Introduction
Prostate cancer occupies the first place of new
cases and the second leading cause of cancer mortality in males,
with an estimated 220,800 new cases and 27,540 deaths in US in 2015
(1). When prostate cancer is at the
early stage, it can be cured by radical prostatectomy or radiation.
However, local recurrence and distant metastasis will occur in most
of these patients later (2).
Androgen ablation can act as an important effective treatment for
the first 1–3 years, after this period it gradually develops into
castration-resistant prostate cancer (CRPC) with the
characteristics of elevated PSA level, higher metastasis rate and
more aggressiveness (3). At
present, the widely used first-line systemic chemotherapy for CRPC
is the combination of docetaxel and prednisone, but it is not a
curative scheme and only prolongs overall survival time for a short
period. In addition, it is associated with severe side-effects
(4). Therefore, the lack of
effective treatment for prostate cancer arouses a great necessity
to develop novel drug therapy to overcome these shortcomings and
work effectively for CRPC.
In recent years, novel cancer therapies aiming to
inhibit angiogenesis and tumor growth have attracted great
interests of researchers among which quercetin
(3,3′,4′,5,7-pentahydroxyflavone) is one of the most important
naturally-occurring flavonoid compounds (5–7). A
large amount of quercetin exists in vegetables and fruits
particularly in onions, red wine, tea and apples, and it has
demonstrated fine anti-prostate cancer property through many
different mechanisms (7). Our
previous study reported preliminary results that quercetin could
effectively inhibit human prostate cancer cell xenograft tumor
growth by inhibiting angiogenesis (8). Thrombospondin-1 (TSP-1) is a 450 kDa
extracellular calcium binding glycoprotein belonging to family of
thrombospondins. It is the first reported endogenous
anti-angiogenic factor and the widely accepted inhibitor of
angiogenesis and tumorigenesis (9).
However, the relationship between quercetin inhibiting angiogenesis
and TSP-1 upregulation in prostate cancer has not been reported
(7). Herein, we explored the
important role of TSP-1 upregulation in the angiogenesis inhibition
and anti-prostate cancer effect of quercetin both in vitro
and in vivo for the first time.
Materials and methods
Ethics statement
All the animal experiment procedures were approved
by the Committee of Animal Experimentation and the Ethics Committee
of Capital Medical University (Permit No. 2013-X-83). All the
operations related with animals were in strict accordance with the
requirements and guidelines of experimental animal ministry.
Chemical reagents
Quercetin, dimethyl sulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and hydroxypropyl-β-cyclodextrin (HPβCD) were purchased from Sigma
(St. Louis, MO, USA). RPMI-1640 medium, 0.25% trypsin-EDTA,
phosphate-buffered saline (PBS) and fetal bovine serum (FBS) were
purchased from HyClone (Logan, UT, USA). VascuLife VEGF medium
complete kit, 0.05% trypsin-EDTA and trypsin neutralizing solution
were purchased from Cascade Biologics (Invitrogen, USA). Matrigel
was from BD Biosciences (Franklin Lakes, NJ, USA).
Cell lines and cell culture
Human prostate cancer androgen-independent PC-3
cells were obtained from Peking Union Medical College and cultured
in RPMI-1640 medium supplemented with 10% FBS. Human umbilical vein
endothelial cells (HUVECs) were purchased from Peking Union Medical
College and grown in VascuLife VEGF medium. PC-3 cells and HUVECs
were maintained at 37°C in the incubator with 95% air and 5%
CO2.
Cell culture and drug treatment
Quercetin was dissolved in DMSO to firstly form a
concentration of 10 mM. It was then diluted using cell culture
medium to reach the required concentrations (25, 50 and 100
µM) and equal volume of variable concentrations was added to
the media. Following culture for the designated time, cell
proliferation rate was determined by MTT assay. Comparable amount
of DMSO without quercetin acted as the vehicle control during the
research process.
Cell proliferation assay
MTT assay was generally used to evaluate the cell
viability in the culture after drug treatment (10). PC-3 cells at a density of
3×104 cells/ml and HUVECs at a density of
5×104 cells/ml were seeded into 96-well plates in
triplicate. After cultured for 24 h allowing both PC-3 cells and
HUVECs to attach, cells were treated with variable concentrations
of quercetin (25, 50 and 100 µM) or DMSO for further 24, 48
and 72 h. Four hours before the termination of each time points, 20
µl MTT with the concentration of 5 mg/ml was added to each
well and co-cultured with PC-3 cells and HUVECs for another 4 h in
the humidified environment containing 5% CO2 at 37°C.
Then culture medium was carefully removed leaving the formazan
crystals preserved completely, which was dissolved with 150
µl me were calculated based on the following formula:
Proliferation inhibition rate = [1 −
(A570treated/A570control)] × 100% (10).
Transwell migration assay
Transwell assay (BD Biosciences) was employed to
analyze the cell migration capability. Cells (1×104) in
serum-free medium with different concentrations of quercetin or
DMSO were seeded in the upper chamber of a 24-well Transwell
cluster plates in triplicate. Cell culture medium containing 10%
FBS was added into the lower chamber that was separated from the
upper one by an 8-µm pore size filter membrane. After
incubated in a 5% CO2 incubator at 37°C for 24 h, cells
in the the upper surface of the filter were discarded, and the
migrated cells on the lower surface were fixed in ethanol and
stained with crystal violet. Finally, migrated cells were
photographed with inverted microscope (magnification, ×40) and
counted from 5 random high powered fields (magnification, ×200).
The data are normalized to control as means ± SD.
Transwell invasion assay
Transwell assay was also performed to analyze the
cell invasion capability. The Matrigel (1 mg/ml) (BD Biosciences)
was well-distributed inside the upper surface of Transwell filter
membrane overnight at 4°C. After it was well formed and washed two
times with medium before use, cells (1×104) in
serum-free medium with different concentrations of quercetin or
DMSO were seeded in the upper compartment of the filter insert that
was already coated with Matrigel. The remaining procedures were
carried out as described in the above migration assay.
Capillary-like tube formation assay
Capillary-like tube formation assay was used to
evaluate the inhibitory effect of quercetin on angiogenesis in
vitro. Experimental procedure was performed as previously
described (10). Briefly, 100
µl Matrigel solution was added to the 96-well plates and
placed in the incubator with controlled conditions to form a
Matrigel gel. HUVECs (1×104/well) were seeded in the
Matrigel layer and cultured in VascuLife VEGF medium with quercetin
(25, 50 and 100 µM) or DMSO for 24 h at 37°C in 5%
CO2 environment. Tube formation was detected under
microscopy and photographed (magnification, ×40). The number was
calculated from 5 random fields and inhibition rate was determined
according to the formula: Tube formation inhibition rate = [1 −
(Tubestreated/tubescontrol)] × 100%.
Animal study
Male BALB/c nude mice 4–6 weeks old were provided by
the experimental animal ministry of Capital Medical University and
raised in pathogen-free environment with controlled constant 12-h
light and 12-h dark cycle, suitable humidity and temperature. Mice
were allowed one week to get accustomed to the new environment
before the experiments.
PC-3 cells ~5×105 were suspended in 100
µl PBS and inoculated subcutaneously into the right back.
