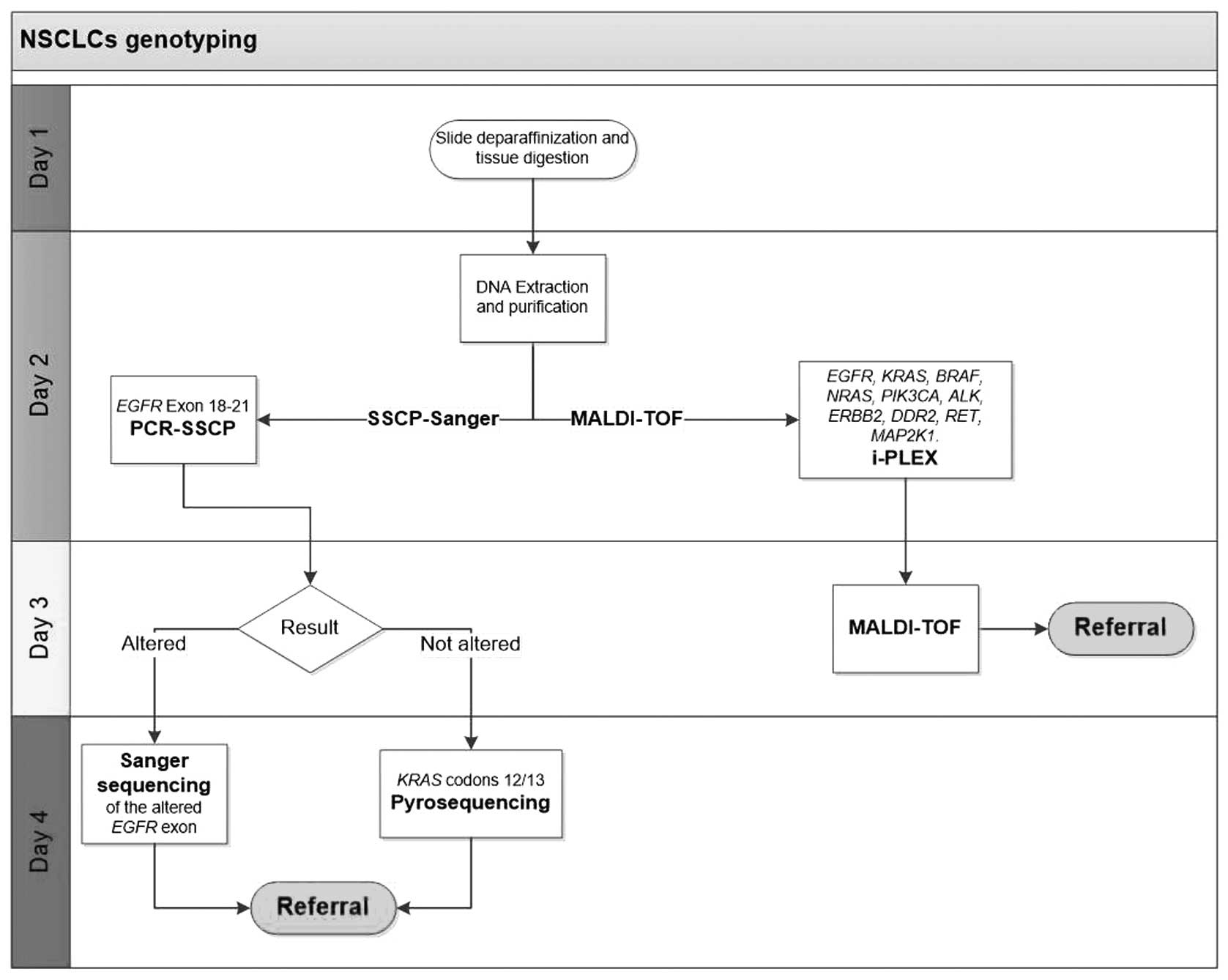
|
1
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al Spanish Lung Cancer Group: Screening for epidermal growth
factor receptor mutations in lung cancer. N Engl J Med.
361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber B, Hager H, Sorensen BS, McCulloch
T, Mellemgaard A, Khalil AA, Nexo E and Meldgaard P: EGFR mutation
frequency and effectiveness of erlotinib: A prospective
observational study in Danish patients with non-small cell lung
cancer. Lung Cancer. 83:224–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krawczyk P, Ramlau R, Chorostowska-Wynimko
J, Powrózek T, Lewandowska MA, Limon J, Wasąg B, Pankowski J,
Kozielski J, Kalinka-Warzocha E, et al: The efficacy of EGFR gene
mutation testing in various samples from non-small cell lung cancer
patients: A multicenter retrospective study. J Cancer Res Clin
Oncol. 141:61–68. 2015. View Article : Google Scholar :
|
|
4
|
Scarpino S, Pulcini F, Di Napoli A,
Giubettini M and Ruco L: EGFR mutation testing in pulmonary
adenocarcinoma: Evaluation of tumor cell number and tumor percent
in paraffin sections of 120 small biopsies. Lung Cancer. 87:8–13.
