Introduction
The control of cell proliferation is a major issue
in the treatment of cancer. In breast cancer, endocrine therapies
targeting the estrogen receptor α (ER) are major therapeutic tools.
Antiestrogens which primarily act by competing with estrogens for
binding to the ER prevent the mitogenic effect of estrogens
(1–3). They act as pure ER antagonist, such as
fulvestrant (Faslodex or ICI182780) or selective ER
modulators, such as tamoxifen (Nolvadex) or raloxifen (Evista). The
major limitation of antiestrogens is due to the acquired resistance
of responsive breast tumors after several years of treatment. Thus,
the sequential or combinatorial use of antiestrogen with other
therapeutics has been proposed to overcome this resistance
(4).
Previous studies have supported the importance of
cyclinkinase inhibitor (CKI) proteins, p21WAF1 (p21) and
p27KIP1 (p27), in ER signaling and in the antiestrogen
response (5–8). Indeed, antiestrogen treatment was
found to induce a G0/G1 arrest in sensitive
ER-positive breast cancer cells due to an upregulation of both p21
and p27 levels (5). This
G0/G1 arrest was abrogated whether p21 and
p27 were depleted. Moreover, low levels of these CKIs indicate a
poor prognosis of breast cancers (9–14).
Proteasome inhibitors (PIs) have likewise been found
to regulate these CKIs (15–20)
and recently, we demonstrated that in ER-positive breast cancer, ER
was a critical mediator of bortezomib-induced inhibition (21). Thus, PIs could be of potential
interest in combination therapeutic strategies with antiestrogens.
MG132 is a reversible peptide aldehyde used in experimental
studies. Bortezomib (formerly known as PS-341 or Velcade) is a
boronic acid, that has been approved by the Food and Drug
Administration for the treatment of relapsed and refractory
multiple myeloma (22,23). Bortezomib and MG132 both inhibit
proteasome chymotrypsin-like activity; however, their effect on ER
is different (24). Therefore,
comparison of these two PIs combined with antiestrogen provided
distinct results.
In the present study, we addressed for the first
time, the growth effects of several PIs in the presence of
antiestrogens in three distinct ER-positive breast cancer cell
lines. We also explored the underlying mechanisms by focusing on
p21 and p27 expression. The effects of PIs associated with
antiestrogens were also assessed in two cellular models of acquired
antiestrogen resistance.
Materials and methods
Cell culture
MCF7, T47D and ZR 75.1 human breast cancer cells
were routinely maintained in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS). LCC2 and
RTx6 cells are sublines of the MCF-7 human breast cancer cell line
respectively selected for their resistance to 4-hydroxytamoxifen
(OHT) and tamoxifen (Tam), kindly provided by R. Clarke (25) and F. Bayard (26), respectively.
Stock solutions of ICI182,780 (also
called fulvestrant), tamoxifen or its active metabolite OHT (a kind
gift from A. Wakeling, AstraZeneca, Cheshire, UK), raloxifen (Eli
Lilly and Company, France) and 17β-estradiol (E2) (Sigma-Aldrich
Chimie, Saint-Quentin Fallavier, France) were prepared in ethanol.
MG132 (Sigma-Aldrich Chimie) was diluted in DMSO. Bortezomib (a
kind gift from Millennium Pharmaceuticals, Cambridge, MA, USA) was
protected from light and prepared in aqueous solution. Treatments
were always equilibrated for vehicle concentration.
Cell growth assay
Steroids were withdrawn from cells by a 6-day
culture in phenol red-free DMEM supplemented with 10%
steroid-stripped serum. Then, the cells were harvested and plated
on 96-well plates. Adherent cells were treated for 5 days with 1 nM
E2 and 0.3 µM MG132 or 26 nM bortezomib combined or not with
1 µM OHT, tamoxifen, raloxifen or 0.1 µM
ICI182,730. Outgrowth assays were performed with 5,000
cells in 96-well plates as previously described (27). After 4 days, the cells were
quantified using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (200 µg/ml MTT for 4 h). Values are expressed as a
percentage of E2-stimulated cells.
Colony formation assay
These assays were carried out according to
Prud'homme et al (28).
Briefly, using a 12-well plate, 4,000 cells in 0.3% agar
(Sigma-Aldrich Chemie, Lyon, France) were layered on preformed 0.7%
agar layer (Costar, Fisher Bioblock Scientific, Illkirch, France).
After plating, the cells in agar were incubated in conditioned
medium and simultaneously treated with 1 nM E2 and 0.3 µM
MG132 or 26 nM bortezomib combined or not with 1 µM OHT or
0.1 µM ICI182,730. Colonies were counted under
the microscope using a low magnification (x5) after 28 days.
Immunofluorescence
Cells grown on coverslips were treated for 24 h as
indicated before fixation with 4% PFA for 12 min, cold methanol for
4 min, cold acetone for 2 min, and then saturated overnight at 4°C
with 3% goat serum in phosphate-buffered saline (PBS) containing 4%
bovine γ-globulin. For immunostaining, the cells were incubated
with the mouse monoclonal p21WAF1 (Oncogene Research
Products, San Diego, CA, USA) or p27KIP1 (Interchim,
Montluçon, France) antibody for 2 h. The cells were extensively
washed and incubated for 1 h with Alexa Fluor 568 anti-mouse
antibody (Fisher Bioblock Scientific), and then washed again and
incubated with 4′,6-diamidino-2-phenylindole (DAPI) (0.5
µg/ml; Sigma-Aldrich Chimie) for 15 min for nuclear
staining. Negative controls were performed with a purified rabbit
IgG1.