When the xenograft tumor volume grew to ~100 mm3, mice
were randomly assigned to vehicle control and three quercetin
treated groups (n=8 each group) and treated intraperitoneally. The
therapeutic dose and regimen, administration manner and dissolution
method were determined according to our preliminary experiments,
our previous research results and numerous other research studies
(8,11–15).
The concrete implementation was as follows: i) vehicle control
group, vehicle of quercetin, namely 25% HPβCD (w/v in
ddH2O) on day 1; ii) quercetin treated groups, quercetin
25, 50 and 75 mg/kg dissolved in 25% HPβCD (w/v in
ddH2O) were injected in the three separate groups on day
1 (8,16); iii) three days was a treatment cycle
during which mice were weighed and xenograft tumors were measured
by slide caliper, and the treatment process was terminated 4 weeks
later. Tumor volume was obtained on the basis of the formula: L ×
S2 × 0.5, where L was the longest diameter and S was the
shortest diameter of xenograft tumor (8,4).
Mental state, food and water intake, health condition and mortality
rate were recorded as well (5). At
the end of the experiments the mice were sacrificed by cervical
dislocation following anesthesia by chloral hydrate. Xenograft
tumors were taken out promptly, measured, weighed and photographed.
Except for the portion preserved for immunohistochemical
examination being kept in 10% neutral buffered formalin, the
remaining was rapidly placed into liquid nitrogen for other
biomarker analysis.
Western blot analysis
Western blot experiments were performed as
previously described (8,11). Briefly, after HUVECs, PC-3 cells and
mice were treated with different concentrations of quercetin,
proteins from cells and xenograft tumor tissues were extracted and
quantified. Equivalent amount (80 µg) of proteins were
loaded, separated by 6–10% SDS-PAGE gels and transferred to
polyvinylidene fluoride (PVDF) membrane (Pall, New York, NY, USA)
which was blocked by 5% non-fat milk, incubated by primary antibody
TSP-1 (1:500, Abcam) overnight at 4°C and GAPDH (1:10,000; Sigma)
for 1 h at room temperature, and incubated with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; Cell
Signaling) for another 1 h at room temperature in turn. Enhanced
chemiluminescence kit (ECL Plus; Amersham Pharmacia Biotech,
Piscataway, NJ, USA) was used to detect the antigen-antibody
complex. GAPDH was used as the loading control.
Reverse transcription-quantitative
real-time polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as previously described
(8). Briefly, Total RNA of HUVECs,
PC-3 cells and xenograft tumor tissues were isolated using TRIzol
reagent (Invitrogen). The first-strand cDNA was synthesized with
oligo(dT) primers by Transcriptor First Strand cDNA Synthesis Kit
(Roche, Germany). Sequences of the PCR primers were as follows:
TSP-1 forward, 5′-CACGCTGCAG GACAGCAT-3′ and reverse,
5′-GGCCGCCTCAGCTCATT-3′. β-actin forward, 5′-CGGGAAATCGTGCGTGAC-3′
and reverse, 5′-GTGGCCATCTCTTGCTCGAA-3′. SYBR-Green PCR master mix
was used to quantify the cDNA under certain controlled conditions
(ViiA 7 Real-Time PCR System) (both from Applied Biosystems, Foster
City, CA, USA) and the samples were in triplicate. TSP-1 mRNA level
was calculated based on the 2−ΔΔCt method and then
normalized to β-actin expression.
Immunohistochemistry
The procedures for immunohistochemistry were carried
out as previously described (8).
Briefly, paraffin was removed and endogenous peroxidase activity
was eliminated. Following antigen retrieval, sections were
incubated with primary antibody CD31 (1:50) and CD34 (1:100) (both
from Abcam) for 24 h at 4°C. The slides were then interacted with
horseradish peroxidase-conjugated secondary antibody (1:100; Cell
Signaling) at room temperature for another 30 min. Diaminobenzidine
reaction was employed to visualize the signals which were
counterstained with hematoxylin. CD31- and CD34-positive vessels
were counted from three random high power fields in each slide. In
addition, at least three sections were analyzed independently in
each group.
Statistical analysis
All the results are represented as means ± SD.
Statistical analysis and plots were performed by SPSS 17.0 and
SigmaPlot 10.0, respectively. Differences between treated groups
and vehicle control were compared using independent-samples t-test.
P-value <0.05 denotes significant difference.
Results
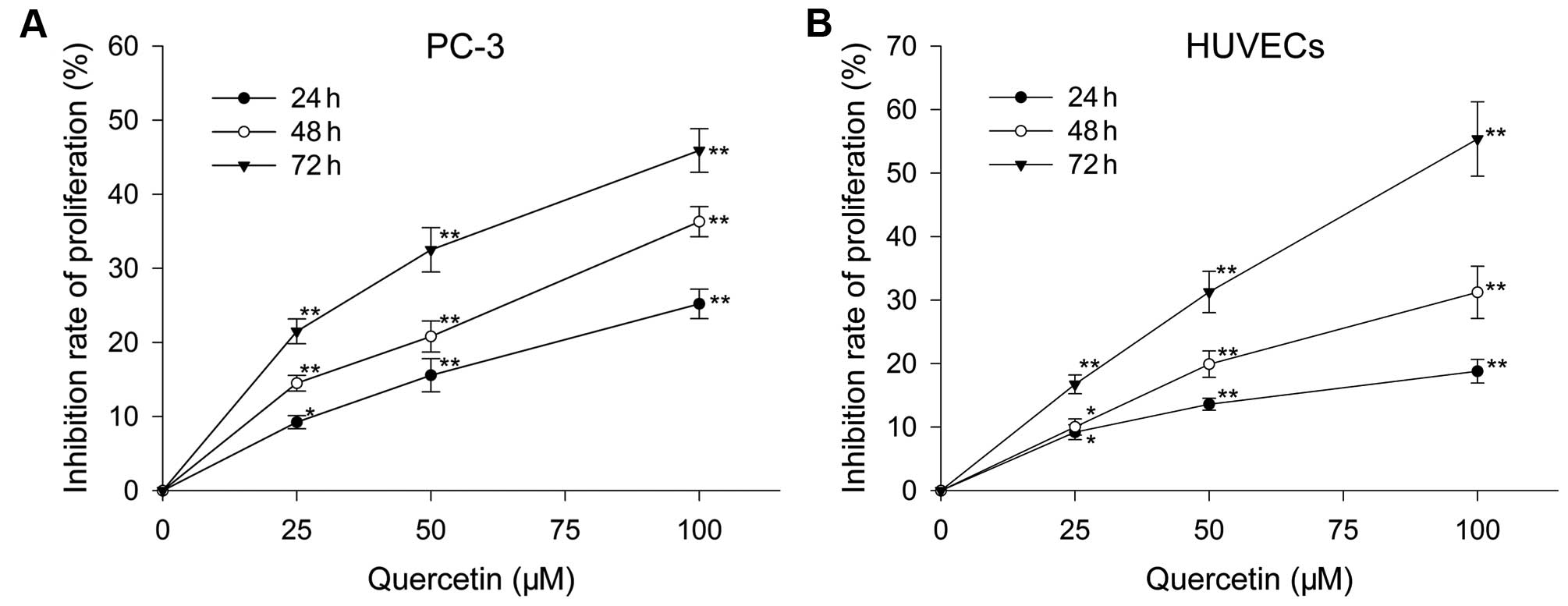
Effect of quercetin on HUVEC and PC-3
cell proliferation
After treatment with varied doses of quercetin for
the certain time, proliferation of HUVECs and PC-3 cells were
inhibited in a dose- and time-dependent manner. Following culture
with quercetin at 25, 50 and 100 µM for 24 h, the inhibition
rate was 9.2, 13.6 and 18.8%, respectively, for HUVECs, and 13.2,
15.57 and 25.2%, respectively, for PC-3 cells. When treated with
above doses for 48 h, HUVECs were inhibited by 12.02, 19.91 and
31.22%, respectively, and PC-3 cells were inhibited by 14.5, 22.8
and 39.3%, respectively. When exposed to the three doses for 72 h,
the inhibition rate was 16.74, 31.28 and 55.36%, respectively, for
HUVECs, and 21.5, 32.5 and 50.9%, respectively, for PC-3 cells
(Fig. 1).
