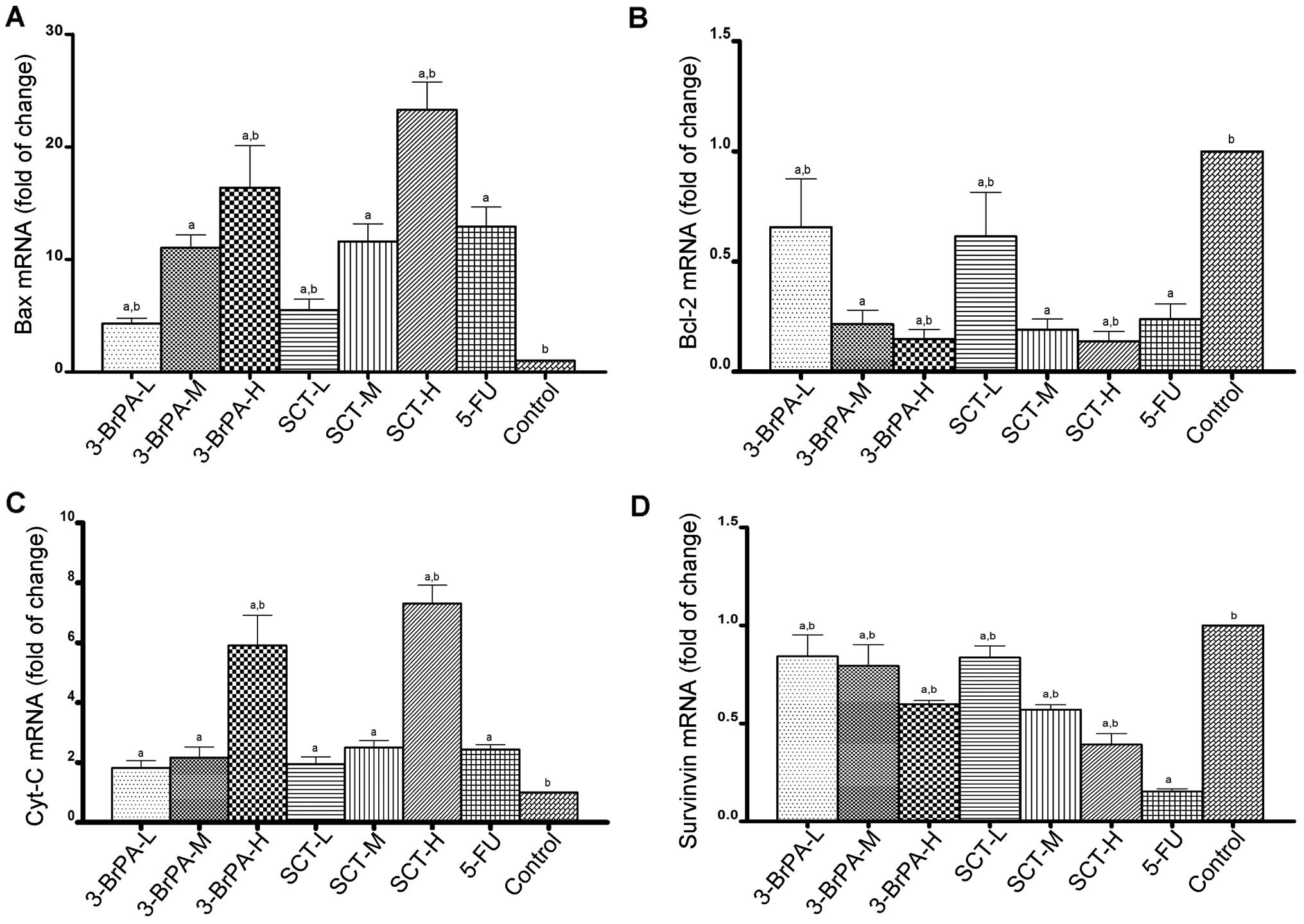
Expression of apoptosis-related genes is
altered by 3-BrPA and SCT
To determine the effect of apoptosis-related genes
induced by 3-BrPA and SCT, the mRNA levels of Bax, Bcl-2, Cyt-C and
survivin were detected by RT-qPCR analysis. As shown in Fig. 6, compared with the control group,
the mRNA levels of Bax and Cyt-C were increased, while the mRNA
levels of Bcl-2 and survivin were decreased after exposure to
3-BrPA, SCT, or 5-FU (P<0.05). Furthermore, we found that 3-BrPA
or SCT produced these effects in a dose-dependent manner. The mRNA
levels of Bax and Cyt-C in the SCT-H group were higher than these
levels in the 3-BrPA-H group (P<0.05). While 5-FU also increased
the mRNA expression of Bax and Cyt-C, and decreased the mRNA
expression of Bcl-2, it was less effective than 3-BrPA-H and SCT-H
(P<0.05). However, the inhibitory effect of 5-FU on survivin
mRNA expression was the highest among all the groups
(P<0.05).