Immunoblotting
For protein expression analysis of whole cell
lysates, the cell lysates were extracted by 3 freeze-thaw cycles in
buffer containing 50 mM HEPES, 150 mM NaCl, 0.1% NP-40, 10%
glycerol, 2.5 mM EGTA and protease inhibitors (Complete, Roche
Diagnostics, Indianapolis, IN, USA), and centrifuged at 10,000 × g
for 15 min. Proteins were quantified by the Bradford assay and a
constant amount was analyzed by immunoblotting. Afterwards, the
samples were mixed with equal amounts of sample buffer (125 mM
Tris/HCl, 288 mM β-mercatoethanol, 20% glycerol, 2% SDS, 10
µg/ml bromphenol blue), boiled for 3 min, and blotted as
previously described (29).
Immunostaining was performed using mouse monoclonal p21 (Oncogene
Research Products) and p27 (Interchim). Polyclonal rabbit antibody
against actin was purchased from Sigma-Aldrich Chimie (A2066).
Immunoblotting was performed with goat anti-rabbit or sheep
anti-mouse secondary antibodies, purchased from Amersham
(Piscataway, NJ, USA). The bound immunoglobulins were visualized
using the ECL detection system (Amersham). Immunoblotting films
were analyzed by densitometry with the PC-Bas 2.0 software (Fuji,
Stanford, CT, USA).
Quantitative real-time (qRT)-PCR
Total RNA was isolated from the cells using the
RNeasy Mini kit from Qiagen (Courtaboeuf, France). For analyzing
the transcription of p21 and p27, 1 µg of total RNA was
reverse-transcribed in 20 µl reaction mix using the
SuperScript II First-Strand Synthesis System (Invitrogen).
qRT-PCR was carried out in Roche lightCycler using
DNA double-strand-specific SYBR-Green I dye for detection (Roche).
P21 primer sequences were: 5′-CTG GTG ACT CTC AGG GTC GAA-3′ (sense
primer) and 5′-GGA TTA GGG CTT CCT CTT GGA-3′ (antisense primer).
Primer sequences for p27 were: 5′-AGA CGG GGT TAG CGG AGC-3′ (sense
primer) and 5′-GAA CCG TCT GAA ACA TTT TCT TCT GT-3′ (antisense
primer). The relative mRNA levels in cells were calculated using
the ∆∆Ct method with endogenous RS9 mRNA as a control (21).
Results
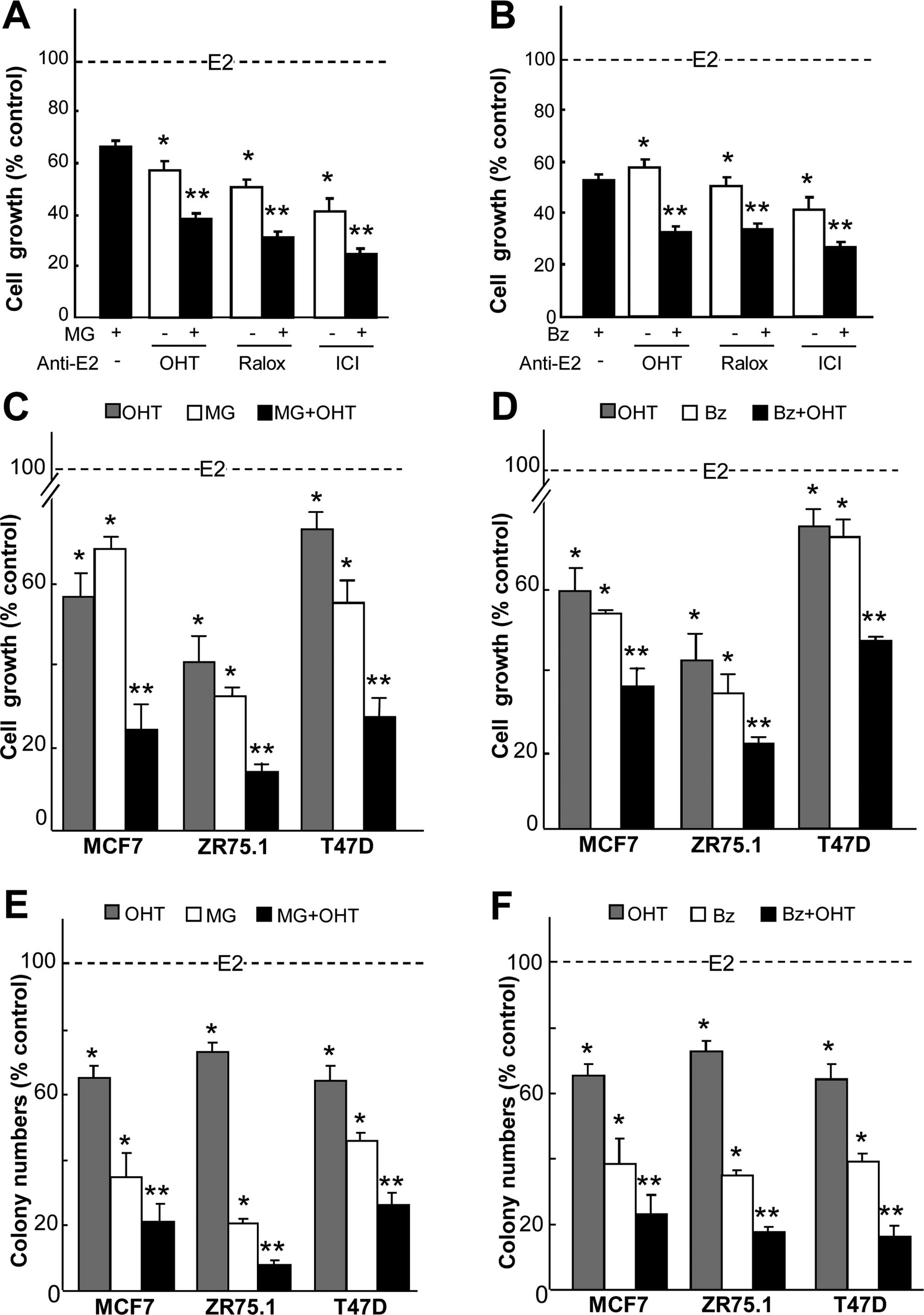
In the present study, we investigated the synergism
between antiestrogens and antiproteasome treatments in cancer cell
lines. At first, we studied the effects of 3 antiestrogens with
partial agonist/antagonist or pure antagonist activities in
combination with PIs at IC50 concentrations [as
previously described (21)] of 0.3
µM MG132 and 26 nM bortezomib. Combinations appeared
significantly more effective at reducing the growth than the
individual treatments (Fig. 1A and
B) in the MCF7 cancer cells. As shown in Fig. 1A, a 4-day treatment with
OH-tamoxifen and MG132 resulted in a 62% inhibition of growth,
whereas the separate treatments inhibited 43% of the growth.
