Introduction
Cholangiocarcinoma (CCA) or bile duct epithelial
cancer associated with the liver fluke (Opisthorchis
viverrini; Ov) infection is the most common cancer in northeast
Thailand (1–3). CCA is a slow progression cancer with
no specific symptoms and most CCA patients usually present with the
advanced incurable stage. Surgical restriction is the best
treatment regimen for CCA (4,5).
However, not all CCA patients are good candidates for curative
surgery and complete surgical restriction is often followed by
local recurrence with a less than satisfactory 5-year survival rate
(6,7). Therefore, the identification of
putative therapeutic targets and/or potential anticancer agents
against this malignancy is urgently needed.
A signal transducer and activator of transcription
(STATs) family of protein kinases play roles in the immune response
mechanism, inflammation and cellular development (8,9).
Conversely, abnormal activation of STATs has been shown to be
involved in the genesis and progression of several types of cancers
as well as CCA (10–12). We have previously reported the
involvement of protein kinases in CCA development and they
represent promising targets for CCA treatment (13,14).
Among the kinases, the STAT protein family particularly STAT3 was
defined as the major STAT which played a role in inflammation that
contributed to CCA carcinogenesis and progression, and was
associated with poor prognosis of CCA (15). Therefore, STAT3 could be a potential
molecular target for CCA prevention and treatment.
During the past decade, the strategy for cancer
prevention and treatment of the identification and
characterizations of dietary phytochemicals that are capable of
blocking or reversing carcinogenesis as well as possessing
anticancer properties has received increased research focus
(16–19). Xanthohumol (XN) has been identified
and suggested to possess chemopreventive and anticancer activities
in every step of carcinogenesis. XN can potently inhibit
pro-carcinogen activating and detoxifying enzymes as well as
exhibiting antioxidant and free-radical scavenging activity
(20). This compound also has an
anti-inflammatory activity by abrogating the expression of several
inflammatory genes, such as cyclo-oxygenase (COX-1, COX-2) and
inducible nitric oxide synthase (iNOS) and it can inhibit cancer
cell growth as well as tumor angiogenesis via the suppression of
Akt and NFκB activation (21–24).
In previous studies, the anticancer potential of XN has been
demonstrated in several types of cancer. However, an inhibitory
effect of XN on STAT3 and CCA development has not been reported.
Therefore, the present study explored the effects of XN on STAT3 as
well as CCA development in both an in vitro and a CCA
xenograft model. Results obtained may assist in evaluating whether
STAT3 is a potential target for CCA treatment and provide data
regarding the effectiveness of XN against CCA.
Materials and methods
Cell culture
Human CCA cells, M214 and M139 were cultured and
maintained as previously described (13).
Antibodies and reagents
Antibodies for western blotting were as follows:
anti-phospho-STAT3 (Cambridge, UK), anti-phospho-STAT3,
phospho-Akt, total Akt (Cell Signaling Technology, Danvers, MA,
USA), anti-p65 NFκB (Santa Cruz Biotechology, Santa Cruz, CA, USA),
anti-β-actin (Sigma-Aldrich, St. Louis, MO, USA). Recombinant human
IL-6 was commercially available and purchased from R&D Systems,
Minneapolis, MN, USA. XN was kindly provided by Hopsteiner,
Mainberge, Germany.
Western blot analysis
Western blot analysis was performed as previously
described (15).
Cell proliferation assay
M214 and M139 CCA cells (2×103/100
µl) were seeded into 96-well plates and incubated overnight
at 37°C and 5% CO2. Then, XN at designated
concentrations was added and incubated for 24, 48 and 72 h. Cell
proliferation assay was performed using sulforhodamine B (SRB;
Sigma-Aldrich, St. Louis, MO, USA) as previously described
(25).
For the XN suppressed IL-6-induced STAT3 activation
experiment, cell proliferation was determined by trypan blue
exclusion assay. Cells were treated with 10 ng/ml recombinant human
IL-6 concomitant with the indicated concentration of XN (0, 10, 20
and 50 µM) for 24 h after that cell was trypsinized and the
viable cells were counted in a cell counting chamber under a light
microscope. The experiment was carried out in duplicate.
Animal study
Six-week-old female BALB/cAJcl-nu/nu mice were
purchased from CLEA Japan (Tokyo, Japan). Animals were housed under
specific pathogen-free conditions at the animal center, Institute
of Medical Science, The University of Tokyo. All animal experiments
were performed according to institutional guidelines. Mice were
subcutaneously injected with 2×106 cells of KKU-M214 at
both flanks. One week after tumors were visible, animals were
divided into two groups; the control group was provided with a
vehicle (0.5% ethanol) whereas treatment groups were administrated
20 and 50 µM of XN in drinking water for 30 days. Drinking
water solutions were secured in the amber bottles to prevent
degradation and renewed on a daily basis. Mice were determined for
water consumption every other day, and body weight and tumor volume
were measured twice a week. The tumor volume was calculated by the
formula: 0.5 × width2 × length and tumor growth was
indicated by relative tumor volume (tumor volume normalized with
tumor volume day 0).
Immunohistochemistry detection of Ki67
proliferation marker
Immunostaining of Ki67, proliferation marker was
performed on paraffin-embedded nude mouse tumor tissues to
determine the antiproliferative effect of XN in a CCA animal model.
Nude mouse tissue sections were deparaffinized in xylene followed
by rehydration in a series of different ethanol concentrations.
Then, the antigen was retrieved using Tris-EDTA buffer, pH 8.8 in
pressure cooker and 0.3% H2O2 was used to
block endogenous peroxidase activity for 30 min with agitation.
Nonspecific binding was blocked by 10% skim milk in
phosphate-buffered saline (PBS) for 30 min. Sections were incubated
with the anti-Ki67 antibody at 4°C overnight in a moisture
chamber.
Sections were then incubated with
peroxidase-conjugated EnVision™ secondary antibody (Dako, Denmark)
followed by washing with working PBS for 5 min, three times. After
that the color was developed with 0.1% diaminobenzidine
tetrahy-drochloride solution for 5 min and followed by
counterstaining with Mayer's hematoxylin. Sections were observed
under a light microscope (Carl Zeiss, Germany). Ki67-positive cells
of each tumor section was counted in at least five of the ×200
power fields.