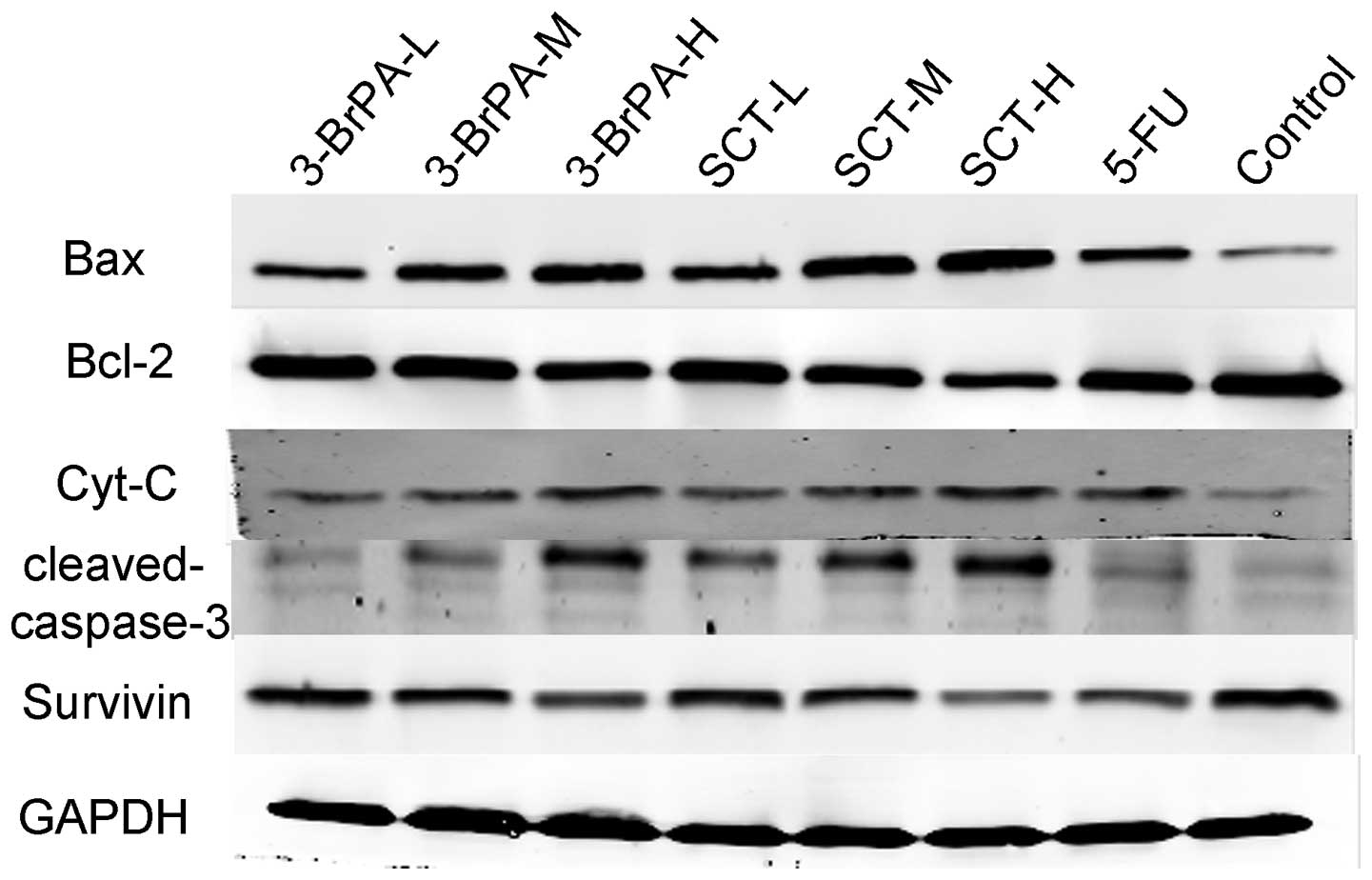
Expression of apoptosis-related proteins
is altered by 3-BrPA and SCT
To further confirm that 3-BrPA and SCT regulate the
expression of apoptosis-related genes, we determined the protein
expression of Bax, Bcl-2, Cyt-C, cleaved caspase-3, and survivin by
western blot analysis. As shown in Fig.
