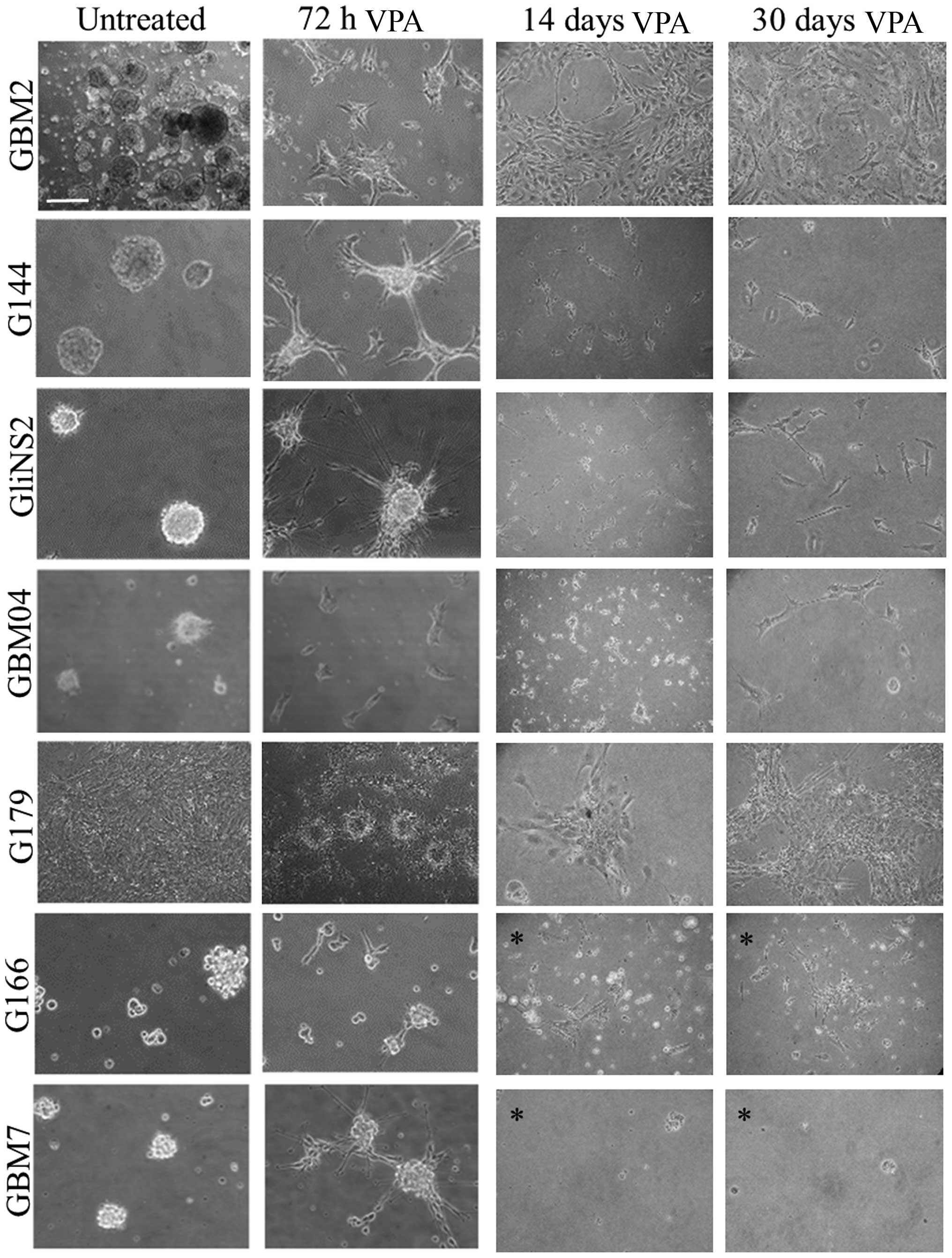
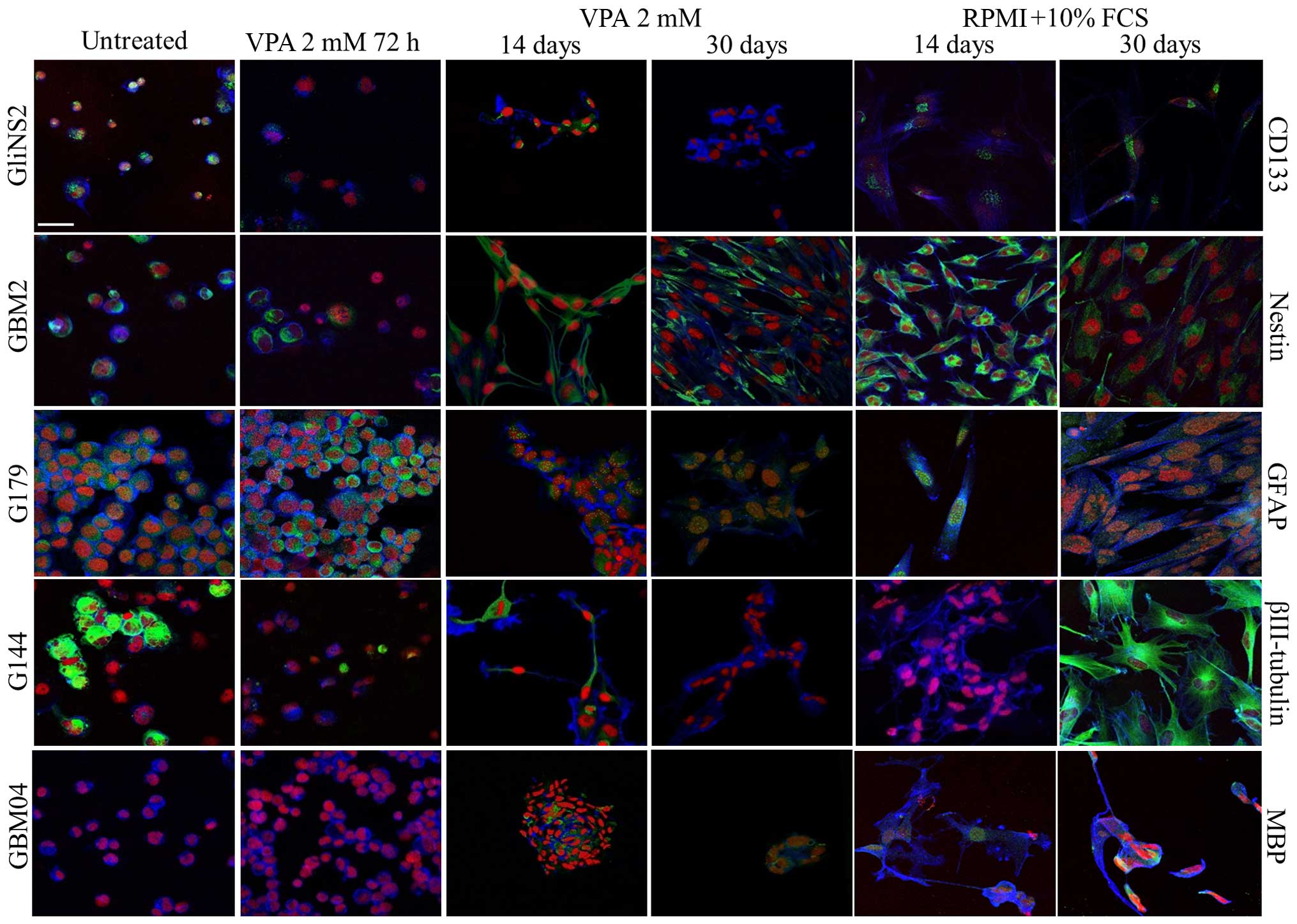
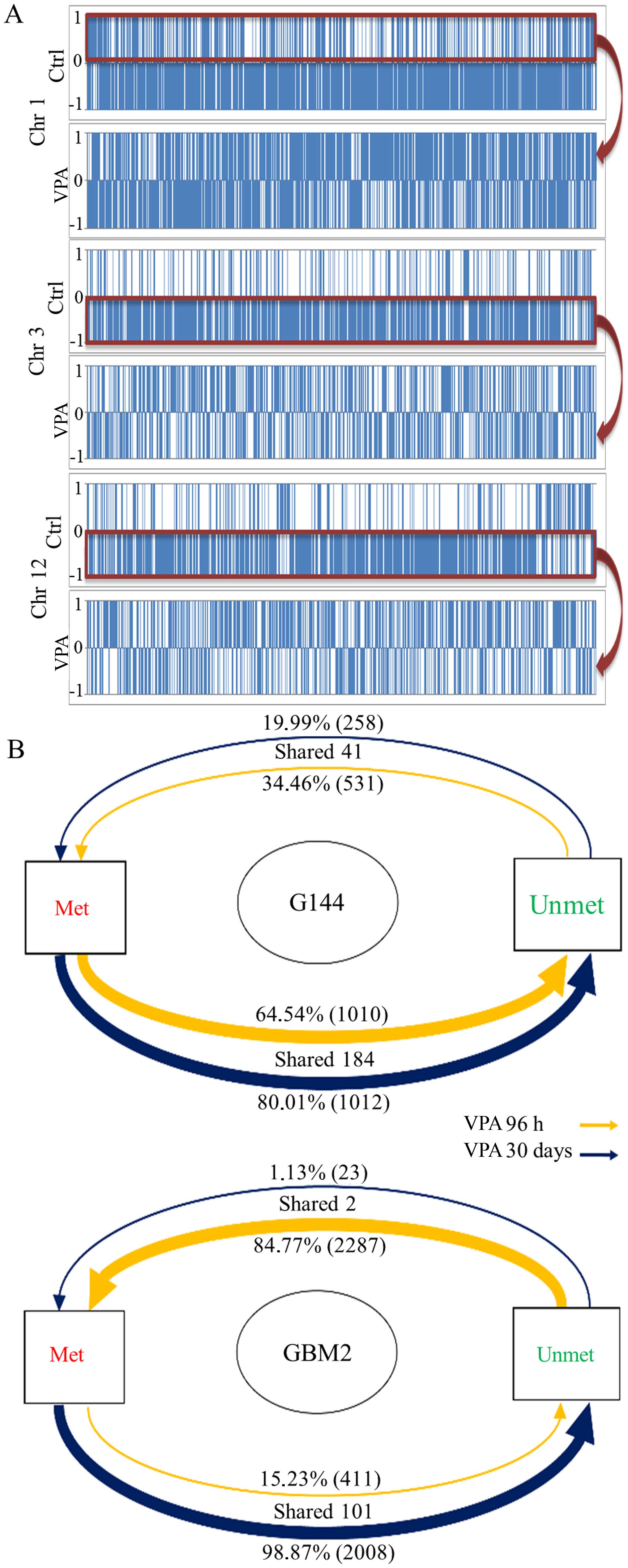
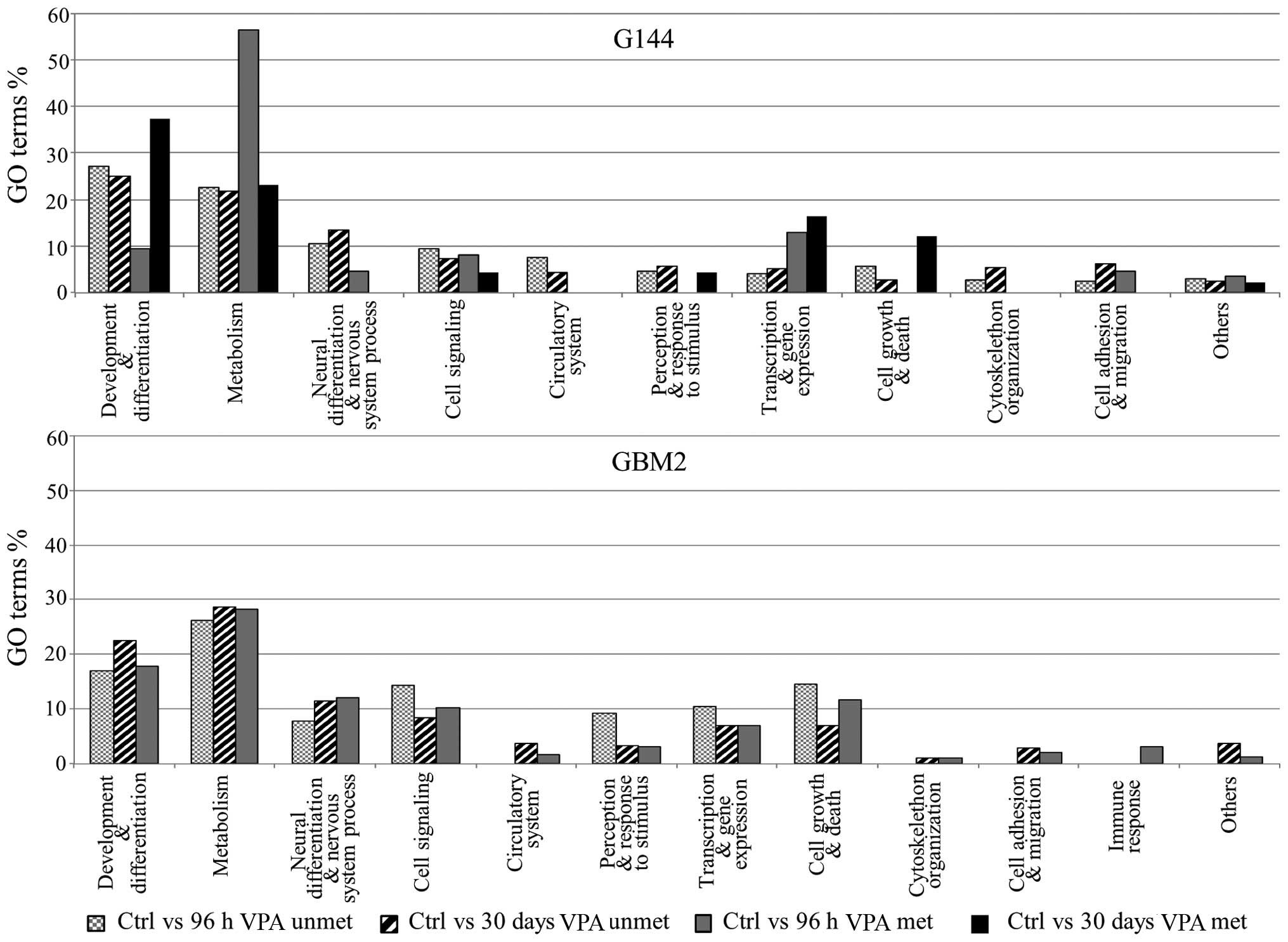
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brandes AA, Franceschi E, Ermani M, Tosoni
A, Albani F, Depenni R, Faedi M, Pisanello A, Crisi G, Urbini B, et
al: Pattern of care and effectiveness of treatment for glioblastoma
patients in the real world: Results from a prospective
population-based registry. Could survival differ in a high-volume
center? Neurooncol Pract. 1:166–171. 2014.
|
|
3
|
Nduom EK, Hadjipanayis CG and Van Meir EG:
Glioblastoma cancer stem-like cells: Implications for pathogenesis
and treatment. Cancer J. 18:100–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sottoriva A, Spiteri I, Piccirillo SG,
Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C and Tavaré
S: Intratumor heterogeneity in human glioblastoma reflects cancer
evolutionary dynamics. Proc Natl Acad Sci USA. 110:4009–4014. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piccirillo SG, Combi R, Cajola L, Patrizi
A, Redaelli S, Bentivegna A, Baronchelli S, Maira G, Pollo B,
Mangiola A, et al: Distinct pools of cancer stem-like cells coexist
within human glioblastomas and display different tumorigenicity and
independent genomic evolution. Oncogene. 28:1807–1811. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatziapostolou M and Iliopoulos D:
Epigenetic aberrations during oncogenesis. Cell Mol Life Sci.
68:1681–1702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagarajan RP and Costello JF: Molecular
epigenetics and genetics in neuro-oncology. Neurotherapeutics.
6:436–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan O and La Thangue NB: HDAC inhibitors
in cancer biology: Emerging mechanisms and clinical applications.