Apoptosis assay
Histologic analysis of DNA fragmentation was used to
identify apoptotic cells in the paraffin sections of CCA nude mouse
tissues. In situ terminal deoxynucleotide
transferase-mediated dUTP nick-end labeling (TUNEL) assay was
carried out using the In situ Cell Death Detection kit, POD
(Roche). TUNEL-positive cells were quantified in at least five of
the x200 power fields of randomly selected tissue sections.
Statistical analysis
Results from cell proliferation, Ki67 staining
analysis, apoptosis assay and animal experiments are represented as
mean ± SD, statistical significance was addressed by independent
samples t-test and a two-way ANOVA (GraphPad Prism 5 software).
P-value of <0.05 was considered to indicate a statistically
significant result.
Results
Antiproliferative effect of XN on CCA
cells
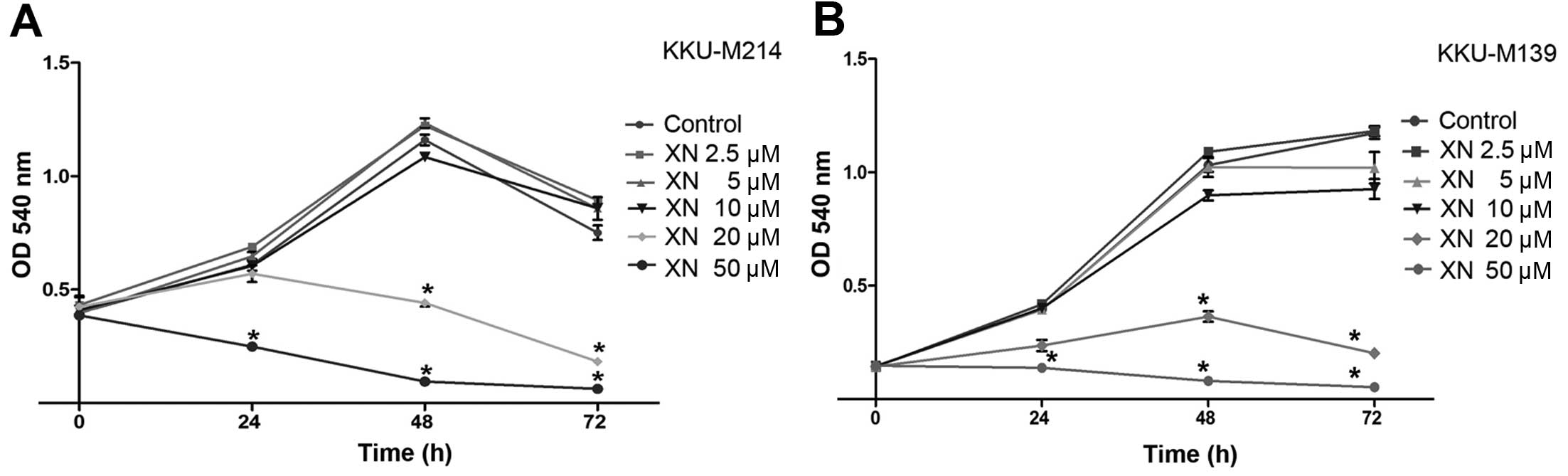
The effects of XN on the growth of CCA cells were
determined in human CCA cell lines established from primary tumors
of Ov-associated CCA patients namely, KKUM214 and KKU-M139. The
results showed that XN inhibited CCA cell growth which occurred in
a dose- and time-dependent manner. A 20 µM concentration of
XN significantly reduced CCA cell growth at 48 and 72 h (P<0.05)
when compared to control cells (Fig. 1A
and B). Moreover, a 50 µM concentration of XN
significantly inhibited CCA cell growth at 24, 48 and 72 h
(P<0.05) in both KKU-M214 and KKU-M139 cell lines (Fig. 1A and B). Low concentrations of XN
caused no evidence or significant effects on cell growth inhibition
even at long exposure times.
Effects of XN on IL-6 induces STAT3
activation and CCA cell growth
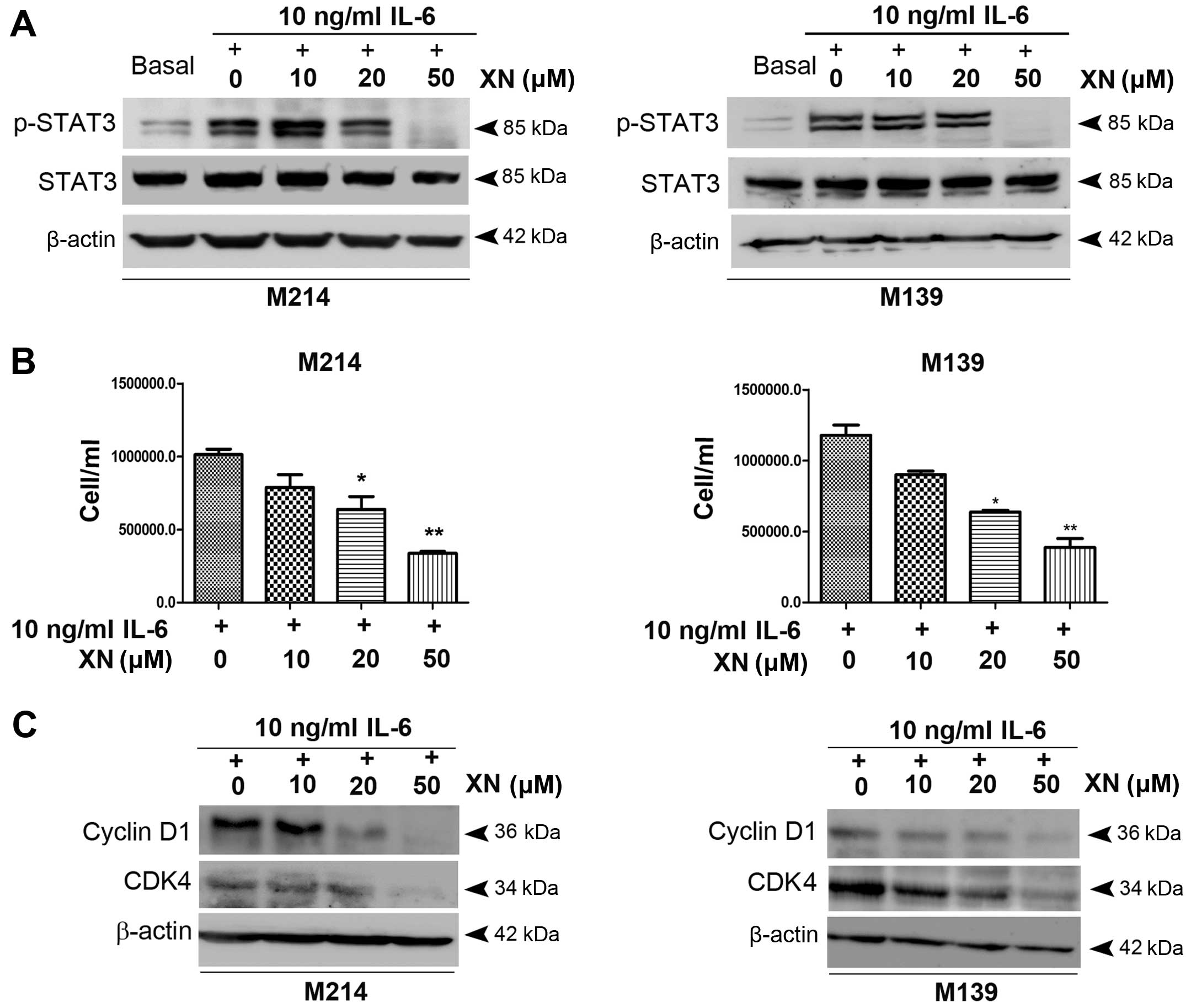
We then evaluated whether inhibiting STAT3
activation leads to growth inhibition as well as apoptosis
induction in CCA cells. CCA cells were exposed to XN upon
stimulation with IL-6. The results showed that a low concentration
of XN (10 µM) caused an elevation of STAT3 activation while
XN at 20 µM concentration partially inhibited STAT3
activation. A 50 µM concentration of XN, however, completely
inhibited STAT3 activity (Fig. 2A).