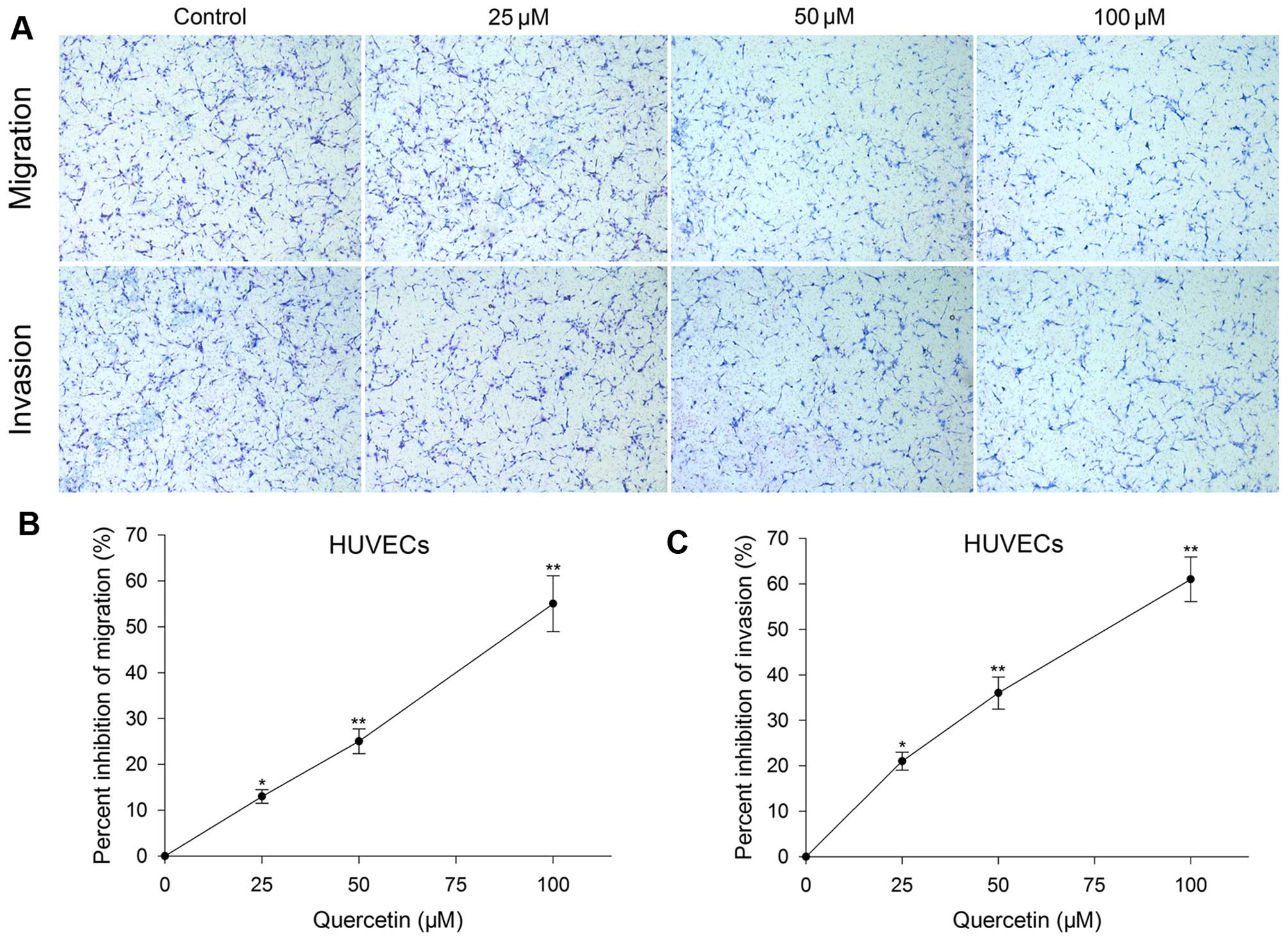
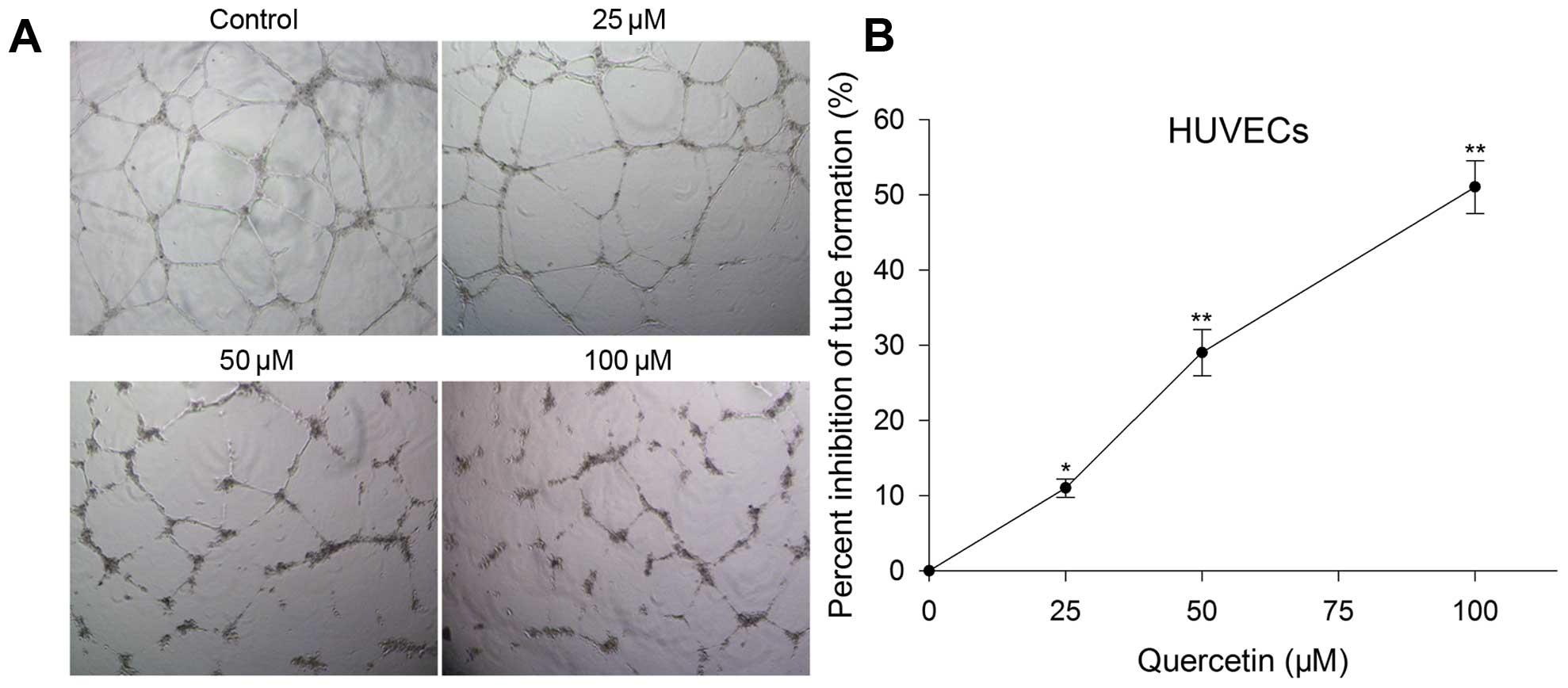
Effect of quercetin on HUVEC migration,
invasion and tube formation
Transwell migration, invasion and tube formation of
HUVECs were performed to evaluate the inhibitory effect of
quercetin on angiogenesis in vitro. After treated with
quercetin at 25, 50 and 100 µM for 24 h, the migration,
invasion and tube formation were also inhibited and the level was
in proportion to the concentration increase. Migration inhibition
rate was 13, 25 and 55%, invasion inhibition rate was 21, 36 and
61% (Fig. 2), and tube formation
inhibition rate was 11, 29 and 51%, respectively (Fig. 4).