Immunol Cell Biol. 90:85–94. 2012. View Article : Google Scholar
|
|
10
|
Song SH, Han SW and Bang YJ:
Epigenetic-based therapies in cancer: Progress to date. Drugs.
71:2391–2403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cinatl J Jr, Cinatl J, Scholz M, Driever
PH, Henrich D, Kabickova H, Vogel JU, Doerr HW and Kornhuber B:
Antitumor activity of sodium valproate in cultures of human
neuroblastoma cells. Anticancer Drugs. 7:766–773. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blaheta RA, Michaelis M, Driever PH and
Cinatl J Jr: Evolving anticancer drug valproic acid: Insights into
the mechanism and clinical studies. Med Res Rev. 25:383–397. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Lint C, Emiliani S and Verdin E: The
expression of a small fraction of cellular genes is changed in
response to histone hyperacetylation. Gene Expr. 5:245–253.
1996.PubMed/NCBI
|
|
14
|
Chateauvieux S, Morceau F, Dicato M and
Diederich M: Molecular and therapeutic potential and toxicity of
valproic acid. J Biomed Biotechnol. 2010:4793642010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bacon CL, O'Driscoll E and Regan CM:
Valproic acid suppresses G1 phase-dependent sialylation of a 65 kDa
glycoprotein in the C6 glioma cell cycle. Int J Dev Neurosci.
15:777–784. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knüpfer MM, Hernáiz-Driever P, Poppenborg
H, Wolff JE and Cinatl J: Valproic acid inhibits proliferation and
changes expression of CD44 and CD56 of malignant glioma cells in
vitro. Anticancer Res. 18:3585–3589. 1998.PubMed/NCBI
|
|
17
|
Chavez-Blanco A, Perez-Plasencia C,
Perez-Cardenas E, Carrasco-Legleu C, Rangel-Lopez E, Segura-Pacheco
B, Taja-Chayeb L, Trejo-Becerril C, Gonzalez-Fierro A, Candelaria
M, et al: Antineoplastic effects of the DNA methylation inhibitor
hydralazine and the histone deacetylase inhibitor valproic acid in
cancer cell lines. Cancer Cell Int. 6:22006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Das CM, Aguilera D, Vasquez H, Prasad P,
Zhang M, Wolff JE and Gopalakrishnan V: Valproic acid induces p21
and topoisomerase-II (alpha/beta) expression and synergistically
enhances etoposide cytotoxicity in human glioblastoma cell lines. J
Neurooncol. 85:159–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Nifterik KA, Van den Berg J, Slotman
BJ, Lafleur MV, Sminia P and Stalpers LJ: Valproic acid sensitizes
human glioma cells for temozolomide and γ-radiation. J Neurooncol.
107:61–67. 2012. View Article : Google Scholar
|
|
20
|
Ryu CH, Yoon WS, Park KY, Kim SM, Lim JY,
Woo JS, Jeong CH, Hou Y and Jeun SS: Valproic acid downregulates
the expression of MGMT and sensitizes temozolomide-resistant glioma
cells. J Biomed Biotechnol. 2012:9874952012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CH, Chang YJ, Ku MS, Chung KT and
Yang JT: Enhancement of temozolomide-induced apoptosis by valproic
acid in human glioma cell lines through redox regulation. J Mol Med
Berl. 89:303–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pollard SM, Yoshikawa K, Clarke ID, Danovi
D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et
al: Glioma stem cell lines expanded in adherent culture have
tumor-specific phenotypes and are suitable for chemical and genetic
screens. Cell Stem Cell. 4:568–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griffero F, Daga A, Marubbi D, Capra MC,
Melotti A, Pattarozzi A, Gatti M, Bajetto A, Porcile C, Barbieri F,
et al: Different response of human glioma tumor-initiating cells to
epidermal growth factor receptor kinase inhibitors. J Biol Chem.