In addition, abrogation of STAT3 activation by XN at 20 and 50
µM concentrations was associated with a significant
reduction of M214 and M139 CCA cell growth which was concordant
with decreasing expression of cell cycle controlling proteins,
cyclin D1 and CDK4 (Fig. 2B and
C).
Apoptosis induction of XN in CCA
cells
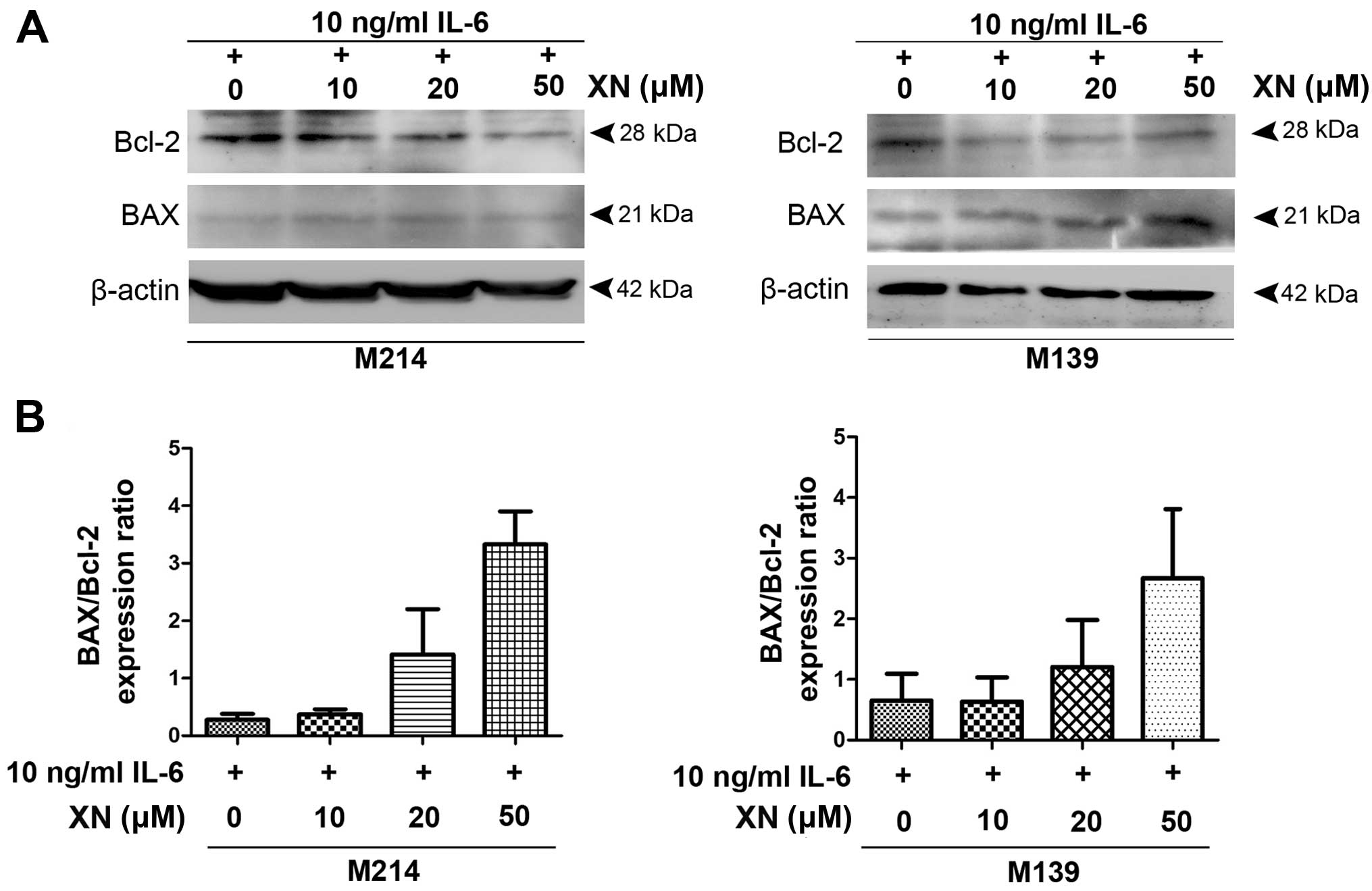
To investigate if suppression of STAT3 activation by
XN inhibited CCA growth resulted from apoptosis induction, we
examined the expression of anti-apoptosis protein, Bcl-2 as well as
BAX, pro-apoptotic protein. The results demonstrated that
decreasing protein levels of Bcl-2 was seen in M214 and M139 CCA
cells after treatment with XN, whereas BAX protein expression was
increased (Fig. 3).