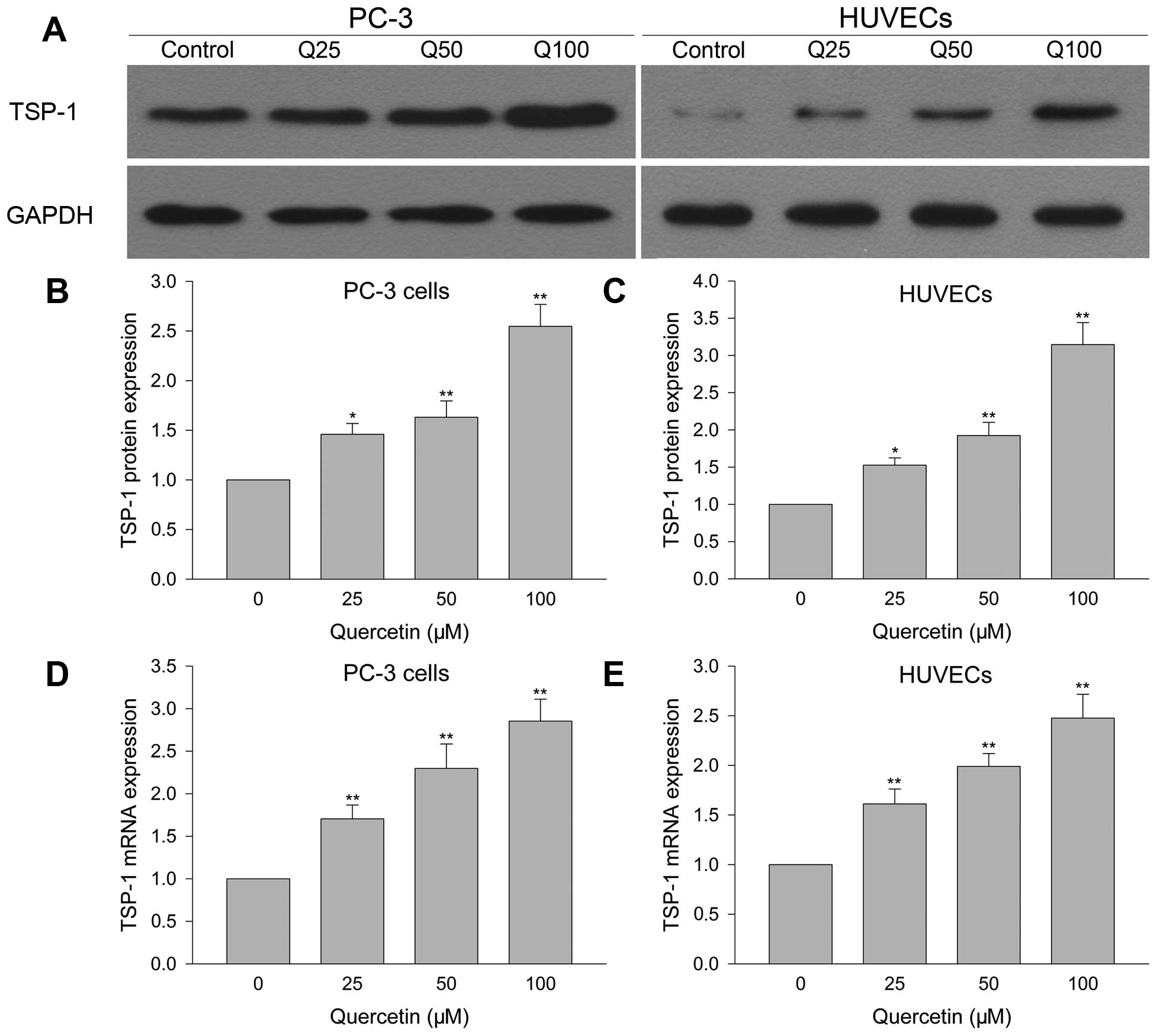
Quercetin upregulates TSP-1 protein and
mRNA expression in HUVECs
Effect of quercetin on TSP-1 protein and mRNA
expression in HUVECs was investigated by western blotting and
Q-PCR. Fig. 5A and C, show that
quercetin 25 µM greatly increased TSP-1 protein expression
compared with vehicle control, and this phenomenon was more obvious
when drug dose was increased. As for TSP-1 mRNA (Fig. 5E), it showed the same pattern as
protein with larger dose of quercetin resulting in more mRNA
expression than the lower dose.