OH-tamoxifen also showed a synergistic effect with bortezomib
(Fig. 1B) where growth inhibition
reached 70%. Ralox and ICI182,780 combination also
obtained an additive antiproliferative activity with MG132 and
bortezomib. However, in the case of ICI182,780, the
benefit attributed to the combination was limited by the strong
effect of ICI182,780 alone. Therefore, we focused on
OH-tamoxifen to test the combinatorial treatments on several
ER-positive breast cancer cell lines. Cell viability assays of
MCF7, ZR75.1 and T47D cells were then carried out with PIs and
OH-tamoxifen. For all cell lines, the antiproliferative activity of
the combined agents was significantly higher than that of the
single treatments (Fig. 1C and D).
Next, we compared the effects of the combination on colony
formation in soft agar. Treatments with MG132 (Fig. 1E) or bortezomib (Fig. 1F) in combination with OH-tamoxifen
were found to drastically decreased cell colony formation as
compared to single treatment. This confirmed, in an
anchorage-independent condition, the synergistic activity of PIs
and antiestrogens as shown by cell viability assays. One can also
note that the importance of the combination also lies in using
minimized concentrations of active compounds to obtain a maximal
inhibition.
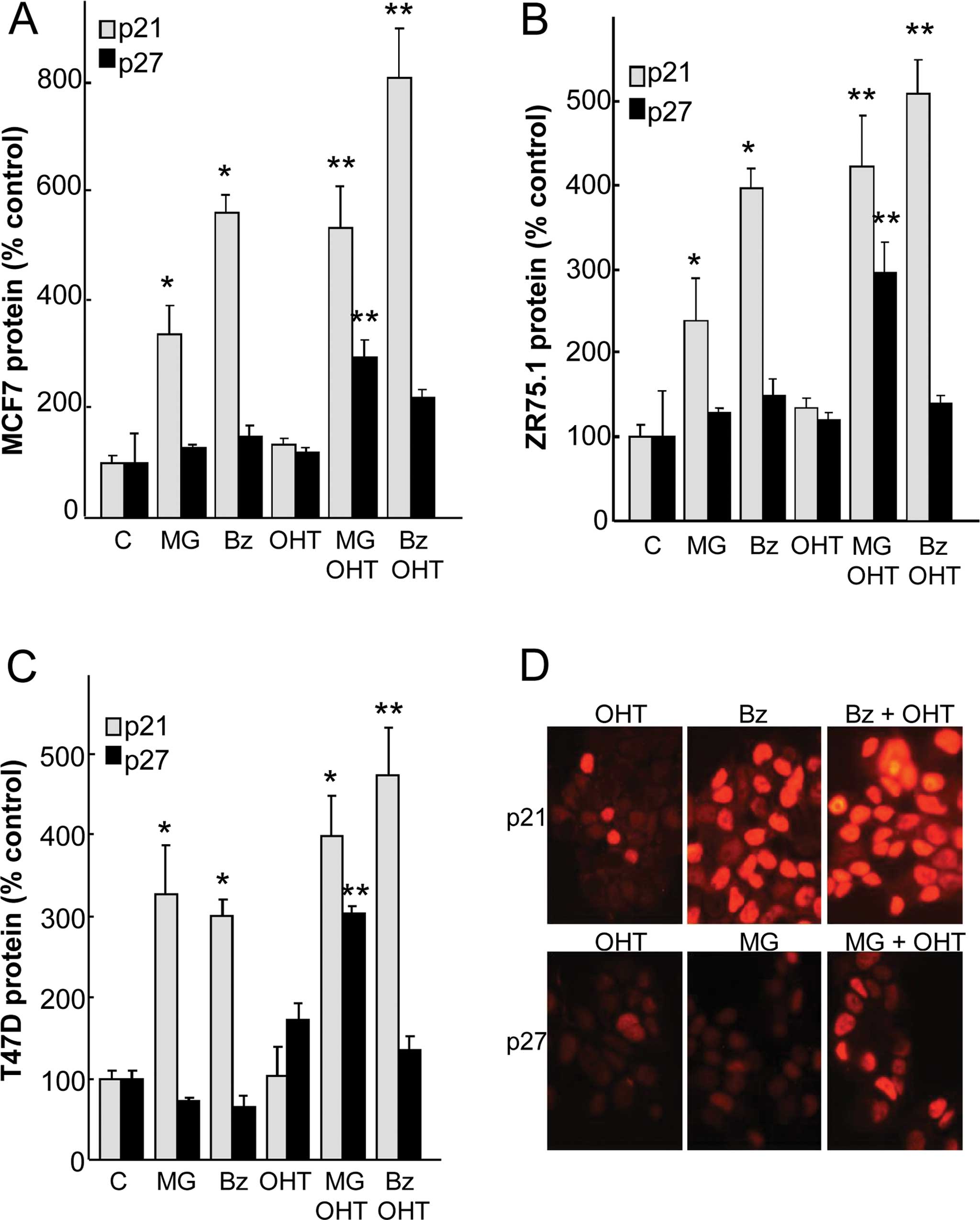
As PIs and OH-tamoxifen are known to regulate cell
growth by controling cell cycle proteins, we investigated their
effects on p27 and p21 expression in three ER-positive cell lines
(Fig. 2A–C). A 24-h treatment with
0.3 µM MG132 or 30 nM bortezomib significantly increased p21
levels in all cell lines in a range of 2.4- to 5.5-fold.