Antitumor activity of XN in CCA
inoculated mice
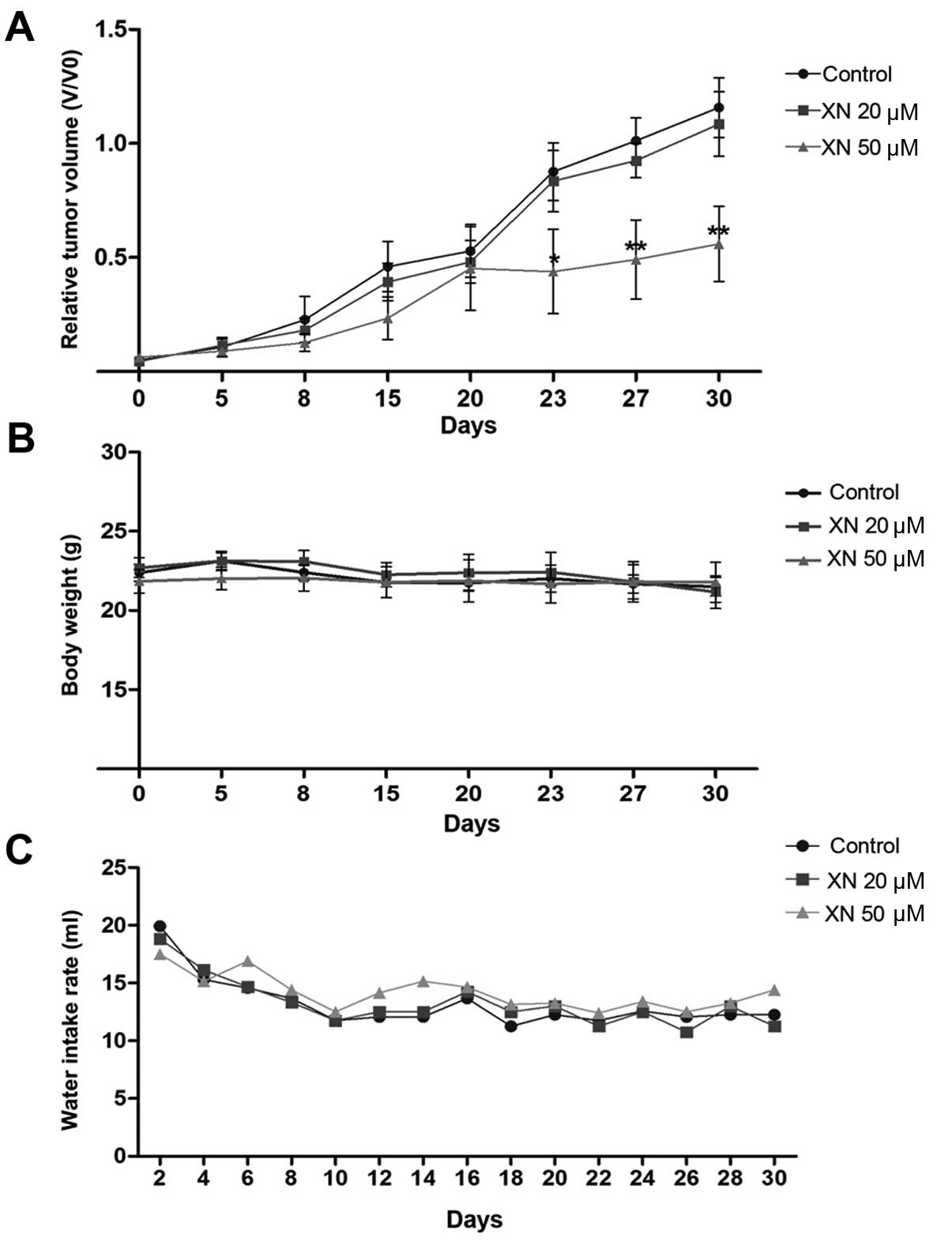
To evaluate an in vivo anticancer activity of
XN, KKU-M214 CCA cells were subcutaneously inoculated into athymic
BALB/c nude mice, then the mice were administrated with 0.5%
ethanol (as control) or 20 and 50 µM concentrations of XN in
drinking water for 30 days and tumor growth was determined. The
results showed that 50 µM concentrations of XN significantly
suppressed the rate of tumor growth when compared with control mice
at day 23 (Fig. 4A). A 20 µM
concentration of XN, however, had no effect on the inhibition of
tumor growth (Fig. 4A). No
side-effects were observed during the treatment. Histological
features of internal organs including liver, spleen and kidney
indicated an absence of toxicity (data not shown). Mice treated
with XN had similar body weight and water intake rate as the
control mice (Fig. 4B and C).