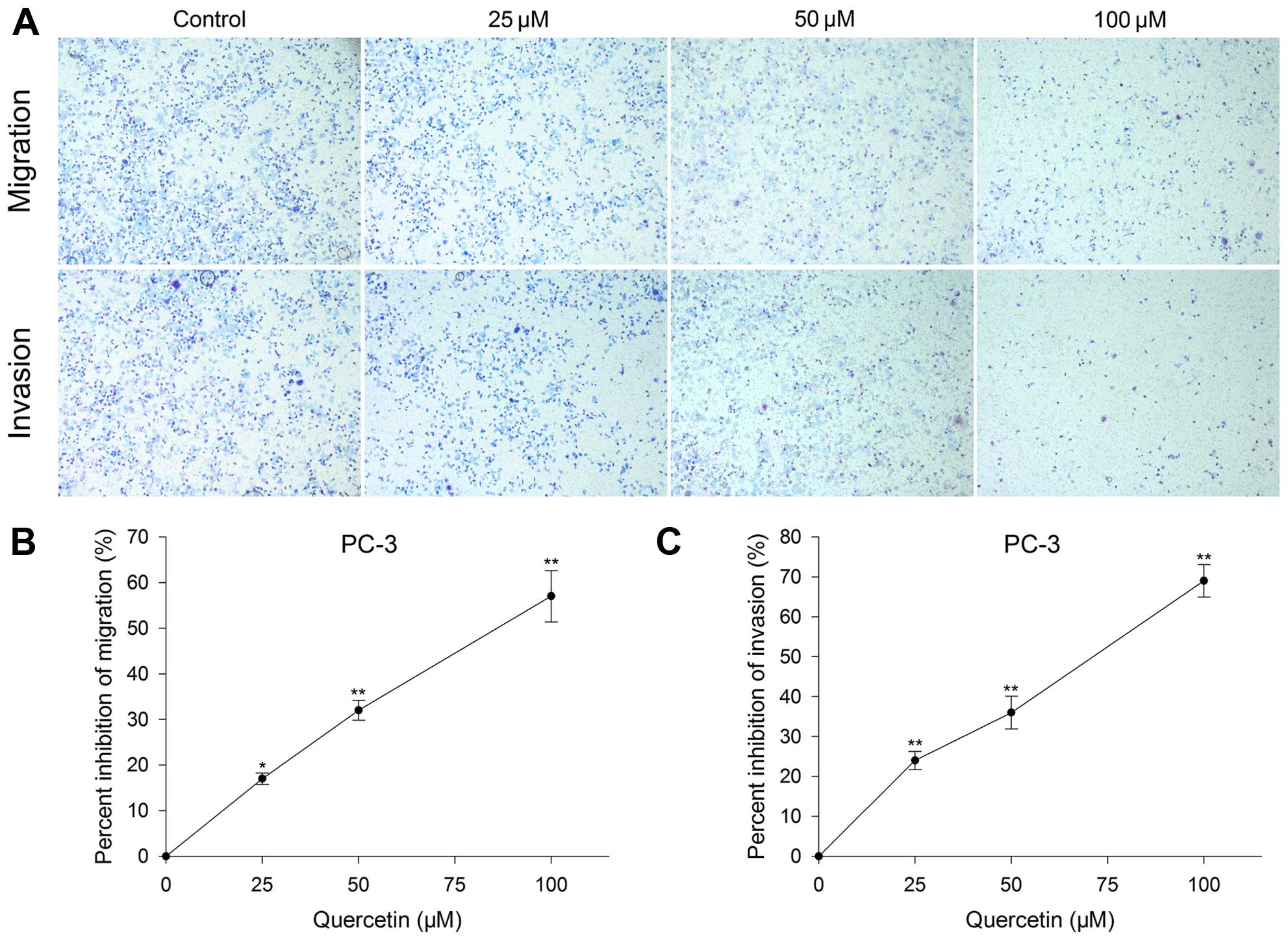
Effect of quercetin on PC-3 cell
migration and invasion
Inhibitory effect of quercetin on PC-3 cells was
assessed by Transwell migration and invasion. After PC-3 cells were
cultured with quercetin at 25, 50 and 100 µM for 24 h, the
migration inhibition rate was 17, 32 and 57%, and invasion
inhibition was 24, 36 and 69%, respectively. The inhibitory degree
had a positive association with the dose increase as well (Fig. 3).
Quercetin upregulates TSP-1 protein and
mRNA expression in PC-3 cells
TSP-1 protein and mRNA expression were detected to
explore the underlying mechanisms of quercetin inhibiting PC-3 cell
growth. After PC-3 cells were treated with quercetin for 24 h, 25
µM greatly increased TSP-1 protein and mRNA expression
compared with vehicle control group. When quercetin reached 50 and
100 µM, TSP-1 protein and mRNA expression was more than that
of the lower dose (Fig. 5A, B and
D).
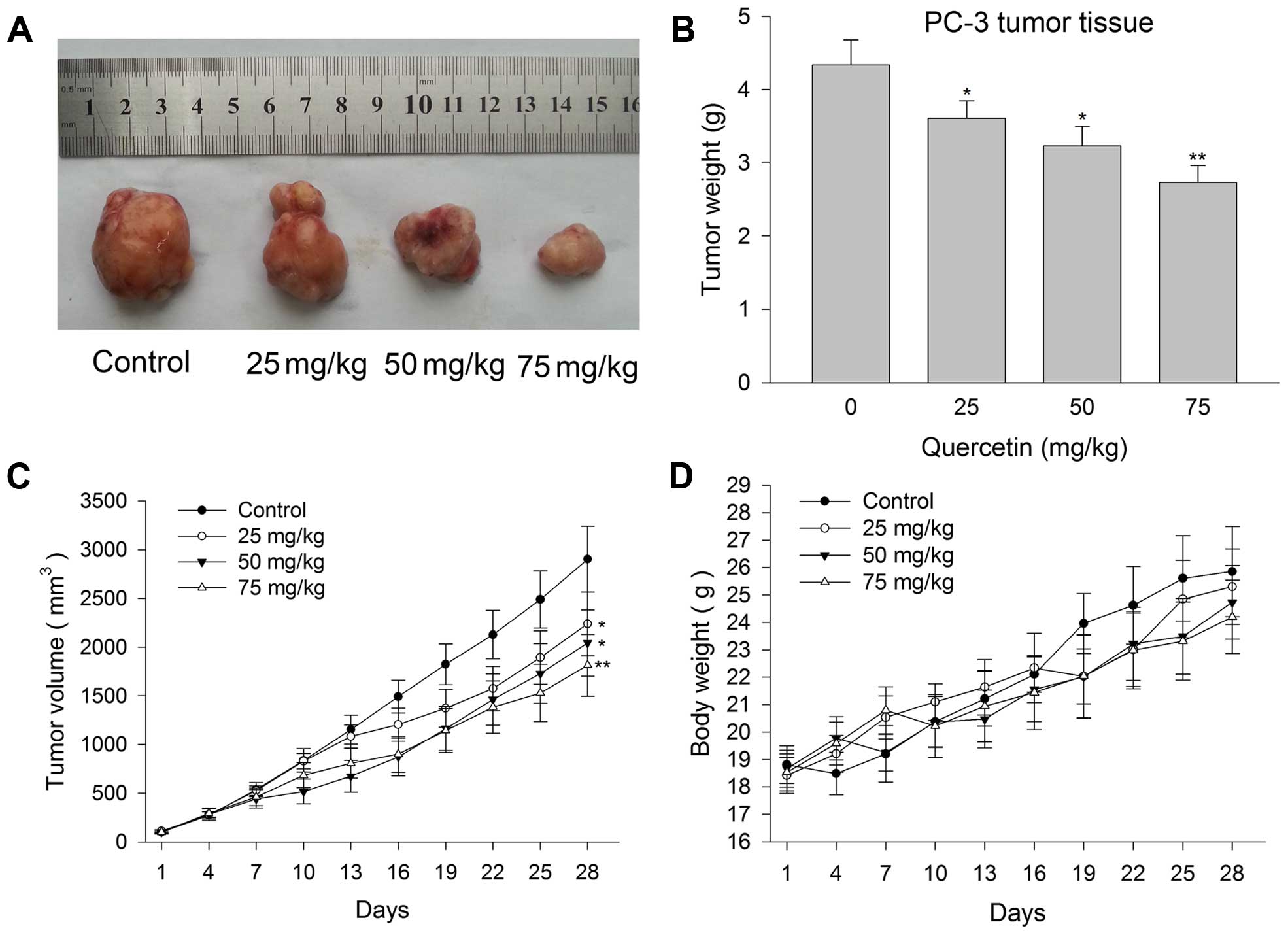
Quercetin inhibits human prostate cancer
PC-3 cell xenograft tumor growth
When PC-3 cell xenografts reached ~100
mm3, male BALB/c nude mice were randomly assigned and
treated with vehicle or quercetin at the dose of 25, 50 and 75
mg/kg. At the end of the intervention procedure lasting 4 weeks,
xenografts of the four groups were harvested and it was found that
PC-3 tumors were smaller in quercetin 25 mg/kg treated groups than
in the control group. The xenografts become much smaller when the
drug dose increased (Fig. 6A). The
inhibition rate of 25, 50 and 75 mg/kg groups were 22.85, 29.6 and
37.5%, respectively (Fig. 6B and
C). In the entire treatment process, even large dose of 75
mg/kg was well tolerated by the nude mice. Drug-related toxic
reaction or side-effects such as poor mental state, convulsion and
hematuria were not observed. Weight loss in the treated groups did
not show significant difference from the control group (Fig. 6D).