7, 3-BrPA and SCT both
upregulated Bax, Cyt-C, and cleaved caspase-3 protein expression,
and downregulated Bcl-2 and survivin protein expression in a
dose-dependent manner (P<0.05). 5-FU also increased the protein
expression of Bax, Cyt-C, cleaved caspase-3 and decreased the
expression of Bcl-2 and survivin, but its ability was weaker than
3-BrPA-M and SCT-M (P<0.05). The increased expression of Bax,
and downregulation of Bcl-2 and survivin, in the 3-BrPA-H group was
less than that in the SCT-H group (P<0.05). Expression levels of
Cyt-C and cleaved caspase-3 in the 3-BrPA-H group were not
significantly different when compared with the SCT-H group
(P>0.05).
3-BrPA and SCT inhibit gastric orthotopic
transplantation tumor growth in vivo
To determine whether 3-BrPA and SCT are effective
against gastric tumors in vivo, we produced a gastric
orthotopic transplantation tumor model and administered an
intraperitoneal injection of 3-BrPA, SCT, 5-FU or PBS for 4 weeks.
As shown in Fig. 8A, models of
human gastric orthotopic transplantation tumors in nude mice were
successfully made, and the tumor formation rate was 85%. As shown
in Fig. 8A and B, tumor volumes in
the 3-BrPA-, SCT-, or 5-FU-treated mice were significantly reduced
compared with the PBS-treated mice (P<0.05). Furthermore, 3-BrPA
and SCT inhibited the orthotopic transplantation tumor growth in a
dose-dependent manner (P<0.05).
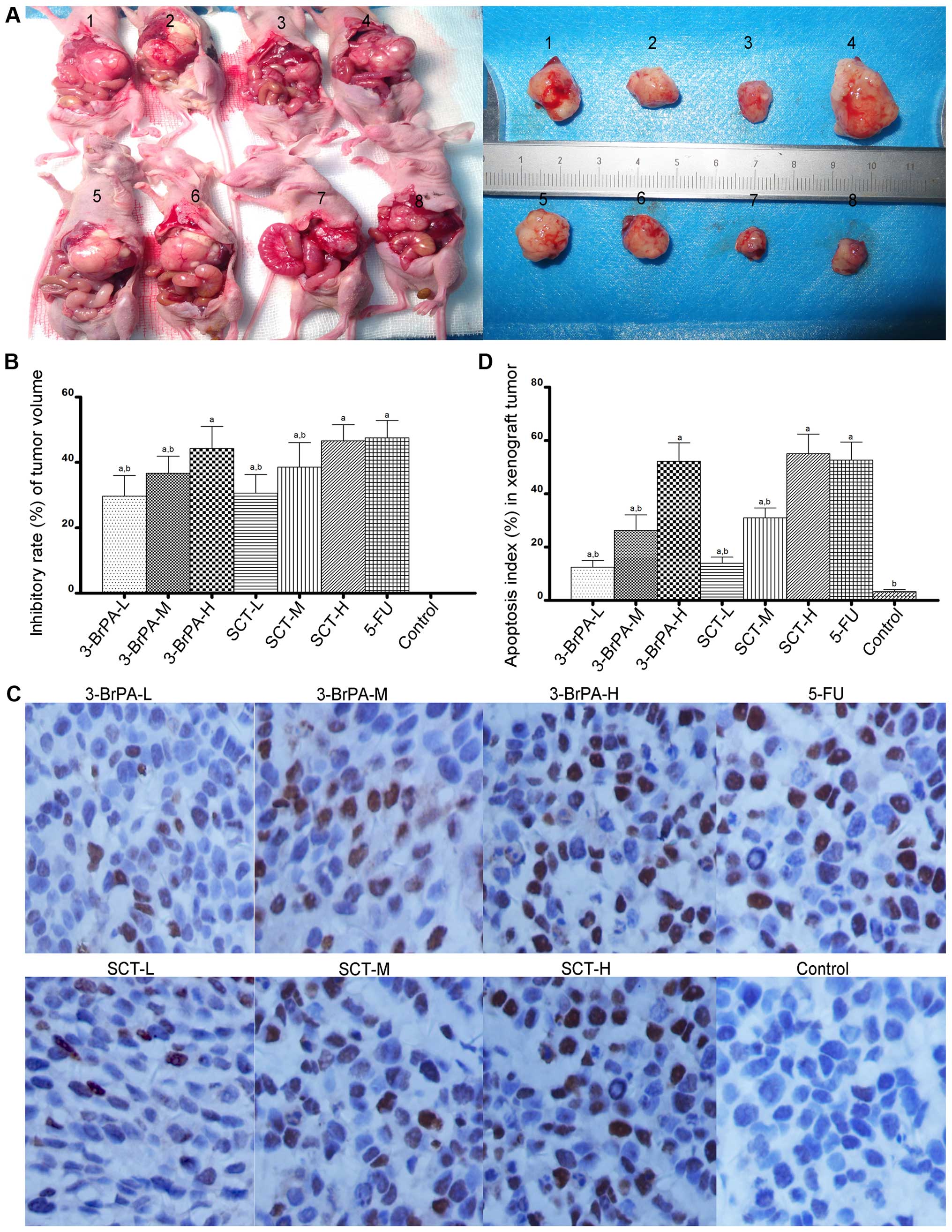 | Figure 83-BrPA and SCT mediate the inhibition
of gastric orthotopic transplantation tumor growth and induction of
apoptosis. (A) The tumor model of gastric orthotopic
transplantation in nude mice was successfully constructed. Image 1,