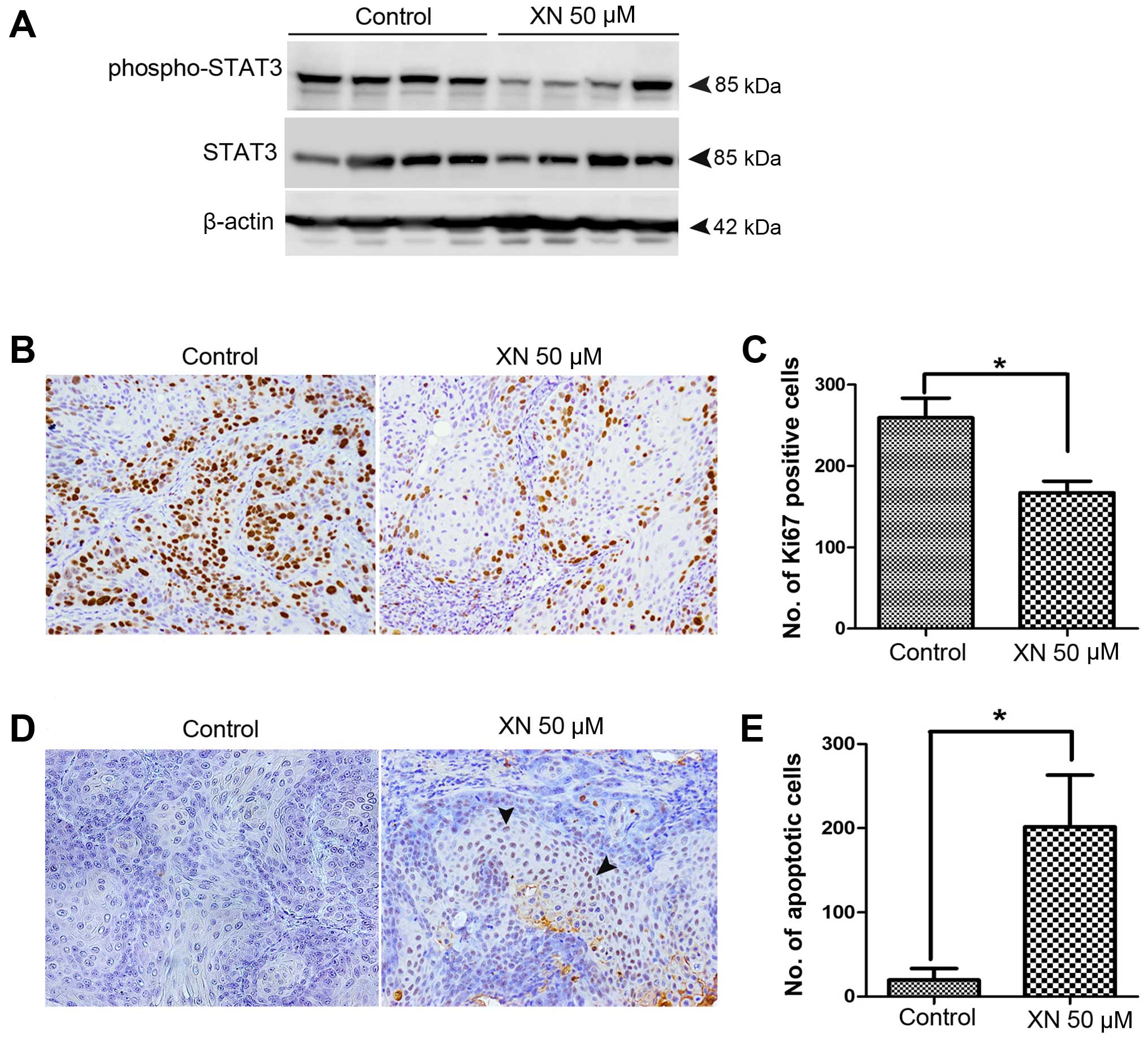
XN inhibits STAT3 activation and tumor
cell proliferation, but induces apoptosis in the CCA mouse
model
As shown in the in vitro results, we found
that XN can inhibit STAT3 activation as well as CCA cell growth and
survival. Moreover, the inhibitory growth effect of XN was observed
for XN 50 µM concentrations in treated CCA xenograft. Thus,
we investigated whether the observed effects were due to an
inhibitory effect of XN on STAT3 activation. We demonstrated that
STAT3 activation was reduced in tumor tissues of XN 50 µM
concentrations in treated mice when compared to control mice
(Fig. 5A). The effects of XN on
tumor cell proliferation inhibition and apoptosis induction were
further evaluated. Immunostaining of Ki67 proliferation marker was
performed to confirm antiproliferation activity of XN (Fig. 5B). Ki67 nuclei stained tumor cells
of XN 50 µM concentrations treated mice was significantly
decreased when compared to control group (Fig. 5C). Moreover, the apoptosis induction
activity of XN was detected by immunohistochemistry of TUNEL
(Fig. 5D). Cell apoptosis was
significantly higher in XN-treated tumors than in the control group
(Fig. 5E).
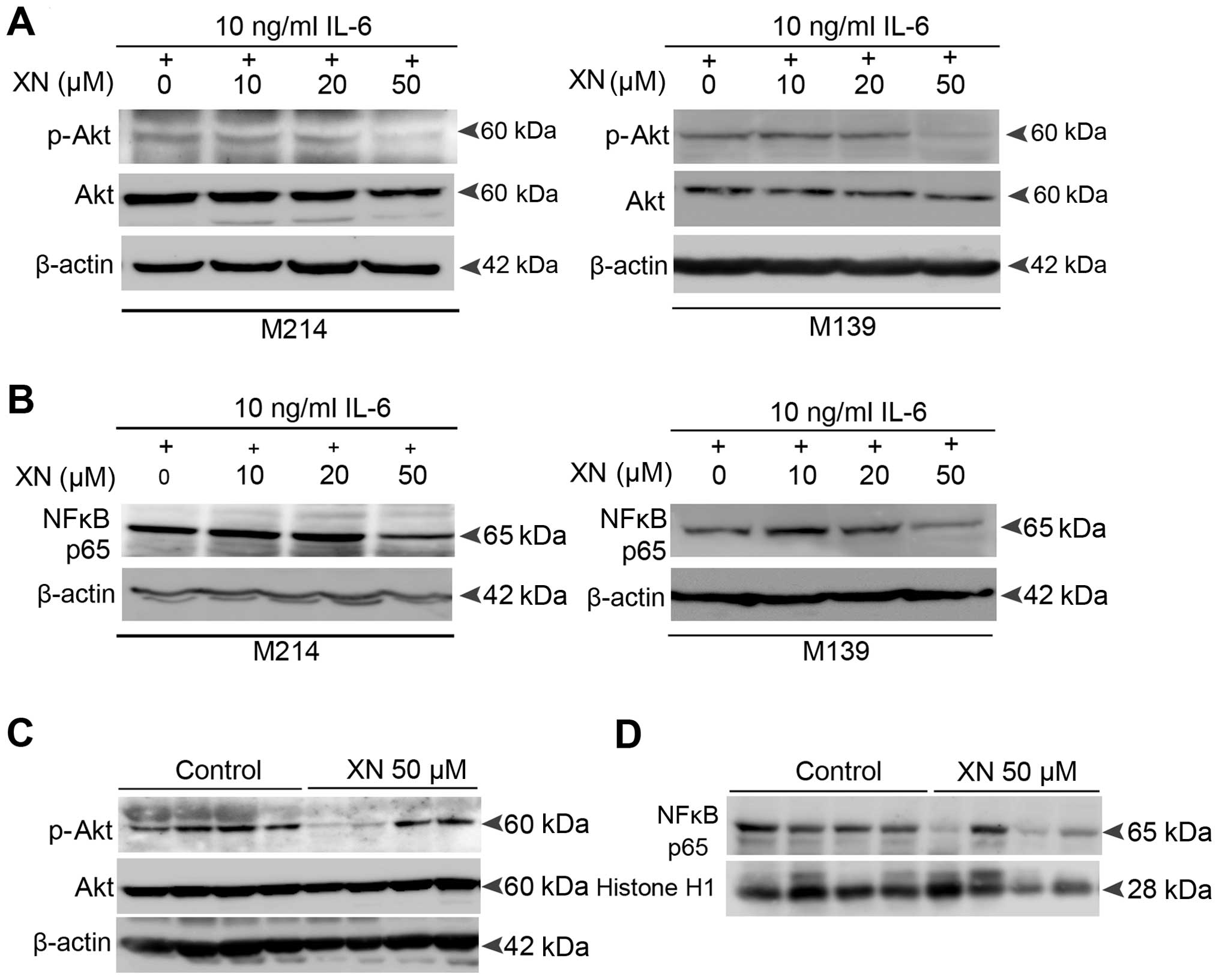
Molecular mechanisms by which XN inhibits
STAT3 activation in CCA
The above data revealed an inhibitory effect of XN
on STAT3 activation both in vitro and in vivo. Thus,
we explored the molecular mechanisms by which XN could inhibit
STAT3 activation in CCA. We focused on Akt and NFκB signaling as
molecular targets of XN as there is mounting evidence that support
the association between Akt, NFκB and STAT3 activation (26–29).