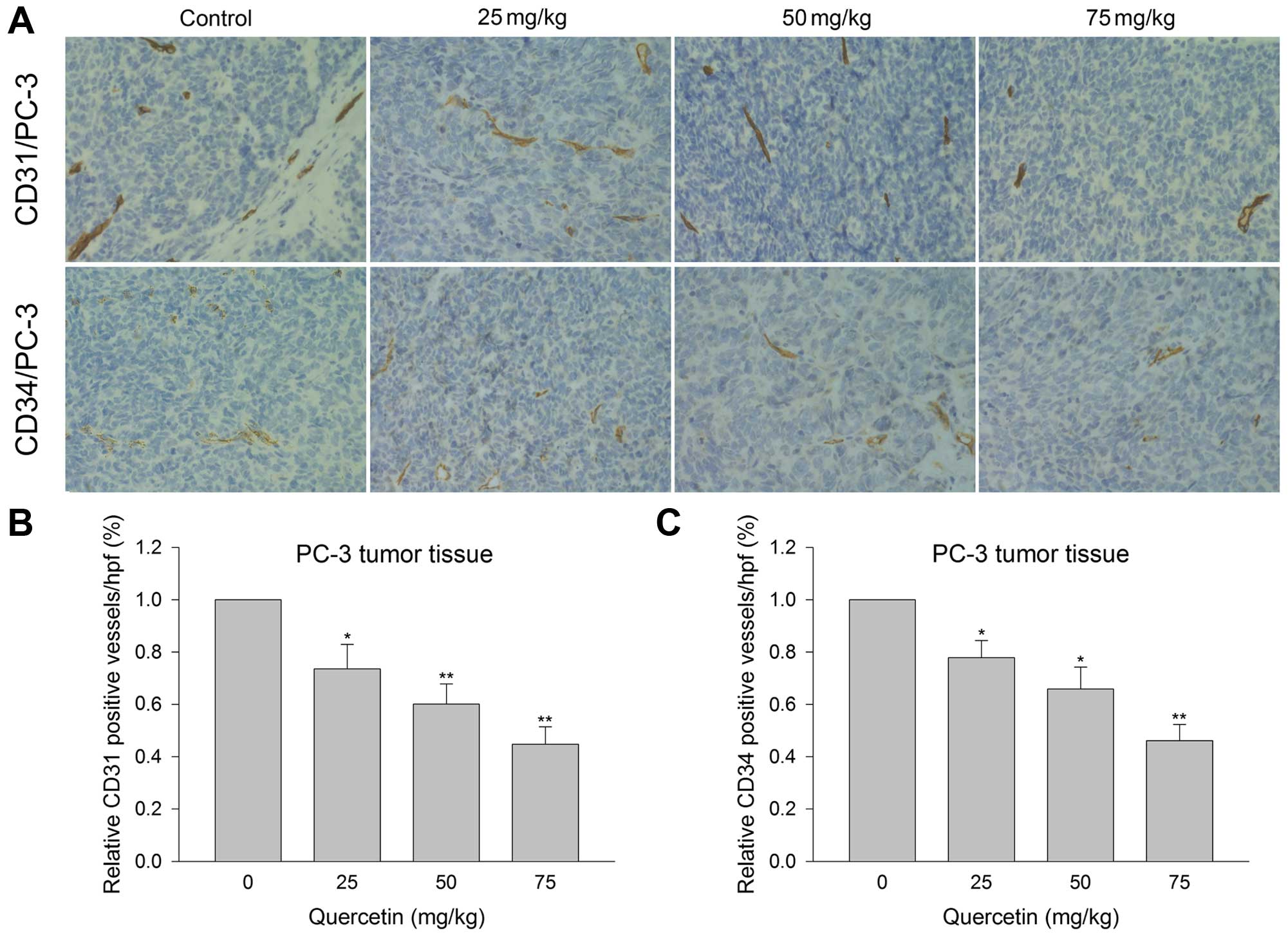
Quercetin inhibits tumor microvessel
density in xenograft tumor tissue
Microvessel density examination was carried out to
explore the inhibitory effect of quercetin on angiogenesis in
vivo. Immunohistochemistry showed that relative CD31- and
CD34-positive vessels in quercetin 25 mg/kg group were 73.5 and
77.8%, respectively, as compared to 100% in the vehicle control
group. The positive rates for CD31 and CD34 were 60.08 and 65.87%,
respectively, in quercetin 50 mg/kg group, and 44.68 and 46.11%,
respectively, in 75 mg/kg group (Fig.
7). The above verified the ability of quercetin to inhibit
angiogenesis in prostate cancer cell xenografts in a dose-dependent
manner.