3-BrPA-L group; 2, 3-BrPA-M group; 3, 3-BrPA-H group; 4, control
(PBS) group; 5, SCT-L group; 6, SCT-M group; 7, SCT-H group; 8,
5-FU group. (A and B) After an intraperitoneal injection of 3-BrPA,
SCT or 5-FU for 4 weeks, tumor volumes in the 3-BrPA-, SCT- or
5-FU-treated mice were significantly reduced
(aP<0.05). (C) Images of TUNEL revealing apoptotic
cells which appeared as brownish granules in the cytoplasm and
nucleus. A greater proportion of apoptotic cells was observed in
the 3-BrPA, SCT and 5-FU groups (magnification, x400). (D) A
significant difference in AI values was noted between the 3-BrPA-,
SCT- or 5-FU-treated group and the PBS-treated group
(aP<0.05). |
3-BrPA and SCT induce apoptosis in the
gastric orthotopic transplantation tumors
We next evaluated whether 3-BrPA and SCT induce
apoptosis in orthotopic transplantation tumors by TUNEL staining.
As shown in Fig. 8C, a greater
proportion of apoptotic cells, appearing with brownish granules in
the cytoplasm and nucleus, were observed in the 3-BrPA, SCT, and
5-FU groups. As shown in Fig. 8D,
the apoptosis index (AI) in each group was as follows: 3-BrPA-L
group 12.50±2.43%; 3-BrPA-M group 26.29±5.76%; 3-BrPA-H group
52.21±6.92%; SCT-L group 13.98±2.28%; SCT-M group 30.93±3.79%;
SCT-H group 55.07±7.38%; 5-FU group 52.71±6.73%; and control group
3.29±0.76%. A significant difference in AI values was identified
between the 3-BrPA-, SCT-, or 5-FU-treated group and the control
group (P<0.05). The AI of the 5-FU group was similar to the
3-BrPA-H and SCT-H groups (P>0.05). Furthermore, 3-BrPA-H and
SCT-H both induced apoptosis of the orthotopic transplantation
tumors in a dose-dependent manner.