Combination with OH-tamoxifen with each PI significantly raised p21
levels over the values of the single treatments. These effects
correspond to a synergistic action of OH-tamoxifen and PI. In
contrast to p21, the regulation of p27 appeared different, since
the expression of this CKI was not significantly affected by
treatments except for OH-tamoxifen plus MG132, which significantly
increased p27 in all cell lines. Taken together, these data suggest
a synergism in the action of antiestrogens and PIs on p21 and p27
expression.
As previous data indicate that CKI overexpression
could be associated with cellular delocalization (30), we verified the nuclear localization
of these two CKIs by immunofluorescence (Fig. 2D). After treatments with PI and/or
OH-tamoxifen, the overexpression of p21 and p27 was found to be
mainly located in the nucleus in the MCF7 cells.
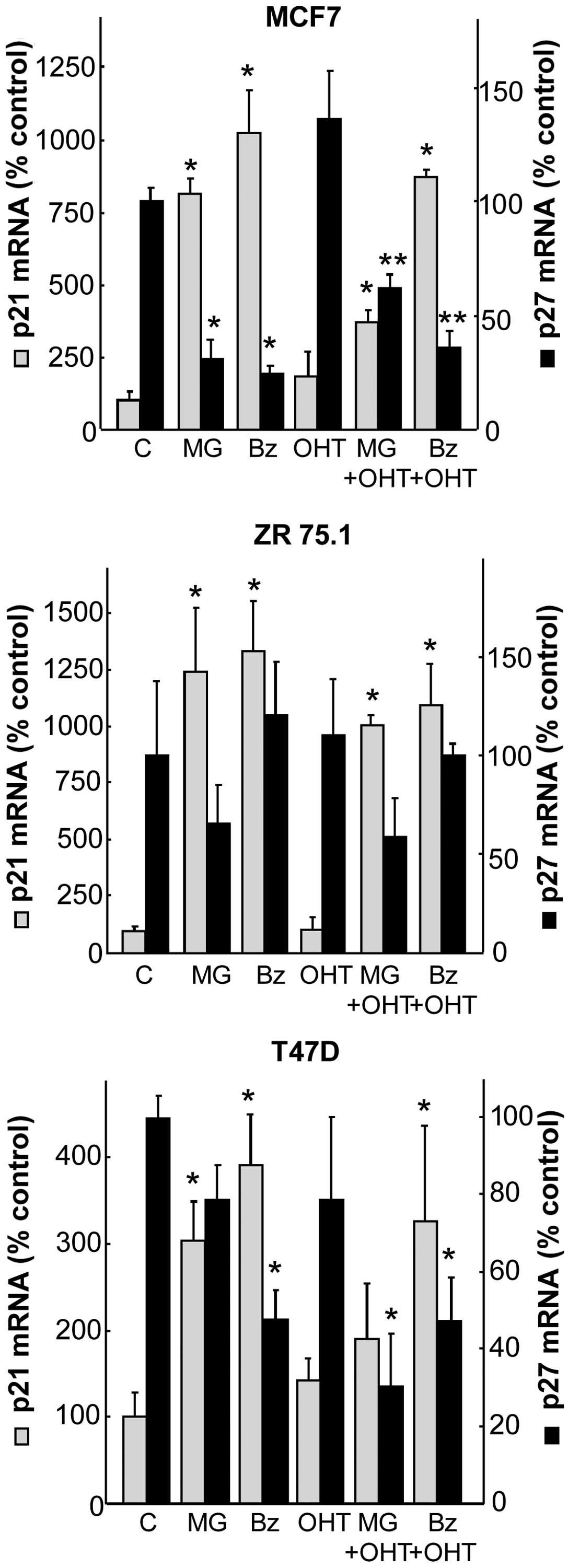
In order to explore the potential mechanism that
increases CKIs, we semi-quantified mRNA levels of p21 and p27 using
qRT-PCR assays after a 24-h treatment of PIs combined or not with
antiestrogens. The two PIs increased p21 mRNA levels in all cell
lines, from 3- to 4-fold in the T47D cells, 8- to 10-fold in the
MCF7 cells and 12- to 13-fold in the ZR75.1 cells (Fig. 3). OH-tamoxifen treatment was not
significantly effective alone on p21 mRNA and did not exhibit
synergism when combined with PI. Considering p27, the results
showed that PI alone or PI in combination with OH-tamoxifen
downregulated p27 mRNA levels. These results indicate that p27
overexpression of CKIs is independent of its mRNA expression,
whereas p21 expression can be regulated at the mRNA level by
PIs.