Our results showed that XN also suppresses Akt
activation as well as the nuclear translocation activity of p65
NFκB in both IL-6-induced CCA cells (Fig. 6A and B) and the CCA xenograft model
(Fig. 6C and D). This suggests that
the mechanisms by which XN suppresses STAT3 activation in CCA
resulted from the inhibition of Akt-NFκB signaling.
Discussion
STAT3 is a protein kinase, which plays various roles
as a signal messenger and as a transcription factor. STAT3
signaling can be triggered by inflammatory cytokines, growth
factors and hormones, particularly IL-6 (8,9). Stat3
knockout mice and tissue-specific gene deletions revealed the
critical roles of STAT3 in the regulation of epithelial cell
apoptosis, involution in skin remodeling, keratinocyte migration,
macrophage inactivation, and reduction of T-helper cell responses
to IL-6 (30–32). Thus indicating diverse functions of
STAT3 both in the immune response and cellular development.
Conversely, sustained activation of STAT3 is implicated in
malignant transformation. Various studies have demonstrated that
constitutive STAT3 signaling was required for oncogenic
transformation (33–35). In CCA, several studies have
investigated whether STAT3 acts as a critical molecule in
carcinogenesis and progression of CCA (11,36–38).
Our previous study showed that the activation of the
STAT protein family occurred in both CCA cells and tissues
(13). Moreover, we demonstrated
that among the members of the STAT protein, STAT3 expression was
associated with shorter survival of CCA patients as well as
prominently activating chronic inflammatory CCA carcinogenesis in a
hamster model and CCA cell lines (15). Furthermore, we showed that
LPS-induced macrophage conditioned media, which contains several
inflammatory cytokines including IL-6 (39), can mediate STAT3 activation in CCA
cells (15). Hence, STAT3 is the
major STAT that is involved in inflammation contributing to CCA
carcinogenesis and progression, and may serve as a molecular marker
for CCA poor prognosis. Therefore, targeting STAT3 could be
beneficial for CCA prevention and treatment. In the present study,
we aimed to inhibit STAT3 activation using a potential
anti-inflammatory agent, xanthohumol (XN) in order to evaluate
whether STAT3 could be a promising target for XN resulting in the
inhibition of CCA growth.
XN, prenylated chalcone which can be isolated from
the hop plant (Humulus lupulus L.), has been identified and
reported as an anti-inflammatory and chemopreventive agent
(20,40). XN provides anti-inflammation and
antitumor potential by interfering with molecules which are
recognized as key mediators in inflammation associated
carcinogenesis and progression including iNOS, COX2, NFκB and Akt.
Previous studies on Kaposi's sarcoma, hematopoietic cancer,
prostate cancer, and breast cancer, have demonstrated apoptosis
induction and an anti-angiogenic effect of XN through Akt and NFκB
signaling inhibition (21,22,24,41,42).
Recently, we demonstrated the inhibitory effect of XN on COX
activity which leads to decreased PGE2 production as well as CCA
cell migration inhibition (43),
suggesting a potential chemopreventive and anticancer activity of
XN against cancers including CCA.
The present study showed that XN can inhibit CCA
cell proliferation in a dose- and time-dependent manner. Moreover,
this is the first time that an inhibitory effect of XN on STAT3
activation has been demonstrated. We revealed that XN at 20
µM concentration could partially suppress IL-6-induced STAT3
activation in CCA cells and a complete inhibitory effect was seen
at 50 µM concentration. In addition, our results revealed
that inhibition of STAT3 activation by XN was associated with not
only growth inhibition but also apoptosis induction of CCA cells.
Abrogation of STAT3 activation by XN caused significant reduction
of CCA cell growth and concurrently suppressed the expression of
the growth-related gene, cyclin D1, which is a specific target gene
of STAT3 (44) as well as CDK4, its
partner protein. We also found that suppression of STAT3 activation
by XN was correlated with CCA cell apoptosis as indicated by
downregulation of the anti-apoptotic protein Bcl-2, which is a
STAT3 target gene (45) while
increasing of the pro-apoptotic protein expression BAX was
seen.
Based on the in vitro results, we next
investigated the inhibitory effects of XN on STAT3 activation and
CCA development in a nude mouse model. Our results showed that oral
administration of XN at 50 µM concentrations to
CCA-inoculated mice attenuated tumor growth without noticeable
toxicity. Conversely, a 20 µM XN concentration had no effect
on tumor growth suppression. This result was similar to in
vitro results which showed that low concentrations of XN (2.5,
5 and 10 µM) cannot inhibit CCA cell growth, however, it
induced CCA cell growth as well as STAT3 activation when compared
to control group. This may result from the compensatory signaling
mechanisms of cancer cells that can overcome an inhibitory effect
of low concentration of XN which can lead to an increase of STAT3
activation as well as tumor proliferation. This phenomenon can be
explained by the acquired resistance mechanism of cancer when
blocked by inhibitor treatment. When signaling is inhibited by the
inhibitor, the signaling loop is disrupted which causes
upregulation or increased activation of target molecules that
mediate signaling redundancy, which is the compensatory signaling
mechanism in cancer treatment (46).