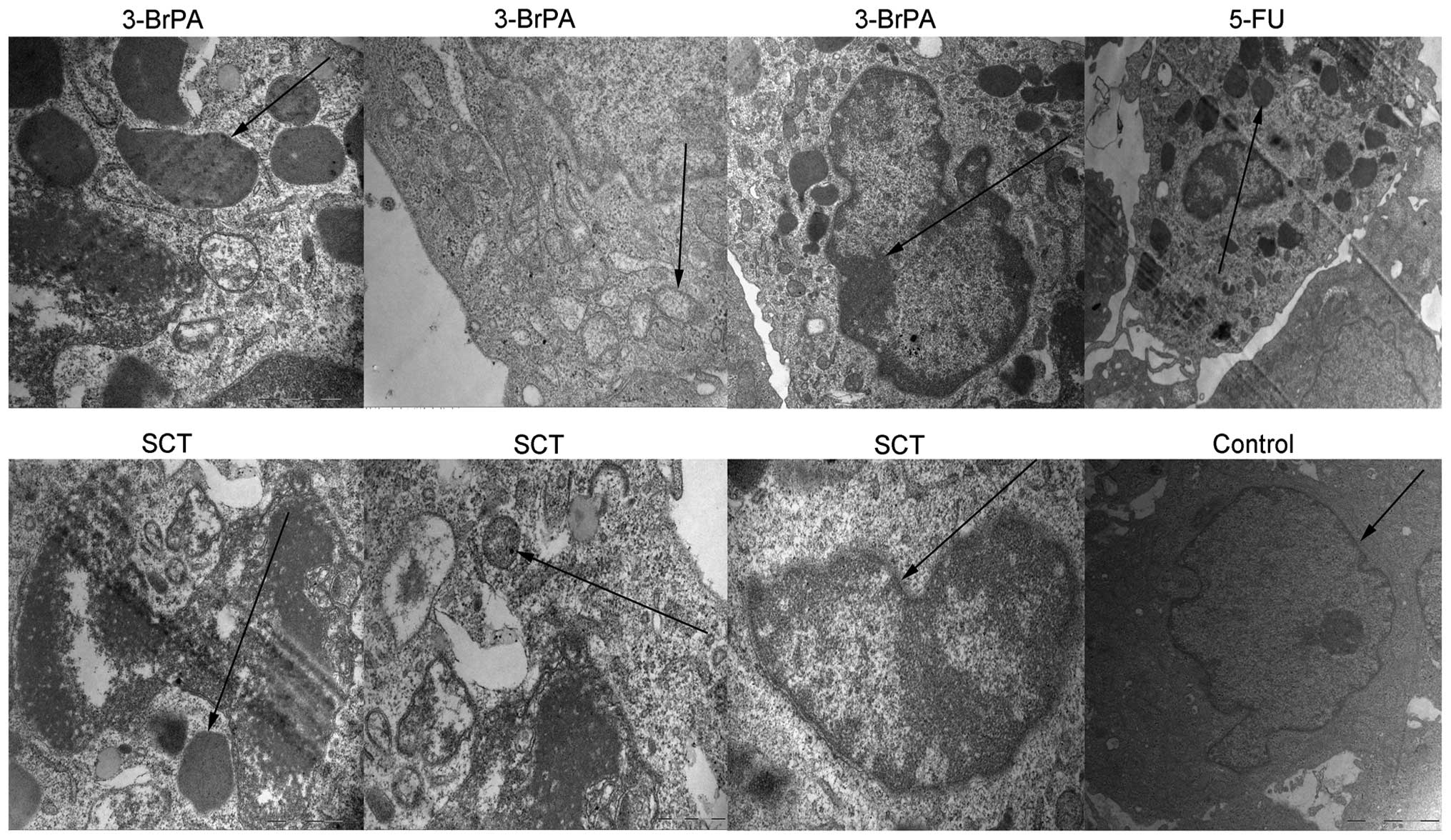
3-BrPA and SCT induce ultrastructure
changes in the tumors typical of apoptosis
To further confirm the apoptotic effects,
ultrastructural changes in the tumors treated by 3-BrPA, SCT, or
5-FU were observed by TEM. As shown in Fig. 9, typical features of apoptosis, such
as the formation of apoptotic bodies, mitochondrial crest fracture,
or disappearance and chromatin concentration or fragmentation, were
observed in the 3-BrPA, SCT and 5-FU groups. However, the control
group had large nuclei, with prominent nucleoli, and no apoptotic
bodies were observed.