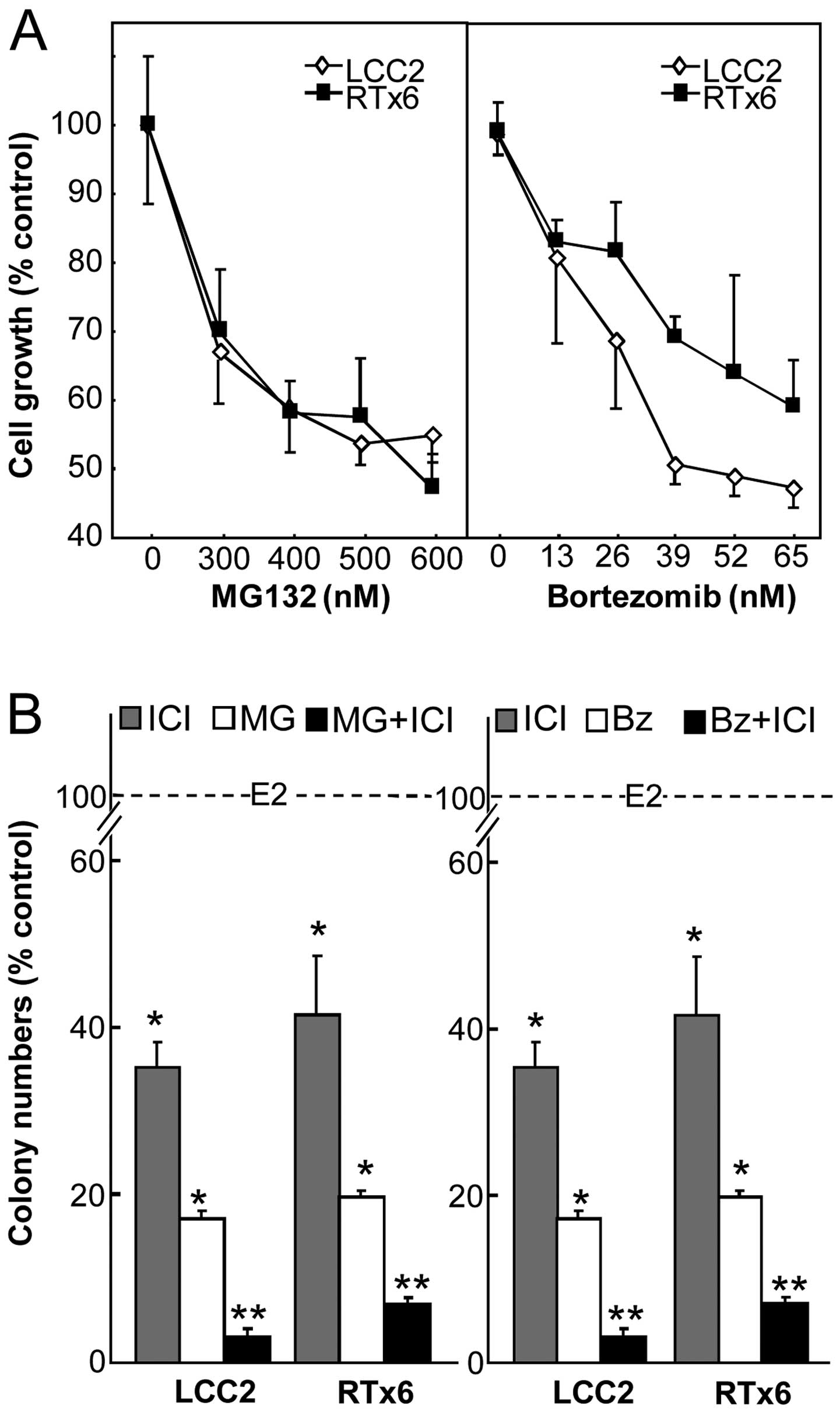
To gain further insight into the study of PIs in
combination with antiestrogens, we investigated the effects of PIs
on tamoxifen- and OH-tamoxifen resistant cells. For this purpose,
the MCF7 sublines lCC2 and RTx6 respectively selected for their
resistance to OH-tamoxifen and tamoxifen were analyzed. At first,
we tested their sensitivity to MG132 and bortezomib by cell growth
assays (Fig. 4A). The
dose-dependent growth inhibition of these cell lines appeared in
the same range after a 4-day treatment. The IC50 value
was 0.35 µM for MG132 and ~40 nM for bortezomib. When
compared under the same experimental conditions, the inhibition
obtained by 65 nM bortezomib was 51 and 40%, respectively, for LCC2
and RTx6 cells whereas the wild-type MCF7 had a 73% inhibition of
growth (21). Therefore, these cell
sublines were less sensitive to bortezomib than the original cell
line. We then analyzed the effects on colony formation in soft agar
with 0.1 µM ICI182,780 combined with PIs (0.3
µM MG132 or 26 nM bortezomib) (Fig. 4B). Treatments were found to
synergistically decrease cell colony formation as compared to
single agent treatment. This indicates the interest of a PI and an
antiestrogen combination even in resistant cell lines. Importantly,
we were surprised to find that the combination restored sensitivity
in the resistant cell lines close to that of the MCF7 parental cell
line.
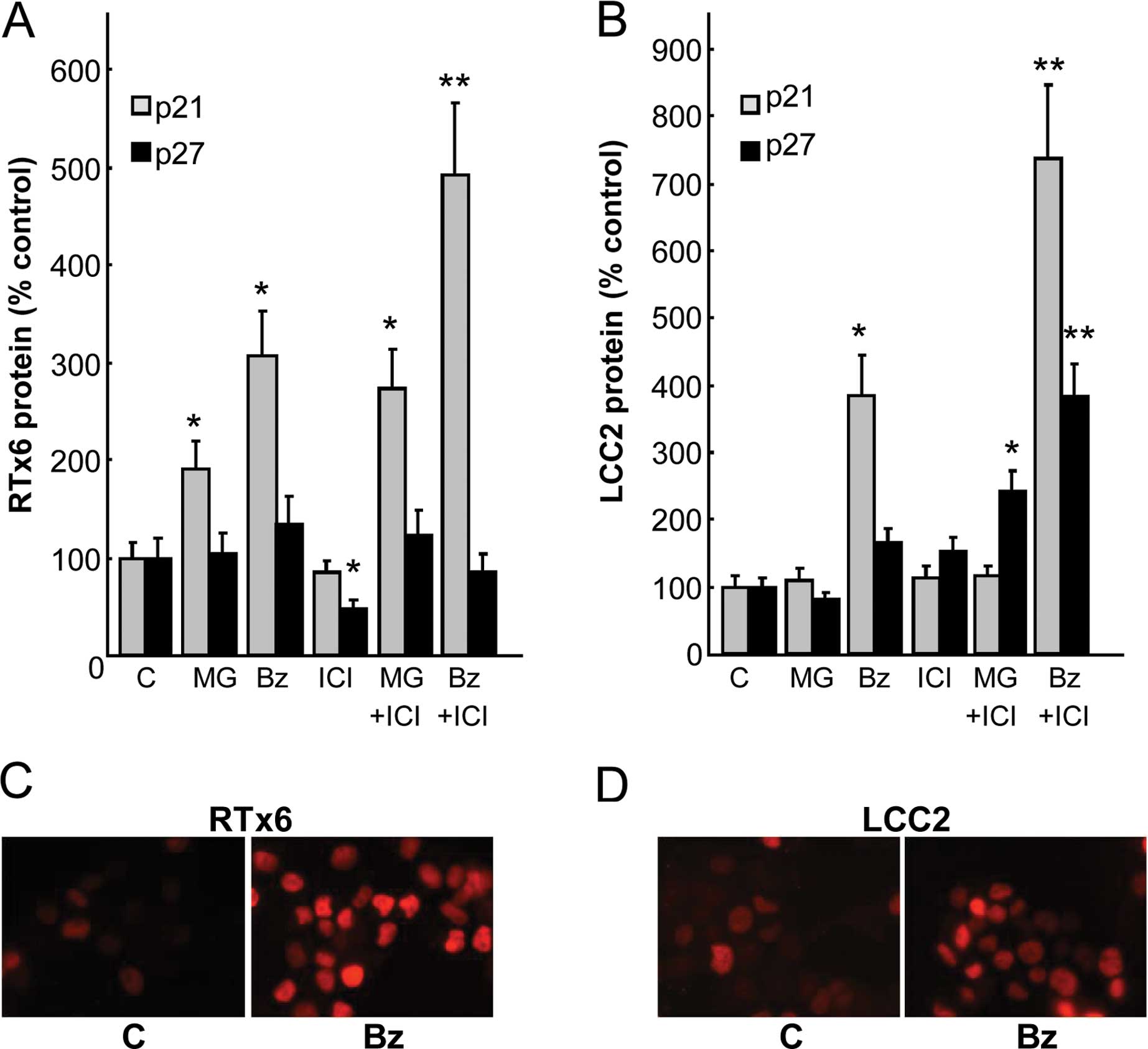
To gain further insight into the ability of
bortezomib to reverse resistance, p21 and p27 expression levels
were analyzed after a 24-h treatment (Fig. 5A and B). In the resistant cell
lines, the PI-induced overexpression of p21 was also noted,
although the increase was inferior to that observed in the parental
MCF7 cells. Notably, the addition of antiestrogens that are poorly
or not effective alone, synergized with bortezomib as in the
parental MCF7 cells. Concerning p27 expression, a synergistic
effect was observed with ICI182,780 but only in the RTx6
cell lysates. We also confirmed that overexpression of p21 occurs
in the nucleus of these cells by immunofluorescence (Fig. 5C and D). The present study also
confirmed that combined therapy could also be utilized on targeting
resistant breast cancers.
Discussion
Since some PIs have been approved by the US Food and
Drug Administration (FDA) for primary treatment of multiple
myeloma, they have emerged as an important therapeutic strategy in
hematologic malignancies and solid tumors. To date, primary
treatment with bortezomib alone in patients with advanced
metastatic breast cancers have shown no positive responses
(31–33). However, bortezomib may be successful
for the treatment of breast cancer patients in combination with
other therapies. Combination effects of bortezomib with