Our in vivo results showed that tumor tissues
from XN-treated mice exhibited reduced STAT3 activation as well as
suppressed tumor proliferation and increased apoptosis induction.
These findings suggest that STAT3 is a promising target of XN and
reveal, antitumor activity of XN against CCA growth and
survival.
Furthermore, we explored the molecular mechanisms by
which XN inhibits STAT3 activation in CCA. Results showed that XN
provided anticancer activities via the suppression of Akt and NFκB,
the molecules that are involved in the proliferation, survival and
angiogenesis of tumor cells. Moreover, the interconnection between
Akt-NFκB and STAT3 signaling has been described (26–29).
Our results showed a decreased activation of Akt and NFκB after
treatment with XN in both the IL-6-induced CCA cells and the CCA
inoculated mice. Therefore, the possible mechanisms by which XN
suppresses STAT3 activation in CCA could be due to Akt-NFκB
signaling inhibition.
In conclusion, we have shown that XN can inhibit
STAT3 activation in human CCA cell lines as well as CCA inoculated
mice. Moreover, XN can effectively suppress the growth of tumor and
induce apoptosis in CCA cells and tumor inoculated mice without any
noticeable side-effects. This is the first time that STAT3 has been
demonstrated as a potential target of XN. Moreover, our results
have shown the potential efficacy of XN for CCA treatment. The
above knowledge can provide the basis to develop new therapeutic
strategies for CCA using XN alone and/or combined with conventional
chemotherapy drugs to improve the efficacy of CCA treatment.
Acknowledgments
We thank the research technicians (Division of
Molecular Pathology, Department of Cancer Biology, Institute of
Medical Science, The University of Tokyo) who kindly assisted us in
the animal experiment. The present study was supported by Liver
Fluke and Cholangiocarcinoma Research Center to H.D., the Research
Assistantship Grant of the Faculty of Medicine, Khon Kaen
University (grant no. AS57202) and the Khon Kaen University Grant
(KKU59), the co-funding from Japan Science and Technology Agency
(JST), Ministry of Education, Culture, Sport, Science and
Technology of Japan, and grant of the Higher Education Research
Promotion and National Research University Project of Thailand,
Office of the Higher Education Commission, through the Center of
Excellence in Specific Health Problems in Greater Mekong Sub-region
cluster (SHeP-GMS), KhonKaen University. We also thank Professor
Ross H. Andrews for editing the initial submission via Publication
Clinic KKU, Thailand.
References
|
1
|
Elkins DB, Haswell-Elkins MR, Mairiang E,
Mairiang P, Sithithaworn P, Kaewkes S, Bhudhisawasdi V and
Uttaravichien T: A high frequency of hepatobiliary disease and
suspected cholangiocarcinoma associated with heavy Opisthorchis
viverrini infection in a small community in north-east Thailand.
Trans R Soc Trop Med Hyg. 84:715–719. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elkins DB, Mairiang E, Sithithaworn P,
Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V and
Haswell-Elkins MR: Cross-sectional patterns of hepatobiliary
abnormalities and possible precursor conditions of
cholangiocarcinoma associated with Opisthorchis viverrini infection
in humans. Am J Trop Med Hyg. 55:295–301. 1996.PubMed/NCBI
|
|
3
|
Sripa B and Pairojkul C:
Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol.
24:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SA, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan SA, Taylor-Robinson SD, Toledano MB,
Beck A, Elliott P and Thomas HC: Changing international trends in
mortality rates for liver, biliary and pancreatic tumours. J
Hepatol. 37:806–813. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khuntikeo N, Pugkhem A, Titapun A and
Bhudhisawasdi V: Surgical management of perihilar
cholangiocarcinoma: A Khon Kaen experience. J Hepatobiliary
Pancreat Sci. 21:521–524. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ihle JN: The Stat family in cytokine
signaling. Curr Opin Cell Biol. 13:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeda K and Akira S: STAT family of
transcription factors in cytokine-mediated biological responses.
Cytokine Growth Factor Rev. 11:199–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smirnova OV, Ostroukhova TY and Bogorad
RL: JAK-STAT pathway in carcinogenesis: Is it relevant to
cholangiocarcinoma progression? World J Gastroenterol.
13:6478–6491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dokduang H, Juntana S, Techasen A, Namwat
N, Yongvanit P, Khuntikeo N, Riggins GJ and Loilome W: Survey of
activated kinase proteins reveals potential targets for
cholangiocarcinoma treatment. Tumour Biol. 34:3519–3528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loilome W, Juntana S, Namwat N,
Bhudhisawasdi V, Puapairoj A, Sripa B, Miwa M, Saya H, Riggins GJ
and Yongvanit P: PRKAR1A is overexpressed and represents a possible
therapeutic target in human cholangiocarcinoma. Int J Cancer.
129:34–44. 2011. View Article : Google Scholar
|
|
15
|
Dokduang H, Techasen A, Namwat N,
Khuntikeo N, Pairojkul C, Murakami Y, Loilome W and Yongvanit P:
STATs profiling reveals predominantly-activated STAT3 in
cholangiocarcinoma genesis and progression. J Hepatobiliary
Pancreat Sci. 21:767–776. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
González-Vallinas M, González-Castejón M,
Rodríguez-Casado A and Ramírez de Molina A: Dietary phytochemicals
in cancer prevention and therapy: A complementary approach with
promising perspectives. Nutr Rev. 71:585–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landis-Piwowar KR and Iyer NR: Cancer
chemoprevention: Current state of the art. Cancer Growth
Metastasis. 7:19–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murakami A, Ohigashi H and Koshimizu K:
Anti-tumor promotion with food phytochemicals: A strategy for
cancer chemoprevention. Biosci Biotechnol Biochem. 60:1–8. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gerhauser C, Alt A, Heiss E, Gamal-Eldeen
A, Klimo K, Knauft J, Neumann I, Scherf HR, Frank N, Bartsch H, et
al: Cancer chemopreventive activity of Xanthohumol, a natural
product derived from hop. Mol Cancer Ther. 1:959–969.