Discussion
In the present study, we explored the mechanisms of
3-BrPA-and SCT-mediated inhibition of in vitro human gastric
cancer cell growth, and in vivo gastric orthotopic
transplantation tumor growth in nude mice. We found that 3-BrPA and
SCT effectively suppressed cancer cell proliferation, arrested the
cell cycle in the G2/M phases, induced apoptosis, and decreased the
production of lactate and ATP, which indicates inhibition of
glycolysis. Moreover, 3-BrPA significantly reduced the activity of
the glycolytic enzyme HK, while SCT selectively inhibited the
activity of the glycolytic enzyme PFK-1 in a time- and
dose-dependent manner. Furthermore, 3-BrPA and SCT upregulated Bax,
Cyt-C, and cleaved caspase-3, but downregulated Bcl-2 and survivin
mRNA and protein expression. Finally, our in vivo animal
study indicated that intraperitoneal injections of 3-BrPA and SCT
suppressed orthotopic transplantation tumor growth and induced
tumor apoptosis.
While glycolysis and apoptosis have previously been
regarded as independent pathways (30,31),
our results indicated that cell apoptosis and cell cycle arrest
were closely associated with glycolytic enzyme inhibition. 3-BrPA
significantly reduced the activity of the glycolytic enzyme HK. By
interacting with the outer membrane protein voltage dependent anion
channel (VDAC), HK can stop the release of proteins from the
mitochondrial intermembrane space, including Bax and Cyt-C. Thus,
HK can enhance cell proliferation and suppress apoptosis by binding
to mitochondria (14). We speculate
that 3-BrPA inhibits HK activity and isolates it from the
mitochondria, allowing the VDAC to open, and release Cyt-C, thereby
inducing caspase-mediated apoptosis. Therefore, there is a link
between glycolysis and the mitochondrial apoptotic pathway, which
can both be targeted by 3-BrPA and/or SCT.
We also found that 3-BrPA and SCT downregulated
survivin expression. Survivin specifically binds caspase-3,
caspase-7, and caspase-9, and inhibits their activity to suppress
apoptosis (33). Therefore, by
downregulating survivin expression, apoptosis was no longer
suppressed in the gastric cancer cells. This result suggests that
3-BrPA and SCT have multiple mechanisms by which they promote
apoptosis and prevent cell proliferation in gastric tumors.
Next, we determined whether 3-BrPA and SCT were
effective against gastric tumors in vivo, by using a gastric
orthotopic transplantation tumor model in nude mice and monitoring
the tumor volume inhibition rate and tumor apoptosis. Our data
showed that 3-BrPA and SCT suppressed the growth and induced the
apoptosis of human gastric cancer tumors in vivo. Moreover,
we determined that intraperitoneal injection is an effective form
of administration of 3-BrPA and SCT.
In conclusion, the present study identified that
cell cycle arrest, glycolytic enzyme inhibition, decreased ATP
production, mitochondrial apoptotic pathway activation, and
survivin suppression may be the mechanisms by which 3-BrPA and SCT
inhibit proliferation and induce apoptosis in gastric cancer cells.
Furthermore, intraperitoneal injections of 3-BrPA and SCT into our
mouse model of gastric cancer suppressed tumor growth and induced
apoptosis in vivo. However, whether selectively targeting
glycolysis to exhaust ATP availability and induce apoptosis also
increases cancer cell sensitivity to radiation and chemotherapy
requires further investigation.
Acknowledgments
This research was supported by the National Natural
Science Foundation of China (grant no. 81260366) and Guangxi
Scientific Research and Technology Development Project.
References
|
1
|
Yamamoto M, Sakaguchi Y, Matsuyama A,
Yoshinaga K, Tsutsui S and Ishida T: Surgery after preoperative
chemotherapy for patients with unresectable advanced gastric
cancer. Oncology. 85:241–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corso S, Ghiso E, Cepero V, Sierra JR,
Migliore C, Bertotti A, Trusolino L, Comoglio PM and Giordano S:
Activation of HER family members in gastric carcinoma cells
mediates resistance to MET inhibition. Mol Cancer. 9:1212010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warburg O: The Metabolism of Tumors.