chemotherapeutic agents, such as taxanes, anthracyclins or
antibodies have already demonstrated significant positive responses
in phase II trials (34–36).
In the present study, the combined treatments of
antiestrogens and PIs were found to synergistically inhibit the
growth of three ER-positive breast cancer cell lines. Furthermore,
these combinations were also active on the growth of two
antiestrogenresistant cell lines. This later result is in agreement
with data from Periyasamy-Thandavan et al, who demonstrated
that bortezomib prevented cell survival of an OHT-resistant MCF7
cell line (37).
Notably, we found that, depending on the PI used,
the CKI recruitment was different. MG132 combination was associated
with a p27 accumulation in breast cancer cells while bortezomib
action was preferentially correlated with p21 overexpression. Such
difference in recruitment of CKIs has been reported for
ubiquitylation and proteasome-mediated degradation (38).
Furthermore, as an antiestrogen, bortezomib also
requires an intact ER expression. Indeed, although the specific
mechanisms of PI-induced growth arrest remain to be elucidated, we
showed that bortezomib efficacy on ER-positive cells relies on a
functional ER (21). Notably,
cancers with endocrine acquired resistance or estrogen-independency
are often associated with the expression of a functional ER
(5,39–42).
Therefore, our data suggest that ER-positive cells resistant to
classical endocrine therapies could be sensitive to PI action.
Collectively, PI and antiestrogen combination offers
a new approach to treat ER-positive breast cancer. As antiestrogen
efficacy in breast cancer treatment is limited by the frequent
development of acquired resistance, the current data provide a
rationale for further insight into the clinical evaluation of
PI-combined therapies.
Acknowledgments
We would like to thank M. Gleizes for the technical
skills. The present study was supported in part by grants of the
'Ligue Régionale de lutte contre le Cancer', the 'Association pour
la Recherche contre le Cancer' (SFI2010-1201906) and BQR Grants
from the University of Montpellier 1.
Abbreviations:
|
Bz
|
bortezomib
|
|
CKI
|
cyclin-dependent kinase inhibitor
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethylsulfoxide
|
|
E2
|
17β-estradiol
|
|
ER
|
estrogen receptor α
|
|
FBS
|
fetal bovine serum
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
OHT
|
4-hydroxytamoxifen
|
|
p21
|
p21WAF1
|
|
p27
|
p27KIP1
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
proteasome inhibitor
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
Ralox
|
raloxifen
|
|
Tam
|
tamoxifen
|
References
|
1
|
Henderson BE, Ross R and Bernstein L:
Estrogens as a cause of human cancer: The Richard and Hinda
Rosenthal Foundation award lecture. Cancer Res. 48:246–253.