284:7138–7148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baronchelli S, Bentivegna A, Redaelli S,
Riva G, Butta V, Paoletta L, Isimbaldi G, Miozzo M, Tabano S, Daga
A, et al: Delineating the cytogenomic and epigenomic landscapes of
glioma stem cell lines. PLoS One. 8:e574622013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riva G, Baronchelli S, Paoletta L, Butta
V, Biunno I, Lavitrano M, Dalprà L and Bentivegna A: In vitro
anticancer drug test: A new method emerges from the model of glioma
stem cells. Toxicol Rep. 1:188–199. 2014. View Article : Google Scholar
|
|
26
|
Aouali N, Palissot V, El-Khoury V, Moussay
E, Janji B, Pierson S, Brons NH, Kellner L, Bosseler M, Van Moer K,
et al: Peroxisome proliferator-activated receptor gamma agonists
potentiate the cytotoxic effect of valproic acid in multiple
myeloma cells. Br J Haematol. 147:662–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Straussman R, Nejman D, Roberts D,
Steinfeld I, Blum B, Benvenisty N, Simon I, Yakhini Z and Cedar H:
Developmental programming of CpG island methylation profiles in the
human genome. Nat Struct Mol Biol. 16:564–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beissbarth T and Speed TP: GOstat: Find
statistically overrepresented Gene Ontologies within a group of
genes. Bioinformatics. 20:1464–1465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanu OO, Hughes B, Di C, Lin N, Fu J,
Bigner DD, Yan H and Adamson C: Glioblastoma multiforme
oncogenomics and signaling pathways. Clin Med Oncol. 3:39–52.
2009.PubMed/NCBI
|
|
30
|
Wardak Z and Choe KS: Molecular pathways
and potential therapeutic targets in glioblastoma multiforme.
Expert Rev Anticancer Ther. 13:1307–1318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Stevens MF and Bradshaw TD:
Temozolomide: Mechanisms of action, repair and resistance. Curr Mol
Pharmacol. 5:102–114. 2012. View Article : Google Scholar
|
|
33
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG,
et al: Valproic acid defines a novel class of HDAC inhibitors
inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phiel CJ, Zhang F, Huang EY, Guenther MG,
Lazar MA and Klein PS: Histone deacetylase is a direct target of
valproic acid, a potent anticonvulsant, mood stabilizer, and
teratogen. J Biol Chem. 276:36734–36741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Osuka S, Takano S, Watanabe S, Ishikawa E,
Yamamoto T and Matsumura A: Valproic acid inhibits angiogenesis in
vitro and glioma angiogenesis in vivo in the brain. Neurol Med Chir
(Tokyo). 52:186–193. 2012. View Article : Google Scholar
|
|
37
|
Knüpfer MM, Pulzer F, Schindler I, Hernaíz
Driever P, Knüpfer H and Keller E: Different effects of valproic
acid on proliferation and migration of malignant glioma cells in
vitro. Anticancer Res. 21:347–351. 2001.PubMed/NCBI
|
|
38
|
Cameron EE, Bachman KE, Myöhänen S, Herman
JG and Baylin SB: Synergy of demethylation and histone deacetylase
inhibition in the re-expression of genes silenced in cancer. Nat
Genet. 21:103–107. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
De la Cruz-Hernández E, Perez-Plasencia C,
Pérez-Cardenas E, Gonzalez-Fierro A, Trejo-Becerril C,
Chávez-Blanco A, Taja-Chayeb L, Vidal S, Gutiérrez O, Dominguez GI,
et al: Transcriptional changes induced by epigenetic therapy with
hydralazine and magnesium valproate in cervical carcinoma. Oncol
Rep. 25:399–407. 2011.
|
|
40
|
Suzuki H, Gabrielson E, Chen W, Anbazhagan
R, van Engeland M, Weijenberg MP, Herman JG and Baylin SB: A
genomic screen for genes upregulated by demethylation and histone
deacetylase inhibition in human colorectal cancer. Nat Genet.