Constable Press; London: 1930
|
|
5
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, Yu J, Chan FK, Sung JJ and Jin HC: Warburg effect
revisited: An epigenetic link between glycolysis and gastric
carcinogenesis. Oncogene. 29:442–450. 2010. View Article : Google Scholar
|
|
6
|
Lu Y, Zhang X, Zhang H, Lan J, Huang G,
Varin E, Lincet H, Poulain L and Icard P: Citrate induces apoptotic
cell death: A promising way to treat gastric carcinoma? Anticancer
Res. 31:797–805. 2011.PubMed/NCBI
|
|
7
|
Xian SL, Wei C and Lu YF: 3 BrPA inhibits
proliferation of human gastric cancer cell. Chin Pract Med J.
12:78–82. 2013.In Chinese.
|
|
8
|
Liu L, Gong L, Zhang Y and Li N:
Glycolysis in Panc-1 human pancreatic cancer cells is inhibited by
everolimus. Exp Ther Med. 5:338–342. 2013.
|
|
9
|
Khatri S, Yepiskoposyan H, Gallo CA,
Tandon P and Plas DR: FOXO3a regulates glycolysis via
transcriptional control of tumor suppressor TSC1. J Biol Chem.
285:15960–15965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Floridi A, Paggi MG and Fanciulli M:
Modulation of glycolysis in neuroepithelial tumors. J Neurosurg
Sci. 33:55–64. 1989.PubMed/NCBI
|
|
11
|
Nwagwu M and Opperdoes FR: Regulation of
glycolysis in Trypanosoma brucei: Hexokinase and
phosphofructokinase activity. Acta Trop. 39:61–72. 1982.PubMed/NCBI
|
|
12
|
Okar DA and Lange AJ:
Fructose-2,6-bisphosphate and control of carbohydrate metabolism in
eukaryotes. Biofactors. 10:1–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rider MH, Bertrand L, Vertommen D, Michels
PA, Rousseau GG and Hue L:
6-phosphofructo-2-kinase/fructose-2,6-bisphos-phatase: Head-to-head
with a bifunctional enzyme that controls glycolysis. Biochem J.
381:561–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gottlob K, Majewski N, Kennedy S, Kandel
E, Robey RB and Hay N: Inhibition of early apoptotic events by
Akt/PKB is dependent on the first committed step of glycolysis and
mitochondrial hexokinase. Genes Dev. 15:1406–1418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vander Heiden MG, Plas DR, Rathmell JC,
Fox CJ, Harris MH and Thompson CB: Growth factors can influence
cell growth and survival through effects on glucose metabolism. Mol
Cell Biol. 21:5899–5912. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
18
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
19
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grosse J, Warnke E, Wehland M, Pietsch J,
Pohl F, Wise P, Magnusson NE, Eilles C and Grimm D: Mechanisms of
apoptosis in irradiated and sunitinib-treated follicular thyroid
cancer cells. Apoptosis. 19:480–490. 2014. View Article : Google Scholar
|
|
21
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim H, Rafiuddin-Shah M, Tu HC, Jeffers
JR, Zambetti GP, Hsieh JJ and Cheng EH: Hierarchical regulation of
mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell
Biol. 8:1348–1358. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko YH, Pedersen PL and Geschwind JF:
Glucose catabolism in the rabbit VX2 tumor model for liver cancer:
Characterization and targeting hexokinase. Cancer Lett. 173:83–91.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pedersen PL: 3-Bromopyruvate (3BP) a fast
acting, promising, powerful, specific, and effective 'small
molecule' anti-cancer agent taken from labside to bedside:
Introduction to a special issue. J Bioenerg Biomembr. 44:1–6. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shoshan MC: 3-Bromopyruvate: Targets and
outcomes. J Bioenerg Biomembr. 44:7–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Killion JJ, Radinsky R and Fidler IJ:
Orthotopic models are necessary to predict therapy of
transplantable tumors in mice. Cancer Metastasis Rev. 17:279–284.
1998–1999. View Article : Google Scholar
|
|
31
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi J, Wei PK, Zhang S, Qin ZF, Li J, Sun
DZ, Xiao Y, Yu ZH, Lin HM, Zheng GJ, et al: OB glue paste technique
for establishing nude mouse human gastric cancer orthotopic
transplantation models. World J Gastroenterol. 14:4800–4804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|