1988.PubMed/NCBI
|
|
2
|
Couse JF and Korach KS: Estrogen receptor
null mice: What have we learned and where will they lead us? Endocr
Rev. 20:358–417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McDonnell DP, Connor CE, Wijayaratne A,
Chang CY and Norris JD: Definition of the molecular and cellular
mechanisms underlying the tissue-selective agonist/antagonist
activities of selective estrogen receptor modulators. Recent Prog
Horm Res. 57:295–316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeda H, Taira N, Nogami T, Shien K, Okada
M, Shien T, Doihara H and Miyoshi S: Combination treatment with
fulvestrant and various cytotoxic agents (doxorubicin, paclitaxel,
docetaxel, vinorelbine, and 5-fluorouracil) has a synergistic
effect in estrogen receptor-positive breast cancer. Cancer Sci.
102:2038–2042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cariou S, Donovan JC, Flanagan WM, Milic
A, Bhattacharya N and Slingerland JM: Down-regulation of
p21WAF1/CIP1 or p27Kip1 abrogates
antiestrogen-mediated cell cycle arrest in human breast cancer
cells. Proc Natl Acad Sci USA. 97:9042–9046. 2000. View Article : Google Scholar
|
|
6
|
Maynadier M, Ramirez JM, Cathiard AM,
Platet N, Gras D, Gleizes M, Sheikh MS, Nirde P and Garcia M:
Unliganded estrogen receptor alpha inhibits breast cancer cell
growth through interaction with a cyclin-dependent kinase inhibitor
(p21WAF1). FASEB J. 22:671–681. 2008. View Article : Google Scholar
|
|
7
|
Planas-Silva MD and Weinberg RA:
Estrogen-dependent cyclin E-cdk2 activation through p21
redistribution. Mol Cell Biol. 17:4059–4069. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varshochi R, Halim F, Sunters A, Alao JP,
Madureira PA, Hart SM, Ali S, Vigushin DM, Coombes RC and Lam EW:
ICI182,780 induces p21WAF1 gene transcription through
releasing histone deacetylase 1 and estrogen receptor alpha from
Sp1 sites to induce cell cycle arrest in MCF-7 breast cancer cell
line. J Biol Chem. 280:3185–3196. 2005. View Article : Google Scholar
|
|
9
|
Catzavelos C, Bhattacharya N, Ung YC,
Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I,
Kapusta L, et al: Decreased levels of the cell-cycle inhibitor
p27Kip1 protein: Prognostic implications in primary
breast cancer. Nat Med. 3:227–230. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan P, Cady B, Wanner M, Worland P, Cukor
B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M and Loda M: The
cell cycle inhibitor p27 is an independent prognostic marker in
small (T1a,b) invasive breast carcinomas. Cancer Res.
57:1259–1263. 1997.PubMed/NCBI
|
|
11
|
Wakasugi E, Kobayashi T, Tamaki Y, Ito Y,
Miyashiro I, Komoike Y, Takeda T, Shin E, Takatsuka Y, Kikkawa N,
et al: p21(Waf1/Cip1) and p53 protein expression in breast cancer.
Am J Clin Pathol. 107:684–691. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tiezzi DG, Andrade JM, Ribeiro-Silva A,
Zola FE, Marana HR and Tiezzi MG: HER-2, p53, p21 and hormonal
receptors proteins expression as predictive factors of response and
prognosis in locally advanced breast cancer treated with
neoadjuvant docetaxel plus epirubicin combination. BMC Cancer.
7:362007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sáez A, Sánchez E, Sánchez-Beato M, Cruz
MA, Chacón I, Muñoz E, Camacho FI, Martínez-Montero JC, Mollejo M,
García JF, et al: p27Kip1 is abnormally expressed in
diffuse large B-cell lymphomas and is associated with an adverse
clinical outcome. Br J Cancer. 80:1427–1434. 1999. View Article : Google Scholar
|
|
14
|
Tsihlias J, Kapusta L and Slingerland J:
The prognostic significance of altered cyclin-dependent kinase
inhibitors in human cancer. Annu Rev Med. 50:401–423. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: A novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
16
|
Uddin S, Ahmed M, Bavi P, El-Sayed R,
Al-Sanea N, AbdulJabbar A, Ashari LH, Alhomoud S, Al-Dayel F,
Hussain AR, et al: Bortezomib (Velcade) induces p27Kip1
expression through S-phase kinase protein 2 degradation in
colorectal cancer. Cancer Res. 68:3379–3388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dulić V, Stein GH, Far DF and Reed SI:
Nuclear accumulation of p21Cip1 at the onset of mitosis:
A role at the G2/M-phase transition. Mol Cell Biol.
18:546–557. 1998. View Article : Google Scholar
|
|
18
|
Hideshima T, Richardson P, Chauhan D,
Palombella VJ, Elliott PJ, Adams J and Anderson KC: The proteasome
inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes
drug resistance in human multiple myeloma cells. Cancer Res.
61:3071–3076. 2001.PubMed/NCBI
|
|
19
|
Ludwig H, Khayat D, Giaccone G and Facon
T: Proteasome inhibition and its clinical prospects in the
treatment of hematologic and solid malignancies. Cancer.
104:1794–1807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F and Harrison LE:
Ciglitazone-induced cellular anti-proliferation increases
p27Kip1 protein levels through both increased
transcriptional activity and inhibition of proteasome degradation.
Cell Signal. 17:809–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maynadier M, Shi J, Vaillant O, Gary-Bobo
M, Basile I, Gleizes M, Cathiard AM, Wah JL, Sheikh MS and Garcia
M: Roles of estrogen receptor and p21Waf1 in
bortezomib-induced growth inhibition in human breast cancer cells.
Mol Cancer Res. 10:1473–1481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richardson PG, Barlogie B, Berenson J,
Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina
M, Alexanian R, et al: A phase 2 study of bortezomib in relapsed,
refractory myeloma. N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orlowski RZ, Stinchcombe TE, Mitchell BS,
Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ,
Pien CS, et al: Phase I trial of the proteasome inhibitor PS-341 in
patients with refractory hematologic malignancies. J Clin Oncol.
20:4420–4427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Powers GL, Ellison-Zelski SJ, Casa AJ, Lee
AV and Alarid ET: Proteasome inhibition represses ERalpha gene
expression in ER+ cells: A new link between proteasome
activity and estrogen signaling in breast cancer. Oncogene.