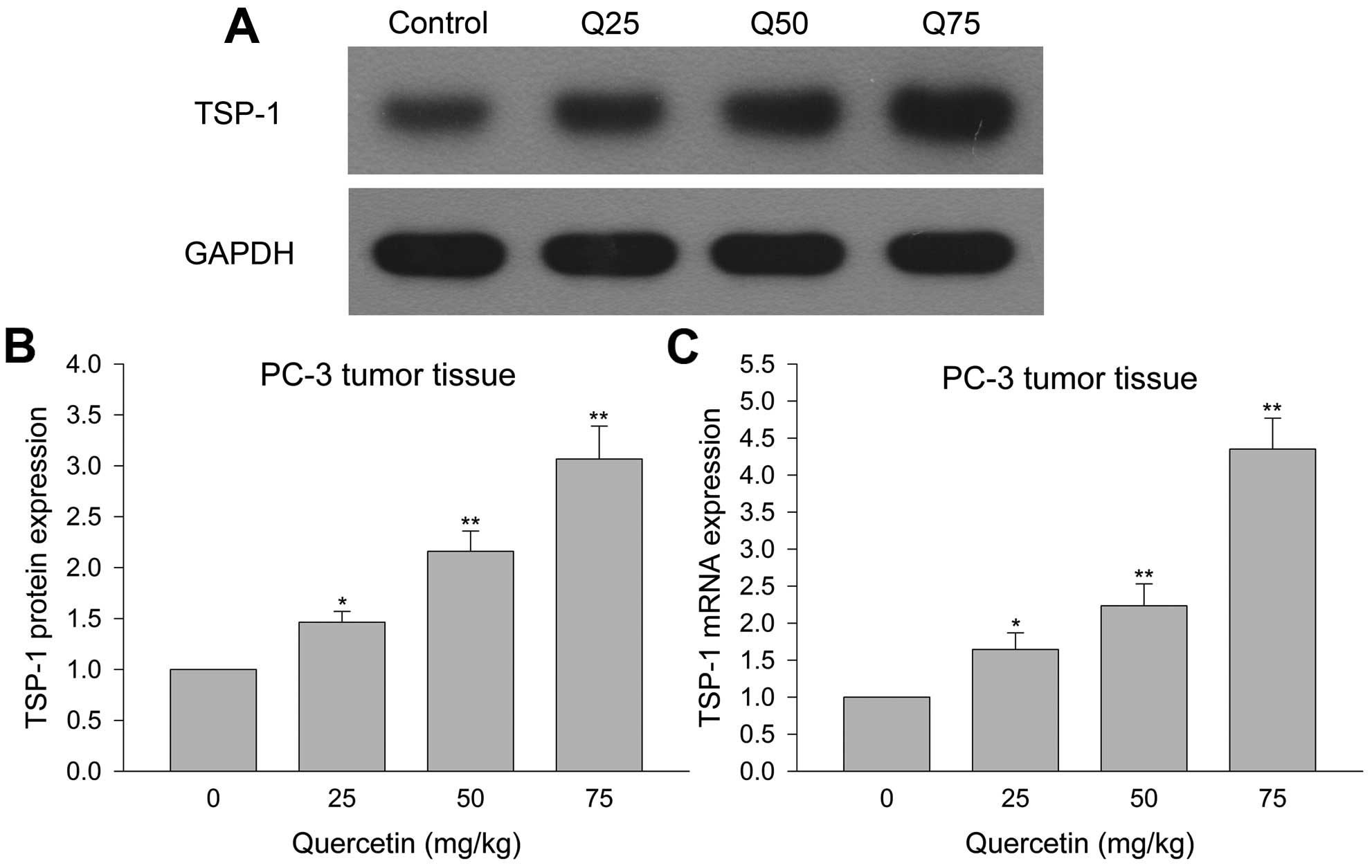
Quercetin inhibits TSP-1 protein and mRNA
expression in PC-3 cell xenograft tumor tissue
Western blotting and Q-PCR detected TSP-1 protein
and mRNA expression in xenograft tumor tissues to investigate the
reason for quercetin inhibition of prostate cancer PC-3 cell growth
in vivo. Fig. 8, shows that
quercetin 25 mg/kg led to increased TSP-1 protein and mRNA
expression in tumor tissues compared with control, whereas larger
doses resulted in greater amount of TSP-1 protein and mRNA. These
results indicated that quercetin increased TSP-1 protein and mRNA
expression to inhibit angiogenesis thereby leading to antagonize
human prostate cancer growth in vivo.
Discussion
The incidence and mortality rate of prostate cancer
have been increasing in recent years, but effective treatments for
prostate cancer are scarce and the present comprehensive
therapeutic measures cannot achieve satisfactory results (1,4). Based
on these disadvantageous situations, researchers are investigating
naturally-occurring substances to solve this thorny problem.
Quercetin has presented its promising anticancer
property in prostate cancer (7).
In vitro, quercetin inhibited prostate cancer cell growth
through reducing androgen receptor (AR) and its inducible genes
expression (6), proapoptotic and
antiproliferative activities (11),
and many other possible mechanisms (7). In vivo, quercetin was able to
antagonize prostate cancer cell xenograft growth to a large degree
by targeting various signal pathways (7). In the present study, it was proven
again that quercetin effectively inhibited androgen-independent
PC-3 human prostate cancer cell growth to a large degree both in
vitro and in vivo, and this anti-prostate cancer action
was manifested in a dose- and time-dependent manner. In addition,
quercetin is non-toxic. It was well tolerated by experimental
animals and did not show any genotoxicity (8,12,13,17).
Recently, two clinical studies have been carried out for quercetin
and verified that 1,000 mg of quercetin was well tolerated by
healthy adults or patients with III chronic prostatitis and this
dosage did not produce any side-effects or drug-related toxicity
(18,19). Furthermore, quercetin is abundant in
various fruits and vegetables and can be sufficiently gained
through diet. Therefore, quercetin is safe and effective and has
broad application prospects in prostate cancer treatment.
Angiogenesis refers to the process of
neovascularization from the existing vascular system and plays an
important role in the tumor growth as new blood vessels can provide
sufficient oxygen and nutrients. At the same time, tumors
themselves can promote more neovascularization to further
facilitate tumor development and progression (7). In the absence of local new blood
vessel formation, tumors will not grow larger than 2–3 mm in
diameter and its metastasis will be hindered as well (20). Thus tumors can be effectively
treated by inhibiting angiogenesis. Moreover, natural inhibitors of
angiogenesis are low toxic and will not harm the pre-existing
vasculature (20). The above
indicated that novel cancer therapies targeting angiogenesis have
great therapeutic potential and have attracted attention long ago
(21). Our present experiment
manifested that quercetin effectively inhibited proliferation,
migration, invasion and tube formation of HUVECs and thus confirmed
the ability of quercetin to inhibit angiogenesis in vitro.
When PC-3 cells were exposed to various doses of quercetin, the
proliferation was inhibited significantly in a dose- and
time-dependent manner. It is speculated that quercetin inhibited
prostate cancer cell growth through angiogenesis inhibition and
this was further confirmed in vivo. When prostate cancer
xenograft tumor bearing nude mice were treated with quercetin, the
volume and weight of xenograft tumors were greatly reduced.
Immunohistochemical examination showed that microvascular density
represented by CD31- and CD34-positive vessels was decreased in
drug-treated groups and it was more obvious when dose increased.
The inhibitory effect of quercetin on angiogenesis in vivo
directly resulted in the inhibition of xenograft tumor growth.
Angiogenesis involves a series of processes and
procedures that are initiated by angiogenic factors with subsequent
relevant signal pathways activated. Vascular endothelial growth
factor (VEGF) and hypoxia inducible factor-1α (HIF-1α) are the two
important angiogenic factors to regulate angiogenesis and have been
deemed as the therapeutic target for many anticancer drugs
(9,22–24).
Thrombospondin-1 (TSP-1) is first isolated from platelet α-granules
and acts as the endogenous antiangiogenic factor (24). Various substances can achieve the
inhibitory effect on angiogenesis and tumor growth through
upregulating TSP-1 (5). The
possible mechanisms are as follows: direct effects on endothelial
cell migration, induction of apoptosis, inhibition of matrix
metalloproteinase-9 activation, and interaction of TSP-1 with CD36
(20,25). Although the present study, and
Pratheeshkumar et al verified that quercetin could inhibit
prostate cancer growth through antagonizing angiogenesis both in
vitro and in vivo (8,23), the
important role of TSP-1 upregulation in the angiogenesis inhibition
and anti-prostate cancer effect of quercetin has not been
previously reported.
We gave different doses of quercetin or DMSO to
HUVECs and then cultured for 24, 48 and 72 h. Migration, invasion
and tube formation were greatly inhibited. Western blotting and
Q-PCR showed that TSP-1 protein and mRNA increased with the dose
increase. It is strongly suggested that quercetin inhibited
angiogenesis through TSP-1 upregulation. Then PC-3 cells were
treated with quercetin, the proliferation was inhibited and TSP-1
protein and mRNA were increased in a dose-dependent manner. This
showed that quercetin effectively antagonized prostate cancer cells
growth by means of increasing TSP-1 expression. It was already
previously clear that quercetin could inhibit human prostate cancer
cell growth through angiogenesis inhibition that was mediated via
raising TSP-1 protein and mRNA level. However, the quercetin
effects on prostate cancer in vivo and the mechanisms
involved are not known.