2015. View Article : Google Scholar
|
|
5
|
Sherwood JL, Müller S, Orr MC, Ratcliffe
MJ and Walker J: Panel based MALDI-TOF tumour profiling is a
sensitive method for detecting mutations in clinical non small cell
lung cancer tumour. PLoS One. 9:e1005662014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
da Cunha Santos G, Saieg MA, Geddie W and
Leighl N: EGFR gene status in cytological samples of nonsmall cell
lung carcinoma: Controversies and opportunities. Cancer Cytopathol.
119:80–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eberhard DA, Giaccone G and Johnson BE;
Non-Small-Cell Lung Cancer Working Group: Biomarkers of response to
epidermal growth factor receptor inhibitors in Non-Small-Cell Lung
Cancer Working Group: Standardization for use in the clinical trial
setting. J Clin Oncol. 26:983–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aisner DL, Deshpande C, Baloch Z, Watt CD,
Litzky LA, Malhotra B, Sepulveda AR, Langer C, Evans T and Van
Deerlin VM: Evaluation of EGFR mutation status in cytology
specimens: An institutional experience. Diagn Cytopathol.
41:316–323. 2013. View
Article : Google Scholar
|
|
9
|
Aisner DL and Marshall CB: Molecular
pathology of non-small cell lung cancer: A practical guide. Am J
Clin Pathol. 138:332–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Helland Å, Skaug HM, Kleinberg L, Iversen
ML, Rud AK, Fleischer T, Sagerup C, Solberg S, Jørgensen L,
Ariansen S, et al: EGFR gene alterations in a Norwegian cohort of
lung cancer patients selected for surgery. J Thorac Oncol.
6:947–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ludovini V, Bianconi F, Pistola L, Pistola
V, Chiari R, Colella R, Bellezza G, Tofanetti FR, Siggillino A,
Baldelli E, et al: Optimization of patient selection for EGFR-TKIs
in advanced non-small cell lung cancer by combined analysis of
KRAS, PIK3CA, MET, and non-sensitizing EGFR mutations. Cancer
Chemother Pharmacol. 69:1289–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rotella V, Fornaro L, Vasile E, Tibaldi C,
Boldrini L, Chella A, D'Incecco A, Cirigliano G, Chioni A, Lupi C,
et al: EGFR and K-Ras mutations in women with lung adenocarcinoma:
Implications for treatment strategy definition. J Exp Clin Cancer
Res. 33:772014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gahr S, Stoehr R, Geissinger E, Ficker JH,
Brueckl WM, Gschwendtner A, Gattenloehner S, Fuchs FS, Schulz C,
Rieker RJ, et al: EGFR mutational status in a large series of
Caucasian European NSCLC patients: Data from daily practice. Br J
Cancer. 109:1821–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchetti A, Martella C, Felicioni L,
Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli
F, Mezzetti A, et al: EGFR mutations in non-small-cell lung cancer:
Analysis of a large series of cases and development of a rapid and
sensitive method for diagnostic screening with potential
implications on pharmacologic treatment. J Clin Oncol. 23:857–865.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smits AJ, Kummer JA, Hinrichs JW, Herder
GJ, Scheidel-Jacobse KC, Jiwa NM, Ruijter TE, Nooijen PT,
Looijen-Salamon MG, Ligtenberg MJ, et al: EGFR and KRAS mutations
in lung carcinomas in the Dutch population: Increased EGFR mutation
frequency in malignant pleural effusion of lung adenocarcinoma.
Cell Oncol (Dordr). 35:189–196. 2012. View Article : Google Scholar
|
|
16
|
Locatelli-Sanchez M, Couraud S, Arpin D,
Riou R, Bringuier PP and Souquet PJ: Routine EGFR molecular
analysis in non-small-cell lung cancer patients is feasible: Exons
18–21 sequencing results of 753 patients and subsequent clinical
outcomes. Lung. 191:491–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Penzel R, Sers C, Chen Y,
Lehmann-Mühlenhoff U, Merkelbach-Bruse S, Jung A, Kirchner T,
Büttner R, Kreipe HH, Petersen I, et al: EGFR mutation detection in
NSCLC - assessment of diagnostic application and recommendations of
the German Panel for Mutation Testing in NSCLC. Virchows Arch.
458:95–98. 2011. View Article : Google Scholar
|
|
18
|
Miyamae Y, Shimizu K, Hirato J, Araki T,
Tanaka K, Ogawa H, Kakegawa S, Sugano M, Nakano T, Mitani Y, et al:
Significance of epidermal growth factor receptor gene mutations in
squamous cell lung carcinoma. Oncol Rep. 25:921–928.
2011.PubMed/NCBI
|
|
19
|
Perez-Moreno P, Brambilla E, Thomas R and
Soria JC: Squamous cell carcinoma of the lung: Molecular subtypes
and therapeutic opportunities. Clin Cancer Res. 18:2443–2451. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boch C, Kollmeier J, Roth A,
Stephan-Falkenau S, Misch D, Grüning W, Bauer TT and Mairinger T:
The frequency of EGFR and KRAS mutations in non-small cell lung
cancer (NSCLC): Routine screening data for central Europe from a
cohort study. BMJ Open. 3:42013. View Article : Google Scholar
|
|
21
|
Unni AM, Lockwood WW, Zejnullahu K,
Lee-Lin SQ and Varmus H: Evidence that synthetic lethality
underlies the mutual exclusivity of oncogenic KRAS and EGFR
mutations in lung adenocarcinoma. eLife. 4:e069072015. View Article : Google Scholar : PubMed/NCBI
|