29:1509–1518. 2010. View Article : Google Scholar
|
|
25
|
Brünner N, Frandsen TL, Holst-Hansen C,
Bei M, Thompson EW, Wakeling AE, Lippman ME and Clarke R:
MCF7/LCC2: A 4-hydroxytamoxifen resistant human breast cancer
variant that retains sensitivity to the steroidal antiestrogen ICI
182,780. Cancer Res. 53:3229–3232. 1993.PubMed/NCBI
|
|
26
|
Faye JC, Jozan S, Redeuilh G, Baulieu EE
and Bayard F: Physicochemical and genetic evidence for specific
antiestrogen binding sites. Proc Natl Acad Sci USA. 80:3158–3162.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gary-Bobo M, Hocine O, Brevet D, Maynadier
M, Raehm L, Richeter S, Charasson V, Loock B, Morère A, Maillard P,
et al: Cancer therapy improvement with mesoporous silica
nanoparticles combining targeting, drug delivery and PDT. Int J
Pharm. 423:509–515. 2012. View Article : Google Scholar
|
|
28
|
Prud'homme GJ, Glinka Y, Toulina A, Ace O,
Subramaniam V and Jothy S: Breast cancer stem-like cells are
inhibited by a non-toxic aryl hydrocarbon receptor agonist. PloS
One. 5:e138312010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maynadier M, Chambon M, Basile I, Gleizes
M, Nirde P, Gary-Bobo M and Garcia M: Estrogens promote cell-cell
adhesion of normal and malignant mammary cells through increased
desmosome formation. Mol Cell Endocrinol. 364:126–133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pérez-Tenorio G, Berglund F, Esguerra
Merca A, Nordenskjöld B, Rutqvist LE, Skoog L and Stål O:
Cytoplasmic p21WAF1/CIP1 correlates with Akt activation
and poor response to tamoxifen in breast cancer. Int J Oncol.
28:1031–1042. 2006.
|
|
31
|
Shah MH, Young D, Kindler HL, Webb I,
Kleiber B, Wright J and Grever M: Phase II study of the proteasome
inhibitor bortezomib (PS-341) in patients with metastatic
neuroendocrine tumors. Clin Cancer Res. 10:6111–6118. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papandreou CN, Daliani DD, Nix D, Yang H,
Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, et al:
Phase I trial of the proteasome inhibitor bortezomib in patients
with advanced solid tumors with observations in
androgen-independent prostate cancer. J Clin Oncol. 22:2108–2121.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blaney SM, Bernstein M, Neville K,
Ginsberg J, Kitchen B, Horton T, Berg SL, Krailo M and Adamson PC:
Phase I study of the proteasome inhibitor bortezomib in pediatric
patients with refractory solid tumors: A Children's Oncology Group
study (ADVL0015). J Clin Oncol. 22:4804–4809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Awada A, Albanell J, Canney PA, Dirix LY,
Gil T, Cardoso F, Gascon P, Piccart MJ and Baselga J:
Bortezomib/docetaxel combination therapy in patients with
anthracycline-pretreated advanced/metastatic breast cancer: A phase
I/II dose-escalation study. Br J Cancer. 98:1500–1507. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JE, Yoon DH, Jang G, Lee DH, Kim S,
Park CS, Huh J, Kim WS, Park J, Lee JH, et al: A phase I/II study
of bortezomib plus CHOP every 2 weeks (CHOP-14) in patients with
advanced-stage diffuse large B-cell lymphomas. Korean J Hematol.
47:53–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Houot R, Le Gouill S, Ojeda Uribe M,
Mounier C, Courby S, Dartigeas C, Bouabdallah K, Alexis Vigier M,
Moles MP, Tournilhac O, et al French GOELAMS group: Combination of
rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil
(RiPAD+C) as first-line therapy for elderly mantle cell lymphoma
patients: Results of a phase II trial from the GOELAMS. Ann Oncol.
23:1555–1561. 2012. View Article : Google Scholar
|
|
37
|
Periyasamy-Thandavan S, Jackson WH,
Samaddar JS, Erickson B, Barrett JR, Raney L, Gopal E, Ganapathy V,
Hill WD, Bhalla KN, et al: Bortezomib blocks the catabolic process
of autophagy via a cathepsin-dependent mechanism, affects
endoplasmic reticulum stress and induces caspase-dependent cell
death in antiestrogen-sensitive and resistant ER+ breast
cancer cells. Autophagy. 6:19–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu Z and Hunter T: Ubiquitylation and
proteasomal degradation of the p21Cip1,
p27Kip1 and p57Kip2 CDK inhibitors. Cell
Cycle. 9:2342–2352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Yau C, Gray JW, Chew K, Dairkee
SH, Moore DH, Eppenberger U, Eppenberger-Castori S and Benz CC:
Enhanced NF kappa B and AP-1 transcriptional activity associated
with antiestrogen resistant breast cancer. BMC Cancer. 7:592007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Regan RM, Osipo C, Ariazi E, Lee ES,
Meeke K, Morris C, Bertucci A, Sarker MA, Grigg R and Jordan VC:
Development and therapeutic options for the treatment of
raloxifene-stimulated breast cancer in athymic mice. Clin Cancer
Res. 12:2255–2263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fox EM, Arteaga CL and Miller TW:
Abrogating endocrine resistance by targeting ERα and PI3K in breast
cancer. Front Oncol. 2:1452012. View Article : Google Scholar
|
|
42
|
Shaw LE, Sadler AJ, Pugazhendhi D and
Darbre PD: Changes in oestrogen receptor-alpha and -beta during
progression to acquired resistance to tamoxifen and fulvestrant
(Faslodex, ICI 182,780) in MCF7 human breast cancer cells. J
Steroid Biochem Mol Biol. 99:19–32. 2006. View Article : Google Scholar : PubMed/NCBI
|