2002.PubMed/NCBI
|
|
21
|
Albini A, Dell Eva R, Vené R, Ferrari N,
Buhler DR, Noonan DM and Fassina G: Mechanisms of the
antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB
and Akt as targets. FASEB J. 20:527–529. 2006.PubMed/NCBI
|
|
22
|
Dell'Eva R, Ambrosini C, Vannini N,
Piaggio G, Albini A and Ferrari N: AKT/NF-kappaB inhibitor
xanthohumol targets cell growth and angiogenesis in hematologic
malignancies. Cancer. 110:2007–2011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harikumar KB, Kunnumakkara AB, Ahn KS,
Anand P, Krishnan S, Guha S and Aggarwal BB: Modification of the
cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by
xanthohumol leads to suppression of NF-kappaB-regulated gene
products and potentiation of apoptosis in leukemia cells. Blood.
113:2003–2013. 2009. View Article : Google Scholar
|
|
24
|
Monteghirfo S, Tosetti F, Ambrosini C,
Stigliani S, Pozzi S, Frassoni F, Fassina G, Soverini S, Albini A
and Ferrari N: Antileukemia effects of xanthohumol in
Bcr/Abl-transformed cells involve nuclear factor-kappaB and p53
modulation. Mol Cancer Ther. 7:2692–2702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Namwat N, Amimanan P, Loilome W,
Jearanaikoon P, Sripa B, Bhudhisawasdi V and Tassaneeyakul W:
Characterization of 5-fluorouracil-resistant cholangiocarcinoma
cell lines. Chemotherapy. 54:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blando JM, Carbajal S, Abel E, Beltran L,
Conti C, Fischer S and DiGiovanni J: Cooperation between Stat3 and
Akt signaling leads to prostate tumor development in transgenic
mice. Neoplasia. 13:254–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kortylewski M, Feld F, Krüger KD,
Bahrenberg G, Roth RA, Joost HG, Heinrich PC, Behrmann I and
Barthel A: Akt modulates STAT3-mediated gene expression through a
FKHR (FOXO1a)-dependent mechanism. J Biol Chem. 278:5242–5249.
2003. View Article : Google Scholar
|
|
28
|
Squarize CH, Castilho RM, Sriuranpong V,
Pinto DS Jr and Gutkind JS: Molecular cross-talk between the
NFkappaB and STAT3 signaling pathways in head and neck squamous
cell carcinoma. Neoplasia. 8:733–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang
Y, Deng J, Margolick JB, Liotta LA, Petricoin E III and Zhang Y:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sano S, Itami S, Takeda K, Tarutani M,
Yamaguchi Y, Miura H, Yoshikawa K, Akira S and Takeda J:
Keratinocyte-specific ablation of Stat3 exhibits impaired skin
remodeling, but does not affect skin morphogenesis. EMBO J.
18:4657–4668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeda K, Clausen BE, Kaisho T, Tsujimura
T, Terada N, Förster I and Akira S: Enhanced Th1 activity and
development of chronic enterocolitis in mice devoid of Stat3 in
macrophages and neutrophils. Immunity. 10:39–49. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeda K, Noguchi K, Shi W, Tanaka T,
Matsumoto M, Yoshida N, Kishimoto T and Akira S: Targeted
disruption of the mouse Stat3 gene leads to early embryonic
lethality. Proc Natl Acad Sci USA. 94:3801–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bromberg JF, Horvath CM, Besser D, Lathem
WW and Darnell JE Jr: Stat3 activation is required for cellular
transformation by v-src. Mol Cell Biol. 18:2553–2558. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao X, Tay A, Guy GR and Tan YH:
Activation and association of Stat3 with Src in v-Src-transformed
cell lines. Mol Cell Biol. 16:1595–1603. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turkson J, Bowman T, Garcia R, Caldenhoven
E, De Groot RP and Jove R: Stat3 activation by Src induces specific
gene regulation and is required for cell transformation. Mol Cell
Biol. 18:2545–2552. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Isomoto H, Mott JL, Kobayashi S, Werneburg
NW, Bronk SF, Haan S and Gores GJ: Sustained IL-6/STAT-3 signaling
in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing.
Gastroenterology. 132:384–396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sia D, Hoshida Y, Villanueva A, Roayaie S,
Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C, et al:
Integrative molecular analysis of intrahepatic cholangiocarcinoma
reveals 2 classes that have different outcomes. Gastroenterology.
144:829–840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sia D, Tovar V, Moeini A and Llovet JM:
Intrahepatic cholangio-carcinoma: Pathogenesis and rationale for
molecular therapies. Oncogene. 32:4861–4870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Techasen A, Loilome W, Namwat N, Dokduang
H, Jongthawin J and Yongvanit P: Cytokines released from activated
human macrophages induce epithelial mesenchymal transition markers
of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 13(Suppl):
S115–S118. 2012.
|
|
40
|
Gerhäuser C: Beer constituents as
potential cancer chemopreventive agents. Eur J Cancer.
41:1941–1954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Colgate EC, Miranda CL, Stevens JF, Bray
TM and Ho E: Xanthohumol, a prenylflavonoid derived from hops
induces apoptosis and inhibits NF-kappaB activation in prostate
epithelial cells. Cancer Lett. 246:201–209. 2007. View Article : Google Scholar
|
|
42
|
Monteiro R, Calhau C, Silva AO,
Pinheiro-Silva S, Guerreiro S, Gärtner F, Azevedo I and Soares R:
Xanthohumol inhibits inflammatory factor production and
angiogenesis in breast cancer xenografts. J Cell Biochem.
104:1699–1707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jongthawin J, Techasen A, Loilome W,
Yongvanit P and Namwat N: Anti-inflammatory agents suppress the
prostaglandin E2 production and migration ability of
cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 13(Suppl):
47–51. 2012.PubMed/NCBI
|
|
44
|
Liu B, Ren Z, Shi Y, Guan C, Pan Z and
Zong Z: Activation of signal transducers and activators of
transcription 3 and over-expression of its target gene CyclinD1 in
laryngeal carcinomas. Laryngoscope. 118:1976–1980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Williams JG: STAT signalling in cell
proliferation and in development. Curr Opin Genet Dev. 10:503–507.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Logue JS and Morrison DK: Complexity in
the signaling network: Insights from the use of targeted inhibitors
in cancer therapy. Genes Dev. 26:641–650. 2012. View Article : Google Scholar : PubMed/NCBI
|