31:141–149. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Breemen MS, Wilms EB and Vecht CJ:
Epilepsy in patients with brain tumours: Epidemiology, mechanisms,
and management. Lancet Neurol. 6:421–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sizoo EM, Koekkoek JA, Postma TJ, Heimans
JJ, Pasman HR, Deliens L, Taphoorn MJ and Reijneveld JC: Seizures
in patients with high-grade glioma: A serious challenge in the
end-of-life phase. BMJ Support Palliat Care. 4:77–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guthrie GD and Eljamel S: Impact of
particular antiepileptic drugs on the survival of patients with
glioblastoma multiforme. J Neurosurg. 118:859–865. 2013. View Article : Google Scholar
|
|
44
|
Vecht CJ, Kerkhof M and Duran-Pena A:
Seizure prognosis in brain tumors: New insights and evidence-based
management. Oncologist. 19:751–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Duenas-Gonzalez A, Candelaria M,
Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E and
Herrera LA: Valproic acid as epigenetic cancer drug: Preclinical,
clinical and transcriptional effects on solid tumors. Cancer Treat
Rev. 34:206–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dai J, Bercury KK, Ahrendsen JT and
Macklin WB: Olig1 function is required for oligodendrocyte
differentiation in the mouse brain. J Neurosci. 35:4386–4402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Z, Li H, Hu X, Yu L, Liu H, Han R,
Colella R, Mower GD, Chen Y and Qiu M: Control of precerebellar
neuron development by Olig3 bHLH transcription factor. J Neurosci.
28:10124–10133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Soda Y, Marumoto T, Friedmann-Morvinski D,
Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari
S, et al: Transdifferentiation of glioblastoma cells into vascular
endothelial cells. Proc Natl Acad Sci USA. 108:4274–4280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu T, Liu PY, Tee AE, Haber M, Norris MD,
Gleave ME and Marshall GM: Over-expression of clusterin is a
resistance factor to the anti-cancer effect of histone deacetylase
inhibitors. Eur J Cancer. 45:1846–1854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Juengel E, Makarević J, Tsaur I, Bartsch
G, Nelson K, Haferkamp A and Blaheta RA: Resistance after chronic
application of the HDAC-inhibitor valproic acid is associated with
elevated Akt activation in renal cell carcinoma in vivo. PLoS One.
8:e531002013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Milutinovic S, D'Alessio AC, Detich N and
Szyf M: Valproate induces widespread epigenetic reprogramming which
involves demethylation of specific genes. Carcinogenesis.
28:560–571. 2007. View Article : Google Scholar
|
|
52
|
Detich N, Bovenzi V and Szyf M: Valproate
induces replication-independent active DNA demethylation. J Biol
Chem. 278:27586–27592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brandes AA, Franceschi E, Tosoni A, Blatt
V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F,
Andreoli A, et al: MGMT promoter methylation status can predict the
incidence and outcome of pseudoprogression after concomitant
radiochemotherapy in newly diagnosed glioblastoma patients. J Clin
Oncol. 26:2192–2197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Nagula V, Scotto KW and Rameshwar P: Temozolomide induces the
production of epidermal growth factor to regulate MDR1 expression
in glioblastoma cells. Mol Cancer Ther. 13:2399–2411. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Ramkissoon SH, Ligon KL and Rameshwar P: Temozolomide resistance in
glioblastoma cells occurs partly through epidermal growth factor
receptor-mediated induction of connexin 43. Cell Death Dis.
5:e11452014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Munoz JL, Walker ND, Scotto KW and
Rameshwar P: Temozolomide competes for P-glycoprotein and
contributes to chemoresistance in glioblastoma cells. Cancer Lett.
367:69–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tivnan A, Zakaria Z, O'Leary C, Kögel D,
Pokorny JL, Sarkaria JN and Prehn JH: Inhibition of multidrug
resistance protein 1 (MRP1) improves chemotherapy drug response in
primary and recurrent glioblastoma multiforme. Front Neurosci.
9:2182015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kitange GJ, Mladek AC, Carlson BL,
Schroeder MA, Pokorny JL, Cen L, Decker PA, Wu W, Lomberk GA, Gupta
SK, et al: Inhibition of histone deacetylation potentiates the
evolution of acquired temozolomide resistance linked to MGMT
upregulation in glioblastoma xenografts. Clin Cancer Res.
18:4070–4079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zighetti ML, Fontana G, Lussana F, Chiesa
V, Vignoli A, Canevini MP and Cattaneo M: Effects of chronic
administration of valproic acid to epileptic patients on
coagulation tests and primary hemostasis. Epilepsia. 56:e49–e52.
2015. View Article : Google Scholar : PubMed/NCBI
|