We performed prostate cancer-bearing nude mouse
model experiments to explore this issue. When xenografts reached
~100 mm3, mice were randomly assigned and intervened for
four weeks. After relevant biochemical examinations were carried
out, it was found that both TSP-1 protein and mRNA in the tumor
tissues were increased, and the level change had a direct
proportional relationship with the drug dose. Microvascular density
examination demonstrated that quercetin significantly reduced
angiogenesis of xenograft tumor tissues. Large dose such as 75
mg/kg gave a much stronger inhibitory effect. Therefore, our in
vivo results further verified the findings of in vitro
experiments that quercetin greatly inhibited angiogenesis by TSP-1
upregulation through which prostate cancer growth was effectively
inhibited. However, we did not conducted a deeper and more detailed
study on the concrete signal pathway of quercetin inhibiting
angiogenesis that mainly involved TSP-1 as well as its upstream and
downstream regulated factors. The present study mainly focuses on
the effect of quercetin and TSP-1 on angiogenesis and prostate
cancer growth both in vitro and in vivo, but
mechanism study is still required.
In conclusion, quercetin can effectively inhibit
angiogenesis through TSP-1 upregulation to antagonize human
prostate cancer PC-3 cell growth in vitro and in
vivo. However, the detailed signal pathway concentrating on
TSP-1 needs further investigation. The present study can provide a
good foundation for the future mechanism research and applying
quercetin to clinical study, and it also raise the possibility of
using quercetin to treat and prevent human prostate cancer in the
near future.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81450027).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fleshner N: Defining high-risk prostate
cancer: Current status. Can J Urol. 12(Suppl 1): S14–S17;
discussion 94–96. 2005.
|
|
3
|
Hotte SJ and Saad F: Current management of
castrate-resistant prostate cancer. Curr Oncol. 17(Suppl 2):
S72–S79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qu S, Wang K, Xue H and Wang Y, Wu R, Liu
C, Gao AC, Gout PW, Collins CC and Wang Y: Enhanced anticancer
activity of a combination of docetaxel and Aneustat (OMN54) in a
patient-derived, advanced prostate cancer tissue xenograft model.
Mol Oncol. 8:311–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olsson A, Björk A, Vallon-Christersson J,
Isaacs JT and Leanderson T: Tasquinimod (ABR-215050), a
quinoline-3-carboxamide anti-angiogenic agent, modulates the
expression of thrombospondin-1 in human prostate tumors. Mol
Cancer. 9:1072010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing N, Chen Y, Mitchell SH and Young CY:
Quercetin inhibits the expression and function of the androgen
receptor in LNCaP prostate cancer cells. Carcinogenesis.
22:409–414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang F, Song L, Wang H, Wang J, Xu Z and
Xing N: Quercetin in prostate cancer: Chemotherapeutic and
chemopreventive effects, mechanisms and clinical application
potential (Review). Oncol Rep. 33:2659–2668. 2015.PubMed/NCBI
|
|
8
|
Yang F, Song L, Wang H, Wang J, Xu Z and
Xing N: Combination of quercetin and 2-methoxyestradiol enhances
inhibition of human prostate cancer LNCaP and PC-3 cells xenograft
tumor growth. PLoS One. 10:e01282772015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi X, Deepak V, Wang L, Ba X, Komori T,
Zeng X and Liu W: Thrombospondin-1 is a putative target gene of
Runx2 and Runx3. Int J Mol Sci. 14:14321–14332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Li XX, Xing NZ and Cao XG:
Quercetin inhibits choroidal and retinal angiogenesis in vitro.
Graefes Arch Clin Exp Ophthalmol. 246:373–378. 2008. View Article : Google Scholar
|
|
11
|
Wang G, Song L, Wang H and Xing N:
Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth
and inducing apoptosis in human prostate cancer cells. Oncol Rep.
30:357–363. 2013.PubMed/NCBI
|
|
12
|
Sharmila G, Bhat FA, Arunkumar R, Elumalai
P, Raja Singh P, Senthilkumar K and Arunakaran J: Chemopreventive
effect of quercetin, a natural dietary flavonoid on prostate cancer
in in vivo model. Clin Nutr. 33:718–726. 2014. View Article : Google Scholar
|
|
13
|
Reiner T, de las Pozas A, Gomez LA and
Perez-Stable C: Low dose combinations of 2-methoxyestradiol and
docetaxel block prostate cancer cells in mitosis and increase
apoptosis. Cancer Lett. 276:21–31. 2009. View Article : Google Scholar
|
|
14
|
Ma ZS, Huynh TH, Ng CP, Do PT, Nguyen TH
and Huynh H: Reduction of CWR22 prostate tumor xenograft growth by
combined tamoxifen-quercetin treatment is associated with
inhibition of angiogenesis and cellular proliferation. Int J Oncol.
24:1297–1304. 2004.PubMed/NCBI
|
|
15
|
Ma Z, Hung Nguyen T, Hoa Huynh T, Tien Do
P and Huynh H: Reduction of rat prostate weight by combined
quercetin-finasteride treatment is associated with cell cycle
deregulation. J Endocrinol. 181:493–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borghetti GS, Lula IS, Sinisterra RD and
Bassani VL: Quercetin/beta-cyclodextrin solid complexes prepared in
aqueous solution followed by spray-drying or by physical mixture.
AAPS PharmSciTech. 10:235–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Utesch D, Feige K, Dasenbrock J, Broschard
TH, Harwood M, Danielewska-Nikiel B and Lines TC: Evaluation of the
potential in vivo genotoxicity of quercetin. Mutat Res. 654:38–44.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cialdella-Kam L, Nieman DC, Sha W, Meaney
MP, Knab AM and Shanely RA: Dose-response to 3 months of
quercetin-containing supplements on metabolite and quercetin
conjugate profile in adults. Br J Nutr. 109:1923–1933. 2013.
View Article : Google Scholar
|
|
19
|
Shoskes DA, Zeitlin SI, Shahed A and
Rajfer J: Quercetin in men with category III chronic prostatitis: A
preliminary prospective, double-blind, placebo-controlled trial.
Urology. 54:960–963. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren B, Yee KO, Lawler J and Khosravi-Far
R: Regulation of tumor angiogenesis by thrombospondin-1. Biochim
Biophys Acta. 1765:178–188. 2006.PubMed/NCBI
|
|
21
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DH and Lee YJ: Quercetin suppresses
hypoxia-induced accumulation of hypoxia-inducible factor-1alpha
(HIF-1alpha) through inhibiting protein synthesis. J Cell Biochem.
105:546–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar
|
|
24
|
Miyata Y and Sakai H: Thrombospondin-1 in
urological cancer: Pathological role, clinical significance, and
therapeutic prospects. Int J Mol Sci. 14:12249–12272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kazerounian S, Yee KO and Lawler J:
Thrombospondins in cancer. Cell Mol Life Sci. 65:700–712. 2008.
View Article : Google Scholar : PubMed/NCBI
|